Abstract
Climate change is likely to influence competitive interactions between native and non-native plant species by altering soil resource availability. The objective of this study was to characterize how shifts in fall precipitation timing due to climate change affect plant community assembly of native and non-native plant communities. We selected common non-native annuals and native perennial species from the Great Basin Desert in western North America and grew them in native, non-native, and native + non-native mixed communities. We tested the responses of these three community types to simulated earlier fall precipitation in a full factorial design. Early fall precipitation dramatically increased the height, density, biomass, seed production per unit biomass, and carbon-to-nitrogen ratio (C:N) of both native and non-native plant communities in comparison with the late precipitation treatment. However, competition with non-native species reduced the positive benefit of early precipitation for the native plant community. When grown in a native-only community, native plant species increased in height (twofold), density (threefold), biomass (13-fold), seed production per unit biomass (18-fold), and C:N (1.3-fold)but not tissue percent nitrogen as compared to a mixed community. In contrast, non-native plant species grown in mixed communities with natives showed little to no reduction in growth and reproduction. While all species benefitted from earlier fall precipitation our data suggest that increased earlier fall precipitation will likely magnify the exclusion of native vegetation by non-native annuals, particularly Bromus tectorum L., which is largely responsible for human-grass-fire cycles in this ecoregion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plant community assembly is influenced by abiotic and biotic interactions that vary across space and time (Kraft et al. 2015; McGill et al. 2006). Plant regeneration is influenced by dispersal potential, abiotic filtering (resource acquisition, stress tolerance), biotic filtering (e.g., competitive exclusion, density dependence), or a combination of these assembly filters (Kraft et al. 2015). Non-native species that successfully spread in their introduced range are increasingly competing for limited resources (Kolb et al. 2002; Pearson et al. 2018) that puts the ecosystem at risk of state changes characterized by loss of native plant community structure and function (Catford et al. 2012; Chambers et al. 2016; Fargione et al. 2003).
Non-native annual grasses introduced from Eurasia drive human-grass-fire cycles and create novel disturbance regimes in desert ecosystems of North America (Brooks et al.2004; Bradley et al. 2018; Fusco et al. 2022). Post-disturbance plant community assembly in these deserts is influenced by competition between non-native annual grasses and native perennial plant communities (St. Clair et al. 2016; Bishop et al. 2020). Plant invasion success is determined in large part by competition for resources that vary in time and space (Chesson 2003; Davis et al. 2000; Vilà and Weiner 2004). The Fluctuating Resource Hypothesis (FRH) (Davis et al. 2000) posits that traits that optimize the capture of fluctuating resources increase invasion success. In deserts where competition for soil resources is high, germination responsiveness, high propagule pressure, and fast growth rates are key traits that tend to optimize resource acquisition (priority effects) (Booth et al. 2003a; Chambers et al. 2007; Germino et al. 2016; Meyer et al. 1997; Vilà and Weiner 2004; Wolkovich and Cleland 2011; Gioria and Pyšek 2017; St. Clair and Bishop 2019). Therefore, changes from historically available resources will likely affect the invasibility of a system and subsequent structuring of the plant community (Goodale and Wilsey 2018; Thomson et al. 2017; Ulrich and Perkins 2014; Kolb et al. 2002).
The magnitude and timing of precipitation strongly determine plant recruitment success, particularly in water-limited systems (Sala and Lauenroth 1982; Schwinning and Sala 2004; Chesson 2003). In cold deserts of North America, precipitation typically comes in winter and early spring as snow and rain (Miller et al. 2013a, b). Forecasts of climate change for these regions include projections of earlier fall precipitation and shifts to less snow and more rain in winter, accompanied by drier summers (Abatzoglou and Kolden 2011). Competitive outcomes between native and invasive vegetation will increasingly be driven by traits that optimize the capture of soil moisture in response to these shifts in precipitation patterns (Vaughn and Young 2015; Bishop et al. 2020). Non-native species with strong priority effects and faster relative growth rates may acquire resources earlier than native species (Gill et al. 2018; Goodale and Wilsey 2018; Horn et al. 2017; Young et al. 2015), potentially driving competitive exclusion of natives. Recent work conducted in the Mojave Desert suggests that earlier fall precipitation is increasing the spread of invasive annual grasses and wildfire (Bishop et al. 2020; Horn et al. 2017; Horn and St. Clair 2017). However, little research has explored community-level interactions between non-native and native plant communities in response to shifts in soil resource availability due to climate change. As disturbance regimes expand and climate change intensifies, understanding patterns of plant community responses and interactions to changing resource availability is critically important (Chambers and Pellant 2008; Pilliod et al. 2017).
Native plant communities and non-native annual grasses often employ contrasting resource acquisition traits and strategies (reviewed in Chesson 2003 and Davidson et al. 2011) that are likely to interact with changes in the magnitude and timing of changing precipitation regimes (Kadmon and Shmida 1990; Gebauer et al. 2002; Walk et al. 2022). Greater soil nitrogen acquisition and nutrient use efficiency by non-native plants may promote rapid re-establishment and competitive advantages over native vegetation (Funk and Vitousek 2007; Slate et al. 2022), particularly in low-resource environments. These traits can be linked to greater responsiveness and mechanistically driving help drive invasion success, particularly with pulse events (James 2008). Therefore, changes in nutrient inputs due to human activities (e.g., disturbance, nutrient deposition, climate change) may affect plant establishment because mismatched resource acquisition strategies of non-native and native species may alter community assembly and subsequent composition (Blank 2010; Callaway 2000; Herget et al. 2015; McGill et al. 2006; Vilà and Weiner 2004 and others).
The dramatic invasion success of non-native invasive annuals such as Bromus tectorum L. and Sisymbrium altissimum L. in the native perennial sagebrush communities of the Great Basin Desert provides an ideal study system for better understanding plant community assembly responses to shifts in resource availability due to climate change and changing fire regimes. Native and invasive regeneration in the Great Basin Desert is strongly influenced by competition for space and limiting soil resources particularly following disturbance (Booth et al. 2003a; Rau et al. 2014; Chambers et al. 2016). The temporal and spatial distribution of precipitation and soil resources are strongly shifting in the Great Basin due to climate change (reviewed in Nielsen and Ball 2015) and changing fire regimes (Allen et al. 2011; Abella and Engle 2013). As changes in precipitation, and subsequently availability of resources, are linked with the human-grass-fire cycle established in the Great Basin, (Pilliod et al. 2017; Fusco et al. 2022) we aim to understand how native plant communities, starting from seed, may interact with invasive plant communities under conditions of precipitation timing shifts linked to climate change.
The objective of this study was to determine how fall precipitation timing influences the establishment patterns of native and invasive plant communities grown independently and together. We examined the following questions: (1) Do non-native invasive plant species generally exhibit more robust initial growth and fecundity than native species? (2) Is the growth and fecundity of Great Basin invasive plant communities more responsive to changes in the timing of fall precipitation pulses than native communities? (3) Is there evidence that Great Basin invasive plant community's responsiveness to early fall precipitation, and its nutrient use, results in a competitive advantage over native communities?
Methods
Experimental system
Our study sites were in the ancestral Núu-agha-tʉvʉ-pʉ̱ (Ute) and Goshute lands at Rush Valley in Tooele County, Utah, USA (40.089300°, − 112.306239°), a latitudinally central location of the sagebrush steppe ecosystem of the Great Basin Desert ecoregion. Soils in the mid-low elevation (1650 m) area classified as silty, mixed mesic Haplic Natrargid Taylors Flat Loam (Soil Survey Staff). There have been no recent fires (decades or longer) or grazing activity evidenced by well-developed biological soil crusts (rugose crusts consisting of lichens, mosses, and dark cyanobacteria described in Aanderud et al. 2019), no evidence of Bromus tectorum invasion and the native plants are mature shrubs and perennial grasses, primarily Artemisia tridentata Nutt. ssp. wyomingensis Beetle and Young (hereafter Artemisia tridentata) and Elymus elymoides (Raf.) Swezey (bottlebrush squirreltail).
Experimental design
We extracted 80 soil cores in PVC cylinders that were 15 cm in diameter and 40 cm deep from intershrub spaces. To keep soils intact but still allow for water flow and root penetration, a mesh screen was wrapped around the bottom of each core. We wetted the area where soil cores were extracted to reduce cracking and breakage of the biological soil crust. Biological soil crusts remained intact, and the cores were devoid of above-ground vegetation. The cores were transported to a common garden site near Brigham Young University (BYU), Provo, Utah, USA (40.2518°, − 111.6493°, elevation 1450 m), 48 km east of Rush Valley, and buried in the soil, with the top 1 cm above the ground. A 2-m tall welded-wire fence was installed around the garden 30 cm below ground to deter herbivores from entering. Cores were watered to saturation to germinate much of the seed bank. Seedlings were then removed, though very few seedlings emerged.
This study was a 2 × 3 full-factorial design replicated in 4 blocks. The simulated precipitation timing treatment included two precipitation start points (early fall precipitation vs. late fall precipitation timing), accompanied by three seeding treatments: non-native seed community, native seed community, and non-native + native seed community grown together. The addition of seeds to measure community composition was conducted using a modified additive design. Seeds for the study were harvested in Rush Valley or within the greater region by authors or received from the Great Basin Research Center, Utah Division of Wildlife Resources (Ephraim, UT). Twenty seeds of each species were added to the three seed mixes (in bold): (1) Non-native mix of five non-native annual species included: [Bromus tectorum L. (cheatgrass), Sisymbrium altissimum L. (tall tumblemustard), Alyssum alyssoides L. (pale madwort), Ceratocephala testiculata (Crantz) Roth (curveseed butterwort), and Halogeton glomeratus (saltlover/halogeton)]; (2) Native mix of six native species included: [Elymus elymoides (Raf.) Swezey (squirreltail), Poa secunda J. Presl (Sandberg bluegrass), Achillea millefolium L. car. occidentalis (western yarrow), Linum lewisii Pursh (Lewis flax), Atriplex canescens (four-wing saltbush), and Artemisia tridentata Nutt. ssp. wyomingensis Beetle and Young (Wyoming big sagebrush; hereafter Artemisia tridentata)]; (3) Non-native + native mix where all native and non-native species were grown together (11 species total). We recognize that the annual (non-native and native) seeded species are usually faster growing with earlier maturation compared with slower growing species (mostly native, perennials). The reason for this design is to understand the ecological dynamics between species that are currently competing with each other in the system. All non-native (St. Clair et al. 2016; Young et al. 1972) and native species included have large distributions across the Great Basin (Chambers et al. 2016; Miller et al. 2013a, b). The native species were chosen due to their ecological prevalence in the experimental system and use in post-disturbance reseeding efforts (Miller et al. 2013a, b; St. Clair et al. 2016). Seeding density was calculated based on seeding recommendations by the BLM field office and Great Basin Research Center and converted to the per unit area of the core itself. Seeding density is recognized as a potential influence on the composition outcomes thus additive designs commonly maintain total seed density but increase species richness (Weigelt and Jolliffe 2003; Gibson et al. 1999). However, total plant densities in landscapes can increase in the early stages of invasion, and increased densities and proportion of non-native species in the soil seed bank often lead to secondary invasions (reviewed in Gioria et al. 2014). We, therefore, chose to combine the native species mix with the non-native species mix without altering individual species densities to more closely mimic field conditions in early invasion stages. Seeds were sown on the top of each core with very little disturbance and only lightly pressed into the soil by hand.
The timing was the main difference between the two precipitation treatments (Bishop et al. 2020). Cores were subject to natural weather conditions from early September 2016 until mid-June 2017, the full period of the study. The mean annual air temperature in Provo, UT, where BYU is located, is 12.3 °C, with a July mean of 24.2 °C and January mean of − 1.1 °C and mean annual precipitation of 411 mm. Precipitation treatments consisted of watering each core with 1.5 mm of water once every morning and afternoon for 14 days (total of 3 mm of water per day), except on days with natural precipitation events. Cores were watered from September 6 to 22 in the early treatment and from October 12 to 26 in the late treatment (Fig. 1). Because it rained on one day during the early treatment and two days during the late treatment, the latter received 3 mm less added water. Added water made up roughly 10% of the total precipitation received during the experiment. There was one other natural rain event on 23–24 September outside of the watering window. The combination of natural events and not adding water to the cores equaled an 18 mm increase in total cumulative water for the early precipitation treatment (Fig. 1). Meaning, the early precipitation treatment received 18 mm more total water than the late precipitation treatment. The total cumulative amount of water the cores received was 413–431 mm from 6 September 2016 until plant collection.
Plant measurements
Herbaceous plant material was collected in mid-June and A. tridentata in late August 2017, and destructively harvested by removing all above-ground biomass and stored in separate paper bags. Biomass was air-dried in a climate-controlled laboratory at room temperature for six months. For B. tectorum and S. altissimum, a random subsample was used to count seeds for individuals, and an allometric equation was derived based on the number of seeds per unit mass of the seed head (R2 = 0.88; y = 719.07x + 39.28) or silique (R2 = 0.97; y = 1344.4x + 37.54) to calculate the total seed per core. All other seed was counted individually. At the time of harvest, many individuals were too immature for seed production, however, given their stage of development they were unlikely to produce seeds in this growing season. Because of the correlation between the overall size of the plant and the seed production, we measured the seed density per unit of plant biomass (seeds g−1 biomass). Other plant measurements included average height (cm), density (m2), and biomass (g m−2) of above-ground plant tissues.
Plant tissue N and C analysis
A homogenized vegetative plant tissue sample from each core (minus seed head) was clipped from dry biomass that had already been weighed. The homogenized plant tissue was analyzed for nitrogen concentration (% N) and carbon-to-nitrogen ratio (C:N) using the combustion method (TruSpec CN Determinator, LECO Instruments, St. Joseph, Mich., USA) at the BYU Environmental Analytical Laboratory.
Statistical analysis
Linear mixed-effects analysis of variance models were used to test the main and interactive effects of precipitation timing and seed mixes (plant community type) on height, density, biomass, seed production, %N and C:N of all planted species and mixes using the ‘nlme’ package in Program R (Pinheiro et al. 2021). Precipitation timing and seed mixes were considered fixed effects with experimental block as a random effect. Homogeneity of variance was achieved by using a varIdent covariance structure for precipitation or seed mix where needed. All model assumptions were investigated; for those models without a normal distribution, a poisson distribution or square root transformation was used.
Results
Plant responses to precipitation timing
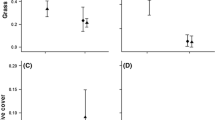
Both non-native and native species had positive responses to earlier fall precipitation. Earlier precipitation increased the mean non-native plant height by 17 cm (twofold), density by 1,407 individuals m−2 (1.5-fold), biomass by 4,905 g m−2 (ninefold), seed per unit biomass production by 185 seeds g−1 biomass (1.5-fold), and C:N (1.5-fold) compared with the late precipitation treatment (Tables 1, 2, Figs. 2, 3). Early precipitation increased the mean native plant height by 3.4 cm (2.4-fold), density by 382 individuals m−2 (1.5-fold), biomass by 168 g m−2 (ninefold), and C:N (1.5-fold) in comparison with the late precipitation treatment (Tables 1, 2, Figs. 2, 3). Early precipitation decreased plant tissue nitrogen concentration for both non-native and native species regardless of seed mix (Fig. 3).
Plant growth, establishment, and seed production response of the non-native plant community and herbaceous native community grown in community mixes modified by precipitation timing. Seed density (m2) are adjusted for overall plant biomass (g m−2) to show how many seeds per gram of plant biomass were produced. Note: scales are different for invasive and native species “Plant Biomass” and “Seed” to fit the data bars. Means presented ± SE. F-statistics and p-values are found in Tables 1
Nitrogen concentrations and C:N of the non-native and native plant communities grown in non-native only, native only, and non-native + native community seed mixes. Letters denote pairwise comparisons and statistical significance for each response variable. The same letters denote no statistical difference. Responses can have multiple letters to illustrate differences across species and seed mix type. Means presented ± SE, F-statistics and p-values are found in Table 2
Plant community composition outcomes
Combining native and non-native plant communities reduced native species growth and seed production more than non-native species (Fig. 2). When native and non-native species were grown together it reduced native plant species height (just over 200%), density (300%), biomass (1300%), seed production (1800%), and C:N (130%) compared to native plant species grown alone (Tables 1, 2 and Figs. 2, 3). The presence of non-natives did not affect tissue nitrogen concentration of the native plant community (Fig. 3). In contrast, the native plant community did not impact the non-native plant height, biomass, seed production, %N or C:N (Tables 1, 2, Fig. 2, 3). The slight reduction in density (P = 0.09) is a result of B. tectorum having a slight reduction in the presence of natives in the early precipitation treatment. Individual species responses to treatments are provided in Supplementary Tables 1, 2 and 3.
The effect of non-native plant species interfering with native plant species growth responses was intensified by early precipitation as indicated by the significant early precipitation interaction terms for the native plant mix density, height, biomass, seed production, and C:N but not % N (Tables 1, 2, Figs. 2, 3). The positive effect of early precipitation on the native plant community density, height, biomass, and C:N decreased or became non-existent when grown with non-native species in a mixed community (Tables 1, 2, Fig. 2, 3). We analyzed the data with and without A. tridentata (the only woody plant that grew) and the overall patterns remained the same, and the magnitude of the difference with and without A. tridentata was small for both precipitation treatments and community composition.
Discussion
Life-history traits of non-native plants have a large influence in determining whether they become invasive or not (Levine et al. 2004; Mack et al. 2000). In this study, Great Basin non-native invasive plants generally exhibited more robust growth than native communities pursuant to our first question (Figs. 2, 3). In accordance with our second question, we found that precipitation timing more strongly impacted non-native species establishment, growth and fecundity than native species (Figs. 2, 3). Precipitation timing is increasingly recognized as a critical driver of native plant establishment and invasibility of ecosystems (Horn et al. 2017; Pilliod et al. 2017; Prevéy and Seastedt 2014). Our results suggest that shifts toward earlier fall precipitation forecasted in the Great Basin will likely favor the establishment and spread of non-native species (Prevéy and Seastedt 2014) due by increasing their competitive advantage over native species (Fig. 2). Our data suggested that key traits that led to this advantage by non-native species included increased growth rates, greater nitrogen use, and uptake, as measured by %N, and C:N (Besaw et al. 2011; Gebauer et al. 2002; Perkins and Hatfield 2014; Wolkovich and Cleland 2011; Slate et al 2022) (Fig. 3).
Both native and non-native communities benefitted from earlier precipitation (Fig. 2). Water is a major limitation constraining plant establishment in more arid systems (Miller et al. 2006; Noy-Meir 1979; Wainwright et al. 2012). Predicted changes in the timing of the precipitation in the Great Basin for wetter winter and fall periods are likely to benefit plants established during those periods (Abatzoglou and Kolden 2011; Pilliod et al. 2017; Prevéy and Seastedt 2015). Earlier fall precipitation can release water limitations for seedling emergence and extend the growing period into more favorable temperature windows in the fall that increases emergence, establishment, and robustness (Bates et al. 2006; Slate et al. 2019; Ulrich and Perkins 2014) potentially decreasing seedling bottlenecks such as winter mortality (Belnap 2002; Pregitzer and King 2005). Longer growing periods created by earlier fall precipitation can also increase nitrogen acquisition which is also a major limitation to the productivity of desert plants (Belnap 2002; Bishop et al. 2020). Nitrogen acquisition during increased water availability could indicate the possibility of a shift in community composition hierarchy depending on nutrient use uptake and efficiency by native or non-native plant species (Slate et al. 2022). For example, non-native plants can exploit available resources when the loss of native vegetation occurs after disturbance (Davis et al. 2000; Levine et al. 2003; Vilà and Weiner 2004).
The reduction of native plant growth in a mixed non-native and native community was intensified by early precipitation timing (Fig. 2). The priority effects hypothesis posits that early-season establishment by non-natives (Germino et al. 2016; Meyer et al. 1997) provides a competitive advantage (Goodale and Wilsey 2018; Thomson et al. 2017; Ulrich and Perkins 2014). Non-native species neutralized the positive effect of early precipitation for native species, while natives had little effect on non-natives (Fig. 2). This suggests that priority effects may be a mechanism driving the community’s composition in our study system (Figs. 2, 3) (Dickson et al. 2012; Perkins and Hatfield 2014; Wainwright et al. 2012). However, with greater resource availability, competition typically decreases (Davies et al. 2007; Davis et al. 2000); therefore, more available water in favorable temperature conditions during the fall period may cause a shift towards nutrient limitation and nitrogen utilization efficiency as the drivers of plant competitive effects (Miller et al. 2006; Slate et al. 2019; Slate et al. 2022).
The higher C:N of the non-native species with earlier precipitation provide supporting evidence that non-native plant species are more effective in rapidly acquiring soil nutrients, particularly nitrogen, coupled with the increased biomass leading us to conclude there is likely higher efficiency in converting those nutrients into increased plant growth compared to native plants in this study (Fig. 3, Supp Tables 1, 2) (Booth et al. 2003a; DeFalco et al. 2007; Huxman et al. 2008). Greater nitrogen utilization efficiency and nitrogen use efficiency have been shown to increase relative growth rates and biomass accumulation (Reich 2014; Zhang et al. 2020) that increase competitive advantage (Figs. 2, 3). Native desert plants commonly exhibit low relative growth rates to maintain high leaf nitrogen concentrations to increase stress tolerance and water use efficiency in response to soil resource limitations (Huxman et al. 2008; Valliere 2019). This can lead to a competitive disadvantage particularly in resource poor environments like deserts (Funk 2013) or in post-disturbance conditions where there are often rapid increases in soil resource pools, providing opportunities for establishment of invasive plant species (fast or slow plant economics; Reich 2014).
Biological invasions are restructuring native plant communities across the Earth’s ecosystems, including the Great Basin Desert (Chambers and Wisdom 2009). Responsiveness of plant communities to changes in precipitation timing and how it modifies plant community composition will be a strong determinant of system vulnerability to vegetation state changes (Chambers et al. 2014b). The dominance of non-native vegetation demonstrated in this study is consistent with shifts in vegetation observed in the Great Basin Desert in recent decades (Chambers et al. 2014b; Prevéy and Seastedt 2014, 2015) and highlights the competitive advantage of non-natives when all plants establish from seed. Even though early precipitation timing positively affected non-native and native plant species (Supp Table 1, Fig. 2), the responsiveness of non-native annual plants to increases in fall precipitation not only increases their competitive edge but also may reduce the potential benefits of early fall moisture to the native plant community (Fig. 2) (Knapp 2002; Schantz et al. 2015; van Kleunen et al. 2010). We conclude that these results highlight the necessity for increased information on within season variation, particularly from a management perspective. Incorporation of weather data has shown positive results in restoration of sage-steppe environments (Simler-Williamson et al. 2022). Utilizing improved forecasts for extreme and/or shifting weather patterns will likely improve allocation of management resources and subsequent success of restoration efforts.
In conclusion, while native plant communities have shown varying degrees of robustness in resisting non-native plant invasions (Humphrey and Schupp 2004; Vaughn and Young 2015; Young et al. 2015), biotic resistance is typically highest in intact, mature native plant communities that have not experienced recent disturbance (Chambers et al. 2017; St. Clair et al. 2016). Therefore, long-term biotic resistance is unlikely to be maintained in the presence of increased disturbance where native seedlings are more likely to be competing against non-native seedlings (Chambers et al. 2014b; Germino et al. 2016; St. Clair and Bishop 2019). Traits that confer an advantage for non-native invasive annuals in the Great Basin are likely linked to an ability to acquire water and use N in low N-environments enabled by rapid relative growth rates much like Bromus tectorum (Poorter et al. 1990; van der Werf et al. 1993). This advantage is problematic as Bromus tectorum-dominated landscapes are directly linked to the increased frequency and size of wildfires (i.e., Bradley et al. 2018) and other forms of disturbance. However, the reduction of growth of B. tectorum grown in the mixed community (Supp Table 1) may offer opportunities to mitigate its success that has contributed to the establishment and spread of invasive grass-fire cycles in the Great Basin Desert (Bradley et al. 2009; St. Clair and Bishop 2019). Reducing disturbances and conserving established native plant communities will increase ecosystem resilience and resistance to invasion by reducing opportunites for priority advantaged non-native annual species that benefit most from climate change and early fall precipitation (Booth et al. 2003a; James et al. 2012; Madsen et al. 2016).
Data availability
Available upon request.
References
Aanderud ZT et al (2019) The burning of biocrusts facilitates the emergence of a bare soil community of poorly-connected chemoheterotrophic bacteria with depressed ecosystem services. Front Ecol Evol 7(4):467. https://doi.org/10.3389/fevo.2019.00467
Abatzoglou JT, Kolden CA (2011) Climate change in western US deserts: potential for increased wildfire and invasive annual grasses. Rangeland Ecol Manag 64:471–478. https://doi.org/10.2111/REM-D-09-00151.1
Abella SR, Engel EC (2013) Influences of wildfires on Organic Carbon, Total Nitrogen, and other properties of desert soils. Soil Sci Soc Am J 77:1806–1817. https://doi.org/10.2136/sssaj2012.0293
Allen EB, Steers RJ, Dickens SJ (2011) Impacts of fire and invasive species on desert soil ecology. Rangel Ecol Manag 64(5):450–462. https://doi.org/10.2111/REM-D-09-00159.1
Bates JD, Svejcar T, Miller RF, Angell RA (2006) The effects of precipitation timing on sagebrush steppe vegetation. J Arid Environ 64:670–697. https://doi.org/10.1016/j.jaridenv.2005.06.026
Belnap J (2002) Nitrogen fixation in biological soil crusts from southeast Utah, USA. Biol Fertil Soils 35:128–135. https://doi.org/10.1007/s00374-002-0452-x
Besaw LM, Thelen GC, Sutherland S et al (2011) Disturbance, resource pulses and invasion: short-term shifts in competitive effects, not growth responses, favour non-native annuals: Fertilization and competition between natives and non-natives. J Appl Ecol 48:998–1006. https://doi.org/10.1111/j.1365-2664.2011.01988.x
Bishop TBB, Nusink BC, Lee Molinari R et al (2020) Earlier fall precipitation and low severity fire impacts on cheatgrass and sagebrush establishment. Ecosphere 11(1):e03019. https://doi.org/10.1002/ecs2.3019
Blank RR (2010) Intraspecific and interspecific pair-wise seedling competition between non-native annual grasses and native perennials: plant–soil relationships. Plant Soil 326:331–343. https://doi.org/10.1007/s11104-009-0012-3
Booth MS, Caldwell MM, Stark JM (2003a) Overlapping resource use in three Great Basin species: implications for community invasibility and vegetation dynamics. J Ecology 91:36–48. https://doi.org/10.1046/j.1365-2745.2003.00739.x
Bradley BA, Oppenheimer M, Wilcove DS (2009) Climate change and plant invasions: restoration opportunities ahead? Global Change Biol 15:1511–1521. https://doi.org/10.1111/j.1365-2486.2008.01824.x
Bradley BA, Curtis CA, Fusco EJ et al (2018) Cheatgrass (Bromus tectorum) distribution in the intermountain Western United States and its relationship to fire frequency, seasonality, and ignitions. Biol Invasions 20:1493–1506. https://doi.org/10.1007/s10530-017-1641-8
Brooks ML, D’Antonion CM, Richardson DM et al (2004) Effects of invasive allien plants on fire regimes. Bioscience 54(7):677–688. https://doi.org/10.1641/0006-3568(2004)054[0677:EOIAPO]2.0.CO;2
Callaway RM (2000) Invasive plants versus their new and old neighbors: a mechanism for non-native invasion. Science 290:(5491)521–523. https://doi.org/10.1126/science.290.5491.521
Catford JA, Daehler CC, Murphy HT et al (2012) The intermediate disturbance hypothesis and plant invasions: Implications for species richness and management. Perspect Plant Ecol 14:231–241. https://doi.org/10.1016/j.ppees.2011.12.002
Chambers JC, Pellant M (2008) Climate Change Impacts on Northwestern and Intermountain United States Rangelands. Rangelands 30:29–33. https://doi.org/10.2111/1551-501X(2008)30[29:CCIONA]2.0.CO;2
Chambers JC, Wisdom MJ (2009) Priority research and management issues for the imperiled great basin of the western United States. Restor Ecol 17:707–714. https://doi.org/10.1111/j.1526-100X.2009.00588.x
Chambers JC, Roundy BA, Blank RR et al (2007) What makes Great Basin sagebrush ecosystems invasible by Bromus tectorum? Ecol Monogr 77:117–145. https://doi.org/10.1890/05-1991
Chambers JC, Miller RF, Board DI et al (2014b) Resilience and resistance of sagebrush ecosystems: implications for state and transition models and management treatments. Rangeland Ecol Manag 67:440–454. https://doi.org/10.2111/REM-D-13-00074.1
Chambers JC, Germino MJ, Belnap J et al (2016) Plant community resistance to invasion by Bromus species: the roles of community attributes, Bromus interactions with plant communities, and Bromus traits. In: Germino MJ, Chambers JC, Brown CS (eds) Non-native brome-grasses in arid and semiarid ecosystems of the western US. Springer, Cham, pp 275–304
Chambers JC, Maestas JD, Pyke DA et al (2017) Using resilience and resistance concepts to manage persistent threats to sagebrush ecosystems and greater sage-grouse. Rangeland Ecol Manag 70:149–164. https://doi.org/10.1016/j.rama.2016.08.005
Chesson P (2003) Quantifying and testing coexistence mechanisms arising from recruitment fluctuations. Theor Popul Biol 64:345–357. https://doi.org/10.1016/S0040-5809(03)00095-9
Condon L, Pyke DA (2016) Filling the interspace- restoring arid land mosses: source populations, organic matter, and overwintering govern success. Ecol Evol 6(21):7623–7632. https://doi.org/10.1002/ece3.2448
Davidson AM, Jennions M, Nicotra AB (2011) Do invasive species show higher phenotypic plasticity than native species and if so, is it adaptive? A meta-analysis. Ecol Lett 14:419–431. https://doi.org/10.1111/j.1461-0248.2011.01596.x
Davies KW, Bates JD, Miller RF (2007) Environmental and vegetation relationships of the Artemisia tridentata spp. wyomingensis alliance. J Arid Environ 70:478–494. https://doi.org/10.1016/j.jaridenv.2007.01.010
Davis MA, Grime JP, Thompson K (2000) Fluctuating resources in plant communities: a general theory of invasibility Summary Journal of Ecology 88(3): 528–534 https://doi.org/10.1046/j.1365-2745.2000.00473.x
DeFalco LA, Fernandez GCJ, Nowak RS (2007) Variation in the establishment of a non-native annual grass influences competitive interactions with Mojave Desert perennials. Biol Invasions 9:293–307. https://doi.org/10.1007/s10530-006-9033-5
Dickson TL, Hopwood JL, Wilsey BJ (2012) Do priority effects benefit invasive plants more than native plants? An experiment with six grassland species. Biol Invasions 14:2617–2624. https://doi.org/10.1007/s10530-012-0257-2
Fargione J, Brown CS, Tilman D (2003) Community assembly and invasion: an experimental test of neutral versus niche processes. P Natl Acad Sci 100:8916–8920. https://doi.org/10.1073/pnas.1033107100
Funk JL (2013) The physiology of invasive plants in low-resource environments. Conserv Physiol 1:cot226. https://doi.org/10.1093/conphys/cot026
Funk JL, Vitousek PM (2007) Resource-use efficiency and plant invasion in low-resource systems. Nature 446:1079–1081. https://doi.org/10.1038/nature05719
Fusco EJ, Balch JK, Mahood AL, Nagy RC, Syphard AD, Bradley BA (2022) The human–grass–fire cycle: how people and invasives co-occur to drive fire regimes. Front Ecol Evol 20(2):117–126. https://doi.org/10.1002/fee.2432
Gebauer RLE, Schwinning S, Ehleringer JR (2002) Interspecific competition and resource pulse utilization in a cold desert community. Ecology 83:2602–2616. https://doi.org/10.1890/0012-9658(2002)083[2602:ICARPU]2.0.CO;2
Germino MJ, Belnap J, Stark JM et al (2016) Ecosystem impacts of non-native annual invaders in the genus Bromus. In: Germino MJ, Chambers JC, Brown CS (eds) Non-native brome-grasses in arid and semiarid ecosystems of the western US. Springer, Cham, pp 61–95
Gibson DJ, Connolly J, Hartnett DC, Weidenhamer JD (1999) Designs for greenhouse studies of interactions between plants Journal of Ecology 87(1): 1–16 https://doi.org/10.1046/j.1365-2745.1999.00321.x
Gill RA, O’Connor RC, Rhodes A et al (2018) Niche opportunities for invasive annual plants in dryland ecosystems are controlled by disturbance, trophic interactions, and rainfall. Oecologia 187:755–765. https://doi.org/10.1007/s00442-018-4137-z
Gioria M, Pyšek P (2017) Early bird catches the worm: germination as a critical step in plant invasion. Biol Invasions 19:1055–1080. https://doi.org/10.1007/s10530-016-1349-1
Gioria M, Jarošik V, Pyšek P (2014) Impact of invasions by alien plants on soil seed bank communities: emerging patterns. Perspect Plant Ecol Evol Syst 16(3):132–142. https://doi.org/10.1016/j.ppees.2014.03.003
Goodale KM, Wilsey BJ (2018) Priority effects are affected by precipitation variability and are stronger in non-native than native grassland species. Plant Ecol 219:429–439. https://doi.org/10.1007/s11258-018-0806-6
Herget ME, Hufford KM, Mummey DL et al (2015) Effects of competition with Bromus tectorum on early establishment of Poa secunda accessions: can seed source impact restoration success?: Seed source impacts on restoration success. Restor Ecol 23:277–283. https://doi.org/10.1111/rec.12177
Horn KJ, St. Clair SB (2017) Wildfire and non-native grass invasion alter plant productivity in response to climate variability in the Mojave Desert. Landscape Ecol 32:635–646. https://doi.org/10.1007/s10980-016-0466-7
Horn KJ et al (2017) Precipitation timing and soil heterogeneity regulate the growth and seed production of the invasive grass red brome. Biol Invasions 19:1339–1350. https://doi.org/10.1007/s10530-016-1348-2
Humphrey LD, Schupp EW (2004) Competition as a barrier to establishment of a native perennial grass (Elymus elymoides) in alien annual grass (Bromus tectorum) communities. J Arid Environ 58:405–422. https://doi.org/10.1016/j.jaridenv.2003.11.008
Huxman TE, Barron-Gafford G, Gerst KL et al (2008) Photosynthetic resource-use efficiency and demographic variability in desert winter annual plants. Ecology 89:1554–1563. https://doi.org/10.1890/06-2080.1
James JJ (2008) Leaf nitrogen productivity as a mechanism driving the success of invasive annual grasses under low and high nitrogen supply. J Arid Environ 72(10):1775–1784. https://doi.org/10.1016/j.jaridenv.2008.05.001
James JJ, Rinella MJ, Svejcar T (2012) Grass seedling demography and sagebrush steppe restoration. Rangeland Ecol Manag 65:409–417. https://doi.org/10.2111/REM-D-11-00138.1
Kadmon R, Shmida A (1990) Competition in a variable environment: an experimental study in a desert annual plant population. Isr J Plant Sci 39(4):403–412. https://doi.org/10.1080/0021213X.1990.10677164
Knapp AK (2002) Rainfall variability, carbon cycling, and plant species diversity in a mesic grassland. Science 298:2202–2205. https://doi.org/10.1126/science.1076347
Kolb A, Alpert P, Enters D, Holzapfel C (2002) Patterns of invasion within a grassland community. J Ecol 90:871–881. https://doi.org/10.1046/j.1365-2745.2002.00719.x
Kraft NJB, Adler PB, Godoy O et al (2015) Community assembly, coexistence and the environmental filtering metaphor. Funct Ecol 29:592–599. https://doi.org/10.1111/1365-2435.12345
Levine JM, Vilà M, Antonio CMD et al (2003) Mechanisms underlying the impacts of non-native plant invasions. Proc R Soc Lond B 270:775–781. https://doi.org/10.1098/rspb.2003.2327
Levine JM, Adler PB, Yelenik SG (2004) A meta-analysis of biotic resistance to non-native plant invasions: biotic resistance to plant invasion. Ecol Lett 7:975–989. https://doi.org/10.1111/j.1461-0248.2004.00657.x
Mack RN, Simberloff D, Mark Lonsdale W et al (2000) Biotic invasions: causes, epidemiology, global consequences, and control. Ecol Appl 10:689–710. https://doi.org/10.1890/1051-0761(2000)010[0689:BICEGC]2.0.CO;2
Madsen MD, Davies KW, Boyd CS et al (2016) Emerging seed enhancement technologies for overcoming barriers to restoration: emerging seed enhancement technologies. Restor Ecol 24:S77–S84. https://doi.org/10.1111/rec.12332
Mcgill B, Enquist B, Weiher E, Westoby M (2006) Rebuilding community ecology from functional traits. Trends Ecol Evol 21:178–185. https://doi.org/10.1016/j.tree.2006.02.002
Meyer SE, Allen PS, Beckstead J (1997) Seed germination regulation in Bromus tectorum (Poaceae) and its ecological significance. Oikos 78:475. https://doi.org/10.2307/3545609
Miller ME, Belnap J, Beatty SW, Reynolds RL (2006) Performance of Bromus tectorum L. in relation to soil properties, water additions, and chemical amendments in calcareous soils of southeastern Utah, USA Plant and Soil 288: 1–18 https://doi.org/10.1007/s11104-006-0058-4
Miller RF, Chambers JC, Pyke DA, et al (2013a) A review of fire effects on vegetation and soils in the Great Basin Region: response and ecological site characteristics. Gen Tech Rep RMRS-GTR-308 Fort Collins, CO: US Department of Agriculture, Forest Service, Rocky Mountain Research Station 126 p 308. https://doi.org/10.2737/RMRS-GTR-308
Miller RF, Chambers JC, Pyke DA, et al (2013b) A review of fire effects on vegetation and soils in the Great Basin Region: response and ecological site characteristics. Gen Tech Rep RMRS-GTR-308 Fort Collins, CO: US Department of Agriculture, Forest Service, Rocky Mountain Research Station 126 p 308:. https://doi.org/10.2737/RMRS-GTR-308
Nielsen UN, Ball BA (2015) Impacts of altered precipitation regimes on soil communities and biogeochemistry in arid and semi-arid ecosystems. Glob Change Biol 21:1407–1421. https://doi.org/10.1111/gcb.12789
Noy-Meir I (1979) Structure and function of desert ecosystems. Israel J Bot 28:1–19
Pearson DE, Ortega YK, Eren Ö, Hierro JL (2018) Community assembly theory as a framework for biological invasions. Trends Ecol Evol 33(5):313–325. https://doi.org/10.1016/j.tree.2018.03.002
Perkins LB, Hatfield G (2014) Competition, legacy, and priority and the success of three invasive species. Biol Invasions 16:2543–2550. https://doi.org/10.1007/s10530-014-0684-3
Pilliod DS, Welty JL, Arkle RS (2017) Refining the cheatgrass-fire cycle in the Great Basin: precipitation timing and fine fuel composition predict wildfire trends. Ecol Evol 7:8126–8151. https://doi.org/10.1002/ece3.3414
Pinheiro J, Bates D, Saikat DebRoy, et al (2021) nlme: Linear and Nonlinear Mixed Effects Models. Version 3.1–152. https://CRAN.R-project.org/package=nlme
Poorter H, Remkes C, Lambers H (1990) Carbon and nitrogen economy of 24 wild species differing in relative growth rate. Plant Physiol 94(2):621–627. https://doi.org/10.1104/pp.94.2.621
Pregitzer KS, King JS (2005) Effects of soil temperature on nutrient uptake. In: BassiriRad H (ed) Nutrient acquisition by plants: an ecological perspective. Ecological Studies. Springer, Berlin, pp 277–310
Prevéy JS, Seastedt TR (2014) Seasonality of precipitation interacts with non-native species to alter composition and phenology of a semi-arid grassland. J Ecol 102:1549–1561. https://doi.org/10.1111/1365-2745.12320
Prevéy JS, Seastedt TR (2015) Effects of precipitation change and neighboring plants on population dynamics of Bromus tectorum. Oecologia 179:765–775. https://doi.org/10.1007/s00442-015-3398-z
Rau BM, Chambers JC, Pyke DA, Roundy BA, Schupp EW, Doescher P, Caldwell TG (2014) Soil resources influence vegetation and response to fire and fire-surrogate treatments in sagebrush-steppe ecosystems. Rangel Ecol Manag 67(5):506–521. https://doi.org/10.2111/07-037.1
Reich PB (2014) The world-wide ‘fast-slow’ plant economics spectrum: a traits manifesto. J Ecol 102:275–301. https://doi.org/10.1111/1365-2745.12211
Sala OE, Lauenroth WK (1982) Small rainfall events: an ecological role in semiarid regions. Oecologia 53:301–304. https://doi.org/10.1007/BF00389004
Schantz MC, Sheley RL, James JJ (2015) Role of propagule pressure and priority effects on seedlings during invasion and restoration of shrub-steppe. Biol Invasions 17:73–85. https://doi.org/10.1007/s10530-014-0705-2
Schwinning S, Sala OE (2004) Hierarchy of responses to resource pulses in arid and semi-arid ecosystems. Oecologia 141:211–220. https://doi.org/10.1007/s00442-004-1520-8
Simler Williamson, A. B., Applestein, C., & Germino, M. J. (2022) Interannual variation in climate contributes to contingency in post fire restoration outcomes in seeded sagebrush steppe. Conservation Science and Practice 4(7): e12737.
Slate ML, Callaway RM, Pearson DE (2019) Life in interstitial space: biocrusts inhibit non-native but not native plant establishment in semi-arid grasslands. J Ecol 107:1317–1327. https://doi.org/10.1111/1365-2745.13117
Slate ML, Matallana-Mejia N, Aromin A et al (2022) Nitrogen addition, but not pulse frequency, shifts competitive interactions in favor of exotic invasive plant species. Biol Invasions 24:3109–3118. https://doi.org/10.1007/s10530-022-02833-3
St. Clair SB, Bishop TBB (2019) Loss of biotic resistance and high propagule pressure promote invasive grass-fire cycles. J Ecol 107:1995–2005. https://doi.org/10.1111/1365-2745.13156
St. Clair SB, O’Connor R, Gill R, McMillan B (2016) Biotic resistance and disturbance: rodent consumers regulate post-fire plant invasions and increase plant community diversity. Ecology 97:1700–1711. https://doi.org/10.1002/ecy.1391
Thomson DM, King RA, Schultz EL (2017) Between invaders and a risky place: Exotic grasses alter demographic tradeoffs of native forb germination timing Abstract Ecosphere 8(10): https://doi.org/10.1002/ecs2.1987
Ulrich E, Perkins L (2014) Bromus inermis and Elymus canadensis but not Poa pratensis demonstrate strong competitive effects and all benefit from priority. Plant Ecol 215:1269–1275. https://doi.org/10.1007/s11258-014-0385-0
Valliere JM (2019) Tradeoffs between growth rate and water-use efficiency in seedlings of native perennials but not invasive annuals. Plant Ecol 220:361–369. https://doi.org/10.1007/s11258-019-00919-y
van der Werf A, van Nuenen M, Visser AJ, Lambers H (1993) Contribution of physiological and morphological plant traits to a species’ competitive ability at high and low nitrogen supply. Oecologia 94:434–440. https://doi.org/10.1007/BF00317120
van Kleunen M, Weber E, Fischer M (2010) A meta-analysis of trait differences between invasive and non-invasive plant species. Ecol Lett 13:235–245. https://doi.org/10.1111/j.1461-0248.2009.01418.x
Vaughn KJ, Young TP (2015) Short-term priority over non-native annuals increases the initial density and longer-term cover of native perennial grasses. Ecol Appl 25:791–799. https://doi.org/10.1890/14-0922.1
Vilà M, Weiner J (2004) Are invasive plant species better competitors than native plant species? Evidence from pair-wise experiments. Oikos 105:229–238. https://doi.org/10.1111/j.0030-1299.2004.12682.x
Wainwright CE, Wolkovich EM, Cleland EE (2012) Seasonal priority effects: implications for invasion and restoration in a semi-arid system: Priority effects and invasion. J Appl Ecol 49:234–241. https://doi.org/10.1111/j.1365-2664.2011.02088.x
Walk JL, Hidayati SN, Dixon KW, Thompson K, Poschlod P (2022) Climate change and plant regeneration from seed. Glob Chang Biol 17:2145–2161. https://doi.org/10.1111/j.1365-2486.2010.02368.x
Weigelt, A., & Jolliffe, P. (2003). Indices of plant competition. Journal of ecology, 707–720.
Soil Survey Staff, Natural Resources Conservation Service, United States Department of Agriculture. Web Soil Survey. https://websoilsurvey.sc.egov.usda.gov/App/WebSoilSurvey.aspx . Accessed 2 Jul 2021
Wolkovich EM, Cleland EE (2011) The phenology of plant invasions: a community ecology perspective. Front Ecol Environ 9:287–294. https://doi.org/10.1890/100033
Young JA, Evans RA, Major J (1972) Alien plants in the great basin. J Range Manage 25:194. https://doi.org/10.2307/3897054
Young TP, Zefferman EP, Vaughn KJ, Fick S (2015) Initial success of native grasses is contingent on multiple interactions among non-native grass competition, temporal priority, rainfall and site effects. AoB PLANTS. https://doi.org/10.1093/aobpla/plu081
Zhang J, He N, Liu C et al (2020) Variation and evolution of C: N ratio among different organs enable plants to adapt to N-limited environments. Glob Change Biol 26:2534–2543. https://doi.org/10.1111/gcb.14973
Acknowledgements
We gratefully acknowledge Justin Taylor, Rebecca Lee Molinari, Rebekah Stanton, Kristina Cass, Brianna Woodbury, Sierra Curtis Nichols, Elianna Bowes Carter, Eliza Jones, Joshua Day, Madelyn McLaughlin, and Carol Belnap for their field assistance and maintenance of this experiment and Danny Summers at the Great Basin Research Center, Utah Division of Wildlife Resources for providing quality seed and help when needed. We thank Dr. Chelcy Miniat for the review and help in refining our manuscript. We also are grateful for the use of ancestral Timpanogos (Shoshone) and Goshute lands historically stewarded by the Timpanogos (Shoshone) people. Learn more about the Timpanogos Nation on their website (http://timpanogostribe.com/index.html). The findings and conclusions in this publication are those of the authors and should not be construed to represent any official USDA determination or policy.
Funding
Funded in part by the USDA Forest Service Rocky Mountain Research Station.
Author information
Authors and Affiliations
Contributions
SBS formulated the study. TBBB and SBS designed and developed methodology. TBBB, SBS, AB, and BCN conducted fieldwork and experimental treatments. TBBB, AB, and BCN collected, processed, and analyzed specimens. TBBB statistically analyzed the data and created figures. TBBB wrote the manuscript with BCN and SBS providing editorial feedback.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bishop, T.B.B., Barnes, A., Nusink, B.C. et al. Earlier fall precipitation increases the competitive advantage of non-native plant communities in a desert ecosystem. Biol Invasions 26, 719–731 (2024). https://doi.org/10.1007/s10530-023-03202-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-023-03202-4