Abstract
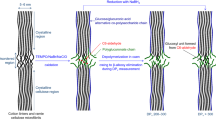
A method for conversion of carboxyl groups present on the surface of TEMPO-oxidized cellulose nanofibrils to N-acylureas using carbodiimide was developed. A TEMPO-oxidized cellulose nanofibril with free carboxyl groups (TOCN–COOH) dispersed in N,N-dimethylformamide (DMF) is prepared from a bleached kraft pulp, and then the TOCN–COOH is reacted with either N,N′-diisopropylcarbodiimide (DIC) or N,N′-dicyclohexylcarbodiimide (DCC) under apparently homogeneous conditions. FT-IR and solid-state 13C NMR analyses showed that the reaction products contained N-acylurea groups, and yields were >80%. Conversion ratios of carboxyl groups to N-acylureas are approximately 80 and 60%, when DIC and DCC, respectively, of 5 mol per mole of carboxyl groups are used as the reagents. X-ray diffraction analysis demonstrated that neither crystallinity nor crystal width of the original wood cellulose I structure was changed by the N-acylurea formation. The isolated and never-dried TOCN-N-acylureas are nano-dispersed in DMF but not in i-PrOH or dioxane. Pellets of the TOCN-N-acylureas had water-contact angles of >70°.










Similar content being viewed by others
References
Angelova P, Kostova K, Tsankov D (2005) A convenient synthesis of long-chain 4-substituted benzyloxycarbonyl alkanethiols for the formation of self assembled monolayers on metal substrates. Cent Eur J Chem 3:658–667
Azzam F, Heux L, Putaux JL, Jean B (2010) Preparation by grafting onto, characterization, and properties of thermally responsive polymer-decorated cellulose nanocrystals. Biomacromolecules 11:3652–3659
Berlioz S, Boisseau SM, Nishiyama Y, Heux L (2009) Gas-phase surface esterification of cellulose microfibrils and whiskers. Biomacromolecules 10:2144–2151
Buchanan GW, Rastegar MF, Enright G (2007) Reaction of RF-palmitic acid-F13 with dicyclohexylcarbodiimide. J Fluor Chem 128:1026–1028
Chazeau L, Cavaille JY, Canova G, Dendievel R, Boutherin B (1999) Viscoelastic properties of plasticized PVC reinforced with cellulose whiskers. J Appl Polym Sci 71:1797–1808
Costanzo PJ, Beyer FL (2007) Thermoresponsive, optically active films based on Diels-Alder chemistry. Chem Matter 19:6168–6173
DeTer DF, Silverstein R (1965) Reactions of carbodiimides. I. The mechanisms of the reactions of acetic acid with dicyclohexylcarbodiimide. J Am Chem Soc 88:1013–1019
Fujisawa S, Okita Y, Fukuzumi H, Saito T, Isogai A (2011) Preparation and characterization of TEMPO-oxidized cellulose nanofibril films with free carboxyl groups. Carbohydr Polym 84:579–583
Fukuzumi H, Saito T, Iwata T, Kumamoto Y, Isogai A (2009) Transparent and high gas barrier films of cellulose nanofibers prepared by TEMPO-mediated oxidation. Biomacromolecules 10:162–165
Heux L, Dinand E, Vignon MR (1999) Structural aspects in ultrathin cellulose microfibrils followed by 13C CP-MAS NMR. Carbohydr Polym 40:115–124
Hirota M, Furihata K, Saito T, Kawada T, Isogai A (2010) Glucose/glucuronic acid alternating co-polysaccharides prepared from TEMPO-oxidized native celluloses by surface peeling. Angew Chem Int Ed 49:7670–7672
Isogai A, Saito T, Fukuzumi H (2011) TEMPO-oxidized cellulose nanofibers. Nanoscale 3:71–85
Iwatake A, Nogi M, Yano H (2008) Cellulose nanofiber-reinforced polylactic acid. Compos Sci Technol 68:2103–2106
Khorana HG (1953) The chemistry of carbodiimides. Chem Rev 53:145–166
Kim DY, Nishiyama Y, Kuga S (2002) Surface acetylation of bacterial cellulose. Cellulose 9:361–367
Lasseuguette E (2008) Grafting onto microfibrils of native cellulose. Cellulose 15:571–580
Mohanty AK, Misra M, Drzal LT (2002) Sustainable bio-composites from renewable resources: opportunities and challenges in the green materials world. J Polym Environ 10:19–26
Okita Y, Saito T, Isogai A (2010) Entire surface oxidation of various cellulose microfibrils by TEMPO-mediated oxidation. Biomacromolecules 11:1696–1700
Okita Y, Fujisawa S, Saito T, Isogai A (2011) TEMPO-oxidized cellulose nanofibrils dispersed in organic solvents. Biomacromolecules 12:518–522
Saito T, Nishiyama Y, Putaux JL, Vignon M, Isogai A (2006) Homogeneous suspensions of individualized microfibrils from TEMPO-catalyzed oxidation of native cellulose. Biomacromolecules 7:1687–1691
Saito T, Kimura S, Nishiyama Y, Isogai A (2007) Cellulose nanofibers prepared by TEMPO-mediated oxidationof native cellulose. Biomacromolecules 8:2485–2491
Saito T, Hirota M, Tamura N, Kimura S, Fukuzumi H, Heux L, Isogai A (2009) Individualization of nano-sized plant cellulose fibrils by direct surface carboxylation using TEMPO catalyst under neutral conditions. Biomacromolecules 1:1992–1996
Sheehan JC, Hess GP (1955) A new method of forming peptide bonds. J Am Chem Soc 77:1067–1068
Siqueira G, Bras J, Dufresne A (2009) New process of chemical grafting of cellulose nanoparticles with a long chain isocyanate. Langmuir 26:402–411
Smith M, Moffatt JG, Khorana HG (1958) Carbodiimides 8. Observations on the reactions of carbodiimides with acids and some new applications in the synthesis of phosphoric acid esters. J Am Chem Soc 80:6204–6212
Viëtor RJ, Newman RH, Ha MA, Apperley DC, Jarvis MC (2002) Conformational features of crystal-surface cellulose from higher plants. Plant J 30:721–731
Yokum TS, Alsina J, Barany G (2000) Solid-phase syntheses of heterocycles containing the 2-aminothiophenol moiety. J Comb Chem 2:282–292
Acknowledgment
This research was supported by the Japan Society for the Promotion of Science (JSPS), Grant-in-Aid for Scientific Research (S, Grant number 21228007).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fujisawa, S., Okita, Y., Saito, T. et al. Formation of N-acylureas on the surface of TEMPO-oxidized cellulose nanofibril with carbodiimide in DMF. Cellulose 18, 1191–1199 (2011). https://doi.org/10.1007/s10570-011-9578-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-011-9578-z