Abstract
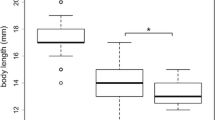
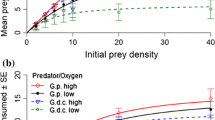
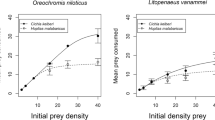
The invasive Ponto–Caspian amphipod Dikerogammarus villosus strongly impairs the structure of recipient freshwater communities, mostly through predation of a wide range of invertebrates. Useful insights on the ecological impact of an invader can be provided by comparing its functional response (FR)—namely the relationship between resource use and resource availability—to that of trophic analogs from the recipient communities. We applied this method and derived the FR of D. villosus, the native Gammarus pulex, and Echinogammarus berilloni, another gammarid that is also undergoing range expansion, feeding on live dipteran larvae. We tested a range of predator densities to account for mutual interference among predators, assuming that predators do not forage alone in their natural environment. We also analysed the predators’ spatial distribution to investigate whether spatial behaviour might be at the origin of interference. For the three gammarid species, the per capita predation rate was a function of the prey-to-predator ratio and showed a decelerating rise to an asymptote. There was no difference in searching efficiency between the three species while prey handling time was significantly lower in G. pulex than in D. villosus and E. berilloni, leading to a higher food intake for the native at high prey densities. Differences in morphology and behaviour between the three gammarids might explain our results. Indeed, D. villosus tended to aggregate more than the other two gammarid species, which promotes conspecific interactions. Our findings suggest that mutual interference between conspecifics is a fundamental shaper of gammarid predation. Time spent interacting with conspecifics is likely to decrease the biotic pressure of the Killer shrimp D. villosus. From a broader perspective, in addition to higher predation risk, parasitism, habitat complexity or climatic variables, the presence of conspecifics of the predator (i.e. predator density) is another factor that has the potential to alter FR comparisons.





Similar content being viewed by others
References
Alexander ME, Dick JT, Weyl OL, Robinson TB, Richardson DM (2014) Existing and emerging high impact invasive species are characterized by higher functional responses than natives. Biol Lett 10:20130946. doi:10.1098/rsbl.2013.0946
Alexander ME, Kaiser H, Weyl OLF, Dick JTA (2015) Habitat simplification increases the impact of a freshwater invasive fish. Environ Biol Fish 98:477–486. doi:10.1007/s10641-014-0278-z
Anholt BR, Werner EE (1995) Interaction between food availability and predation mortality mediated by activity. Ecology 76:2230–2234. doi:10.2307/1941696
Arditi R, Akçakaya HR (1990) Underestimation of the mutual interference of predators. Oecologia 83:358–361
Arditi R, Ginzburg LR (1989) Coupling in predator prey dynamics: ratio dependence. J Theor Biol 139:311–326
Arditi R, Ginzburg LR (2012) How species interact: altering the standard view on trophic ecology. Oxford University Press, New York
Arditi R, Perrin N, Saïah H (1991) Functional responses and heterogeneities: an experimental test with cladocerans. Oikos 60:69–75. doi:10.2307/3544994
Arditi R, Tyutyunov Y, Morgulis A, Govorukhin V, Senina I (2001) Directed movement of predators and the emergence of density dependence in predator–prey models. Theor Popul Biol 59:207–221. doi:10.1006/tpbi.2001.1513
Arditi R, Callois JM, Tyutyunov Y, Jost C (2004) Does mutual interference always stabilize predator–prey dynamics? A comparison of models. C R Biologies 327:1037–1057. doi:10.1016/j.crvi.2004.06.007
Bacela-Spychalska K, Rigaud T, Wattier RA (2014) A co-invasive microsporidian parasite that reduces the predatory behaviour of its host Dikerogammarus villosus (Crustacea, Amphipoda). Parasitology 141:254–258. doi:10.1017/S0031182013001510
Barrios-O’Neill D, Dick JTA, Emmerson MC, Ricciardi A, MacIsaac HJ (2014a) Predator-free space, functional responses and biological invasions. Funct Ecol. doi:10.1111/1365-2435.12347
Barrios-O’Neill D, Dick JTA, Emmerson MC, Ricciardi A, MacIsaac HJ, Alexander ME, Bovy HC (2014b) Fortune favours the bold: a higher predator reduces the impact of a native but not an invasive intermediate predator. J Anim Ecol 83:693–701. doi:10.1111/1365-2656.12155
Barrios-O’Neill D, Dick JTA, Ricciardi A, MacIsaac HJ, Emmerson MC (2014c) Deep impact: in situ functional responses reveal context-dependent interactions between vertically migrating invasive and native mesopredators and shared prey. Freshw Biol 59:2194–2203. doi:10.1111/fwb.12423
Beale EML (1960) Confidence regions in non-linear estimation. J Roy Statist Soc Ser B 22:41–88
Beddington JR (1975) Mutual interference between parasites or predators and its effect on searching efficiency. J Anim Ecol 51:331–340
Bollache L, Dick JTA, Farnsworth KD, Montgomery WI (2008) Comparison of the functional responses of invasive and native amphipods. Biol Lett 4:166–169. doi:10.1098/rsbl.2007.0554
Bovy HC, Barrios-O’Neill D, Emmerson MC, Aldridge DC, Dick JTA (2014) Predicting the predatory impacts of the “demon shrimp” Dikerogammarus haemobaphes, on native and previously introduced species. Biol Inv. doi:10.1007/s10530-014-0751-9
Buřič M, Koči L, Petrusek A, Kouba A, Kozák P (2009) Invaders eating invaders: potential trophic interactions between the amphipod Dikerogammarus villosus and juvenile crayfish Orconectes limosus. Knowl Manag Aquat Ecosyst 5:394–395. doi:10.1051/kmae/2009015
Burnham KP, Anderson DR (1998) Model selection and inference: a practical information—theoretic approach, 2nd edn. Springer, New York
Casellato S, Visentin A, La Piana G (2007) The predatory impact of Dikerogammarus villosus, a danger for fish. In: Gherardi F (ed) Biological invaders in inland waters: profiles, distribution, and threats. Invading nature: Springer series in invasion ecology. Springer, Dordrecht, pp 495–506
Chapple DG, Simmonds SM, Wong BB (2012) Can behavioral and personality traits influence the success of unintentional species introductions? Trends Ecol Evol 27:57–64. doi:10.1016/j.tree.2011.09.010
Cosner C, DeAngelis DL, Ault JS, Olson DB (1999) Effects of spatial grouping on the functional response of predators. Theor Popul Biol 56:65–75. doi:10.1006/tpbi.1999.1414
Covich AP, Palmer MA, Crowl TA (1999) The role of benthic invertebrate species in freshwater ecosystems. Bioscience 49:119–127. doi:10.2307/1313537
Crowley PH, Martin EK (1989) Functional responses and interference within and between year classes of a dragonfly population. J North Am Benthol Soc 8:211–221
DeAngelis DL, Goldstein RA, O'neill RV (1975) A model for tropic interaction. Ecol 56:881–892. doi:10.2307/1936298
Dick JTA (1995) The cannibalistic behaviour of two Gammarus species (Crustacea: Amphipoda). J Zool 237:697–706. doi:10.1111/j.1469-7998.1995.tb02740.x
Dick JTA (2008) Role of behaviour in biological invasions and species distributions; lessons from interactions between the invasive Gammarus pulex and the native G. duebeni (Crustacea: Amphipoda). Contrib Zool 77:91–98
Dick JTA, Platvoet D (2000) Invading predatory crustacean Dikerogammarus villosus eliminates both native and exotic species. Proc Roy Soc B Biol Sci 267:977–983. doi:10.1098/rspb.2000.1099
Dick JTA, Platvoet D, Kelly DW (2002) Predatory impact of the freshwater invader Dikerogammarus villosus (Crustacea: Amphipoda). Can J Fish Aquat Sci 59:1078–1084. doi:10.1139/f02-074
Dick JTA, Armstrong M, Clarke HC, Farnsworth KD, Hatcher MJ, Ennis M, Kelly A, Dunn AM (2010) Parasitism may enhance rather than reduce the predatory impact of an invader. Biol Lett 66:36–638. doi:10.1098/rsbl.2010.0171
Dick JTA, Gallagher K, Avlijas S, Clarke HC, Lewis SE, Leung S, Minchin D, Caffrey J, Alexander ME, Maguire C, Harrod C, Reid N, Haddaway NR, Farnsworth KD, Penk M, Ricciardi A (2013) Ecological impacts of an invasive predator explained and predicted by comparative functional responses. Biol Inv 15:837–846. doi:10.1007/s10530-012-0332-8
Dick JTA, Alexander ME, Jeschke JM, Ricciardi A, MacIsaac HJ, Robinson TB, Kumschick S, Weyl OLF, Dunn AM, Hatcher MJ, Paterson RA, Farnsworth KD, Richardson DM (2014) Advancing impact prediction and hypothesis testing in invasion ecology using a comparative functional response approach. Biol Inv 16:735–753. doi:10.1007/s10530-013-0550-8
Dodd JA, Dick JTA, Alexander ME, MacNeil C, Dunn AM, Aldridge DC (2014) Predicting the ecological impacts of a new freshwater invader: functional responses and prey selectivity of the ‘killer shrimp’, Dikerogammarus villosus, compared to the native Gammarus pulex. Freshw Biol 59:337–352. doi:10.1111/fwb.12268
Durieux R, Rigaud T, Médoc V (2012) Parasite-induced suppression of aggregation under predation risk in a freshwater amphipod: sociality of infected amphipods. Behav Process 91:207–213. doi:10.1016/j.beproc.2012.08.002
Furse MT, Wright JF, Armitage PD, Moss D (1981) An appraisal of pond-net samples for biological monitoring of lotic macro-invertebrates. Wat Res 15:679–689. doi:10.1016/0043-1354(81)90160-3
Gentleman WC, Neuheimer AB (2008) Functional responses and ecosystem dynamics: how clearance rates explain the influence of satiation, food-limitation and acclimation. J Plankton Res 30:1215–1231. doi:10.1093/plankt/fbn078
Griffen BD, Delaney DG (2007) Species invasion shifts the importance of predator dependence. Ecology 88:3012–3021. doi:10.1890/07-0172.1
Haddaway NR, Wilcox RH, Heptonstall RE, Griffiths HM, Mortimer RJ, Christmas M, Dunn AM (2012) Predatory functional response and prey choice identify predation differences between native/invasive and parasitised/unparasitised crayfish. PLoS ONE 7:e32229. doi:10.1371/journal.pone.0032229
Hassell MP, Varley GC (1969) New inductive population model for insect parasites and its bearing on biological control. Nature 223:1133–1136
Hassell MP, Lawton JH, Beddington JR (1977) Sigmoid functional responses by invertebrate predators and parasitoids. J Anim Ecol 46:249–262
Holling CS (1959) Some characteristics of simple types of predation and parasitism. Can Entomol 91:385–398
Hudina S, Hock K, Žganec K (2014) The role of aggression in range expansion and biological invasions. Curr Zool 60:401–409
Juliano SA (2001) Non-linear curve fitting: predation and functional response curves. In: Scheiner SM, Gurevitch J (eds) Design and analysis of ecological experiments. Oxford University Press, UK, pp 178–196
Kalinkat G, Schneider FD, Digel C, Guill C, Rall BC, Brose U (2013) Body masses, functional responses and predator–prey stability. Ecol Lett 16:1126–1134. doi:10.1111/ele.12147
Kestrup AM, Dick JTA, Ricciardi A (2011) Interaction between invasive and native crustaceans: differential functional responses of intraguild predators towards juvénile hetero-specifics. Biol Inv 13:731–737. doi:10.1007/s10530-010-9863-z
Kinzler W, Maier G (2003) Asymmetry in mutual predation: possible reason for the replacement of native gammarids by invasives. Archiv Hydrobiol 157:473–481. doi:10.1127/0003-9136/2003/0157-0473
Krisp H, Maier G (2005) Consumption of macroinvertebrates by invasive and native gammarids: a comparison. J Limnol 64:55–59. doi:10.4081/jlimnol.2005.55
Kullmann H, Thünken T, Baldauf SA, Bakker TCM, Frommen JG (2008) Fish odour triggers conspecific attraction behaviour in an aquatic invertebrate. Biol Lett 4:458–460. doi:10.1098/rsbl.2008.0246
Laverty C, Dick JT, Alexander ME, Lucy FE (2015) Differential ecological impacts of invader and native predatory freshwater amphipods under environmental change are revealed by comparative functional responses. Biol Inv 17:1761–1770. doi:10.1007/s10530-014-0832-9
Maazouzi C, Masson G, Izquierdo MS, Pihan JC (2007) Fatty acid composition of the amphipod Dikerogammarus villosus: feeding strategies and trophic links. Comp Biochem Physiol A Mol Integr Physiol 147:868–875
MacNeil C, Platvoet D (2005) The predatory impact of the freshwater invader Dikerogammarus villosus on native Gammarus pulex (Crustacea: Amphipoda); influences of differential microdistribution and food resources. J Zool 267:31–38. doi:10.1017/S0952836905007351
MacNeil C, Platvoet D, Dick JTA, Fielding N, Constable A, Hall N, Aldridge D, Renals T, Diamond M (2010) The Ponto–Caspian ‘killer shrimp’, Dikerogammarus villosus (Sowinsky, 1894), invades the British Isles. Aquat Inv 5:441–445. doi:10.3391/ai.2010.5.4.15
MacNeil C, Dick JTA, Platvoet D, Briffa M (2011) Direct and indirect effects of species displacements: an invading freshwater amphipod can disrupt leaf-litter processing and shredder efficiency. J North Am Benthol Soc 30:38–48. doi:10.1899/10-056.1
MacNeil C, Boets P, Lock K, Goethals PLM (2013) Potential effects of the invasive ‘killer shrimp’ (Dikerogammarus villosus) on macroinvertebrate assemblages and biomonitoring indices. Freshwat Biol 58:171–182. doi:10.1111/fwb.12048
Mayer G, Maier G, Maas A, Waloszek D (2008) Mouthparts of the Ponto–Caspian invader Dikerogammarus villosus (Amphipoda: Pontogammaridae). J Crust Biol 28:1–15. doi:10.1651/07-2867R.1
Médoc V, Spataro T (2015) Predicting the impact of invasive species: a look forward on the comparative functional response approach. Rev Ecol (Terre Vie) 70(supp. 12). in press
Médoc V, Spataro T, Arditi R (2013) Prey: predator ratio dependence in the functional response of a freshwater amphipod. Freshw Biol 58:858–865. doi:10.1111/fwb.12091
Murdoch WW, Oaten A (1975) Predation and population stability. Adv Ecol Res 9:1–131
Paterson RA, Dick JTA, Pritchard DW, Ennis M, Hatcher MJ, Dunn AM (2014) Predicting invasive species impacts: a community module functional response approach reveals context dependencies. J Anim Ecol. doi:10.1111/1365-2656.12292
Piscart C, Mermillod-Blondin F, Maazouzi C, Merigoux S, Marmonier P (2011) Potential impact of invasive amphipods on leaf litter recycling in aquatic ecosystems. Biol Inv 13:2861–2868. doi:10.1007/s10530-011-9969-y
Platvoet D, Dick JTA, Konijnendijk N, van der Velde G (2006) Feeding on micro-algae in the invasive Ponto–Caspian amphipod Dikerogammarus villosus (Sowinsky, 1894). Aquat Ecol 40:237–245. doi:10.1007/s10452-005-9028-9
Platvoet D, van der Velde G, Dick JTA, Li SQ (2009) Flexible omnivory in Dikerogammarus villosus (Sowinsky, 1894) (Amphipoda)—amphipod pilot species project (Ampis) report 5. Crustaceana 82:703–720. doi:10.1163/156854009X423201
Posada D, Crandall KA (2001) Selecting the best-fit model of nucleotide substitution. Syst Biol 50:580–601. doi:10.1080/10635150118469
Rall BC, Guill B, Brose U (2008) Food-web connectance and predator interference dampen the paradox of enrichment. Oikos 117:202–213. doi:10.1111/j.2007.0030-1299.15491.x
Rewicz T, Grabowski M, MacNeil C, Bącela-Spychalska K (2014) The profile of a ‘perfect’ invader—the case of killer shrimp, Dikerogammarus villosus. Aquat Inv 9:267–288
Rothschild M, Clay T (1952) Fleas, flukes and cuckoos: a study of bird parasites. Collins, London
Solomon ME (1949) The natural control of animal populations. J Anim Ecol 18:1–35
Stillman RA, Goss-Custard JD, Caldow RWG (1997) Modelling interférence from basic foraging behaviour. J Anim Ecol 66:692–703. doi:10.2307/5922
Thünken T, Baldauf SA, Bersau N, Bakker TCM, Kullmann H, Frommen JG (2010) Impact of olfactory nonhost predator cues on aggregation behaviour and activity in Polymorphus minutus infected Gammarus pulex. Hydrobiologia 654:137–145. doi:10.1007/s10750-010-0377-6
Tregenza T (1995) Building on the ideal free distributions. Adv Ecol Res 26:253–307
Truhlar AM, Aldridge DC (2015) Differences in behavioural traits between two potentially invasive amphipods, Dikerogammarus villosus and Gammarus pulex. Biol Inv 17:1569–1579. doi:10.1007/s10530-014-0816-9
Truhlar AM, Dodd JA, Aldridge DC (2014) Differential leaf-litter processing by native (Gammarus pulex) and invasive (Dikerogammarus villosus) freshwater crustaceans under environmental extremes. Aquat Conserv Marine Freshw Ecosyst 24:56–65. doi:10.1002/aqc.2375
Tyutyunov Y, Titova L, Arditi R (2007) A minimal model of pursuit-evasion in a predator–prey system. Math Model Nat Phenom 2:122–134. doi:10.1051/mmnp:2008028
U.S. EPA (Environmental Protection Agency) (2008) Predicting future introductions of nonindigenous species to the Great Lakes. National Center for Environmental Assessment, Washington, DC; EPA/600/R-08/066F. Available from the National Technical Information Service, Springfield, VA, and http://www.epa.gov/ncea
Van der Velde G, Rajagopal S, Kelleher B, Muskó IB, Bij De Vaate A (2000) Ecological impact of crustacean invaders: general considerations and examples from the Rhine River. Biodivers Crisis Crustac 12:3–33
Vucic-Pestic O, Rall BC, Kalinkat G, Brose U (2010) Allometric functional response model: body masses constrain interaction strengths. J Anim Ecol 79:249–256. doi:10.1111/j.1365-2656.2009.01622.x
Weis JS (2010) The role of behavior in the success of invasive crustaceans. Mar Freshw Behav Physiol 43:83–98. doi:10.1080/10236244.2010.480838
Acknowledgments
We are grateful to Marion Guillaumin, Marie-Claire Danner and Samuel Perret for their help during field sampling and during the experiments. We thank the Foljuif biological station for providing excellent working conditions. We also thank three anonymous referees for their constructive comments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Médoc, V., Albert, H. & Spataro, T. Functional response comparisons among freshwater amphipods: ratio-dependence and higher predation for Gammarus pulex compared to the non-natives Dikerogammarus villosus and Echinogammarus berilloni . Biol Invasions 17, 3625–3637 (2015). https://doi.org/10.1007/s10530-015-0984-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-015-0984-2