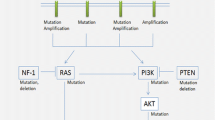
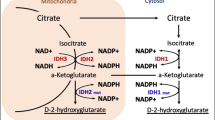
Abstract
Nearly 20 years ago, the concept of targeting the proteasome for cancer therapy began gaining momentum. This concept was driven by increased understanding of the biology/structure and function of the 26S proteasome, insight into the role of the proteasome in transformed cells, and the synthesis of pharmacological inhibitors with clinically favorable features. Subsequent in vitro, in vivo, and clinical testing culminated in the FDA approval of three proteasome inhibitors—bortezomib, carfilzomib, and ixazomib—for specific hematological malignancies. However, despite in vitro and in vivo studies pointing towards efficacy in solid tumors, clinical responses broadly have been evasive. For brain tumors, a malignancy in dire need of new approaches both in adult and pediatric patients, this has also been the case. Elucidation of proteasome-dependent processes in specific types of brain tumors, the evolution of newer proteasome targeting strategies, and the use of proteasome inhibitors in combination strategies will clarify how these agents can be leveraged more effectively to treat central nervous system malignancies. Since brain tumors represent a heterogeneous subset of solid tumors, and in particular, pediatric brain tumors possess distinct biology from adult brain tumors, tailoring of proteasome inhibitor-based strategies to specific subtypes of these tumors will be critical for advancing care for affected patients, and will be discussed in this review.


Similar content being viewed by others
References
Howlander N, Noone AM, Krapcho M, Garshell J, Miller D, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (2014) (eds). in National Cancer Institute, Bethesda, MD.
Hershko, A., & Ciechanover, A. (1998). The ubiquitin system. Annual Review of Biochemistry, 67, 425–479.
Hochstrasser, M. (2009). Origin and function of ubiquitin-like proteins. Nature, 458, 422–429.
Hough, R., Pratt, G., & Rechsteiner, M. (1987). Purification of two high molecular weight proteases from rabbit reticulocyte lysate. The Journal of Biological Chemistry, 262, 8303–8313.
Waxman, L., Fagan, J. M., Tanaka, K., & Goldberg, A. L. (1985). A soluble ATP-dependent system for protein degradation from murine erythroleukemia cells. Evidence for a protease which requires ATP hydrolysis but not ubiquitin. The Journal of Biological Chemistry, 260, 11994–12000.
Driscoll, J., & Goldberg, A. L. (1990). The proteasome (multicatalytic protease) is a component of the 1500-kDa proteolytic complex which degrades ubiquitin-conjugated proteins. The Journal of Biological Chemistry, 265, 4789–4792.
Ciechanover, A., Heller, H., Katz-Etzion, R., & Hershko, A. (1981). Activation of the heat-stable polypeptide of the ATP-dependent proteolytic system. Proceedings of the National Academy of Sciences of the United States of America, 78, 761–765.
Pickart, C. M., & Rose, I. A. (1985). Functional heterogeneity of ubiquitin carrier proteins. The Journal of Biological Chemistry, 260, 1573–1581.
Hershko, A., Heller, H., Elias, S., & Ciechanover, A. (1983). Components of ubiquitin-protein ligase system. Resolution, affinity purification, and role in protein breakdown. The Journal of Biological Chemistry, 258, 8206–8214.
van Nocker, S., & Vierstra, R. D. (1993). Multiubiquitin chains linked through lysine 48 are abundant in vivo and are competent intermediates in the ubiquitin proteolytic pathway. The Journal of Biological Chemistry, 268, 24766–24773.
Ye, Y., & Rape, M. (2009). Building ubiquitin chains: E2 enzymes at work. Nature Reviews. Molecular Cell Biology, 10, 755–764.
Eytan, E., Ganoth, D., Armon, T., & Hershko, A. (1989). ATP-dependent incorporation of 20S protease into the 26S complex that degrades proteins conjugated to ubiquitin. Proceedings of the National Academy of Sciences of the United States of America, 86, 7751–7755.
Glickman, M. H., & Ciechanover, A. (2002). The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiological Reviews, 82, 373–428.
Glickman, M. H., et al. (1998). A subcomplex of the proteasome regulatory particle required for ubiquitin-conjugate degradation and related to the COP9-signalosome and eIF3. Cell, 94, 615–623.
Groll, M., et al. (1999). The catalytic sites of 20S proteasomes and their role in subunit maturation: a mutational and crystallographic study. Proceedings of the National Academy of Sciences of the United States of America, 96, 10976–10983.
Orlowski, M., & Wilk, S. (2000). Catalytic activities of the 20 S proteasome, a multicatalytic proteinase complex. Archives of Biochemistry and Biophysics, 383, 1–16.
Kisselev, A. F., & Goldberg, A. L. (2001). Proteasome inhibitors: from research tools to drug candidates. Chemistry & Biology, 8, 739–758.
Rechsteiner, M., Realini, C., & Ustrell, V. (2000). The proteasome activator 11 S REG (PA28) and class I antigen presentation. Biochemical Journal, 345(Pt 1), 1–15.
Whitby, F. G., et al. (2000). Structural basis for the activation of 20S proteasomes by 11S regulators. Nature-London, 408, 115–120.
Rechsteiner, M., & Hill, C. P. (2005). Mobilizing the proteolytic machine: cell biological roles of proteasome activators and inhibitors. Trends in Cell Biology, 15, 27–33.
Noda, C., Tanahashi, N., Shimbara, N., Hendil, K. B., & Tanaka, K. (2000). Tissue distribution of constitutive proteasomes, immunoproteasomes, and PA28 in rats. Biochemical and Biophysical Research Communications, 277, 348–354.
Chen, X., Barton, L. F., Chi, A., Clurman, B. E., & Roberts, J. M. (2007). Ubiquitin-independent degradation of cell-cycle inhibitors by the REGgamma proteasome. Molecular Cell, 26, 843–852.
Groettrup, M., et al. (1995). The interferon-gamma-inducible 11 S regulator (PA28) and the LMP2/LMP7 subunits govern the peptide production by the 20 S proteasome in vitro. The Journal of Biological Chemistry, 270, 23808–23815.
Cascio, P., Hilton, C., Kisselev, A. F., Rock, K. L., & Goldberg, A. L. (2001). 26S proteasomes and immunoproteasomes produce mainly N-extended versions of an antigenic peptide. The EMBO Journal, 20, 2357–2366.
Toes, R., et al. (2001). Discrete cleavage motifs of constitutive and immunoproteasomes revealed by quantitative analysis of cleavage products. The Journal of Experimental Medicine, 194, 1–12.
Piccinini, M., et al. (2005). Characterization of the 20S proteasome in human glioblastomas. Anticancer Research, 25, 3203–3210.
Swartling, F. J. (2012). Myc proteins in brain tumor development and maintenance. Upsala Journal of Medical Sciences, 117, 122–131.
Bondy, M. L., et al. (2008). Brain tumor epidemiology: consensus from the Brain Tumor Epidemiology Consortium. Cancer, 113, 1953–1968.
Sturm, D., et al. (2012). Hotspot mutations in H3F3A and IDH1 define distinct epigenetic and biological subgroups of glioblastoma. Cancer Cell, 22, 425–437.
Sturm, D., et al. (2014). Paediatric and adult glioblastoma: multiform (epi)genomic culprits emerge. Nature Reviews. Cancer, 14, 92–107.
Korshunov, A., et al. (2015). Integrated analysis of pediatric glioblastoma reveals a subset of biologically favorable tumors with associated molecular prognostic markers. Acta Neuropathologica, 129, 669–678.
Liang, M. L., et al. (2008). Tyrosine kinase expression in pediatric high grade astrocytoma. Journal of Neuro-Oncology, 87, 247–253.
Puputti, M., et al. (2006). Amplification of KIT, PDGFRA, VEGFR2, and EGFR in gliomas. Molecular Cancer Research, 4, 927–934.
Peschard, P., & Park, M. (2003). Escape from Cbl-mediated downregulation: a recurrent theme for oncogenic deregulation of receptor tyrosine kinases. Cancer Cell, 3, 519–523.
Kuchay, S., et al. (2013). FBXL2- and PTPL1-mediated degradation of p110-free p85beta regulatory subunit controls the PI(3)K signalling cascade. Nature Cell Biology, 15, 472–480.
Mao, J. H., et al. (2008). FBXW7 targets mTOR for degradation and cooperates with PTEN in tumor suppression. Science, 321, 1499–1502.
Andrae, J., Gallini, R., & Betsholtz, C. (2008). Role of platelet-derived growth factors in physiology and medicine. Genes & Development, 22, 1276–1312.
Assanah, M. C., et al. (2009). PDGF stimulates the massive expansion of glial progenitors in the neonatal forebrain. Glia, 57, 1835–1847.
Clarke, I. D., & Dirks, P. B. (2003). A human brain tumor-derived PDGFR-alpha deletion mutant is transforming. Oncogene, 22, 722–733.
Paugh, B. S., et al. (2010). Integrated molecular genetic profiling of pediatric high-grade gliomas reveals key differences with the adult disease. Journal of Clinical Oncology, 28, 3061–3068.
Dai, C., et al. (2001). PDGF autocrine stimulation dedifferentiates cultured astrocytes and induces oligodendrogliomas and oligoastrocytomas from neural progenitors and astrocytes in vivo. Genes & Development, 15, 1913–1925.
Maxwell, M., et al. (1990). Coexpression of platelet-derived growth factor (PDGF) and PDGF-receptor genes by primary human astrocytomas may contribute to their development and maintenance. The Journal of Clinical Investigation, 86, 131–140.
N. Cancer Genome Atlas Research. (2008). Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature, 455, 1061–1068.
Thorarinsdottir, H. K., et al. (2008). Protein expression of platelet-derived growth factor receptor correlates with malignant histology and PTEN with survival in childhood gliomas. Clinical Cancer Research, 14, 3386–3394.
Shamah, S. M., Stiles, C. D., & Guha, A. (1993). Dominant-negative mutants of platelet-derived growth factor revert the transformed phenotype of human astrocytoma cells. Molecular and Cellular Biology, 13, 7203–7212.
Ohgaki, H., & Kleihues, P. (2007). Genetic pathways to primary and secondary glioblastoma. The American Journal of Pathology, 170, 1445–1453.
Bredel, M., Pollack, I. F., Hamilton, R. L., & James, C. D. (1999). Epidermal growth factor receptor expression and gene amplification in high-grade non-brainstem gliomas of childhood. Clinical Cancer Research, 5, 1786–1792.
Frederick, L., Wang, X. Y., Eley, G., & James, C. D. (2000). Diversity and frequency of epidermal growth factor receptor mutations in human glioblastomas. Cancer Research, 60, 1383–1387.
MacDonald, T. J., Aguilera, D., & Kramm, C. M. (2011). Treatment of high-grade glioma in children and adolescents. Neuro-Oncology, 13, 1049–1058.
Hartman, Z., Zhao, H., & Agazie, Y. M. (2013). HER2 stabilizes EGFR and itself by altering autophosphorylation patterns in a manner that overcomes regulatory mechanisms and promotes proliferative and transformation signaling. Oncogene, 32, 4169–4180.
Koochekpour, S., et al. (1997). Met and hepatocyte growth factor/scatter factor expression in human gliomas. Cancer Research, 57, 5391–5398.
Mosesson, Y., et al. (2003). Endocytosis of receptor tyrosine kinases is driven by monoubiquitylation, not polyubiquitylation. The Journal of Biological Chemistry, 278, 21323–21326.
Marmor, M. D., & Yarden, Y. (2004). Role of protein ubiquitylation in regulating endocytosis of receptor tyrosine kinases. Oncogene, 23, 2057–2070.
Yuan, T. L., & Cantley, L. C. (2008). PI3K pathway alterations in cancer: variations on a theme. Oncogene, 27, 5497–5510.
Zoncu, R., Efeyan, A., & Sabatini, D. M. (2011). mTOR: from growth signal integration to cancer, diabetes and ageing. Nature Reviews. Molecular Cell Biology, 12, 21–35.
Fang, D., & Liu, Y. C. (2001). Proteolysis-independent regulation of PI3K by Cbl-b-mediated ubiquitination in T cells. Nature Immunology, 2, 870–875.
Fan, Q. W., & Weiss, W. A. (2012). Inhibition of PI3K-Akt-mTOR signaling in glioblastoma by mTORC1/2 inhibitors. Methods in Molecular Biology, 821, 349–359.
Zhao, Y., & Sun, Y. (2012). Targeting the mTOR-DEPTOR pathway by CRL E3 ubiquitin ligases: therapeutic application. Neoplasia, 14, 360–367.
Olovnikov, I. A., Kravchenko, J. E., & Chumakov, P. M. (2009). Homeostatic functions of the p53 tumor suppressor: regulation of energy metabolism and antioxidant defense. Seminars in Cancer Biology, 19, 32–41.
Haupt, Y., Maya, R., Kazaz, A., & Oren, M. (1997). Mdm2 promotes the rapid degradation of p53. Nature, 387, 296–299.
Momand, J., Zambetti, G. P., Olson, D. C., George, D., & Levine, A. J. (1992). The mdm-2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivation. Cell, 69, 1237–1245.
Kruse, J. P., & Gu, W. (2009). Modes of p53 regulation. Cell, 137, 609–622.
Wang, X., & Jiang, X. (2012). Mdm2 and MdmX partner to regulate p53. FEBS Letters, 586, 1390–1396.
Love, I. M., & Grossman, S. R. (2012). It takes 15 to tango: making sense of the many ubiquitin ligases of p53. Genes & Cancer, 3, 249–263.
Pomeroy, S. L. (1994). The p53 tumor suppressor gene and pediatric brain tumors. Current Opinion in Pediatrics, 6, 632–635.
Zhukova, N., et al. (2013). Subgroup-specific prognostic implications of TP53 mutation in medulloblastoma. Journal of Clinical Oncology, 31, 2927–2935.
Pollack, I. F., et al. (1997). The relationship between TP53 mutations and overexpression of p53 and prognosis in malignant gliomas of childhood. Cancer Research, 57, 304–309.
Kunkele, A., et al. (2012). Pharmacological activation of the p53 pathway by nutlin-3 exerts anti-tumoral effects in medulloblastomas. Neuro-Oncology, 14, 859–869.
Knoepfler, P. S., Cheng, P. F., & Eisenman, R. N. (2002). N-myc is essential during neurogenesis for the rapid expansion of progenitor cell populations and the inhibition of neuronal differentiation. Genes & Development, 16, 2699–2712.
Korshunov, A., et al. (2012). Biological and clinical heterogeneity of MYCN-amplified medulloblastoma. Acta Neuropathologica, 123, 515–527.
Bjerke, L., et al. (2013). Histone H3.3. mutations drive pediatric glioblastoma through upregulation of MYCN. Cancer Discovery, 3, 512–519.
Kawauchi, D., et al. (2012). A mouse model of the most aggressive subgroup of human medulloblastoma. Cancer Cell, 21, 168–180.
Pei, Y., et al. (2012). An animal model of MYC-driven medulloblastoma. Cancer Cell, 21, 155–167.
Choi, S. H., Wright, J. B., Gerber, S. A., & Cole, M. D. (2010). Myc protein is stabilized by suppression of a novel E3 ligase complex in cancer cells. Genes & Development, 24, 1236–1241.
Popov, N., Schulein, C., Jaenicke, L. A., & Eilers, M. (2010). Ubiquitylation of the amino terminus of Myc by SCF(beta-TrCP) antagonizes SCF(Fbw7)-mediated turnover. Nature Cell Biology, 12, 973–981.
von der Lehr, N., et al. (2003). The F-box protein Skp2 participates in c-Myc proteosomal degradation and acts as a cofactor for c-Myc-regulated transcription. Molecular Cell, 11, 1189–1200.
Adhikary, S., et al. (2005). The ubiquitin ligase HectH9 regulates transcriptional activation by Myc and is essential for tumor cell proliferation. Cell, 123, 409–421.
Zhao, X., et al. (2008). The HECT-domain ubiquitin ligase Huwe1 controls neural differentiation and proliferation by destabilizing the N-Myc oncoprotein. Nature Cell Biology, 10, 643–653.
Penas, C., Ramachandran, V., & Ayad, N. G. (2011). The APC/C ubiquitin ligase: from cell biology to tumorigenesis. Frontiers in Oncology, 1, 60.
Hsu, J. Y., Reimann, J. D., Sorensen, C. S., Lukas, J., & Jackson, P. K. (2002). E2F-dependent accumulation of hEmi1 regulates S phase entry by inhibiting APC(Cdh1). Nature Cell Biology, 4, 358–366.
Lehman, N. L., Verschuren, E. W., Hsu, J. Y., Cherry, A. M., & Jackson, P. K. (2006). Overexpression of the anaphase promoting complex/cyclosome inhibitor Emi1 leads to tetraploidy and genomic instability of p53-deficient cells. Cell Cycle, 5, 1569–1573.
Margottin-Goguet, F., et al. (2003). Prophase destruction of Emi1 by the SCF(betaTrCP/Slimb) ubiquitin ligase activates the anaphase promoting complex to allow progression beyond prometaphase. Developmental Cell, 4, 813–826.
Guardavaccaro, D., et al. (2003). Control of meiotic and mitotic progression by the F box protein beta-Trcp1 in vivo. Developmental Cell, 4, 799–812.
Carrano, A. C., Eytan, E., Hershko, A., & Pagano, M. (1999). SKP2 is required for ubiquitin-mediated degradation of the CDK inhibitor p27. Nature Cell Biology, 1, 193–199.
Marti, A., Wirbelauer, C., Scheffner, M., & Krek, W. (1999). Interaction between ubiquitin-protein ligase SCFSKP2 and E2F-1 underlies the regulation of E2F-1 degradation. Nature Cell Biology, 1, 14–19.
Peart, M. J., et al. (2010). APC/C(Cdc20) targets E2F1 for degradation in prometaphase. Cell Cycle, 9, 3956–3964.
Visintin, R., Prinz, S., & Amon, A. (1997). CDC20 and CDH1: a family of substrate-specific activators of APC-dependent proteolysis. Science, 278, 460–463.
Puram, S. V., & Bonni, A. (2011). Novel functions for the anaphase-promoting complex in neurobiology. Seminars in Cell & Developmental Biology, 22, 586–594.
Lasorella, A., et al. (2006). Degradation of Id2 by the anaphase-promoting complex couples cell cycle exit and axonal growth. Nature, 442, 471–474.
Vlachostergios, P. J., Voutsadakis, I. A., & Papandreou, C. N. (2012). The ubiquitin-proteasome system in glioma cell cycle control. Cell Div, 7, 18.
Schiffer, D., Cavalla, P., Fiano, V., Ghimenti, C., & Piva, R. (2002). Inverse relationship between p27/Kip.1 and the F-box protein Skp2 in human astrocytic gliomas by immunohistochemistry and Western blot. Neuroscience Letters, 328, 125–128.
Veeriah, S., et al. (2010). Somatic mutations of the Parkinson’s disease-associated gene PARK2 in glioblastoma and other human malignancies. Nature Genetics, 42, 77–82.
Ben-Neriah, Y., & Karin, M. (2011). Inflammation meets cancer, with NF-kappaB as the matchmaker. Nature Immunology, 12, 715–723.
Gilmore, T. D. (2006). Introduction to NF-kappaB: players, pathways, perspectives. Oncogene, 25, 6680–6684.
Harhaj, E. W., & Dixit, V. M. (2011). Deubiquitinases in the regulation of NF-kappaB signaling. Cell Research, 21, 22–39.
Arabi, A., et al. (2012). Proteomic screen reveals Fbw7 as a modulator of the NF-kappaB pathway. Nature Communications, 3, 976.
Busino, L., et al. (2012). Fbxw7alpha- and GSK3-mediated degradation of p100 is a pro-survival mechanism in multiple myeloma. Nature Cell Biology, 14, 375–385.
Fukushima, H., et al. (2012). SCF(Fbw7) modulates the NFkB signaling pathway by targeting NFkB2 for ubiquitination and destruction. Cell Reports, 1, 434–443.
Bredel, M., et al. (2011). NFKBIA deletion in glioblastomas. The New England Journal of Medicine, 364, 627–637.
Wang, H., et al. (2004). Analysis of the activation status of Akt, NFkappaB, and Stat3 in human diffuse gliomas. Laboratory Investigation, 84, 941–951.
Hussain, S. F., et al. (2006). The role of human glioma-infiltrating microglia/macrophages in mediating antitumor immune responses. Neuro-Oncology, 8, 261–279.
Dhall, G. (2009). Medulloblastoma. Journal of Child Neurology, 24, 1418–1430.
Kool, M., et al. (2012). Molecular subgroups of medulloblastoma: an international meta-analysis of transcriptome, genetic aberrations, and clinical data of WNT, SHH, Group 3, and Group 4 medulloblastomas. Acta Neuropathologica, 123, 473–484.
DeSouza, R. M., Jones, B. R., Lowis, S. P., & Kurian, K. M. (2014). Pediatric medulloblastoma - update on molecular classification driving targeted therapies. Frontiers in Oncology, 4, 176.
Schuller, U., et al. (2008). Acquisition of granule neuron precursor identity is a critical determinant of progenitor cell competence to form Shh-induced medulloblastoma. Cancer Cell, 14, 123–134.
Gibson, P., et al. (2010). Subtypes of medulloblastoma have distinct developmental origins. Nature, 468, 1095–1099.
Kool, M., et al. (2014). Genome sequencing of SHH medulloblastoma predicts genotype-related response to smoothened inhibition. Cancer Cell, 25, 393–405.
Yauch, R. L., et al. (2009). Smoothened mutation confers resistance to a hedgehog pathway inhibitor in medulloblastoma. Science, 326, 572–574.
Zurawel, R. H., Chiappa, S. A., Allen, C., & Raffel, C. (1998). Sporadic medulloblastomas contain oncogenic beta-catenin mutations. Cancer Research, 58, 896–899.
Huang, H., et al. (2000). APC mutations in sporadic medulloblastomas. The American Journal of Pathology, 156, 433–437.
Rausch, T., et al. (2012). Genome sequencing of pediatric medulloblastoma links catastrophic DNA rearrangements with TP53 mutations. Cell, 148, 59–71.
Lehman, N. L. (2009). The ubiquitin proteasome system in neuropathology. Acta Neuropathologica, 118, 329–347.
Vriend, J., Ghavami, S., & Marzban, H. (2015). The role of the ubiquitin proteasome system in cerebellar development and medulloblastoma. Molecular Brain, 8, 64.
Alvarez-Rodriguez, R., Barzi, M., Berenguer, J., & Pons, S. (2007). Bone morphogenetic protein 2 opposes Shh-mediated proliferation in cerebellar granule cells through a TIEG-1-based regulation of Nmyc. The Journal of Biological Chemistry, 282, 37170–37180.
Nelson, W. J., & Nusse, R. (2004). Convergence of Wnt, beta-catenin, and cadherin pathways. Science, 303, 1483–1487.
Wodarz, A., & Nusse, R. (1998). Mechanisms of Wnt signaling in development. Annual Review of Cell and Developmental Biology, 14, 59–88.
Mikesch, J. H., Steffen, B., Berdel, W. E., Serve, H., & Muller-Tidow, C. (2007). The emerging role of Wnt signaling in the pathogenesis of acute myeloid leukemia. Leukemia, 21, 1638–1647.
Couffinhal, T., Dufourcq, P., & Duplaa, C. (2006). Beta-catenin nuclear activation: common pathway between Wnt and growth factor signaling in vascular smooth muscle cell proliferation? Circulation Research, 99, 1287–1289.
Northcott, P. A., et al. (2011). Pediatric and adult sonic hedgehog medulloblastomas are clinically and molecularly distinct. Acta Neuropathologica, 122, 231–240.
Fan, X., & Eberhart, C. G. (2008). Medulloblastoma stem cells. Journal of Clinical Oncology, 26, 2821–2827.
Scotting, P. J., Walker, D. A., & Perilongo, G. (2005). Childhood solid tumours: a developmental disorder. Nature Reviews. Cancer, 5, 481–488.
Yue, S., Chen, Y., & Cheng, S. Y. (2009). Hedgehog signaling promotes the degradation of tumor suppressor Sufu through the ubiquitin-proteasome pathway. Oncogene, 28, 492–499.
Kim, J. J., et al. (2011). Suppressor of fused controls mid-hindbrain patterning and cerebellar morphogenesis via GLI3 repressor. The Journal of Neuroscience, 31, 1825–1836.
Gulino, A., Di Marcotullio, L., Canettieri, G., De Smaele, E., & Screpanti, I. (2012). Hedgehog/Gli control by ubiquitination/acetylation interplay. Vitamins and Hormones, 88, 211–227.
Lau, A. W., Fukushima, H., & Wei, W. (2012). The Fbw7 and betaTRCP E3 ubiquitin ligases and their roles in tumorigenesis. Front Biosci (Landmark Ed), 17, 2197–2212.
Forget, A., et al. (2014). Shh signaling protects Atoh1 from degradation mediated by the E3 ubiquitin ligase Huwe1 in neural precursors. Developmental Cell, 29, 649–661.
Chen, D., et al. (2005). ARF-BP1/Mule is a critical mediator of the ARF tumor suppressor. Cell, 121, 1071–1083.
Zhao, H., Ayrault, O., Zindy, F., Kim, J. H., & Roussel, M. F. (2008). Post-transcriptional down-regulation of Atoh1/Math1 by bone morphogenic proteins suppresses medulloblastoma development. Genes & Development, 22, 722–727.
Cao, Y., et al. (2014). Selective small molecule compounds increase BMP-2 responsiveness by inhibiting Smurf1-mediated Smad1/5 degradation. Scientific Reports, 4, 4965.
Babaei-Jadidi, R., et al. (2011). FBXW7 influences murine intestinal homeostasis and cancer, targeting Notch, Jun, and DEK for degradation. The Journal of Experimental Medicine, 208, 295–312.
Davis, R. J., Welcker, M., & Clurman, B. E. (2014). Tumor suppression by the Fbw7 ubiquitin ligase: mechanisms and opportunities. Cancer Cell, 26, 455–464.
Wang, Z., Liu, P., Inuzuka, H., & Wei, W. (2014). Roles of F-box proteins in cancer. Nature Reviews. Cancer, 14, 233–247.
Hede, S. M., Savov, V., Weishaupt, H., Sangfelt, O., & Swartling, F. J. (2014). Oncoprotein stabilization in brain tumors. Oncogene, 33, 4709–4721.
Hartmann, W., et al. (2006). Phosphatidylinositol 3′-kinase/AKT signaling is activated in medulloblastoma cell proliferation and is associated with reduced expression of PTEN. Clinical Cancer Research, 12, 3019–3027.
Wlodarski, P., Grajkowska, W., Lojek, M., Rainko, K., & Jozwiak, J. (2006). Activation of Akt and Erk pathways in medulloblastoma. Folia Neuropathologica, 44, 214–220.
Yang, F., et al. (2012). Bortezomib induces apoptosis and growth suppression in human medulloblastoma cells, associated with inhibition of AKT and NF-kB signaling, and synergizes with an ERK inhibitor. Cancer Biology & Therapy, 13, 349–357.
Van Waes, C. (2007). Nuclear factor-kappaB in development, prevention, and therapy of cancer. Clinical Cancer Research, 13, 1076–1082.
Prasad, S., Ravindran, J., & Aggarwal, B. B. (2010). NF-kappaB and cancer: how intimate is this relationship. Molecular and Cellular Biochemistry, 336, 25–37.
Northcott, P. A., Dubuc, A. M., Pfister, S., & Taylor, M. D. (2012). Molecular subgroups of medulloblastoma. Expert Review of Neurotherapeutics, 12, 871–884.
Sunwoo, J. B., et al. (2001). Novel proteasome inhibitor PS-341 inhibits activation of nuclear factor-kappa B, cell survival, tumor growth, and angiogenesis in squamous cell carcinoma. Clinical Cancer Research, 7, 1419–1428.
Adams, J. (2004). The development of proteasome inhibitors as anticancer drugs. Cancer Cell, 5, 417–421.
Spiller, S. E., Logsdon, N. J., Deckard, L. A., & Sontheimer, H. (2011). Inhibition of nuclear factor kappa-B signaling reduces growth in medulloblastoma in vivo. BMC Cancer, 11, 136.
Hoesel, B., & Schmid, J. A. (2013). The complexity of NF-kappaB signaling in inflammation and cancer. Molecular Cancer, 12, 86.
Juvekar, A., et al. (2011). Bortezomib induces nuclear translocation of IkappaBalpha resulting in gene-specific suppression of NF-kappaB—dependent transcription and induction of apoptosis in CTCL. Molecular Cancer Research, 9, 183–194.
Jariel-Encontre, I., Bossis, G., & Piechaczyk, M. (2008). Ubiquitin-independent degradation of proteins by the proteasome. Biochimica et Biophysica Acta, 1786, 153–177.
Pagano, M., et al. (1995). Role of the ubiquitin-proteasome pathway in regulating abundance of the cyclin-dependent kinase inhibitor p27. Science, 269, 682–685.
Maddika, S., et al. (2007). Cell survival, cell death and cell cycle pathways are interconnected: implications for cancer therapy. Drug Resistance Updates, 10, 13–29.
Epstein, F. H., Mitch, W. E., & Goldberg, A. L. (1996). Mechanisms of muscle wasting—the role of the ubiquitin–proteasome pathway. The New England Journal of Medicine, 335, 1897–1905.
Groll, M., Berkers, C. R., Ploegh, H. L., & Ovaa, H. (2006). Crystal structure of the boronic acid-based proteasome inhibitor bortezomib in complex with the yeast 20S proteasome. Structure, 14, 451–456.
Berkers, C. R., et al. (2005). Activity probe for in vivo profiling of the specificity of proteasome inhibitor bortezomib. Nature Methods, 2, 357–362.
Hideshima, T., et al. (2001). The proteasome inhibitor PS-341 inhibits growth, induces apoptosis, and overcomes drug resistance in human multiple myeloma cells. Cancer Research, 61, 3071–3076.
Jagannath, S., et al. (2004). A phase 2 study of two doses of bortezomib in relapsed or refractory myeloma. British Journal of Haematology, 127, 165–172.
Richardson, P. G., et al. (2007). Extended follow-up of a phase 3 trial in relapsed multiple myeloma: final time-to-event results of the APEX trial. Blood, 110, 3557–3560.
Kane, R. C., Bross, P. F., Farrell, A. T., & Pazdur, R. (2003). Velcade: U.S. FDA approval for the treatment of multiple myeloma progressing on prior therapy. The Oncologist, 8, 508–513.
Kane, R. C., Farrell, A. T., Sridhara, R., & Pazdur, R. (2006). United States Food and Drug Administration approval summary: bortezomib for the treatment of progressive multiple myeloma after one prior therapy. Clinical cancer research : an official journal of the American Association for Cancer Research, 12, 2955–2960.
Kane, R. C., et al. (2007). Bortezomib for the treatment of mantle cell lymphoma. Clinical cancer research : an official journal of the American Association for Cancer Research, 13, 5291–5294.
Potts, B. C., et al. (2011). Marizomib, a proteasome inhibitor for all seasons: preclinical profile and a framework for clinical trials. Current Cancer Drug Targets, 11, 254–284.
Millward, M., et al. (2011). Phase 1 clinical trial of the novel proteasome inhibitor marizomib with the histone deacetylase inhibitor vorinostat in patients with melanoma, pancreatic and lung cancer based on in vitro assessments of the combination. Investigational New Drugs, 30, 2303–2317.
Williams, P. G., et al. (2005). New cytotoxic salinosporamides from the marine Actinomycete Salinispora tropica. The Journal of Organic Chemistry, 70, 6196–6203.
Corey, E. J., & Li, W. D. (1999). Total synthesis and biological activity of lactacystin, omuralide and analogs. Chemical & Pharmaceutical Bulletin, 47, 1–10.
Feling, R. H., et al. (2003). Salinosporamide A: a highly cytotoxic proteasome inhibitor from a novel microbial source, a marine bacterium of the new genus Salinospora. Angewandte Chemie (International Ed. in English), 42, 355–357.
Groll, M., Huber, R., & Potts, B. C. M. (2006). Crystal structures of Salinosporamide A (NPI-0052) and B (NPI-0047) in complex with the 20S proteasome reveal important consequences of beta-lactone ring opening and a mechanism for irreversible binding. Journal of the American Chemical Society, 128, 5136–5141.
Manam, R. R., et al. (2008). Leaving groups prolong the duration of 20S proteasome inhibition and enhance the potency of salinosporamides. Journal of Medicinal Chemistry, 51, 6711–6724.
Miller, C. P., et al. (2011). Specific and prolonged proteasome inhibition dictates apoptosis induction by marizomib and its analogs. Chemico-Biological Interactions, 194, 58–68.
Chauhan, D., et al. (2005). A novel orally active proteasome inhibitor induces apoptosis in multiple myeloma cells with mechanisms distinct from bortezomib. Cancer Cell, 8, 407–419.
Kuhn, D. J., et al. (2009). Targeted inhibition of the immunoproteasome is a potent strategy against models of multiple myeloma that overcomes resistance to conventional drugs and nonspecific proteasome inhibitors. Blood, 113, 4667–4676.
Muchamuel, T., et al. (2009). A selective inhibitor of the immunoproteasome subunit LMP7 blocks cytokine production and attenuates progression of experimental arthritis. Nature Medicine, 15, 781–787.
Basler, M., Dajee, M., Moll, C., Groettrup, M., & Kirk, C. J. (2010). Prevention of experimental colitis by a selective inhibitor of the immunoproteasome. Journal of Immunology, 185, 634–641.
Ohshima-Hosoyama, S., Davare, M. A., Hosoyama, T., Nelon, L. D., & Keller, C. (2011). Bortezomib stabilizes NOXA and triggers ROS-associated apoptosis in medulloblastoma. Journal of Neuro-Oncology, 105, 475–483.
Samano, A. K., et al. (2010). Functional evaluation of therapeutic response for a mouse model of medulloblastoma. Transgenic Research, 19, 829–840.
Taniguchi, E., et al. (2009). Bortezomib reverses a post-translational mechanism of tumorigenesis for patched1 haploinsufficiency in medulloblastoma. Pediatric Blood & Cancer, 53, 136–144.
Grasso, C. S., et al. (2015). Functionally defined therapeutic targets in diffuse intrinsic pontine glioma. Nature Medicine, 21, 555–559.
Dimopoulos, M. A., et al. (2017). Carfilzomib or bortezomib in relapsed or refractory multiple myeloma (ENDEAVOR): an interim overall survival analysis of an open-label, randomised, phase 3 trial. The Lancet Oncology, 18, 1327–1337.
Abbott, N. J., Patabendige, A. A. K., Dolman, D. E. M., Yusof, S. R., & Begley, D. J. (2010). Structure and function of the blood-brain barrier. Neurobiology of Disease, 37, 13–25.
Zünkeler, B., et al. (1996). Quantification and pharmacokinetics of blood-brain barrier disruption in humans. Journal of Neurosurgery, 85, 1056–1065.
Balyasnikova, I. V., Ferguson, S. D., Han, Y., Liu, F., & Lesniak, M. S. (2011). Therapeutic effect of neural stem cells expressing TRAIL and bortezomib in mice with glioma xenografts. Cancer Letters, 310, 148–159.
Asklund, T., et al. (2012). Synergistic killing of glioblastoma stem-like cells by bortezomib and HDAC inhibitors. Anticancer Research, 32, 2407–2413.
Premkumar, D. R., Jane, E. P., Agostino, N. R., DiDomenico, J. D., & Pollack, I. F. (2013). Bortezomib-induced sensitization of malignant human glioma cells to vorinostat-induced apoptosis depends on reactive oxygen species production, mitochondrial dysfunction, Noxa upregulation, Mcl-1 cleavage, and DNA damage. Molecular Carcinogenesis, 52, 118–133.
Friday, B. B., et al. (2012). Phase II trial of vorinostat in combination with bortezomib in recurrent glioblastoma: a north central cancer treatment group study. Neuro-Oncology, 14, 215–221.
Labussiere, M., Pinel, S., Delfortrie, S., Plenat, F., & Chastagner, P. (2008). Proteasome inhibition by bortezomib does not translate into efficacy on two malignant glioma xenografts. Oncology Reports, 20, 1283–1287.
Vlashi, E., et al. (2010). Differential effects of the proteasome inhibitor NPI-0052 against glioma cells. Translational Oncology, 3, 50–55.
Singh, A. V., et al. (2010). Pharmacodynamic and efficacy studies of the novel proteasome inhibitor NPI-0052 (marizomib) in a human plasmacytoma xenograft murine model. British Journal of Haematology, 149, 550–559.
Di, K., et al. (2016). Marizomib activity as a single agent in malignant gliomas: ability to cross the blood-brain barrier. Neuro-Oncology, 18, 840–848.
Manton, C. A., et al. (2016). Induction of cell death by the novel proteasome inhibitor marizomib in glioblastoma in vitro and in vivo. Scientific Reports, 6, 18953.
Berkowitz, A., & Walker, S. (2012). Bortezomib-induced peripheral neuropathy in patients with multiple myeloma. Clinical Journal of Oncology Nursing, 16, 86–89.
Argyriou, A. A., Iconomou, G., & Kalofonos, H. P. (2008). Bortezomib-induced peripheral neuropathy in multiple myeloma: a comprehensive review of the literature. Blood, 112, 1593–1599.
Wolf, S., Barton, D., Kottschade, L., Grothey, A., & Loprinzi, C. (2008). Chemotherapy-induced peripheral neuropathy: prevention and treatment strategies. European journal of cancer (Oxford, England : 1990), 44, 1507–1515.
Delforge, M., et al. (2010). Treatment-related peripheral neuropathy in multiple myeloma: the challenge continues. The Lancet. Oncology, 11, 1086–1095.
Zou, W., et al. (2006). Vitamin C inactivates the proteasome inhibitor PS-341 in human cancer cells. Clinical cancer research : an official journal of the American Association for Cancer Research, 12, 273–280.
Richardson PG et al., paper presented at the American Society of Hematology Meeting Abstract, Nov 01 2011.
Yoo, J. Y., et al. (2014). Bortezomib-induced unfolded protein response increases oncolytic HSV-1 replication resulting in synergistic antitumor effects. Clinical Cancer Research, 20, 3787–3798.
Yoo, J. Y., et al. (2016). Bortezomib treatment sensitizes oncolytic HSV-1-treated tumors to NK cell immunotherapy. Clinical Cancer Research, 22, 5265–5276.
Leestemaker, Y., et al. (2017). Proteasome activation by small molecules. Cell Chemical Biology, 24, 725–736 e727.
Phuphanich, S., et al. (2010). Phase 1 clinical trial of bortezomib in adults with recurrent malignant glioma. Journal of Neuro-Oncology, 100, 95–103.
Blaney, S. M., et al. (2004). Phase I study of the proteasome inhibitor bortezomib in pediatric patients with refractory solid tumors: a Children’s Oncology Group study (ADVL0015). Journal of Clinical Oncology, 22, 4804–4809.
Portnow, J., et al. (2012). A phase I study of bortezomib and temozolomide in patients with advanced solid tumors. Cancer Chemotherapy and Pharmacology, 69, 505–514.
Yin, D., et al. (2005). Proteasome inhibitor PS-341 causes cell growth arrest and apoptosis in human glioblastoma multiforme (GBM). Oncogene, 24, 344–354.
Riordan, B., Yu, L. J., Hatsis, P., Brockman, A., Daniels, S., Stagliano, N., Finklestein, S., Ren, J., Milton, M., & Miwa, G. (2006). Study of brain and whole blood PK/PD of bortezomib in rat models. Journal of Clinical Oncology, 24, 12036.
Muscal, J. A., et al. (2013). A phase I trial of vorinostat and bortezomib in children with refractory or recurrent solid tumors: a Children’s Oncology Group phase I consortium study (ADVL0916). Pediatric Blood & Cancer, 60, 390–395.
McCracken, D. J., Celano, E. C., Voloschin, A. D., Read, W. L., & Olson, J. J. (2016). Phase I trial of dose-escalating metronomic temozolomide plus bevacizumab and bortezomib for patients with recurrent glioblastoma. Journal of Neuro-Oncology, 130, 193–201.
Bota, D. A., et al. (2013). Proteasome inhibition with bortezomib induces cell death in GBM stem-like cells and temozolomide-resistant glioma cell lines, but stimulates GBM stem-like cells’ VEGF production and angiogenesis. Journal of Neurosurgery, 119, 1415–1423.
Acknowledgements
The authors gratefully acknowledge research support from the Center for Cancer Epigenetics at MD Anderson, NIH (P30 CA016672, P50 CA127001, R21 NS093387 (to J.C.) and is R01 NS079715 (to V.G.)), and the Thomas Scott Family Foundation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Contribution for Cancer and Metastasis Reviews Special Issue: “The Proteasome: an important player in tumor progression and a novel target for cancer therapy.”
Rights and permissions
About this article
Cite this article
Zaky, W., Manton, C., Miller, C.P. et al. The ubiquitin-proteasome pathway in adult and pediatric brain tumors: biological insights and therapeutic opportunities. Cancer Metastasis Rev 36, 617–633 (2017). https://doi.org/10.1007/s10555-017-9700-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-017-9700-2