Abstract
As the most abundant biopolymer in nature, cellulose has become a fascinating building block for the design of functional nanomaterials. Owing to the presence of numerous hydroxyl groups, cellulose provides a unique platform for the preparation of new materials via versatile chemical modifications. This critical review aims to present the advances about nanomaterials based on cellulose derivatives with the focus on cellulose esters within the last two decades, including the chemistry and application of these nanostructured materials. This review starts with the introduction on first fundamental aspects about diverse esterification techniques used up to now to modify cellulose. The in situ esterification for the isolation of nanocelluloses and diverse post esterification methods of nanocelluloses for the surface functionalization were highlighted in the following description. Various esterification strategies and further nanostructure constructions have been developed aiming to confer specific properties to cellulose esters, extending therefore their feasibility for highly sophisticated applications, which were summarized with respect to the categories of the introduced ester moieties. Thus, this review assembles and emphasizes the state-of-art knowledge of functional nanomaterials derived from diverse esterified cellulose compounds.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
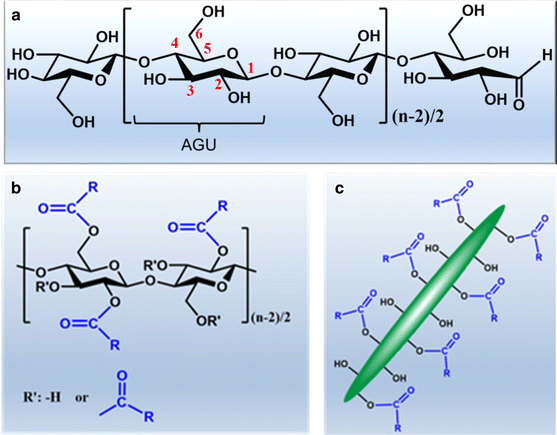
Cellulose is the most abundant, renewable, and sustainable biopolymer in the biosphere, representing around 1.5 × 1012 tons of the annual biomass production (Klemm et al. 1998b, 2005). It is present in diverse ecosystems, ranging from the kingdoms of plants, animals, algae, fungi, bacteria (Habibi 2014; Moon et al. 2011). The term cellulose was first recorded in 1839 by French chemist Anselme Payen, who first isolated cellulose from plants (Eyley and Thielemans 2014; Habibi et al. 2010; Roy et al. 2009). Since then, multiple physical and chemical aspects of cellulose have been extensively studied. The polymeric structure of cellulose was later determined by Staudinger in the 1920s (Eyley and Thielemans 2014; Klemm et al. 2011). He discovered that cellulose is a linear syndiotactic homopolymer composed of D-anhydroglucose units linked by β-(1 → 4)-glycosidic bonds (Fig. 1a) (Klemm et al. 1998b). Cellulose possesses several advantageous properties, such as excellent biocompatibility, nontoxicity, biodegradability and great mechanical strength (Klemm et al. 1998b; Qiu and Hu 2013). It was used as a precursor for mechanical/chemical modifications even before its polymeric nature was confirmed and well understood, such as synthesis of cellulose nitrates for the applications in various fields including plastics, lacquers, coatings, explosives and propellants (Heinze et al. 2006; Klemm et al. 1998a). Natural cellulose-based materials in the form of wood, hemp, and cotton have been used as engineering materials for thousands of years and the tradition still maintains today in the fields of forest products, paper, textiles, etc. (Klemm et al. 2005; Moon et al. 2011; Roman 2009). In recent years, environmental awareness has driven research in using and transforming more naturally occurring sustainable biomaterials, such as cellulose, hemicellulose and lignin from plant sources (Moon et al. 2011). Despite that the naturally occurring cellulose is already outstanding, it still lacks versatile properties, in order to chemically and dimensionally meet the demands of modern society for high performance materials. This point explains the continuing research interest focusing on dimensional transition to nanoscale and chemical modifications with desired functions, which can improve the given features and can be used to tailor advanced materials.
In nature, cellulose is preferentially biosynthesized as fibers via assembly of individual cellulose chains through both intra- and intermolecular hydrogen bonds (Habibi 2014; Somerville 2006). These hydrogen bonds give rise to various three-dimensional (3D) arrangements of the cellulose chains, leading to coexisting of crystalline and amorphous regions within cellulose fibers (John and Thomas 2008; Klemm et al. 2005; Moon et al. 2011; Nada and Hassan 2003). To be more specific in the case of cellulose from plant sources, approximately 36 cellulose chains arrange as a basic fibrillar unit known as elementary fibrils, which have a characteristic lateral dimension of 1.5–3.5 nm with the length up to 100 nm (Chinga-Carrasco 2011; Klemm et al. 2005; Krassig 1990; Yuan and Cheng 2015). These elementary fibrils are further assembled as microfibrils with widths in the range of 10–30 nm, which in turn further assemble into the familiar cellulose macrofibers. However, cellulose from different sources may exhibit different assembling morphologies (Williamson et al. 2002). According to these morphological features, cellulose fibers can be dissociated transversely at the amorphous regions leading to nanoscaled and highly crystalline rod-like fragments, which are referred to as cellulose nanocrystals (CNCs). Similarly, cellulose fibers also can be laterally disintegrated by applying high shear force resulting in nanofibrillated cellulose (NFC) (Habibi 2014). Nanocellulose can also be obtained as bacterial nanocellulose (BNC) after the biosynthesis by bacterial species, such as Gluconoacetobacter xylinum (Brown and Montezinos 1976).
With the presence of three hydroxyl groups per AGU within cellulose chains and on the surface of nanocelluloses, cellulose represents a unique platform for versatile chemical modifications to introduce required functional groups using various techniques to extend their use in a wide range of highly sophisticated applications. All three hydroxyl groups in the AGU including primary hydroxyl group at C6 and secondary hydroxyl groups at C2 and C3 (Fig. 1a) can participate in almost all the reactions as the alcoholic hydroxyl groups do, such as esterification, etherification, oxidation, silylation and polymer grafting (Braun and Dorgan 2009; Braun et al. 2012; Coseri et al. 2013; Dong and Roman 2007; Duan et al. 2016; Filpponen and Argyropoulos 2010; Habibi et al. 2010; Hasani et al. 2008; Ma et al. 2010; Mormann and Demeter 1999; Mormann and Wagner 1997; Pang et al. 2016; Qiu and Hu 2013; Xu et al. 2010; Yoo and Youngblood 2016). Among diverse chemical modifications, esterification represents one of the most promising technique, which was first adopted to synthesize cellulose derivatives (Klemm et al. 1998a). Over the past several decades, there has been extensive research in esterification of cellulose at both polymeric backbone and surface of nancelluloses (Fig. 1b, c). The fundamental aspects of the cellulose esterification, together with highlights of the recent advances about the functionalization of nanocelluloses are considered at first. Then, the potential applications of cellulose esters in the fields of nanomaterials are described. They are by no means a comprehensive summary of all the vast number of research results available, but only of selected pertinent aspects relating to the attached ester moieties primarily of the last two decades.
Esterification
During the esterification, the reaction either occurs on the whole cellulose polymer chains to form conventional cellulose esters or occurs at the outer of cellulose fibers leaving the cellulose crystalline structure in the interior intact. Both homogenous and heterogeneous esterification can be applied for the synthesis of a vast number of cellulose esters. Moreover, the reactions under heterogeneous conditions can be carried out almost exclusively for the surface modification of native cellulose, which also represents one of the main strategies for the isolation and chemical modification of nanocelluloses.
Fundamental aspects
Over the past several decades there has been extensive research in cellulose esterification. The cellulose esters are usually classified into inorganic and organic cellulose esters. Among the numerous inorganic acids known today, only a few have been employed to synthesize inorganic cellulose esters, such as cellulose nitrate, cellulose sulfate, cellulose phosphate and cellulose xanthate (Heinze et al. 2006, 2018).
Cellulose nitrate is by far the oldest and one of the most important inorganic cellulose esters, which have been produced on an industrial scale for more than one century (Klemm et al. 1998a). Cellulose nitrate is used in many application fields including plastics, lacquers, coatings, explosives and propellants (Heinze et al. 2006; Wertz et al. 2010). The industrial production of cellulose nitrate is generally based on the fast heterogenous equilibrium reaction between cellulose and the classical nitrating acid mixture containing nitric acid and sulfuric acid. The degree of substitution (DS) with rang from 1.8 to 2.8 can be controlled by adjusting the composition of the nitrating acid mixture to meet the various requirements (Klemm et al. 1998a). Using this technique, the maximum DS is limited to around 2.9 due to the side reaction of cellulose with sulfuric acid. Cellulose trinitrate can be achieved using nitrating agent systems of nitric acid/phosphoric acid/phosphorus pentoxide or nitric acid/acetic acid/acetic anhydride (Alexander and Mitchell 1949; Klemm et al. 1998a; Heinze et al. 2006). Furthermore, there are some other nitrating agent systems including dinitrogen pentoxide/tetrachloromethane, nitric acid aqueous and nitric acid/dichloromethane that can be used for the production of cellulose nitrates (Klemm et al. 1998a).
Cellulose sulfate is synthesized by the direct esterification of cellulose using sulfuric acid. Besides sulfuric acid, sulfur trioxide, chlorosulfonic acid, sulfuryl chloride, fluorosulfuric acid, ethyl chlorosulfonate and sulfoacetic acid were employed to produce cellulose sulfates (Klemm et al. 1998a). Cellulose sulfates generally can be prepared by three sulfation routes. The first is sulfation of hydroxyl groups from native cellulose. This usually occurs in a heterogeneous system, which results in non-uniformly distributed substitution, leading to poor solvability in water. To obtain uniformly distributed substitution, partially modified cellulose derivatives can be adopted as starting materials. Using this route, the primary substituent acts as a protecting group. During the sulfation under suitable conditions, the sulfating agents solely react with the free hydroxyl groups (Heinze et al. 2006; Zhang et al. 2010, 2011). Cellulose sulfates with a regioselective distribution of substituents have been synthesized via this rout by partial or complete displacement of a labeled group of a cellulose derivative, usually ester (e.g., nitrite) or ether (e.g., trimethylsilyl) (Fox et al. 2011; Klemm et al. 2005; Richter and Klemm 2003; Zhang et al. 2013). Moreover, the cellulose sulfates can also be synthesized by means of displacement of an ester or ether group already present in cellulose using sulfating agents. A wide variety of cellulose sulfates with regioselective substitution patterns also can be realized via this route (Klemm et al. 1998a; Fox et al. 2011).
The introduction of phosphoric acid ester moieties to form cellulose phosphates can be accomplished by means of pentavalent phosphorus reagents including phosphoryl chloride, phosphorus pentoxide and phosphoric acid (Illy et al. 2015). Similar to sulfation, phosphorylation of cellulose is usually carried out either by reaction with unmodified cellulose, or with cellulose derivatives containing specific substituents (Klemm et al. 1998a). Using the former route, the reaction usually occurs in a heterogeneous system or employs a cellulose solution in non-derivatizing solvent systems, such as N-methylmorpholine N-oxide, lithium chloride (LiCl)/dimethylacetamide (DMAc) and dinitrogen tetroxide/dimethylformamide (DMF) (Klemm et al. 1998a). In the latter route, a homogeneous system is generally preferred using completely or partially substituted cellulose esters or ethers in order to arrive at soluble products (Klemm et al. 1998a; Heinze et al. 2006). In comparison to sulfating agents, most of phosphorylating agents show a lower reactivity in esterification and lead to much less chain degradation. Moreover, cellulose phosphates tend to cross-linking due to the formation oligo-phosphate side chains, which impedes products solubility (Heinze et al. 2006).
Esterification of cellulose for the introduction of organic functional groups is among the most versatile transformations of chemical modifications of cellulose. It gives ready synthetic access to a wide range of valuable products. Esterification of cellulose is acylation procedure using carboxylic acids as acylating agents under strong-acid catalysis or by using an activated derivative such as an anhydride or acid chloride, either with base or with a Lewis acid (Heinze et al. 2006). Due to the low reactivity of carboxylic acids, it is not capable to esterify cellulose to a significant extent using the former esterification procedure. The most traditional method for the acylation of cellulose is the reaction with carboxylic acid anhydrides or acid chlorides.
A typical example of the cellulose carboxylate esters is cellulose acetate, which was described as the first organic cellulose ester more than 150 years ago (Klemm et al. 1998a). Cellulose acetate is commonly prepared by conversion of cellulose with a mixture of acetic acid and acetic anhydride in the presence of sulfuric acid as catalyst. During acylation reactions with carboxylic acid anhydrides under acidic catalysis, cellulose hydrolysis generally occurs simultaneously, which causes chain degradation (Heinze et al. 2006). To suppress the degradation, a tertiary base, such as pyridine and triethylamine, is recommended as the solvent medium as well as the acylation catalyst for the esterification with acid anhydrides (Heinze et al. 2006). Söyler et al. (2018) reported a rapid and efficient dissolution and activation of cellulose for the subsequent esterification with succinic anhydride in a CO2 based switchable solvent for only 30 min at room temperature (Fig. 2a). Cellulose was successfully converted to cellulose succinates with DS ranging from 1.51 to 2.59, depending on the reaction conditions and the molar ratio of succinic anhydride. An alternative method for esterification of cellulose using acid anhydrides is the impeller technique. In this case, the carboxylic acids or their anhydrides are converted in situ to reactive mixtures of symmetric and mixed anhydrides using impeller reagents, such as chloroacetyl, methoxyacetyl, and trifluoroacetyl moieties (Heinze et al. 2006). Diverse cellulose triesters including triacetate, tripropanoate, and tributanoate are attainable using trifluoroacetic anhydride as impeller reagent (Iwata et al. 1997).
a Dissolution and activation of cellulose for the subsequent derivatization with succinic anhydride using CO2-based switchable solvent. Repinted from Söyler et al. (2018). Copyright 2018, Royal Society of Chemistry. b Schematic illustration of the esterification involving in situ activation of carboxylic acids with DMT-MM. Reprinted from Hasani and Westman (2007). Copyright 2007, Springer. c Transesterification of vinyl esters under the catalysis of NaOH or KOH in DMSO. Reproduced from Cao et al. (2013). Copyright 2013, American Chemical Society
It should be noted that the introduction of more complex carboxylic acid moieties including fatty acid moieties and aromatic groups, anhydrides are not reactive enough. In this case, acid chlorides in combination with a tertiary base, i.e. pyridine and triethylamine, are applied (Heinze et al. 2006). This procedure is widely used for the preparation of cellulose fatty acid esters with different lengths of aliphatic chains (Crepy et al. 2011; de Menezes et al. 2009; Granstrom et al. 2011; Kulomaa et al. 2015; Zhang et al. 2015a, b). In the case of the esterification in pyridine, pyridine not only acts as the solvent, but also acts as a catalyst via forming a reactive intermediate driving the reaction forward. Cellulose esters with aromatic groups are basically accessible via the same path, but the relating studies are still rare (Garces et al. 2003).
It should be noted that a few new synthesis pathways have been developed over the past years for more effective esterification to introduce new functional groups with more complex chemical structures. One of these synthetic approaches is the in situ activation for the conversion of cellulose with carboxylic acids (Heinze et al. 2006). These reactions are normally carried out in the mild reaction conditions, which avoids the common side reactions including pericyclic reactions, hydrolysis, and oxidation. During these reactions, the carboxylic acids are activated by a reagent, which leads to an intermediately formed highly reactive carboxylic acid derivative. The activation of carboxylic acids with p-toluenesulfonyl chloride (TosCl) (Heinze and Liebert 2001; Heinze et al. 2003; Shimizu et al. 1991; Tosh et al. 2000; Xu et al. 2011; Zheng et al. 2015) and N,N′-dicyclohexylcarbodiimide in combination with 4-pyrrolidinopyridine or 4-dimethylaminopyridine (Fujisawa et al. 2011; Samaranayake and Glasser 1993; Wang et al. 2014; Wu et al. 2004; Yue and Cowie 2002) are typical examples of this synthetic technique (Grabner et al. 2002; Heinze et al. 2018). Homogeneous esterification of cellulose was carried out via in situ activation with TosCl in DMAc/LiCl for the synthesis of 3-(hydroxyphenylphosphinyl)-prop-anoic acid esters of cellulose (Zheng et al. 2015). It was found that the DS range from 0.62 to 1.42 could be adjusted by changing the reaction conditions. While the high toxicity and presence of cellulose-degrading side reactions impeded the wide application of these activating agents. Hasani and Westman reported a new commercially available, non-toxic activating agent, namely 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methyl-morpholinium chloride (DMT-MM), for the esterification of cellulose via in situ activation (Fig. 2b) (Hasani and Westman 2007). The resulting cellulose ester has a low DS of 0.67 due to the low activation efficiency. Among others, N,N′-carbonyldiimidazole (CDI) is the most frequently used non-toxic activating agent for the in situ activation esterification of cellulose (Boufi et al. 2008; Heinze and Liebert 2001; Heinze et al. 2006; Liebert and Heinze 2005; Peng et al. 2016). In this case, the acylating agent is N-acylimidazol that readily reacts with cellulose for the synthesis of cellulsoe ester and regeneration of to imidazole (Heinze et al. 2018).
Moreover, transesterification under the presence of catalysts has been used for the formation of cellulose esters. The preparation of cellulose esters with long aliphatic chains via transesterification with methyl esters have been studied (Antova et al. 2004). In the new transesterification approach, the vinyl esters of the carboxylic acids are predominantly investigated (Cao et al. 2014; Cetin et al. 2009; Ding et al. 2017). Heinze et al. (2000) reported that cellulose could dissolve in dimethyl sulfoxide (DMSO)/tetrabutylammonium fluoride and reacted with vinyl esters homogeneously with or without catalyst. Cetin et al. (2009) demonstrated that CNCs could react with vinyl acetate in DMF under the catalysis of K2CO3, producing acetylated CNCs. However, long pretreatment and/or reaction times from hours to days were required for most of the above-mentioned transesterification reactions, leading to relatively low DS of lower than 2 for many of them even under homogeneous conditions. Cao et al. (2013) developed a new reaction system composed of DMSO, aqueous NaOH or KOH, and vinyl esters to rapidly synthesize cellulose esters by transesterification (Fig. 2c). Remarkably, cellulose could react with vinyl acetate, vinyl propionate, and vinyl butyrate, leading to corresponding cellulose acetate, cellulose propionate, and cellulose butyrate with a high DS of higher than 2 in 5 min under heterogeneous conditions. The authors claimed that the fast reaction is due to the volatile acetaldehydes formed by tautomerization of the produced vinyl alcohol. This can effectively prevent the occurrence of the reverse reaction and, promote the formation of cellulose esters. This type of reaction with very short reaction time is in sharp contrast to the required reaction time of hours in previously existing methods. With the development of ionic liquids, transesterification is also applied in diverse ionic liquid systems heterogeneously for the modification of cellulose (Brand et al. 2017; Hufendiek et al. 2016; Söyler and Meier 2017; Schenzel et al. 2014; Wen et al. 2017).
Esterification of nanocelluloses
Table 1 lists main methodologies applied for the esterification of nanocelluloses along with the isolation process or post modification. Most esterification techniques are generally capable for the esterification of nanocelluloses. The main challenge is to conduct the reaction in such a way that it mainly esterifies hydroxyl groups on the surface of nanocelluloses, while maintaining the integrity of the crystalline cellulose structure in the interior (Habibi et al. 2010). Thus, the reaction should be carried out under mild heterogeneous conditions to avoid severe polymorphic conversion or disintegration of nanocelluloses.
During the esterification of nanocelluloses, the reaction either solely occurs on the surface of nanocelluloses or occurs inside crystal as bulk reaction, which highly depends on the esterification strategies and reaction conditions. Sassi and Chanzy studied the structural aspects of acetylation of cellulose using a mixture of acetic acid and acetic anhydride, and using toluene as non-swelling agent to stop swelling and dissolution of acetylated chains (Sassi and Chanzy 1995). With the presence of the toluene as non-swelling agent, acetylated chains remain insoluble and surrounded the crystalline core of unreacted cellulose chains, leading to great degrees of acetylation without imparting the morphological features. In contrast, without the presence of non-swelling agent, acetylated chains are stripped from the surface of the crystal into solution, leading to severe morphological change (Sassi and Chanzy 1995). Eyley and Thielemans applied a quantitative strategy using a term of surface degree of substitution with the maxium value of 1.5 to assess the level of modification carried out on CNCs (Eyley and Thielemans 2014). In contrast to CNCs, quantification of surface modification on NFC and BNC is more challenging using this mehod due to diverse of the crystalline structures and difficulty to measure the size of the naofibers precisely. Furthermore, the modification level can be, to some extent, qualitatively verified by examining the changes of crystallinity structure and morphology before and after the modification reactions. This review emphasizes more particularly on the diverse functional groups introduced on nanocelluloses via esterification routes in order to confer to specific properties. While, it will not be discussed specificity whether the reaction solely occurs on the surface of nanocelluloses.
In situ esterification during the isolation of nanocelluloses
The main in situ esterification reactions for the isolation of CNCs are sulfation and phosphorylation that occur during the hydrolysis process (Chen et al. 2014; Espinosa et al. 2013; Klemm et al. 2011; Lu et al. 2015b; Revol et al. 1994). During the isolation of CNCs via hydrolysis, sulfuric acid or phosphoric acid reacts with the surface hydroxyl groups via an esterification process allowing the introduction of anionic sulfate ester groups or phosphate ester groups. The sulfation and phosphorylation levels depend highly on diverse parameters including temperature, acid concentration, reaction time, and ratio of acid to cellulose. Compared with phosphorylation, sulfation results in a much higher content of sulfate groups on the surface of resulting CNCs (Espinosa et al. 2013).
The other example of in situ esterification is the production of surface modified CNCs via one-pot reaction methodology, which combines organic acid-catalyzed Fischer esterification and concurrent cellulose acid hydrolysis of amorphous cellulose chains (Fig. 3a) (Braun and Dorgan 2009; Braun et al. 2012; Sobkowicz et al. 2009; Spinella et al. 2016; Yu et al. 2016). For example, acetylated and butylated CNCs were synthesized using acetic or butyric acid that both serves as the reaction solvent and reagent for the esterification at 105 °C with the presence of hydrochloric acid as catalyst and hydrolyzing agent (Braun and Dorgan 2009). The resulting acetylated and butylated CNCs with size of 200–300 nm in length and about 20–50 nm in width are of similar dimensions compared to those obtained by hydrochloric acid hydrolysis alone. Meanwhile, via this one-pot technique using a catalytic quantity of hydrochloric acid and a bio-based organic acid, such as, citric, malic or malonic acid, various anionic carboxylated CNCs were extracted (Spinella et al. 2016). Thus, this one-pot reaction methodology is quite versatile because the organic acids used for the Fischer esterification can be selected to introduce the desired functionalities. However, it is still challenging to strictly control the esterification degrees. A high degree of esterification would result in significant depletion of the interchain hydrogen bonding network, which severely impact morphological and crystalline structure of CNCs. So, this approach is still not widely applied for the isolation and modification of CNCs.
a Reaction scheme illustrating the one-pot methodology for the production of acetylated CNCs using a mixture of acetic and hydrochloric acid. Reproduced from Braun and Dorgan (Braun and Dorgan 2009) Copyright 2009, American Chemical Society. b Schematic illustration for the fabrication of surface-stearoylated cellulose NPs from microcrystalline cellulose. Reprinted from Wang et al. (2015b) Copyright 2015, Wiley
This one-pot strategy also has been applied to produce surface-esterified NFC together with mechanical shearing. Herrick et al. (1983) presented a method for the isolation of acetylated NFC using a mixture of acetic acid and acetic anhydride with sulfuric acid as catalyst. Huang and coworkers reported a similar one-step procedure via mechanochemical strategy in an organic solvent aiming to esterify and defibrillate cellulose fibers simultaneously (Huang et al. 2012, 2013, Huang et al. 2016; Kang et al. 2017; Rao et al. 2015). The method consists of ball milling solid cellulose in a non-aqueous solvent loaded with an esterifying agent. The authors claim that the organic solvents and esterifying agents have dramatic effects on nanoscale dispersion and surface derivatization of NFC. Milling cellulose with hexanoyl chloride in DMF gave hexanoylated NFC with excellent dispersibility in several organic solvents according to the redispersing results in diverse solvents, and milling cellulose with pentafluorobenzoyl chloride in the mixture of pyridine and DMF resulted in hydrophobic fluorinated NFC (Huang et al. 2012, 2013; Rao et al. 2015). Water-dispersible succinylated CNF was also obtained by milling cellulose fiber with succinic anhydride in DMSO for 20 h (Huang et al. 2012, 2016). The produced CNFs are around 20 nm wide and several micrometers long.
Moreover, a new method leading to novel surface-esterified cellulose nanoparticles (NPs) after a one-step esterification of cellulose fibers under heterogeneous conditions was developed using fatty acid chlorides in pyridine and a follow-up purification process (Fig. 3b) (Wang et al. 2015b, 2017). The obtained surface-stearoylated cellulose NPs and surface-undecenoated cellulose NPs have sphere-like morphology with a relatively high size distribution from a few dozens to hundreds of nanometer. Both surface-esterified cellulose NPs have high DS of around 1.4, which would result in significant depletion of the interchain hydrogen bonds, leading to complete conversion of surface hydroxyl groups to esters. With the presence of numerous fatty acid ester groups on the surfaces, they were well dispersible in various non-polar organic solvents, such as, tetrahydrofuran, dichloromethane, cyclohexane, which significantly promoted their compatibility with non-polar compounds for the formation of functional composites.
Post esterification of nanocelluloses
Owing to its ease and straightforwardness, modification of hydroxyl groups present at the surface of nanocelluloses through esterification is widely used. Sulfation has been conducted to introduce stable electrostatic charges on the surface of nanocelluloses for more stable aqueous dispersions. In addition to the in situ sulfation during the isolation of nanocelluloses via sulfuric acid-catalyzed hydrolysis, CNCs produced by hydrochloric acid hydrolysis could also be post-sulfated using sulfuric acid to introduce sulfate moieties in a controlled fashion (Araki et al. 1999, 2000).
Nanocelluloses and functionalized nanocelluloses are excellent reinforcing components for the construction of materials with diverse shapes, such as films, fibers and aerogels (Eichhorn 2011; Klemm et al. 2011; Lam et al. 2012; Moon et al. 2011; Olsson et al. 2010; Walther et al. 2011). The dispersibility of nanocelluloses within the matrix and their interfacial interaction with other matrix components play pivotal roles for the final properties of the obtained nanocomposite materials (Fujisawa et al. 2013). The poor dispersibility of nanocelluloses in non-polar solvents and weak interactions with non-polar synthetic polymers are the main drawbacks limiting the full performance of nanocelluloses. In order to improve all these issues, nanocelluloses are generally surface-modified with functional groups, such as alkyl groups, synthetic polymer chains via “grafting to” or “grafting from” techniques (Fujisawa et al. 2011; Habibi et al. 2008; Johnson et al. 2011; Kan et al. 2013; Siqueira et al. 2009).
Surface-modified nanocelluloses by alkylacyl chains are supposed to be well miscible with other synthetic polymers and exist as reinforcing nanofillers in diverse materials, including films and foams (Blaker et al. 2009; Fujisawa et al. 2011; Habibi et al. 2010; Johnson et al. 2011; Siqueira et al. 2009). Generally, a post esterification of hydroxyl groups on nanocelluloses surface has been used for the immobilization of alkylacyl groups on nanocelluloses surface. Among diverse post esterification reactions for the introduction of alkyl groups, acetylation of nanocelluloses is the most widely investigated approach. The acetylation of nanocelluloses could be conducted using acetic anhydride in the presence of catalyst such as sulfuric acid, perchloric acid and pyridine. These procedures have been applied to produce surface-acetylated nanocelluloses using CNCs (Kim and Song 2016; Naeli et al. 2017; Sassi and Chanzy 1995; Yang et al. 2013), NFC (Fahma et al. 2014; Mashkour et al. 2015; Rodionova et al. 2011) and BNC (Kim et al. 2002; Tome et al. 2011). Furthermore, a novel straightforward route using citric acid as catalyst for the surface esterification of CNCs was proposed (Ramirez et al. 2017). Only the acetic anhydride was used in sufficient excess to allow CNCs dispersion and proper suspension agitation, while no additional solvent was required. By tuning the amount of loaded catalyst, surface-acetylated CNCs with different DS (i.e. DS = 0.18 and 0.34) were obtained. Under the moderate conditions at 120 °C for 3 h, only the surfaces of CNCs were esterified, while the initial crystalline structure of CNCs remained unaffected during the chemical treatment.
Furthermore, transesterification has been adopted in the modification of nanocelluloses using vinyl esters for the attachment of diverse acyl moieties including acetyl (Cetin et al. 2009; Sebe et al. 2013), cinnamoyl (Sebe et al. 2013) and maleyl (Yuwawech et al. 2017). The group of Gilles Sèbe studied the effect of reaction time on the acetylation of CNCs by transesterification of vinyl acetate in DMF at 94 °C using potassium carbonate as catalyst (Cetin et al. 2009). During the first stage of the reaction (less than 2 h), only the surface of the CNCs was modified, while their dimensions and crystallinity remained unchanged. By increasing the reaction time, the inner crystallites were increasingly attacked by the vinyl acetate, leading to an erosion of the CNCs structure and loss of crystallinity. But, the DS of the acetylated CNCs under these reactions has not been reported. Wei et al. (2017) esterified the CNCs successfully by a sustainable and green transesterification approach using vegetable oil fatty acid methyl ester for the first time. After transesterification, the degree of crystallinity and crystalline structure of nanocrystals were not changed, but the esterified CNCs showed higher thermal stability and smaller particle size than unmodified CNCs.
Esterification of nanocelluloses with acid chlorides usually resulted in significant bulk modification, leading to a severe loss of crystallinity and high degree of substitutions. The esterification of nanocelluloses with acid chlorides under vapor phase has also been achieved at 150 °C using vacuum to remove hydrochloric acid (Berlioz et al. 2009; Fumagalli et al. 2013a, b; Rodionova et al. 2013). CNCs were modified with palmitoyl chloride vapors through this gas-phase esterification process, and the palmitoylated CNCs showed the feasibility to form gels in toluene (Berlioz et al. 2009; Fumagalli et al. 2013a). By varying the process parameters including palmitoyl chloride quantity, reaction time and pressure, palmitoylated CNCs presenting DS ranging from 0.1 to nearly 2. The authors claimed that the esterification proceeds essentially from the surface to the core of the CNCs (Fig. 4a). Indeed, with the DS in the range of 0.3–0.8, palmitoylated CNCs still kept their integrity and the modification occurred only at their surface. For higher DS values, the palmitoylation progress had damaged the crystallinity of the sample because the remaining surface shells of highly modified cellulose chains easily soluble or swollen in non-polar solvent. Though this route improved significantly the compatibility of CNCs with non-polar substances for use in nanocomposites, the authors did not evaluate the effect of such extensive esterification and damage of crystalline structure on the mechanical properties and thermal stability of the resulting nanocrystals.
a Scheme of the progress of the gas-phase esterification of CNCs with palmitoyl chloride with (a) DS = 0, (b) DS = 0.25, (c) DS = 0.75, and (d) DS = 1.5. Reprinted from Berlioz et al. (2009) Copyright 2009, American Chemical Society. b SolReact strategy and chemical mechanism for the functionalization of CNCs with aromatic carboxylic acids. Reprinted from Espino-Perez et al. (2014) Copyright 2014, American Chemical Society
Peng et al. (2016) demonstrated a comparative study of various surface esterification methods of CNCs via acid anhydrides, acid chlorides, acid catalyzed carboxylic acids and in situ activated carboxylic acids to introduce acetyl-, hexanoyl-, dodecanoyl-, oleoyl-, and methacryloyl-functions. Acid anhydrides exhibited better grafting efficiency than other reagents as low molecular weight moieties with short aliphatic chains. In addition, utilizing in situ activated carboxylic acids was more viable approach for long aliphatic chain grafts. The preservation of structural morphology and crystallinity of grafted CNCs were confirmed using transmission electron microscopy and X-ray diffraction. The dispersibility of such surface-esterified CNCs in organic solvents was generally improved. In addition, the surface hydrophobization of CNCs by fatty acids, biodiesel, or plant oils was conducted via a green process using an organic solvent as a one-pot method (Yoo and Youngblood 2016).
An environmental friendly surface esterification route was presented by Yuan et al. (2006) using alkenyl succinic anhydride emulsions in water to compatibilize the CNCs with non-polar media. The emulsions were simply mixed with CNCs aqueous suspensions, freeze dried, and the resulting solid was heated to 105 °C. Due to the low DS of around 0.02, the obtained surface-esterified CNCs retained their morphological and crystalline integrity. They were also well dispersible solvents with widely different polarities, such as, DMSO with a very high dielectric constant of 46.45 and 1,4-dioxane with a quite low dielectric constant of 2.21. Another environmental friendly and simple approach, named SolReact, has been developed for a solvent-free esterification of CNCs using aromatic carboxylic acids (Fig. 4b) (Espino-Perez et al. 2014, 2016). In this process, the critical point is the use of an in situ solvent exchange strategy for utilizing the aromatic carboxylic acids as grafting agent as well as solvent media. Furthermore, the reactant can be easily recycled and does not suffer from chemical degradation due to moderate reaction temperatures.
Grafting of polymer chains on the surface of nanocelluloses can be achieved by esterification directly or indirectly via “grafting to” or “grafting from” approaches. The “grafting to” approach was used to graft maleated polypropylene by esterification onto the surface of CNCs in the suspension of toluene (Ljungberg et al. 2005). The resulting grafted CNCs showed very good compatibility and high adhesion when dispersed in atactic polypropylene. A similar approach was described by Mulyadi et al. who grafted maleated styrene block copolymers on the surface of NFC through esterification (Mulyadi and Deng 2016). The grafted polymer fraction of 25 wt% by gravimetric measurement was obtained. The presence of the grafted polymer promoted the surface hydrophobicity and better thermal stability. Furthermore, a significant number of surface esterification reactions were used as precursors for further polymerization on the surface of nanocelluloses via “grafting from” approach. For instance, 2-bromoisobutyryl bromide (BriBB) as the initiator agent was attached to the hydroxyl groups of nanocellulose by esterification for further polymerization (Majoinen et al. 2011; Wu et al. 2015; Yi et al. 2008). This strategy has been used extensively for the creation of initiating sites for the polymerization on the surface of nanocellulose. Moreover, Wang et al. (2016a) developed a multi-step approach using esterification as first step to attach bis(acyl)phosphane oxide photoinitiators on CNCs surface for polymer grafting.
Potential applications
Esterification represents one of the most versatile transformation strategy of cellulose as it provides easy access to a variety of functional cellulose-based materials. In the past few decades, the investigation and utilization of esterified cellulose compounds in functional nanomaterials have attracted a tremendous level of attention because of their exceptional properties. Various functional nanomaterials using esterified cellulose compounds have been developed for a broad range of applications, which include but not limited to sensors, mechanical reinforcement, biomedical materials and interfacial materials (e.g. superhydrophobic surfaces) (Dong et al. 2014; Geissler et al. 2013; Heinze et al. 2006; Mulyadi and Deng 2016; Sehaqui et al. 2014; Zhang et al. 2015b). The properties and potential applications mainly depend on the introduced ester moieties.
Esterified cellulose containing charged moieties
The charged ester moieties, such as sulfate ester groups, can be introduced in cellulose chains using inorganic acids. The cellulose sulfates show excellent rheological and gel-forming properties, allowing themselves for potential applications as film-forming materials, anionic polyelectrolytes, and biologically active compounds (Klemm et al. 2005; Klemm et al. 1998a). Thus, over the past few decades, cellulose sulfates have undergone intensive study (Kamide and Saito 1994; Mestechkina and Shcherbukhin 2010; Zhang et al. 2015c). Due to the presence of negative charged sulfate ester groups, cellulose sulfates exhibit unique biological properties (Zhang et al. 2015c), leading to a wide use in biotechnology and pharmaceutics to encapsulate enzymes and cells (Bucko et al. 2005; Vikartovska et al. 2007), as inhibitors for HIV viruses and anticoagulant effectors (Agarwal et al. 2010; Van Damme et al. 2008).
In combination with polycations, e.g., chitosan and poly(dimethyldiallyl-ammonium chloride) (PolyDADMAC), polyanionic cellulose sulfates can form polyelectrolyte catanionic complexes that possess huge potential for the encapsulation and immunoisolation of biological matters (Dautzenberg et al. 1999; Gericke et al. 2009a; Schaffellner et al. 2005; Stiegler et al. 2014; Zhu et al. 2010). For example, research showed that chitosan-cellulose sulfate complex films can be used as controlled drug release systems (Zhu et al. 2010). Cellulose sulfate/PolyDADMAC complexes showed diverse advantages including stable physiochemical properties, robust mechanical strength and biocompatibility (Zhang et al. 2015c). Gericke et al. demonstrated a typical encapsulation process with water-soluble cellulose sulfate and PolyDADMAC (Gericke et al. 2009a). The entrapped glucose oxidase within the resulting cellulose sulfate/PolyDADMAC capsules retained its activity. Gericke et al. (2009b) also synthesized water-insoluble and ionic liquid-soluble cellulose sulfate with a low DS of 0.16 for the formation of spherical capsules with PolyDADMAC (Fig. 5a). The stable spherical capsules with encapsulated glucose oxidase were obtained via a one-pot procedure. Similar polymeric capsules also prepared using cellulose sulfate and PolyDADMAC (Zeng et al. 2013). These polymeric capsules are of particular interest for microalgae and microorganisms cultivation. Moreover, cellulose sulfates possess osteogenic activity which exceeds that of heparin if derivatization takes place predominantly at the C6 position, with lower sulfation at the C2 position (Peschel et al. 2012). Thus, cellulose sulfates represent a highly effective alternative to heparin in tissue culture applications as component of scaffolds, able to bind, protect and control the release of growth factors. Highly sulfated celluloses enhanced cell growth remarkably without any additional growth factor at lower concentrations (Peschel et al. 2010).
a Polyelectrolyte complex spherical capsules prepared from cellulose sulfate and PolyDADMAC. Reprinted from Gericke et al. (2009b) Copyright 2009, American Chemical Society. b Flammability test of (b1) filter paper and (b2) flame-retardant nanopaper sheets prepared from phosphorylated NFC. Reprinted from Ghanadpour et al. (2015) Copyright 2015, American Chemical Society
Moreover, the negative charge of sulfated cellulose provides accessibility to electrostatic adsorption and conjunction with cationic groups or molecules (Huang et al. 2014). Horikawa et al. prepared a highly conductive poly(3,4-ethylene-dioxythiophene) (PEDOT) system using sulfated cellulose as dopants (Horikawa et al. 2015). PEDOT/sulfated cellulose composite films were prepared via in situ oxidative polymerization of 3,4-ethylene-dioxythiophene in an aqueous solution of sulfated cellulose, followed by the formation of films via spin-coating. It was confirmed that the electrical conductivity of PEDOT was enhanced by doping with sulfated cellulose. Novel oriented surfaces were prepared to promote skeletal muscle myogenesis (Dugan et al. 2013). The orientation was achieved by depositing a monolayer of negatively charged sulfated CNCs on a positively charged polyelectrolyte surface using a flexible and facile spin-coating method. Wang et al. (2016b) reported that sulfated BNC is a promising material for the preconcentration and separation of heavy metals. Furthermore, Thielemans et al. (2009) prepared nanostructured thin films of sulfated CNCs using a simple drop-coating procedure. The negatively charged sulfate groups inhibit the transfer of negatively charged species through the film, while the diffusion of neutral species is only slightly hindered. More specifically, the positively charged species including Ru(NH3) 3+6 was adsorbed by the film, whereas the negatively charged species, such as, IrCl63−, were excluded by the film.
The other charged ester group is phosphate ester group. The introduction of phosphate groups to cellulose chains via the formation of ester bonds significantly decreases the inflammability of cellulose. During combustion, phosphorus generates a polymeric form of phosphoric acid as a char layer, which acts as a shield protecting the material from oxygen (van der Veen and de Boer 2012). Thus, cellulose phosphates have potential to be used as flame-retardant materials (Aoki and Nishio 2010; Cullis et al. 1992; Ghanadpour et al. 2015; Pan et al. 2014). Ghanadpour et al. (2015) prepared thermal stable and flame-retardant nanopaper sheets using phosphorylated NFC (Fig. 5b). The resulting nanopaper sheets showed self-extinguishing properties after consecutive applications of a methane flame for 3 s and did not ignite under a heat flux of 35 kW/m2. By introducing the anionic phosphate groups into the cellulose backbone, cation-exchange properties are conveyed to the polymer chains, showing excellent chelating properties. Thus, cellulose phosphates were used as metal-chelating polymers, as cation exchange materials and as adsorbents for the treatment of pollution (Bezerra et al. 2014; Illy et al. 2015; Li et al. 2002; Oshima et al. 2008; Padilha et al. 1995). Furthermore, phosphorylated BNC was found to be effective as an adsorbent for proteins with a high adsorption capacity via electrostatic interaction (Oshima et al. 2011).
Moreover, cationic amino groups were introduced to cellulose backbone by ring-opening esterification reaction using various lactams (Zarth et al. 2011). The resulting cationic esters are capable of forming polyelectrolyte complexes as capsules for drug delivery. Cationic pyridinium groups were grafted onto CNCs via a one-pot simultaneous esterification using 4-(bromomethyl)benzoic acid or 4-(1-bromoethyl)benzoic acid and TosCl in pyridine (Vandamme et al. 2015). Resulting positively charged CNCs were relatively insensitive to the inhibition of flocculation by algal organic matter showing potential application for microalgae harvesting. Imidazole-grafted CNCs with a low DS of 0.06 were successfully synthesized by in situ esterification with 4-(1-bromo-methyl)benzoic acid activated by TosCl (Eyley et al. 2015). The resulting imidazole-grafted CNCs were shown to have a pH-responsive flocculation property due to switching of the surface charge, which can be adjusted using CO2.
Esterified cellulose containing aliphatic moieties
Cellulose acetate is the commercially most important cellulose ester due to its wide potential applications in fibers, plastics, films, membranes, and coatings (Bifari et al. 2016; Dias and de Pinho 1999; Hou et al. 2012; Kochkodan and Hilal 2015; Lu et al. 2015a; Qasim et al. 2015; Shibata 2004). Moreover, diverse cellulose acetate nanocomposites have potential applications as packaging, separation media, biomedical technologies and sensing (Bifari et al. 2016; Konwarh et al. 2013; Shibata 2004). For instance, Saha et al. (2016) prepared nanocomposite films using cellulose acetate, polyethylene glycol and cetyltrimethylammonium bromide modified montmorillonite. These cellulose acetate-based nanocomposites can be used as active packaging material due to their good antimicrobial activity as well as non-toxicity. Yliniemi et al. (2014) reported composite membranes by means of spin-coating technique containing cellulose acetate and poly(N,N-dimethylaminoethyl methacrylate) (PDMAEMA) for dissolution control of magnesium, which is critical for using magnesium as temporal medical implants. The dissolution control is achieved by the limited ion and H2 flow through the membranes, and the permeability of the membrane can be adjusted by altering the cellulose acetate/PDMAEMA ratio (Fig. 6a). A class of ultrafiltration membrane for separating proteins was developed by Nagendran and Mohan by comprising cellulose acetate and sulfonated poly(ether imide) (Nagendran and Mohan 2008). Wongsasulak et al. (2010) have proposed the use of cellulose acetate and egg albumin as an edible nanofibrous thin films, which could aid new functionalities regarding the in vivo controlled release of pharmaceuticals and nutraceuticals in the gastrointestinal tract. Kulterer et al. (2012) demonstrated an in situ nanoprecipitation technique for preparing composite NPs from cellulose acetate and hydrophilic polysaccharides including hydroxyethyl cellulose, carboxymethyl cellulose, low molecular weight chitosan and amino cellulose. The functional composite NPs exhibited great potential for the dispersion and delivery of hydrophobic substances in aqueous systems. A new system for the delivery of naproxen as nonsteroidal anti-inflammatory drug was developed by using electrospinning cellulose acetate nanofibers loaded with ester prodrugs (Wu et al. 2010b). The in vitro release experiment indicated that sustained drug release from nanofibers mats based on cellulose acetate was observed for a long duration of time. Glassy carbon electrode, fabricated after coverage with cellulose acetate and following modification with prussian blue, could be used as a novel hydrogen peroxide sensor (Wu et al. 2010a). Such electrode exhibited excellent stability in weak acidic and neutral media as well as good catalytic ability for the reduction of hydrogen peroxide. In particular, the microporous structure of cellulose acetate at the surface provided a protective environment to improve the operational stability of prussian blue. Wang et al. (2012) designed two prototypes of highly sensitive and selective solid state biocompatible fluorescence sensing materials for Cu2+ and Cr3+ based on 1,4-dihydroxyanthraquinone (1,4-DHAQ, a fluorophore) doped cellulose nanofiber. 1,4-DHAQ-doped nanofibers have been achieved via electrospinning with subsequent deacetylation, and used for the detection of Cu2+ and Cr3+ in the nanomolar range with higher selectivity than other common metal ions. In addition, an organic vapor-sensitive composite film comprising cellulose acetate and a representative compound 1-n-butyl-2,3-dimethylimidazolium hexafluorophosphate was developed using a solvent precipitation method by Regmi et al. (2012).
a Dissolution control of magnesium by cellulose acetate (CA)/PDMAEMA membranes. Reprinted from Yliniemi et al. (2014) Copyright 2014, American Chemical Society. b Superhydrophobic nanofibrous mat from surface modified cellulose acetate as oil–water separation materials. Reprinted from Arslan et al. (2016) Copyright 2016, American Chemical Society
Furthermore, as a commercial product, cellulose acetate can be used as a starting material for further modification to form advanced materials. For example, superhydrophobic nanofibrous mat is obtained via electrospinning technique of surface-modified cellulose acetate using perfluoroalkoxysilanes (Arslan et al. 2016). The introduction of the perfluoroalkyl groups tailored their chemical and physical features as oil–water separation materials (Fig. 6b). Chen et al. (2009) found that asymmetric ultrafiltration membranes of cellulose acetate-graft-polyacrylonitrile copolymers exhibited remarkably high water permeability of about 100 times higher than the pure cellulose acetate membranes, leading to excellent oil/water separation performance.
Cellulose esters with longer aliphatic moieties other than acetic ones, such as cellulose fatty acid esters, were also attempted to construct advanced nanomaterials. Cellulose fatty acid esters exhibit plasticized polymer behavior (Crepy et al. 2011). Thus, these esters with hydrophobic nature show the potential to be used as film and coating material with unique wetting ability. Bras et al. (2007) studied the water vapor permeability of fully substituted cellulose esters with long aliphatic chains. Fully substituted cellulose esters with acyl substituents ranging in size from C2 to C18 were synthesized using the acid chloride method. These esters were further transferred into film via solvent-casting or compression-molding at elevated temperatures. The resulting films were found to represent effective barriers to water vapor transport. Zhang et al. (2015b) reported the fabrication of moisture-responsive films using cellulose stearoyl esters with a low DS of 0.3. In the presence of a local moisture gradient, such films could reversibly fold and unfold as rhythmical bending motions due to the absorption and desorption of water molecules at the film surface. Geissler et al. (2013) demonstrated an efficient path to superhydrophobize diverse surfaces with non-uniform shapes, such as metal spoon, plastic fork or textile fabric using NPs from cellulose tristearate (Fig. 7). In addition, slippery surfaces were fabricated using nanoporous cellulose lauroyl ester films comprising lubrication fluid. Such surfaces exhibited both excellent liquid repellency upon liquid impact and anti-icing properties (Chen et al. 2014). Furthermore, a highly water-repellent aerogel was prepared using stearoylated NFC with a very low DS (< 0.1) by supercritical CO2 drying process (Granstrom et al. 2011). Such aerogels maintained stable in water for 24 h without disintegration or collapse after drying.
a Schematic illustration of the synthesis of cellulose tristearate. b Scanning electron microscope images of surface and side profile of a nanostructured superhydrophobic surface consisting of cellulose tristearate NPs. c Superhydrophobization of various surfaces by deposition of cellulose tristearate NPs via spray coating. Reprinted from Geissler et al. (2013) Copyright 2013, Royal Society of Chemistry
Esterified cellulose containing aromatic moieties
Espino-Perez et al. (2016) esterified CNCs surfaces with aromatic functions using phenylacetic acid or hydrocinnamic acid. These CNCs with aromatic functionalities at surface showed macroscopically hydrophobic and water-repellent characters, while the water vapour sorption isotherms were only slightly affected. Moreover, such CNCs were able to reversibly take up large quantities of the volatile aromatic compound anisole, while the non-aromatic compound cyclohexane was much less absorbed. Furthermore, diverse hydrophobic dye molecules could be incorporated into NPs prepared by self-assembly of hydrophobic cellulose acetate phthalate (Schulze et al. 2016). The thermal reactive carboxyl moieties in phthaloyl groups were further employed for coupling C-reactive protein anti-bodies. These composite NPs based on cellulose acetate phthalate were well suitable as dye labels in immunoassay applications.
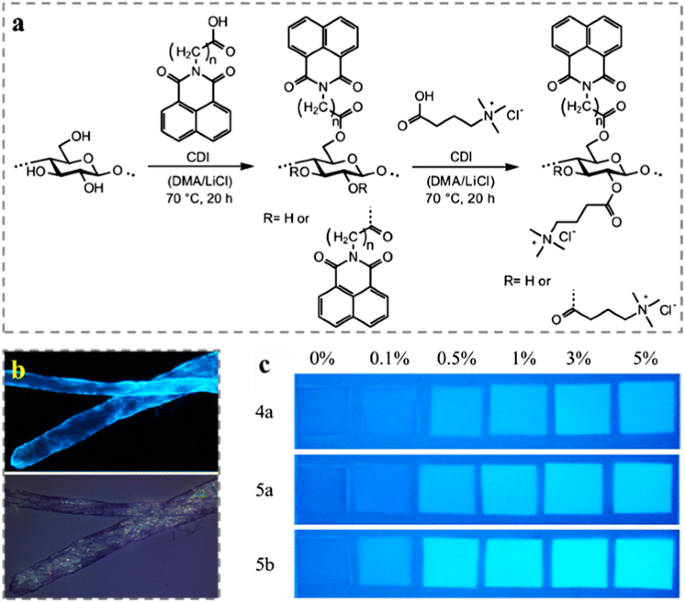
Furthermore, many aromatic moieties show unique photo activities, such as fluorescence. Grigoray et al. (2017) synthesized fluorescent cellulose derivatives, namely N-(3-propanoic acid)-1,8-naphthalimide and N-(4-butanoic acid)-1,8-naphthalimide cellulose esters with various DS (Fig. 8a). The derivatives contained a cationic moiety, namely (3-carboxypropyl)trimethylammonium chloride. While fluorescent cellulose esters were used as surface modifying agents adsorbed irreversibly onto the pulp fibers mainly via irreversibly charge-directed self-assembly. As the result of surface modification, the fibers became fluorescent and they emitted visible light under UV light exposure (Fig. 8b). Under black light illumination, the modified fibers fluoresced and were visually distinguishable from the reference fibers, which made them be potential as authenticity indicator in packaging materials (Fig. 8c). Wondraczek et al. (2012) decorated the cellulose with high amounts of photochemically active chromene moieties after the homogeneous reactions with of 2-[(4-methyl-2-oxo-2Hchromen-7-yl)oxy]acetic acid and cationic (3-carboxypropyl) trimethylammonium chloride via activation with CDI. The obtained water soluble photoactive cellulose-based polyelectrolytes are of interest for the design of smart materials. The 6-O-phthalocyanine cellulose derivative, 2,3-di-O-myristyl-6-O-[p-(9(10),16(17),23(24)-tri-tert-butyl-2-zinc(II)phthalocyaninyl-benzoyl)cellulose was synthesized via the esterification of 2,3-di-O-myristyl cellulose with the mono-substituted phthalocyanine derivative containing phenolic carboxyl groups (Saito et al. 2014). Langmuir–Blodgett monolayer films from the phthalocyanine-containing cellulose derivatives exhibited a photocurrent generation performance in the range of 600–700 nm. This property led such compound to potential application as solar cell materials. Grigoray et al. (2015) used the same synthesis approach to produce coumarin-containing cellulose polyelectrolytes, which were used to decorate pulp fibers to prepare light-responsive pulp fibers/fibrous materials with light-controllable mechanical properties. In addition, fluorescent CNCs with carbazole and coumarin functionalities were synthesized via a one-step esterification reaction using carbazole-9-yl-acetic acid and coumarin-3-carboxylic acid respectively (Sirbu et al. 2016).
a Synthesis scheme of N-(3-propanoic acid)-1,8-naphthalimide and N-(4-butanoic acid)-1,8-naphthalimide esters of cellulose and the corresponding mixed naphthalimide (3-carboxypropyl)trimethylammonium chloride esters of cellulose via in situ activation. b Visualization of fluorescent modified pulp fibers by epi-fluorescence microscope under exposure of UV light (up) and white light (down). c Picture of fiber hand-sheets using mixture of neat pulp fibers and modified fibers with different ratios under black light illumination. Reprinted from Grigoray et al. (2017) Copyright 2016, American Chemical Society
Esterified cellulose containing terminal active moieties
Some ester moieties contain terminal active groups acting as precursors for further modifications. BriBB has been extensively used for the esterification of cellulose to form a macroinitiator for further atom transfer radical polymerization (ATRP) (Eyley and Thielemans 2014). CNCs grafted with fluorescent and thermo-responsive poly(N-isopropylacrylamide) (PNIPAAM) brushes were prepared via ATRP using BriBB as initiator in the methanol/water mixtures with various volume ratios (Fig. 9a) (Wu et al. 2015). Obtained surface-grafted CNCs showed thermo-enhanced fluorescence owing to the thermal-driven chain dehydration of the grafted PNIPAAM brushes (Fig. 9b, c). Liu et al. (2014) showed a new method to synthesize anti-adhesive surfaces based on cellulose-derived materials by grafting the surfaces with zwitterionic polymers through surface-initiated ATRP after the immobilization of BriBB on cellulose membrane surface. These cellulose membrane substrates after the modification with zwitterionic brushes exhibited excellent anti-biofouling ability with low non-specific adsorption of proteins, platelet adhesion and cell attachment. Sui et al. (2008) utilized ionic liquid and DMF as solvent system to synthesize cellulose macroinitiator by esterification with BriBB, and the macroinitiator was further grafted with PDMAEMA via ATRP to obtain pH- and temperature-responsive cellulose-g-PDMAEMA copolymers. Using similar approach, Xu et al. (2008) prepared azo polymers-grafted CNCs and the modified CNCs showed two types of liquid crystal formation, thermotropic and lyotropic properties. Navarro et al. (2016) converted NFC into fluorescently labeled probes using the NFC-based macroinitiators that were synthesized via esterification of the hydroxyl groups on NFC using 2-bromo-2-methylpro-pionic acid. Such NFC-based macroinitiators initiated radical polymerization of methyl acrylate and acrylic acid N-hydroxysuccinimide ester, resulting in NFC with surface-attached block copolymers. A luminescent probe was further coupled to the modified NFC through an amidation reaction, leading to an excellent biomarker sensing property.
a Synthesis route for the immobilization of the initiator on CNCs and subsequent surface grafting of poly(NIPAAM-co-EANI). b Conformation of grafted PNIPAAM brushes below the lower critical solution temperature (LCST) and above the LCST. c Fluorescence emission spectroscopy of surface-grafted CNCs (0.02 wt% in H2O) and EANI (10−6 M in H2O). Reprinted from Wu et al. (2015) Copyright 2015, Royal Society of Chemistry
In addition to BriBB, the double bond and thiol groups have been reported as active sites for further modification. Nielsen et al. (2010) demonstrated a versatile synthetic strategy to obtain fluorescent CNCs for pH sensing. The double bond was introduced onto CNCs by esterification, followed by the thiol-ene Michael addition to introduce amine functionality, which further coupled with the succinimidyl ester dyes containing fluorescein-5′-isothiocyanate (FITC) and rhodamine B isothiocyanate (RBITC) (Fig. 10a). Rosilo et al. (2013) presented the functionalization of rigid native CNCs by esterification using 10-undecenoyl chloride to introduce a dense hydrocarbon chain brush containing cross-linkable terminal double bonds. Composite films with 0–80 wt% of such modified CNCs within a poly(butadiene) rubber matrix were prepared via cross-linking by UV-light initiated thiol-ene click reaction to mimic biological nanocomposites involving self-assembled and space-filed structures of hard reinforcing and soft toughening domains. Furthermore, pH-responsive NPs with switchable sizes using a modified nanoprecipitation method were prepared using cellulose 10-undecenoyl ester (CUE) as precursor (Wang et al. 2016c). The CUE with a DS of 3 was synthesized after the esterification of cellulose with 10-undecenoyl chloride under heterogenous condition. Then, CUE was modified by photo-induced thiol-ene reaction using 2-(diethylamino)ethanethiol hydrochloride and 2-(dimethylamino)ethanethiol hydrochloride, in order to introduce amine groups. The obtained cellulose derivatives containing tertiary amines were further transformed into NPs with average diameters in the range of 90–180 nm, which exhibited pH-responsive, size-switchable properties as shown by alternately changing the pH value between 7 and 4. Moreover, cellulose esters with thiol groups were also applied to manufacture reversibly fluorescent NPs (Wang et al. 2015a). First, the thiol groups were introduced into cellulose chains after the esterification by 3,3′-dithiodipropionic acid and further reductive cleavage of disulfide bonds. Then, rhodamine spiroamide was immobilized via thiol-ene reaction between cellulose thiopropionyl ester and rhodamine B methacrylamide. The rhodamine spiroamide endows the NPs from this cellulose ester reversible fluorescence in response to UV-illumination, temperature and pH, which allows such NPs for potential applications in biomedical sensing and imaging.
a Dual fluorescent labelled CNCs for pH sensing. (a1) Synthesis of fluorescent labeled CNCs with succinimidyl ester dyes; (a2) AFM image of fluorescent labeled CNCs; (a3) suspensions of pH responsive fluorescent labeled CNCs (0.1 wt%) at increasing pH values; (a4) emission spectra of pH responsive fluorescent labeled CNCs at different pH values (λex = 490 and 540 nm for FITC and RBITC, respectively); (a5) plot of intensity ratios versus pH values. Reprinted from Nielsen et al. (2010) Copyright 2010, Royal Society of Chemistry. b Multicolor fluorescent labeled NFC by click chemistry. (b1) Surface chemical structure of the multicolored NFC; (b2) Combined overlay fluorescence-bright-field images of multicolored NFC using confocal laser scanning microscopy. Reprinted from Navarro et al. (2015) Copyright 2015, American Chemical Society
In addition, Navarro et al. have chemically modified NFC with furan and maleimide groups through esterification with 2-furoyl chloride and a Diels–Alder cycloaddition with 1,1′-(methylenedi-4,1-phenylene)bismaleimide (Navarro et al. 2015). The modified NFC fibers were selectively labeled with fluorescent probes, i.e. 7-mercapto-4-methylcoumarin and fluorescein diacetate 5-maleimide, through two specific click chemistry reactions as Diels–Alder cycloaddition and Thiol-Michael reaction. These two luminescent dyes could be selectively labeled onto NFC, yielding a multicolored NFC that could be imaged using a confocal laser scanning microscope (Fig. 10b). In addition, Kim et al. (2015) prepared a novel group of robust aerogels based on maleic acid-grafted NFC, which exhibited good network stability in water and springiness after compression. Such advantageous mechanical properties are derived from the grafted maleic acid that reacted with hypophosphite forming a chemically cross-linked network.
Esterified cellulose containing other more complex structures
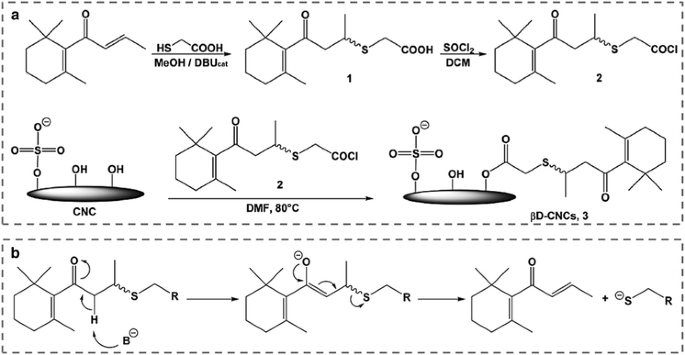
Various substitutes with more complex chemical structures and desired functions have been introduced to esterify cellulose with the aim of constructing high-performance advanced materials. Kuhnt et al. (2015) created a group of new release systems by decorating CNCs with thiol-ene adduct of β-damascone by esterification (Fig. 11a). A short linker serves to bind the fragrance molecules to the CNCs surface and permits their slow release via a retro 1,4-Michael-type reaction (Fig. 11b). The data showed that β-damascone is indeed slowly released, and that the quantity of fragrance released after 3 days is up to 80 times higher than reference experiments, where the tissue was treated with the neat fragrance under identical conditions. Rosin-grafted CNCs were synthesized via esterification of CNCs using nontoxic abietic acid (de Castro et al. 2016). Such rosin-grafted CNCs exhibited a high antibacterial activity against gram-negative bacteria and a modest antibacterial activity against gram-positive bacteria. Recently, CNCs were surface-functionalized with β-cyclodextrin (β-CD) using succinic acid or fumaric acid as bridging agents by esterification (Castro et al. 2016). The resulting β-CD-grafted CNCs showed promising potential to be used as bioactive materials that are able to release antibacterial molecules over a prolonged period of time.
a Synthesis of β-damascone decorated CNCs (β-CD-CNCs). b Reaction mechanism of the base-induced retro 1,4-Michael-type reaction of thiol-ene adducts of β-damascone. Reprinted from Kuhnt et al. (2015) Copyright 2015, Royal Society of Chemistry
Conclusion
Esterification of cellulose is among the most versatile modifications leading to a wide range of structural and functional types with valuable properties. The current review attempts to provide a general overview of chemical transformations of cellulose via esterification for the functional applications. We emphasized various methodologies, materials and achievements for esterified cellulose compounds and provided an overview of their applications as functional materials on a large scale. From the scientific point of view, esterification can yield a broad spectrum of cellulose ester derivatives with DS in the range of 0–3, which were promoted and expanded continually due to the introduction of new esterification methodologies. The maintained challenges are the precise esterification for the introduction of functionalities onto cellulose in diverse size scales including cellulose polymeric chains, nanocellulose, and cellulose microfibers and at the same time the persistence of cellulose polymeric chains or supramolecular morphologies.
Remarkably, nanocelluloses including CNCs, NFC, BNC and other unconventional nanocelluloses are currently the objects of intense scientific curiosity and have been intensively studied over the last 10 years. A wide variety of esterification approaches have been carried out on nanocelluloses ranging from simple in situ esterification to sophisticated post surface modifications, which all impart desired functions to the surface of nanocelluloses. Most of these approaches carried out in nanocelluloses have concentrated on the compatibilization of nanocelluloses with other matrices via turning their hydrophilic nature for the formation of composite functional materials. Esterification has leads to the highest reported surface DS at around 1.5, but the average DS is usually much lower to avoid any damage either to the morphology or to the native crystalline structure of nanocelluloses.
To achieve a broad understanding of the application of esterified cellulose compounds, the review touched upon selected important ester moieties that can lead to advanced materials in many fields including drug delivery, tissue engineering, water purification, catalysis, electrical devices, sensing and more. To be more specific, the conventional cellulose esters, such as cellulose sulfates, cellulose acetates and cellulose fatty acid esters, have been intensively studied to develop new advanced functional materials. Meanwhile, a wide variety of new functional ester moieties, such as pyridinium, chromene, coumarin, rhodamine spiroamide and polymeric chains, have been introduced by esterification in cellulose directly or indirectly to import new properties.
Abbreviations
- 3D:
-
Three-dimensional
- ATRP:
-
Atom transfer radical polymerization
- BNC:
-
Bacterial nanocellulose
- BriBB:
-
2-Bromoisobutyryl bromide
- CDI:
-
N,N′-Carbonyldiimidazole
- CNCs:
-
Cellulose nanocrystals
- CUE:
-
Cellulose 10-undecenoyl ester
- DMAc:
-
Dimethylacetamide
- DMF:
-
Dimethylformamide
- DMSO:
-
Dimethyl sulfoxide
- DS:
-
Degree of substitution
- FITC:
-
Fluorescein-5′-isothiocyanate
- LCST:
-
Lower critical solution temperature
- LiCl:
-
Lithium chloride
- NFC:
-
Nanofibrillated cellulose
- NPs:
-
Nanoparticles
- PDMAEMA:
-
Poly(N,N-dimethylaminoethyl methacrylate)
- PEDOT:
-
Poly(3,4-ethylene-dioxythiophene)
- PNIPAAM:
-
Poly(N-isopropylacrylamide)
- PolyDADMAC:
-
Poly(dimethyldiallyl-ammonium chloride)
- RBITC:
-
Rhodamine B isothiocyanate
- TEMPO:
-
2,2,6,6-Tetramethylpiperidin-1-oxyl
- TosCl:
-
p-Toluenesulfonyl chloride
References
Agarwal HK, Kumar A, Doncel GF, Parang K (2010) Synthesis, antiviral and contraceptive activities of nucleoside-sodium cellulose sulfate acetate and succinate conjugates. Bioorg Med Chem Lett 20:6993–6997
Alexander WJ, Mitchell RL (1949) Rapid measurement of cellulose viscosity by the nitration method. Anal Chem 21:1497–1500
Antova G, Vasvasova P, Zlatanov M (2004) Studies upon the synthesis of cellulose stearate under microwave heating. Carbohydr Polym 57:131–134
Aoki D, Nishio Y (2010) Phosphorylated cellulose propionate derivatives as thermoplastic flame resistant/retardant materials: influence of regioselective phosphorylation on their thermal degradation behaviour. Cellulose 17:963–976
Araki J, Wada M, Kuga S, Okana T (1999) Influence of surface charge on viscosity behavior of cellulose microcrystal suspension. J Wood Sci 45:258–261
Araki J, Wada M, Kuga S, Okano T (2000) Birefringent glassy phase of a cellulose microcrystal suspension. Langmuir 16:2413–2415
Arslan O, Aytac Z, Uyar T (2016) Superhydrophobic, hybrid, electrospun cellulose acetate nanofibrous mats for oil/water separation by tailored surface modification. ACS Appl Mater Interfaces 8:19747–19754
Berlioz S, Molina-Boisseau S, Nishiyama Y, Heux L (2009) Gas-phase surface esterification of cellulose microfibrils and whiskers. Biomacromolecules 10:2144–2151
Bezerra RDS, Silva MMF, Morais AIS, Osajima JA, Santos MRMC, Airoldi C, Silva EC (2014) Phosphated cellulose as an efficient biomaterial for aqueous drug ranitidine removal. Materials 7:7907–7924
Bifari EN, Khan SB, Alamry KA, Asiri AM, Akhtar K (2016) Cellulose acetate based nanocomposites for biomedical applications: a review. Curr Pharm Des 22:3007–3019
Blaker JJ, Lee K-Y, Li X, Menner A, Bismarck A (2009) Renewable nanocomposite polymer foams synthesized from Pickering emulsion templates. Green Chem 11:1321–1326
Boufi S, Vilar MR, Parra V, Ferraria AM, do Rego AMB (2008) Grafting of porphyrins on cellulose nanometric films. Langmuir 24:7309–7315
Brand J, Pecastaings G, Sèbe G (2017) A versatile method for the surface tailoring of cellulose nanocrystal building blocks by acylation with functional vinyl esters. Carbohydr Polym 169:189–197
Bras J, Vaca-Garcia C, Borredon ME, Glasser W (2007) Oxygen and water vapor permeability of fully substituted long chain cellulose esters (LCCE). Cellulose 14:367–374
Braun B, Dorgan JR (2009) Single-step method for the isolation and surface functionalization of cellulosic nanowhiskers. Biomacromolecules 10:334–341
Braun B, Dorgan JR, Hollingsworth LO (2012) Supra-molecular ecobionanocomposites based on polylactide and cellulosic nanowhiskers: synthesis and properties. Biomacromolecules 13:2013–2019
Brown RM, Montezinos D (1976) Cellulose microfibrils—visualization of biosynthetic and orienting complexes in association with plasma-membrane. Proc Natl Acad Sci USA 73:143–147
Bucko M, Vikartovska A, Lacik I, Kollarikova G, Gemeiner P, Patoprsty V, Brygin M (2005) Immobilization of a whole-cell epoxide-hydrolyzing biocatalyst in sodium alginate–cellulose sulfate-poly(methylene-co-guanidine) capsules using a controlled encapsulation process. Enzyme Microb Technol 36:118–126
Cao XF, Sun SN, Peng XW, Zhong LX, Sun RC, Jiang D (2013) Rapid synthesis of cellulose esters by transesterification of cellulose with vinyl esters under the catalysis of NaOH or KOH in DMSO. J Agric Food Chem 61:2489–2495
Cao XF, Peng XW, Zhong LX, Sun SN, Yang D, Zhang XM, Sun RC (2014) A novel transesterification system to rapidly synthesize cellulose aliphatic esters. Cellulose 21:581–594
Castro DO, Tabary N, Martel B, Gandini A, Belgacem N, Bras J (2016) Effect of different carboxylic acids in cyclodextrin functionalization of cellulose nanocrystals for prolonged release of carvacrol. Mater Sci Eng, C 69:1018–1025
Cetin NS, Tingaut P, Ozmen N, Henry N, Harper D, Dadmun M, Sebe G (2009) Acetylation of cellulose nanowhiskers with vinyl acetate under moderate conditions. Macromol Biosci 9:997–1003
Chen WJ, Su YL, Zheng LL, Wang LJ, Jiang ZY (2009) The improved oil/water separation performance of cellulose acetate-graft-polyacrylonitrile membranes. J Membr Sci 337:98–105
Chen LQ, Geissler A, Bonaccurso E, Zhang K (2014) Transparent slippery surfaces made with sustainable porous cellulose lauroyl ester films. ACS Appl Mater Interfaces 6:6969–6976
Chinga-Carrasco G (2011) Cellulose fibres, nanofibrils and microfibrils: the morphological sequence of MFC components from a plant physiology and fibre technology point of view. Nanoscale Res Lett 6:417
Coseri S, Biliuta G, Simionescu BC, Stana-Kleinschek K, Ribitsch V, Harabagiu V (2013) Oxidized cellulose—survey of the most recent achievements. Carbohydr Polym 93:207–215
Crepy L, Miri V, Joly N, Martin P, Lefebvre JM (2011) Effect of side chain length on structure and thermomechanical properties of fully substituted cellulose fatty esters. Carbohydr Polym 83:1812–1820
Cullis CF, Hirschler MM, Madden RG (1992) Studies of the effects of phosphorus and its compounds on the combustion of cellulose. Eur Polym J 28:493–497
Dautzenberg H et al (1999) Development of cellulose sulfate-based polyelectrolyte complex microcapsules for medical applications. Ann N Y Acad Sci 875:46–63
de Castro DO, Bras J, Gandini A, Belgacem N (2016) Surface grafting of cellulose nanocrystals with natural antimicrobial rosin mixture using a green process. Carbohydr Polym 137:1–8
de Menezes AJ, Siqueira G, Curvelo AAS, Dufresne A (2009) Extrusion and characterization of functionalized cellulose whiskers reinforced polyethylene nanocomposites. Polymer 50:4552–4563
Dias CR, de Pinho MN (1999) Water structure and selective permeation of cellulose-based membranes. J Mol Liq 80:117–132
Ding JJ, Li CG, Liu J, Lu YQ, Qin GH, Gan LH, Long M (2017) Time and energy-efficient homogeneous transesterification of cellulose under mild reaction conditions. Carbohydr Polym 157:1785–1793
Dong SP, Roman M (2007) Fluorescently labeled cellulose nanocrystals for bioimaging applications. J Am Chem Soc 129:13810–13811
Dong C, Qian LY, Zhao GL, He BH, Xiao HN (2014) Preparation of antimicrobial cellulose fibers by grafting beta-cyclodextrin and inclusion with antibiotics. Mater Lett 124:181–183
Duan HT, Shao ZQ, Zhao M, Zhou ZW (2016) Preparation and properties of environmental-friendly coatings based on carboxymethyl cellulose nitrate ester & modified alkyd. Carbohydr Polym 137:92–99
Dugan JM, Collins RF, Gough JE, Eichhorn SJ (2013) Oriented surfaces of adsorbed cellulose nanowhiskers promote skeletal muscle myogenesis. Acta Biomater 9:4707–4715
Eichhorn SJ (2011) Cellulose nanowhiskers: promising materials for advanced applications. Soft Matter 7:303
Espino-Perez E, Domenek S, Belgacem N, Sillard C, Bras J (2014) Green process for chemical functionalization of nanocellulose with carboxylic acids. Biomacromolecules 15:4551–4560
Espino-Perez E, Bras J, Almeida G, Relkin P, Belgacem N, Plessis C, Domenek S (2016) Cellulose nanocrystal surface functionalization for the controlled sorption of water and organic vapours. Cellulose 23:2955–2970
Espinosa SC, Kuhnt T, Foster EJ, Weder C (2013) Isolation of thermally stable cellulose nanocrystals by phosphoric acid hydrolysis. Biomacromolecules 14:1223–1230
Eyley S, Thielemans W (2014) Surface modification of cellulose nanocrystals. Nanoscale 6:7764–7779
Eyley S, Vandamme D, Lama S, Van den Mooter G, Muylaert K, Thielemans W (2015) CO2 controlled flocculation of microalgae using pH responsive cellulose nanocrystals. Nanoscale 7:14413–14421
Fahma F, Takemura A, Saito Y (2014) Acetylation and stepwise solvent-exchange to modify hydrophilic cellulose whiskers to polychloroprene-compatible nanofiller. Cellulose 21:2519–2527
Filpponen I, Argyropoulos DS (2010) Regular linking of cellulose nanocrystals via click chemistry: synthesis and formation of cellulose nanoplatelet gels. Biomacromolecules 11:1060–1066
Fox SC, Li B, Xu DQ, Edgar KJ (2011) Regioselective esterification and etherification of cellulose: a review. Biomacromolecules 12:1956–1972
Fujisawa S, Okita Y, Saito T, Togawa E, Isogai A (2011) Formation of N-acylureas on the surface of TEMPO-oxidized cellulose nanofibril with carbodiimide in DMF. Cellulose 18:1191–1199
Fujisawa S, Saito T, Kimura S, Iwata T, Isogai A (2013) Surface engineering of ultrafine cellulose nanofibrils toward polymer nanocomposite materials. Biomacromolecules 14:1541–1546
Fumagalli M, Ouhab D, Boisseau SM, Heux L (2013a) Versatile gas-phase reactions for surface to bulk esterification of cellulose microfibrils aerogels. Biomacromolecules 14:3246–3255
Fumagalli M, Sanchez F, Boisseau SM, Heux L (2013b) Gas-phase esterification of cellulose nanocrystal aerogels for colloidal dispersion in apolar solvents. Soft Matter 9:11309–11317
Garces J, Franco P, Oliveros L, Minguillon C (2003) Mixed cellulose-derived benzoates bonded on allylsilica gel as HPLC chiral stationary phases: influence of the introduction of an aromatic moiety in the fixation substituent. Tetrahedron Asymmetry 14:1179–1185
Geissler A, Chen LQ, Zhang K, Bonaccurso E, Biesalski M (2013) Superhydrophobic surfaces fabricated from nano- and microstructured cellulose stearoyl esters. Chem Commun 49:4962–4964
Gericke M, Liebert T, Heinze T (2009a) Interaction of ionic liquids with polysaccharides, 8-synthesis of cellulose sulfates suitable for polyelectrolyte complex formation. Macromol Biosci 9:343–353
Gericke M, Liebert T, Heinze T (2009b) Polyelectrolyte synthesis and in situ complex formation in ionic liquids. J Am Chem Soc 131:13220–13221
Ghanadpour M, Carosio F, Larsson PT, Wagberg L (2015) Phosphorylated cellulose nanofibrils: a renewable nanomaterial for the preparation of intrinsically flame-retardant materials. Biomacromolecules 16:3399–3410
Grabner D, Liebert T, Heinze T (2002) Synthesis of novel adamantoyl cellulose using differently activated carboxylic acid derivatives. Cellulose 9:193–201
Granstrom M, Paakko MKN, Jin H, Kolehmainen E, Kilpelainen I, Ikkala O (2011) Highly water repellent aerogels based on cellulose stearoyl esters. Polym Chem 2:1789–1796
Grigoray O et al (2015) Photocontrol of mechanical properties of pulp fibers and fiber-to-fiber bonds via self-assembled polysaccharide derivatives. Macromol Mater Eng 300:277–282
Grigoray O, Wondraczek H, Pfeifer A, Fardim P, Heinze T (2017) Fluorescent multifunctional polysaccharides for sustainable supramolecular functionalization of fibers in water. ACS Sustain Chem Eng 5:1794–1803
Habibi Y (2014) Key advances in the chemical modification of nanocelluloses. Chem Soc Rev 43:1519–1542
Habibi Y, Goffin A-L, Schiltz N, Duquesne E, Dubois P, Dufresne A (2008) Bionanocomposites based on poly(ε-caprolactone)-grafted cellulose nanocrystals by ring-opening polymerization. J Mater Chem 18:5002–5010
Habibi Y, Lucia LA, Rojas OJ (2010) Cellulose nanocrystals: chemistry, self-assembly, and applications. Chem Rev 110:3479–3500
Hasani MM, Westman G (2007) New coupling reagents for homogeneous esterification of cellulose. Cellulose 14:347–356
Hasani M, Cranston ED, Westman G, Gray DG (2008) Cationic surface functionalization of cellulose nanocrystals. Soft Matter 4:2238–2244
Heinze T, Liebert T (2001) Unconventional methods in cellulose functionalization. Prog Polym Sci 26:1689–1762
Heinze T, Dicke R, Koschella A, Kull AH, Klohr EA, Koch W (2000) Effective preparation of cellulose derivatives in a new simple cellulose solvent. Macromol Chem Phys 201:627–631
Heinze T, Liebert TF, Pfeiffer KS, Hussain MA (2003) Unconventional cellulose esters: synthesis, characterization and structure–property relations. Cellulose 10:283–296
Heinze T, Liebert T, Koschella A (2006) Esterification of polysaccharides. Springer, Berlin
Heinze T, Seoud OAE, Koschella A (2018) Cellulose derivatives: synthesis, structure, and properties. Springer, Cham
Herrick FW, Casebier RL, Hamilton JK, Sandberg KR (1983) Microfibrillated cellulose: morphology and accessibility. J Appl Polym Sci Appl Polym Symp 37:797–813
Horikawa M, Fujiki T, Shirosaki T, Ryu N, Sakurai H, Nagaoka S, Ihara H (2015) The development of a highly conductive PEDOT system by doping with partially crystalline sulfated cellulose and its electric conductivity. J Mater Chem C 3:8881–8887
Hou JZ, Zhang WX, Guan DB, Sun XP, Li LL (2012) Electrospinning in preparation of modified cellulose acetate. Prog Chem 24:2359–2366
Huang P, Wu M, Kuga S, Wang DQ, Wu DY, Huang Y (2012) One-step dispersion of cellulose nanofibers by mechanochemical esterification in an organic solvent. Chemsuschem 5:2319–2322
Huang P, Wu M, Kuga S, Huang Y (2013) Aqueous pretreatment for reactive ball milling of cellulose. Cellulose 20:2175–2178
Huang J, Chang P, Lin N, Dufresne A (2014) Polysaccharide-based nanocrystals: chemistry and applications. Chemical Industry Press, Weinheim
Huang P, Zhao Y, Kuga S, Wu M, Huang Y (2016) A versatile method for producing functionalized cellulose nanofibers and their application. Nanoscale 8:3753–3759
Hufendiek A, Carlmark A, Meier MAR, Barner-Kowollik C (2016) Fluorescent covalently cross-linked cellulose networks via light-induced ligation. ACS Macro Lett 5:139–143
Illy N, Fache M, Menard R, Negrell C, Caillol S, David G (2015) Phosphorylation of bio-based compounds: the state of the art. Polym Chem 6:6257–6291
Iwata T, Fukushima A, Okamura K, Azuma J (1997) DSC study on regioselectively substituted cellulose heteroesters. J Appl Polym Sci 65:1511–1515
John MJ, Thomas S (2008) Biofibres and biocomposites. Carbohydr Polym 71:343–364
Johnson RK, Zink-Sharp A, Glasser WG (2011) Preparation and characterization of hydrophobic derivatives of TEMPO-oxidized nanocelluloses. Cellulose 18:1599–1609
Kamide K, Saito M (1994) Recent advances in molecular and supermolecular characterization of cellulose and cellulose derivatives. Macromol Symp 83:233–271
Kan KH, Li J, Wijesekera K, Cranston ED (2013) Polymer-grafted cellulose nanocrystals as pH-responsive reversible flocculants. Biomacromolecules 14:3130–3139
Kang XY, Sun PP, Kuga S, Wang C, Zhao Y, Wu M, Huang Y (2017) Thin cellulose nanofiber from corncob cellulose and its performance in transparent nanopaper. ACS Sustain Chem Eng 5:2529–2534
Kim DH, Song YS (2016) Cellulose nanocrystal-embedded triacetate cellulose composite films. Polym Compos 37:3228–3233
Kim DY, Nishiyama Y, Kuga S (2002) Surface acetylation of bacterial cellulose. Cellulose 9:361–367
Kim C, Youn H, Lee H (2015) Preparation of cross-linked cellulose nanofibril aerogel with water absorbency and shape recovery. Cellulose 22:3715–3724
Klemm D, Philipp B, Heinze T, Heinze U, Wagenknecht W (1998a) Comprehensive cellulose chemistry: functionalization of cellulose, vol 2. Wiley-VCH, Weinheim
Klemm D, Philipp B, Heinze T, Heinze U, Wagenknecht W (1998b) Comprehensive cellulose chemistry: fundamentals and analytical methods, vol 1. Wiley-VCH, Weinheim
Klemm D, Heublein B, Fink HP, Bohn A (2005) Cellulose: fascinating biopolymer and sustainable raw material. Angew Chem Int Ed 44:3358–3393
Klemm D, Kramer F, Moritz S, Lindstrom T, Ankerfors M, Gray D, Dorris A (2011) Nanocelluloses: a new family of nature-based materials. Angew Chem Int Ed 50:5438–5466
Kochkodan V, Hilal N (2015) A comprehensive review on surface modified polymer membranes for biofouling mitigation. Desalination 356:187–207
Konwarh R, Karak N, Misra M (2013) Electrospun cellulose acetate nanofibers: the present status and gamut of biotechnological applications. Biotechnol Adv 31:421–437
Krassig H (1990) Cellulose—morphology, structure, accessibility and reactivity. Papier 44:617–623
Kuhnt T, Herrmann A, Benczedi D, Foster EJ, Weder C (2015) Functionalized cellulose nanocrystals as nanocarriers for sustained fragrance release. Polym Chem 6:6553–6562
Kulomaa T, Matikainen J, Karhunen P, Heikkila M, Fiskari J, Kilpelainen I (2015) Cellulose fatty acid esters as sustainable film materials—effect of side chain structure on barrier and mechanical properties. RSC Adv 5:80702–80708
Kulterer MR et al (2012) Functional polysaccharide composite nanoparticles from cellulose acetate and potential applications. Adv Funct Mater 22:1749–1758
Lam E, Male KB, Chong JH, Leung AC, Luong JH (2012) Applications of functionalized and nanoparticle-modified nanocrystalline cellulose. Trends Biotechnol 30:283–290
Li W, Zhao H, Teasdale PR, John R, Zhang S (2002) Application of a cellulose phosphate ion exchange membrane as a binding phase in the diffusive gradients in thin films technique for measurement of trace metals. Anal Chim Acta 464:331–339
Liebert TF, Heinze T (2005) Tailored cellulose esters: synthesis and structure determination. Biomacromolecules 6:333–340
Liu PS, Chen Q, Li L, Lin SC, Shen J (2014) Anti-biofouling ability and cytocompatibility of the zwitterionic brushes-modified cellulose membrane. J Mater Chem B 2:7222–7231
Ljungberg N, Bonini C, Bortolussi F, Boisson C, Heux L, Cavaille JY (2005) New nanocomposite materials reinforced with cellulose whiskers in atactic polypropylene: effect of surface and dispersion characteristics. Biomacromolecules 6:2732–2739
Lu P et al (2015a) Recent advances in cellulose-based forward osmosis membrane. Sci Adv Mater 7:2182–2192
Lu QL, Lin WY, Tang LR, Wang SQ, Chen XR, Huang B (2015b) A mechanochemical approach to manufacturing bamboo cellulose nanocrystals. J Mater Sci 50:611–619
Ma L, Kang HL, Liu RG, Huang Y (2010) Smart assembly behaviors of hydroxypropylcellulose-graft-poly(4-vinyl pyridine) copolymers in aqueous solution by thermo and pH stimuli. Langmuir 26:18519–18525
Majoinen J et al (2011) Polyelectrolyte brushes grafted from cellulose nanocrystals using Cu-mediated surface-initiated controlled radical polymerization. Biomacromolecules 12:2997–3006
Mashkour M, Afra E, Resalati H, Mashkour M (2015) Moderate surface acetylation of nanofibrillated cellulose for the improvement of paper strength and barrier properties. RSC Adv 5:60179–60187
Mestechkina NM, Shcherbukhin VD (2010) Sulfated polysaccharides and their anticoagulant activity: a review. Appl Biochem Microbiol 46:267–273
Moon RJ, Martini A, Nairn J, Simonsen J, Youngblood J (2011) Cellulose nanomaterials review: structure, properties and nanocomposites. Chem Soc Rev 40:3941–3994
Mormann W, Demeter J (1999) Silylation of cellulose with hexamethyldisilazane in liquid ammonia—first examples of completely trimethylsilylated cellulose. Macromolecules 32:1706–1710
Mormann W, Wagner T (1997) Silylation of cellulose and low-molecular-weight carbohydrates with hexamethyldisilazane in liquid ammonia. Macromol Rapid Commun 18:515–522
Mulyadi A, Deng YL (2016) Surface modification of cellulose nanofibrils by maleated styrene block copolymer and their composite reinforcement application. Cellulose 23:519–528
Nada AAMA, Hassan ML (2003) Phosphorylated cation-exchangers from cotton stalks and their constituents. J Appl Polym Sci 89:2950–2956
Naeli MH, Farmani J, Zargaraan A (2017) Rheological and physicochemical modification of trans-free blends of palm stearin and soybean oil by chemical interesterification. J Food Process Eng 40:12409
Nagendran A, Mohan D (2008) Protein separation by cellulose acetate/sulfonated poly(ether imide) blend ultrafiltration membranes. J Appl Polym Sci 110:2047–2057
Navarro JRG, Conzatti G, Yu Y, Fall AB, Mathew R, Eden M, Bergstrom L (2015) Multicolor fluorescent labeling of cellulose nanofibrils by click chemistry. Biomacromolecules 16:1293–1300
Navarro JRG, Wennmalm S, Godfrey J, Breitholtz M, Edlund U (2016) Luminescent nanocellulose platform: from controlled graft block copolymerization to biomarker sensing. Biomacromolecules 17:1101–1109
Nielsen LJ, Eyley S, Thielemans W, Aylott JW (2010) Dual fluorescent labelling of cellulose nanocrystals for pH sensing. Chem Commun 46:8929–8931
Olsson RT et al (2010) Making flexible magnetic aerogels and stiff magnetic nanopaper using cellulose nanofibrils as templates. Nat Nanotechnol 5:584–588
Oshima T, Kondo K, Ohto K, Inoue K, Baba Y (2008) Preparation of phosphorylated bacterial cellulose as an adsorbent for metal ions. React Funct Polym 68:376–383
Oshima T, Taguchi S, Ohe K, Baba Y (2011) Phosphorylated bacterial cellulose for adsorption of proteins. Carbohydr Polym 83:953–958
Padilha PM, Campos JTS, Moreira JC, Federici CC (1995) Study on ion-exchange and adsorption properties of cellulose and modified cellulose. Quim Nova 18:529–533
Pan HF, Song L, Ma LY, Pan Y, Liew KM, Hu Y (2014) Layer-by-layer assembled thin films based on fully biobased polysaccharides: chitosan and phosphorylated cellulose for flame-retardant cotton fabric. Cellulose 21:2995–3006
Pang JH et al (2016) Synthesis of highly polymerized water-soluble cellulose acetate by the side reaction in carboxylate ionic liquid 1-ethyl-3-methylimidazolium acetate. Sci Rep 6:33725
Peng SX, Chang HB, Kumar S, Moon RJ, Youngblood JP (2016) A comparative guide to controlled hydrophobization of cellulose nanocrystals via surface esterification. Cellulose 23:1825–1846
Peschel D, Zhang K, Aggarwal N, Brendler E, Fischer S, Groth T (2010) Synthesis of novel celluloses derivatives and investigation of their mitogenic activity in the presence and absence of FGF2. Acta Biomater 6:2116–2125
Peschel D, Zhang K, Fischer S, Groth T (2012) Modulation of osteogenic activity of BMP-2 by cellulose and chitosan derivatives. Acta Biomater 8:183–193
Qasim M, Darwish NA, Sarp S, Hilal N (2015) Water desalination by forward (direct) osmosis phenomenon: a comprehensive review. Desalination 374:47–69
Qiu XY, Hu SW (2013) “Smart” materials based on cellulose: a review of the preparations, properties, and applications. Materials 6:738–781
Ramirez JAA, Fortunati E, Kenny JM, Torre L, Foresti ML (2017) Simple citric acid-catalyzed surface esterification of cellulose nanocrystals. Carbohydr Polym 157:1358–1364
Rao XM, Kuga S, Wu M, Huang Y (2015) Influence of solvent polarity on surface-fluorination of cellulose nanofiber by ball milling. Cellulose 22:2341–2348
Regmi BP, Monk J, El-Zahab B, Das S, Hung FR, Hayes DJ, Warner IM (2012) A novel composite film for detection and molecular weight determination of organic vapors. J Mater Chem 22:13732–13741
Revol JF, Godbout L, Dong XM, Gray DG, Chanzy H, Maret G (1994) Chiral nematic suspensions of cellulose crystallites—phase-separation and magnetic-field orientation. Liq Cryst 16:127–134
Richter A, Klemm D (2003) Regioselective sulfation of trimethylsilyl cellulose using different SO3-complexes. Cellulose 10:133–138
Rodionova G, Lenes M, Eriksen O, Gregersen O (2011) Surface chemical modification of microfibrillated cellulose: improvement of barrier properties for packaging applications. Cellulose 18:127–134
Rodionova G, Hoff B, Lenes M, Eriksen O, Gregersen O (2013) Gas-phase esterification of microfibrillated cellulose (MFC) films. Cellulose 20:1167–1174
Roman M (2009) Model cellulosic surfaces. ACS symposium series, vol 1019. American Chemical Society: Distributed by Oxford University Press, Washington
Rosilo H, Kontturi E, Seitsonen J, Kolehmainen E, Ikkala O (2013) Transition to reinforced state by percolating domains of intercalated brush-modified cellulose nanocrystals and poly(butadiene) in cross-linked composites based on thiol-ene click chemistry. Biomacromolecules 14:1547–1554
Roy D, Semsarilar M, Guthrie JT, Perrier S (2009) Cellulose modification by polymer grafting: a review. Chem Soc Rev 38:2046–2064
Saha NR et al (2016) Nanocomposite films based on cellulose acetate/polyethylene glycol/modified montmorillonite as nontoxic active packaging material. RSC Adv 6:92569–92578
Saito Y, Kamitakahara H, Takano T (2014) Preparation of Langmuir-Blodgett monolayer films of (zinc(II) phthalocyanine)-containing cellulose derivative; the use of 2,3-di-O-myristyl cellulose as a scaffold. Cellulose 21:1885–1896
Samaranayake G, Glasser WG (1993) Cellulose derivatives with low DS. 1. A novel acylation system. Carbohydr Polym 22:1–7
Sassi JF, Chanzy H (1995) Ultrastructural aspects of the acetylation of cellulose. Cellulose 2:111–127
Schaffellner S et al (2005) Porcine islet cells microencapsulated in sodium cellulose sulfate. Transplant Proc 37:248–252
Schenzel A, Hufendiek A, Kowollik CB, Meier MAR (2014) Catalytic transesterification of cellulose in ionic liquids: sustainable access to cellulose esters. Green Chem 16:3266–3271
Schulze P, Gericke M, Scholz F, Wondraczek H, Miethe P, Heinze T (2016) Incorporation of hydrophobic dyes within cellulose acetate and acetate phthalate based nanoparticles. Macromol Chem Phys 217:1823–1833
Sebe G, Ham-Pichavant F, Pecastaings G (2013) Dispersibility and emulsion-stabilizing effect of cellulose nanowhiskers esterified by vinyl acetate and vinyl cinnamate. Biomacromolecules 14:2937–2944
Sehaqui H, Zimmermann T, Tingaut P (2014) Hydrophobic cellulose nanopaper through a mild esterification procedure. Cellulose 21:367–382
Shibata T (2004) Cellulose acetate in separation technology. Macromol Symp 208:353–369
Shimizu Y, Nakayama A, Hayashi J (1991) Acylation of cellulose with carboxylate salts. Cell Chem Technol 25:275–281
Siqueira G, Bras J, Dufresne A (2009) Cellulose whiskers versus microfibrils: influence of the nature of the nanoparticle and its surface functionalization on the thermal and mechanical properties of nanocomposites. Biomacromolecules 10:425–432
Sirbu E, Eyley S, Thielemans W (2016) Coumarin and carbazole fluorescently modified cellulose nanocrystals using a one-step esterification procedure. Can J Chem Eng 94:2186–2194
Sobkowicz MJ, Braun B, Dorgan JR (2009) Decorating in green: surface esterification of carbon and cellulosic nanoparticles. Green Chem 11:680–682
Somerville C (2006) Cellulose synthesis in higher plants. Annu Rev Cell Dev Biol 22:53–78
Söyler Z, Meier MAR (2017) Sustainable functionalization of cellulose and starch with diallyl carbonate in ionic liquids. Green Chem 19:3899–3907
Söyler Z, Onwukamike KN, Grelier S, Grau E, Cramail H, Meier MAR (2018) Sustainable succinylation of cellulose in a CO2-based switchable solvent and subsequent Passerini 3-CR and Ugi 4-CR modification. Green Chem 20:214–224
Spinella S et al (2016) Concurrent cellulose hydrolysis and esterification to prepare a surface-modified cellulose nanocrystal decorated with carboxylic acid moieties. ACS Sustain Chem Eng 4:1538–1550
Stiegler P et al (2014) Biocompatibility of sodium cellulose sulfate (SCS) microencapsulated porcine islet cells in five beagle dogs. Am J Transpl 14:419
Sui XF et al (2008) Synthesis of cellulose-graft-poly(N,N-dimethylamino-2-ethyl methacrylate) copolymers via homogeneous ATRP and their aggregates in aqueous media. Biomacromolecules 9:2615–2620
Thielemans W, Warbey CR, Walsh DA (2009) Permselective nanostructured membranes based on cellulose nanowhiskers. Green Chem 11:531–537
Tome LC, Freire MG, Rebelo LPN, Silvestre AJD, Neto CP, Marrucho IM, Freire CSR (2011) Surface hydrophobization of bacterial and vegetable cellulose fibers using ionic liquids as solvent media and catalysts. Green Chem 13:2464–2470
Tosh B, Saikia CN, Dass NN (2000) Homogeneous esterification of cellulose in the lithium chloride-N,N-dimethylacetamide solvent system: effect of temperature and catalyst. Carbohydr Res 327:345–352
Van Damme L et al (2008) Lack of effectiveness of cellulose sulfate gel for the prevention of vaginal HIV transmission. N Engl J Med 359:877
van der Veen I, de Boer J (2012) Phosphorus flame retardants: properties, production, environmental occurrence, toxicity and analysis. Chemosphere 88:1119–1153
Vandamme D, Eyley S, Van den Mooter G, Muylaert K, Thielemans W (2015) Highly charged cellulose-based nanocrystals as flocculants for harvesting Chlorella vulgaris. Bioresour Technol 194:270–275
Vikartovska A, Bucko M, Mislovicova D, Patoprsty V, Lacik I, Gemeiner P (2007) Improvement of the stability of glucose oxidase via encapsulation in sodium alginate–cellulose sulfate-poly(methylene-co-guanidine) capsules. Enzyme Microb Technol 41:748–755
Walther A, Timonen JV, Diez I, Laukkanen A, Ikkala O (2011) Multifunctional high-performance biofibers based on wet-extrusion of renewable native cellulose nanofibrils. Adv Mater 23:2924–2928. https://doi.org/10.1002/adma.201100580
Wang ML, Meng GW, Huang Q, Qian YW (2012) Electrospun 1,4-DHAQ-doped cellulose nanofiber films for reusable fluorescence detection of trace Cu2+ and further for Cr3+. Environ Sci Technol 46:367–373
Wang GF, Chu HJ, Wei HL, Liu XQ, Zhao ZX, Zhu J (2014) Click synthesis by Diels–Alder reaction and characterisation of hydroxypropyl methylcellulose-based hydrogels. Chem Pap 68:1390–1399
Wang W, Li W, Zhang K (2015a) Redox-responsive, reversibly fluorescent nanoparticles from sustainable cellulose derivatives. J Mater Chem A 3:10610
Wang Y, Heim LO, Xu Y, Buntkowsky G, Zhang K (2015b) Transparent, stimuli-responsive films from cellulose-based organogel nanoparticles. Adv Funct Mater 25:1434–1441
Wang J et al (2016a) Synthesis of new bis(acyl)phosphane oxide photoinitiators for the surface functionalization of cellulose nanocrystals. Chem Commun 52:2823–2826
Wang Y, Sun FL, Zhang XD, Tao H, Yang YQ (2016b) Microwave-assisted synthesis of esterified bacterial celluloses to effectively remove Pb(II). Acta Phys Chim Sin 32:753–762
Wang Y, Heinze T, Zhang K (2016c) Stimuli-responsive nanoparticles from ionic cellulose derivatives. Nanoscale 8:648–657
Wang Y, Groszewicz PB, Rosenfeldt S, Schmidt H, Volkert CA, Vana P, Gutmann T, Buntkowsky G, Zhang K (2017) Thermoreversible self-assembly of perfluorinated core-coronas cellulose-nanoparticles in dry state. Adv Mater 29:1702473
Wei L, Agarwal UP, Hirth KC, Matuana LM, Sabo RC, Stark NM (2017) Chemical modification of nanocellulose with canola oil fatty acid methyl ester. Carbohydr Polym 169:108–116
Wen X, Wang H, Wei Y, Wang X, Liu C (2017) Preparation and characterization of cellulose laurate ester by catalyzed transesterification. Carbohydr Polym 168:247–254
Wertz JL, Bédué O, Mercier JP (2010) Cellulose science and technology. EPFL Press, Lausanne
Williamson RE, Burn JE, Hocart CH (2002) Towards the mechanism of cellulose synthesis. Trends Plant Sci 7:461–467
Wondraczek H, Pfeifer A, Heinze T (2012) Water soluble photoactive cellulose derivatives: synthesis and characterization of mixed 2-[(4-methyl-2-oxo-2H-chromen-7-yl)oxy]acetic acid-(3-carboxypropyl)trimethylammonium chloride esters of cellulose. Cellulose 19:1327–1335
Wongsasulak S, Patapeejumruswong M, Weiss J, Supaphol P, Yoovidhya T (2010) Electrospinning of food-grade nanofibers from cellulose acetate and egg albumen blends. J Food Eng 98:370–376
Wu CC, Gu QC, Zhang RB, Huang Y, Chen SX (2004) Synthesis and lyotropic behavior of mesogen-linked cellulose acetates. J Appl Polym Sci 92:2693–2697
Wu SG, Liu JP, Bai X, Tan WG (2010a) Stability improvement of prussian blue by a protective cellulose acetate membrane for hydrogen peroxide sensing in neutral media. Electroanalysis 22:1906–1910
Wu XM, Branford-White CJ, Zhu LM, Chatterton NP, Yu DG (2010b) Ester prodrug-loaded electrospun cellulose acetate fiber mats as transdermal drug delivery systems. J Mater Sci Mater Med 21:2403–2411
Wu WB et al (2015) Thermo-responsive and fluorescent cellulose nanocrystals grafted with polymer brushes. J Mater Chem A 3:1995–2005
Xu QX, Yi J, Zhang XF, Zhang HL (2008) A novel amphotropic polymer based on cellulose nanocrystals grafted with azo polymers. Eur. Polym. J. 44:2830–2837
Xu FJ, Zhu Y, Liu FS, Nie J, Ma J, Yang WT (2010) Comb-shaped conjugates comprising hydroxypropyl cellulose backbones and low-molecular-weight poly(N-isopropylacryamide) side chains for smart hydrogels: synthesis, characterization, and biomedical applications. Bioconjugate Chem 21:456–464
Xu YZ, Stark NM, Cai ZY, Jin LW, Wang CP, Chu FX (2011) Preparation of internally plasticized ester of cellulose irradiated by microwave and its properties. Int J Polym Mater 60:1152–1163
Yang ZY, Wang WJ, Shao ZQ, Zhu HD, Li YH, Wang FJ (2013) The transparency and mechanical properties of cellulose acetate nanocomposites using cellulose nanowhiskers as fillers. Cellulose 20:159–168
Yi J, Xu QX, Zhang XF, Zhang HL (2008) Chiral-nematic self-ordering of rodlike cellulose nanocrystals grafted with poly(styrene) in both thermotropic and lyotropic states. Polymer 49:4406–4412
Yliniemi K, Wilson BP, Singer F, Hohn S, Kontturi E, Virtanen S (2014) Dissolution control of Mg by cellulose acetate–polyelectrolyte membranes. ACS Appl Mater Interfaces 6:22393–22399
Yoo YM, Youngblood JP (2016) Green one-pot synthesis of surface hydrophobized cellulose nanocrystals in aqueous medium. ACS Sustain Chem Eng 4:3927–3938
Yu HY, Zhang DZ, Lu FF, Yao JM (2016) New approach for single-step extraction of carboxylated cellulose nanocrystals for their use as adsorbents and flocculants. ACS Sustain Chem Eng 4:2632–2643
Yuan XM, Cheng G (2015) From cellulose fibrils to single chains: understanding cellulose dissolution in ionic liquids. Phys Chem Chem Phys 17:31592–31607
Yuan HH, Nishiyama Y, Wada M, Kuga S (2006) Surface acylation of cellulose whiskers by drying aqueous emulsion. Biomacromolecules 7:696–700
Yue Z, Cowie JMG (2002) Synthesis and characterization of ion conducting cellulose esters with PEO side chains. Polymer 43:4453–4460
Yuwawech K, Wootthikanokkhan J, Wanwong S, Tanpichai S (2017) Polyurethane/esterified cellulose nanocrystal composites as a transparent moisture barrier coating for encapsulation of dye sensitized solar cells. J Appl Polym Sci 134:45010
Zarth CSP, Koschella A, Pfeifer A, Dorn S, Heinze T (2011) Synthesis and characterization of novel amino cellulose esters. Cellulose 18:1315–1325
Zeng XH, Danquah MK, Potumarthi R, Cao J, Chen XD, Lu YH (2013) Characterization of sodium cellulose sulphate/poly-dimethyl-diallyl-ammonium chloride biological capsules for immobilized cultivation of microalgae. J Chem Technol Biotechnol 88:599–605
Zhang K, Peschel D, Klinger T, Gebauer K, Groth T, Fischer S (2010) Synthesis of carboxyl cellulose sulfate with various contents of regioselectively introduced sulfate and carboxyl groups. Carbohydr Polym 82:92–99
Zhang K, Peschel D, Baucker E, Groth T, Fischer S (2011) Synthesis and characterisation of cellulose sulfates regarding the degrees of substitution, degrees of polymerisation and morphology. Carbohydr Polym 83:1659–1664
Zhang K, Fischer S, Geissler A, Brendler E, Gebauer K (2013) Synthesis of carboxyl cellulose sulfates with regioselective sulfation and regiospecific oxidation using cellulose trifluoroacetate as intermediates. Cellulose 20:2069–2080
Zhang K, Geissler A, Heinze T (2015a) Reversibly crystalline nanoparticles from cellulose alkyl esters via nanoprecipitation. Part Part Syst Charact 32:258–266
Zhang K, Geissler A, Standhardt M, Mehlhase S, Gallei M, Chen LQ, Thiele CM (2015b) Moisture-responsive films of cellulose stearoyl esters showing reversible shape transitions. Sci Rep 5:12390
Zhang QL, Lin DQ, Yao SJ (2015c) Review on biomedical and bioengineering applications of cellulose sulfate. Carbohydr Polym 132:311–322
Zheng Y, Song J, Cheng B, Fang X, Yuan Y (2015) Preparation and flame retardancy of 3-(hydroxyphenylphosphinyl)-propanoic acid esters of cellulose and their fibers. Cellulose 22:229–244
Zhu LY, Lin DQ, Yao SJ (2010) Biodegradation of polyelectrolyte complex films composed of chitosan and sodium cellulose sulfate as the controllable release carrier. Carbohydr Polym 82:323–328
Acknowledgments
Y.W. thanks the Northeast Forestry University for a startup grant with the project number of 000/41113296. K.Z. thanks German Research Foundation (DFG) with the Project Number of ZH546/2-1 for the financial support. X.W. thanks the China Scholarship Council (CSC) for financial support.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Wang, Y., Wang, X., Xie, Y. et al. Functional nanomaterials through esterification of cellulose: a review of chemistry and application. Cellulose 25, 3703–3731 (2018). https://doi.org/10.1007/s10570-018-1830-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-018-1830-3