Abstract
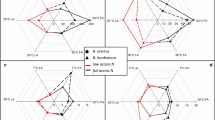
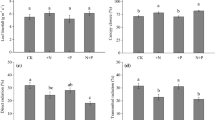
Why do inherently fast-growing species from productive habitats generally have a higher rate of biomass production in short-term low-nitrogen experiments than slow-growing species from unproductive habitats, whereas the opposite is found in long-term experiments? Is this mainly due to inherent differences in biomass allocation, leaf characteristics or the plants' physiology? To analyse these questions we grew five monocotyledonous species from productive and unproductive habitats in a climate chamber at both high and low nitrogen supply. Nitrate was supplied exponentially, enabling us to compare inherent differences in morphological and physiological traits between the species, without any interference due to differences in the species' ability to take up nutrients. At high nitrogen supply, we found major inherent differences in specific leaf area and nitrogen productivity, i.e. daily biomass increment per unit plant nitrogen, where-as there were only small differences in net assimilation rate, i.e. daily biomass increment per unit leaf area, and biomass partitioning. We propose that the higher specific leaf area and nitrogen productivity of inherently fast-growing species are the key factors explaining their high abundance in productive habitats compared with inherently slow-growing ones. At low nitrogen supply, the net assimilation rate was decreased to a similar extent for all species, compared with that at high nitrogen supply. The nitrogen productivity of the inherentlyfast-growing species decreased with decreasing nitrogen supply, whereas that of the inherently slow-growing species remained constant. There were no inherent differences in nitrogen productivity in this treatment. At this low nitrogen supply, the inherently fast-growing species invested relatively more biomass in their roots that the slow-growing ones did. The inherently fast-growing species still had a higher specific leaf area at low nitrogen supply, but the difference between species was less than that at high nitrogen supply. Based on the present results and our optimization model for carbon and nitrogen allocation (Van der Werf et al. 1993a), we propose that the relatively large investment in root biomass of fast-growing species is the key factor explaining their higher biomass production in short-term experiments. We also propose that in the long run the competitive ability of the slow-growing species will increase due to a lower turnover rate of biomass. It is concluded that the plant's physiology (net assimilation rate and nitrogen productivity), only plays a minor role in the species' competitive ability in low-nitrogen environments.
Similar content being viewed by others
References
Aerts R (1989) Plant strategies and nutrient cycling in heathland ecosystems. PhD thesis, University of Utrecht
Berendse F (1985) The effect of grazing on the outcome of competition between plant populations with different nutrient requirements. Oikos 44: 35–39
Berendse F, Elberse WT (1989) Competition and nutrient losses from the plant. In: Lambers H, Cambridge ML, Konings H, Pons TL (eds) Causes and consequences of variation in growth rate and productivity of higher plants. SPB Academic, The Hague, pp 269–284
Berendse F, Elberse WT (1990) Competition and nutrient availability in heathland and grassland ecosystems. In: Grace JB, Tilman D (eds) Perspectives on plant competition. Academic, San Diego, pp 93–116
Berendse F, Jonasson S (1992) Nutrient use and nutrient cycling in northern ecosystems. In: Chapin FS III, Jeffries RL, Reynolds JF, Shaver GR, Svoboda J (eds) Arctic ecosystems in changing climate. An ecophysiological perspective. Academic, San Diego, pp 337–358
Berendse F, Oudhof H, Bol J (1987) A comparitive study on nutrient cycling in wet heathland ecosystems. I. Litter production and nutrient losses from the plant. Oecologia 74: 174–184
Berendse F, Elberse WT, Geerts RHME (1992) Competition and nitrogen loss from plants in grassland ecosystems. Ecology 73: 46–53
Bobbink R, Willems JH (1987) Increasing dominance of Brachypodium pinnatum (L.) Beauv. in chalk grasslands: a threat to a species-rich ecosystems. Biol Conserv 40: 301–314
Boot RGA (1989) The significance of size and morphology of root systems for nutrient acquisition and competition In: Lambers H, Cambridge ML, Konings H, Pons TL (eds) Causes and consequences of variation in growth rate and productivity of higher plants. SPB Academic, The Hague, pp 299–312
Boot RGA, Mensink M (1991) The influence of nitrogen availability on growth parameters of fast- and slow-growing perennial grasses. In: Atkinson D (ed) Plant root growth. An ecological perspective. Blackwell Scientific, Edinburgh, pp 161–168
Bradshaw ADM, Chadwick MJ, Jowett D, Snaydon RW (1964) Experimental investigations into the mineral nutrition of several grass species: IV. Nitrogen level. J Ecol 52: 665–676
Campbell BD, Grime JP, Mackey JML, Jalili A (1991) The quest for a mechanistic understanding of resource competition in plant communities: the role of experiments. Funct Ecol 5: 241–253
Chapin FS III (1980) The mineral nutrition of wild plants. Annu Rev Ecol Syst 11: 233–260
Chapin FS III, Johnson DA, McKendrick JD (1980) Seasonal movement of nutrients in plants of different growth forms in Alaskan tundra ecosystems. Implications for herbivory. J Ecol 68: 189–209
Coley PD (1987) Interspecific variation in anti-herbivore properties: The role of habitat quality and rate of disturbance. New Phytol 106: 251–263
Donald CM (1958) The interaction of competition for light and nutrients. Aust J Agr Res 9: 421–432
Freijsen AHJ, Veen B (1990) Phenotypic variation in growth rate as affected by N-supply: nitrogen productivity. In: Lambers H, Cambridge ML, Konings H, Pons TL (eds) Causes and consequences of variation in growth rate and productivity of higher plants. SPB Academic, The Hague, pp 19–34
Grime JP (1979) Plant strategies and vegetation processes. Wiley, Chichester
Grime JP, Hunt R (1975) Relative growth rate: its range and adaptive significance in a local flora. J Ecol 63: 393–422
Grubb PJ (1986) Sclerophylls, pachyphylls and pycnophylls: the nature and significance of hard leaf surfaces. In: Juniper B, Southwood R (eds) Insects and plant surfaces. Edaward Arnold, London, pp 5–30
Harper J (1989) The value of a leaf. Oecologia 80: 53–58
Hirose T (1987) A vegetative growth model: adaptive significance of phenotypic plasticity in matter partitioning. Funct Ecol 1: 195–202
Hirose T, Werger MGA (1987) Nitrogen use efficiency in instantaneous and daily photosynthesis of leaves in the canopy of a Solidago altissima stand. Physiol Plant 70: 215–222
Hull JC, Mooney HA (1990) Effects of nitrogen on photosynthesis and growth rate of four California annual grasses. Acta Oecol 11: 453–468
Lambers H, Dijkstra P (1987) A physiological analysis of genotypic variation in relative growth rate: Can growth rate confer an ecological advantage? In: Van Andel J, Bakker JP, Snaydon RW (eds) Disturbance in grasslands. Junk, Dordrecht, pp 237–252
Lambers H, Poorter H (1992) Inherent variation in growth rate between higher plants: a search for physiological causes and ecological consequences. Adv Ecol Res 23: 188–261
Lambers H, Freijsen AHJ, Poorter H, Hirose T, Van der Werf A (1989) Analyses of growth based on net assimilation rate and nitrogen productivity. Their physiological background. In: Lambers H, Cambridge ML, Konings H, Pons TL (eds) Causes and consequences of variation in growth rate and productivity of higher plants. SPB Academic, The Hague, pp 1–18
Mahmoud A, Grime JP (1976) An analysis of competitive ability in three perennial grasses. New Phytol 77: 431–435
Makino A, Osmond B (1991) Effects on nitrogen nutrition on nitrogen partitioning between chloroplast and mitochondria in pea and wheat. Plant Physiol 96: 355–362
Muller B, Garnier E (1990) Components of relative growth rate and sensitivity to nitrogen availability in annual and perennial species of Bromus. Oecologia 84: 513–518
Niemann GJ, Pureveen JBM, Eijkel GB, Poorter H, Boon JJ (1992) Differences in relative growth rate in 11 grasses correlate with differences in chemical composition as determined by pyrolysis mass spectrometry. Oecologia 89: 567–573
Olff H (1992a) On the mechanisms of vegetation succession. PhD thesis, University of Groningen
Olff H (1992b) Effects of light and nutrient availability on dry matter and N allocation in six successional grassland species. Testing for resource ratio effects. Oecologia 89: 412–421
Pons TL, Schieving F, Hirose T, Werger MJA (1989) Optimization of leaf nitrogen allocation for canopy photosynthesis in Lysimachia vulgaris. In: Lambers H, Cambridge ML, Konings H, Pons TL (eds) Causes and consequences of variation in growth rate and productivity of higher plants. SPB Academic, The Hague, pp 175–186
Poorter H, Bergkotte M (1992) Chemical composition of 24 wild species differing in relative growth rate. Plant Cell Environ 15: 221–229
Poorter H, Remkes C (1990) Leaf area ratio and net assimilation rate of 24 wild species differing in relative growth rate. Oecologia 83: 553–559
Poorter H, Remkes C, Lambers H (1990) Carbon and nitrogen economy of 24 wild species differing in relative growth rate. Plant Physiol 94: 621–627
Schieving F, Pons TL, Werger MJA, Hirose T (1992) The vertical distribution of nitrogen and photosynthetic activity at different plant densities in Carex acutiformis. Plant Soil 14: 9–17
Shipley B, Keddy PA (1988) The relationship between relative growth rate and sensitivity to nutrient stress in twenty-eight species of emergent macrophytes. J Ecol 76: 1101–1110
Schulze E-D, Chapin FS III (1987) Plant specialization to environments of different resources availability. In: Schulze E-D, Zwolfer H (eds) Potentials and limitations of ecosystem analysis. Springer, Berlin, pp 120–148
Tilman D (1988) Plant strategies and the dynamics and structure of plant communities. Princeton University Press, Princeton
Tilman D (1990) Constraints and tradeoffs: toward a predictive theory of competition and succession. Oikos 58: 3–15
Van der Werf A, Hirose T, Lambers H (1989) Variation in root respiration; causes and consequences for growth. In: Lambers H, Cambridge ML, Konings H, Pons TL (eds) Causes and consequences of variation in growth rate and productivity of higher plants. SPB Academic, The Hague, pp 227–240
Van der Werf A, Welschen R, Lambers H (1992) Respiratory losses increase with decreasing inherent growth rate of a species and with decreasing nitrate supply. In: Lambers H, Van der Plas LHW (eds) Molecular, biochemical and physiological aspects of plant respiration. SPB Academic, The Hague, pp 421–432
Van der Werf A, Visser AJ, Schieving F, Lambers H (1993a) Evidence for optimal partitioning of biomass and nitrogen at a range of nitrogen availabilities for a fast- and slow-growing species. Funct Ecol, 7: 63–74
Van der Werf A, Poorter H, Lambers H (1993b) Respiration as dependent on a species' inherent growth rate and on the nitrogen supply to the plant. In: Roy J, Garnier E (eds) A whole-plant perspective of carbon nitrogen interactions. SPB Academic, The Hague (in press)
Wedin DA (1990) Nitrogen cycling and competition among grass species. PhD Thesis, University of Minnesota
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
van der Werf, A., van Nuenen, M., Visser, A.J. et al. Contribution of physiological and morphological plant traits to a species' competitive ability at high and low nitrogen supply. Oecologia 94, 434–440 (1993). https://doi.org/10.1007/BF00317120
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00317120