Abstract
Invasion ecology urgently requires predictive methodologies that can forecast the ecological impacts of existing, emerging and potential invasive species. We argue that many ecologically damaging invaders are characterised by their more efficient use of resources. Consequently, comparison of the classical ‘functional response’ (relationship between resource use and availability) between invasive and trophically analogous native species may allow prediction of invader ecological impact. We review the utility of species trait comparisons and the history and context of the use of functional responses in invasion ecology, then present our framework for the use of comparative functional responses. We show that functional response analyses, by describing the resource use of species over a range of resource availabilities, avoids many pitfalls of ‘snapshot’ assessments of resource use. Our framework demonstrates how comparisons of invader and native functional responses, within and between Type II and III functional responses, allow testing of the likely population-level outcomes of invasions for affected species. Furthermore, we describe how recent studies support the predictive capacity of this method; for example, the invasive ‘bloody red shrimp’ Hemimysis anomala shows higher Type II functional responses than native mysids and this corroborates, and could have predicted, actual invader impacts in the field. The comparative functional response method can also be used to examine differences in the impact of two or more invaders, two or more populations of the same invader, and the abiotic (e.g. temperature) and biotic (e.g. parasitism) context-dependencies of invader impacts. Our framework may also address the previous lack of rigour in testing major hypotheses in invasion ecology, such as the ‘enemy release’ and ‘biotic resistance’ hypotheses, as our approach explicitly considers demographic consequences for impacted resources, such as native and invasive prey species. We also identify potential challenges in the application of comparative functional responses in invasion ecology. These include incorporation of numerical responses, multiple predator effects and trait-mediated indirect interactions, replacement versus non-replacement study designs and the inclusion of functional responses in risk assessment frameworks. In future, the generation of sufficient case studies for a meta-analysis could test the overall hypothesis that comparative functional responses can indeed predict invasive species impacts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Invasion biology faces two major challenges with respect to increasing our ability to forecast the ecological impacts of invasive species. Firstly, the discipline needs to move beyond describing and cataloguing case studies of impact towards the development of a mechanistic understanding of impact that would allow for more predictive power, and this in the context of global change (Walther et al. 2009; Dick et al. 2013; Simberloff et al. 2013). Secondly, robust tests of major hypotheses in invasion ecology are often lacking, as evidenced by equivocal support for many such hypotheses (e.g. Catford et al. 2009; Davis 2011; Jeschke et al. 2012; Ricciardi et al. 2013). This may, in part, be due to a lack of rigour in defining these hypotheses (Heger et al. 2013) and lack of focus on demographic processes. These two major challenges need to be simultaneously addressed to advance the fundamental science of invasion ecology and to provide practical methodologies that prioritize and mitigate invasion threats by, for example, refining risk assessment protocols (Ricciardi and Rasmussen 1998; Parker et al. 1999; Byers et al. 2002; Andersen et al. 2004; Kumschick et al. 2012; Leung et al. 2012) and managing biological communities to provide maximum biotic resistance (Taylor and Duggan 2012).
There have been several attempts to develop frameworks for conceptualizing the mechanisms whereby invasive species cause ecological impacts, with a common theme being how invaders alter communities and ecosystems through resource use (Vitousek 1990; Chapin et al. 1996; Parker et al. 1999). In particular, Parker et al. (1999) opined the need for ‘operational generalizations’ about impact and stressed the difficulty of assessing the per capita effects of invaders. Not all invaders have a major impact because of their per capita effects; for example, many invasive plants, through their great abundance or biomass, affect fire regimes (Brooks et al. 2004). Nonetheless, many invaders do generate impacts directly because of per capita effects, and a major obstacle to testing impact theories is the lack of standardized methods for determining such effects on use of resources, such as native prey (Ricciardi et al. 2013). Furthermore, we require methods that can reliably explain the ecological impacts of existing invaders, and predict impacts of emerging and future invaders under different or changing environmental circumstances; understanding the corollary, patterns of resistance of natives towards invaders, would also be welcome. Ideally, such methods should be rapid, reliable, inexpensive and applicable across taxonomic and trophic groups, with data collection possible from a variety of laboratory and field-based studies, as appropriate to the organisms and systems involved.
Here, we review and provide a framework for a promising emerging field in invasion ecology that can address these issues: the use of comparative functional responses, whereby the relationship between resource consumption rate (e.g. by a predator) and resource density (e.g. prey) is compared between invader and native species to reveal ecological impact (e.g. see Dick et al. 2013; Fig. 1a–d). Specifically, we: (1) examine species trait comparisons in invasion ecology and explore advantages of the functional response method in this context; (2) review the historical use of functional responses in invasion ecology and its major hypotheses; (3) introduce our comparative functional response framework and its advantages as a predictive tool in invasion ecology; and (4) outline future challenges of implementing this framework in predicting invader impacts and testing hypotheses, and identify research priorities.
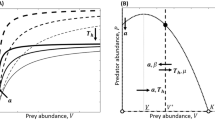
a Functional response types and hypothetical invader/native comparisons; b–d Differences in functional responses between an invasive mysid shrimp (Hemimysis anomala, closed circles, solid line) and a native comparator (Mysis salemaai, open circles, dashed line) explains and predicts known field impacts of the invader on zooplankton prey species (redrawn from Dick et al. 2013)
Species traits comparisons and the utility of functional responses
Comparisons of species traits between invaders and natives (or unsuccessful/less successful invaders) have in some circumstances been successful in identifying broad determinants of invasiveness in terms of establishment and spread (e.g. Mack 1996; Remanjek and Richardson 1996; Sakai et al. 2001; van Kleunen et al. 2010), however, numerous exceptions to any derived rule dilute the predictive power of such techniques for any one known or potential invader. Trait-based predictions have had some success in predicting plant establishment, invasiveness and impact (e.g. Pyšek et al. 2009; but see Palacio-Lopez and Gianoli 2011), but the distribution of success of such trait comparisons is patchy among animal taxa (Hayes and Barry 2008), with some good predictors of invasion success for birds (Sol et al. 2002; Blackburn et al. 2009), fishes (Marchetti et al. 2004a, b) and mammals (Jeschke and Strayer 2006), often based on propagule pressure and human affiliation. However, consistent predictors of invasion success across animal and plant taxa remain elusive (Hayes and Barry 2008). There has been even less progress in the prediction of the ecological impacts of invaders (Branch and Steffani 2004; Ricciardi et al. 2013; but see Nentwig et al. 2009; Kumschick et al. 2013), with invasion history emerging as a useful but restricted indicator of likely future impact (Kulhanek et al. 2011; Kumschick and Richardson 2013), especially since the method only applies to known invaders with sufficient existing studies of impact. However, we require forecasting methods that can be applied to new and emerging invaders, for example, where a new species has just arrived or is known to be spreading, or indeed potential new invasive species from known donor region ‘hotspots’ such as the Ponto-Caspian region (Ricciardi and MacIsaac 2000).
Invasive species are characteristically more able to rapidly and efficiently utilise resources than native species (Vitousek 1990; Strayer et al. 1999; Byers et al. 2002; Funk and Vitousek 2007; Johnson et al. 2008; Weis 2010; Morrison and Hay 2011; Chapple et al. 2012) and thus resources, such as native prey species, are vulnerable to potentially severe declines or extinctions (Clavero and Garcia-Berthou 2005; Snyder and Evans 2006; Salo et al. 2007; Cucherousset and Olden 2011; Roy et al. 2012). Indeed, difference in resource use is a major tenet of 28 of 29 invasion hypotheses identified by Catford et al. (2009). It follows that comparing resource utilisation rates and patterns among known invaders and trophically analogous natives, and perhaps among emerging or new invaders and such natives, could allow more reliable predictions of invader impact. The relationship between resource availability and resource consumption rate is the ‘functional response’ (Solomon 1949; Holling 1959a, b; Juliano 2001), which usually takes three forms (Fig. 1a), and can be derived for consumers and compared among them (e.g. Fig. 1b–d). Although the functional response is a standard measure utilized in classic behavioural, population and community ecology (e.g. Sabelis 1992; Soluk 1993; Barbeau et al. 1998; Jeschke et al. 2002; van Leeuwen et al. 2007), and also a familiar technique in assessing the potential and efficacy of biological control agents (O’Neil 1990; Van Drische and Bellows 2011; but see criticisms by Lester and Harmsen 2002), the uptake of functional responses in invasion biology has been very limited (see below). Indeed, despite the use of functional responses in testing the efficacy of biocontrol agents towards invasive species (e.g. Carrillo and Pena 2012), it has been employed surprisingly rarely by invasion ecologists who are essentially asking very similar questions about ecological impact. In general, it is reasonable to expect that the functional responses of invaders will determine their impact on resources, as the functional response quantifies the strength of primary ecological interactions (e.g. predator–prey). Further, if invaders show greater functional responses than natives, with which the affected native resources (e.g. prey species) have an evolutionary history, then the difference in magnitude of invader/native functional responses may explain and predict invader impacts (as with Fig. 1b–d).
There are several advantages to deriving the functional responses of invasive species and comparing them with native species, or among invaders, as a measure of ecological impact. First, the method quantifies the rate of resource uptake and provides parameter estimates for the functional response curves (attack rate, handling time and maximum feeding rate) that describe the mechanisms driving their shape and magnitude (see, for example, Dick et al. 2013). Secondly, however, and perhaps most importantly, the shape and magnitude of the functional response can inform whether the consumer (e.g. introduced predator) will likely regulate, stabilise or de-stabilise the resource (e.g. prey) populations, with implications for population viability. Here, we use a predator–prey relationship to illustrate this point. In a sigmoidal, positive density-dependent Type III functional response (see Fig. 1a), although the predator may regulate the prey population, prey experience a low density refuge with a reduction in risk of mortality as prey densities fall below a threshold level, thus potentially imparting stability to predator–prey dynamics and facilitating prey persistence (Murdoch and Oaten 1975). This is in contrast to the potentially population de-stabilising, inversely density-dependent Type II functional response where most, if not all prey are consumed at low prey densities, potentially leading to prey extinction at a range of spatial and temporal scales (Hassell 1978; Taylor and Collie 2003; Ward et al. 2008; Rindone and Eggleston 2011). Further, counter to the traditional view, functional responses are not fixed for predator–prey species pairs, and functional responses can change from Type II to Type III or vice versa under different circumstances. For example, it is well established that functional responses may change under the influence of a number of environmental variables, such as temperature, light levels and habitat structure (Lipcius and Hines 1986; Eggleston 1990; Koski and Johnson 2002; Jeschke et al. 2004; Alexander et al. 2012, in press). Also, predators may have different functional response types with different prey species (e.g. Moustahfid et al. 2010). Thus, comparative functional response studies in invasion ecology may have a further predictive advantage in that relevant environmental contexts (e.g. global climate change), and differences in abiotic factors such as temperature or salinity regime, can be incorporated into experiments to reveal differences in the type and/or magnitude of the functional response, and hence likely population outcomes for a variety of impacted native species (e.g. see Kestrup et al. 2011). Further, since factors such as parasitism of invaders/natives can be included in study designs, the methodology allows for the robust testing of other major hypotheses in invasion ecology, such as ‘enemy release’ (see Dick et al. 2010; Fig. 2 as discussed below).
A full assessment of the impact of a consumer on a resource, such as for predators and prey as discussed above, would include alongside the functional response an assessment of the numerical response, which can involve the demographic and/or aggregative response of the predator to prey density; this combination gives the total response (Solomon 1949; Holling 1959a, b). In practice, the functional response is relatively straightforward to derive, whereas empirical derivation of the demographic numerical response is more challenging, and might add little to the predictive power of functional responses if the latter prove overwhelmingly robust in explaining and predicting the ecological impacts of invasive species. Thus, whilst acknowledging the potential to include numerical responses in our framework, we make the case here for a focus on functional responses; however, we make some suggestions as to inclusion of numerical responses and their proxies in impact scoring in the “Challenges, future research and applications” section below.
History of functional responses in invasion ecology
We conducted a systematic search for the use of functional responses in invasion ecology using Web of Science to search for the following: “functional response(s)” AND invader(s); “functional response(s)” AND invasive(s); “functional response(s)” AND exotic(s). We also then utilised each paper’s reference list to bolster and track down more obscure literature. What emerges is that, whilst functional responses have a long history in classical ecology and biological control, their use in invasion ecology is more recent and surprisingly sparse (Table 1). The use of functional responses in a comparative approach, whereby invasive and native predators are compared against one another, features even more rarely and, often, the functional responses of invaders are derived for reasons other than explaining or predicting ecological impact (Table 1). For example, the functional response of the invasive round goby Neogobius melanostomus was compared to that of the native mottled sculpin Cottus bairdi, with the invader showing a higher functional response, but this was done only as a prelude to another experiment on behavioural interactions between these fish species (Dubs and Corkum 1996; Table 1). A more common use of functional responses in an invasion context, however, comes from studies with either invasive or native predators considered separately, with comparisons made of consumption of native and invasive prey (see Table 1). Such studies have been used to deduce whether a native predator is likely to impact a native prey species more than an invasive prey species, or vice versa, with similar conclusions drawn for invasive predators (Table 1).
Although functional responses have been used to evaluate the biological control potential of introduced parasitoids (Greenberg et al. 2001; Jones et al. 2003), the first explicit test of the hypothesis that a known ecologically damaging invader might display a higher functional response than native and other less damaging invaders was by Bollache et al. (2008), who showed that the invasive ‘killer shrimp’, Dikerogammarus villosus, had a higher Type II functional response than other native and introduced comparator species in Europe. Dick et al. (2010) then used the comparative functional response method within an invasive species, showing that an invasive predatory amphipod, Gammarus pulex, had a higher Type II functional response when parasitized with an acanthocephalan worm, counter to the enemy release hypothesis, illustrating the utility of the method in tests of major invasion biology hypotheses (see below). It is again noteworthy that the biological control literature uses comparative functional responses to make such comparisons, but with different questions in mind. Thus, while Dick et al. (2010) explicitly tested the enemy release hypothesis, a study by Farrokhi et al. (2010) on Wolbachia-infected parasitoid wasps essentially did the same, but the context was to test the effects of parasitism on the biocontrol efficacy of a control agent (see also Bayoumy 2011).
Jones et al. (2011) used functional responses of an invasive stoat to examine the dynamics of this predator and its mammal prey. In the same year, the co-existence patterns of two intra-guild predators, a native amphipod and an invasive amphipod, were partly explained using the comparative functional response method, which revealed that the native species withstood replacement by the invader by preying more heavily on the invader’s juveniles (Kestrup et al. 2011). Authors then began explicitly comparing invader and native species with functional responses, such as Haddaway et al. (2012), who showed an invasive crayfish has a higher functional response than a native. However, this study, as with most others (see Bollache et al. 2008; Dick et al. 2010), did not explicitly link differential functional responses to actual field patterns of impact on particular prey species; rather, the prey species were chosen to illustrate the methodology and general pattern of higher functional responses of invaders compared to natives. More recently, however, Dick et al. (2013) demonstrated that the invasive ‘bloody red’ shrimp Hemimysis anomala has a higher functional response to several prey species than trophically analogous native species (that are also themselves invasive in some regions) and, more intriguingly, that the greatest invader/native differentials in functional responses were associated with the greatest field impacts of the invader (Fig. 1b–d). Further, Dick et al. (2013) showed that differential functional responses are consistent across the invader’s geographical range (see also Lohrer et al. 2000), thus demonstrating that this technique offers advantages over other trait-based predictions, since other traits often vary across an invader’s range (Olden et al. 2006; Rossong et al. 2012; Parker et al. 2013).
At present, there are insufficient studies to perform a formal meta-analysis to test the overall hypothesis that ecologically damaging invasive species have higher functional responses than comparator native species (see Table 1). However, of the n = 4 studies in Table 1 that have such a comparison, all support the hypothesis. Further, if we include from those studies the comparison of one invader with multiple native comparators (Bollache et al. 2008) and invader/native comparisons using multiple prey species (Dick et al. 2013), we have n = 12 comparisons, of which 11 support the hypothesis; the one comparison of the invasive Hemimysis anomala with the native Mysis diluviana that showed no difference in functional responses involved a prey species that is not impacted by the invader in the field (Dick et al. 2013). Further data, from both functional response comparisons and actual field corroborations of levels of impacts on natives, will allow more powerful tests of the hypothesis and we encourage research in this area.
Functional responses are increasingly being incorporated into tests of major hypotheses in invasion ecology, such as ‘enemy release’ (Dick et al. 2010; see above) and ‘biotic resistance’ (e.g. Twardochleb et al. 2012). Further, we agree with Heger et al. (2013) that many of these hypotheses need to be “branched” into more specific and testable hypotheses. Thus, whilst functional responses have been used in testing the ‘biotic resistance’ hypothesis and studies of the impacts of resident on invasive species (e.g. Zuharah and Lester 2011; Twardochleb et al. 2012), MacNeil et al. (in press) argue that true support for this hypothesis requires: (1) demonstration by field studies that resident species restrict the range, density or abundance of an invader; and (2) that some form of population regulation or de-stabilising interaction occurs between resident and invading species (e.g. in their predator/prey relationship). This was shown to be the case with a North American invasive amphipod, which is strongly negatively associated with two resident predatory amphipods in Europe (MacNeil et al. in press). In the laboratory, both resident species displayed potentially population de-stabilising Type II functional responses towards the invasive prey, even in the presence of habitat complexity, which often drives more stabilising Type III responses (see Alexander et al. 2012, in press). Additionally, as the functional response methodology examined predation rates over a range of prey densities (see below), it was able to demonstrate that the resident predator with the stronger negative field association with the invader had a significantly higher functional response than the other resident predator (MacNeil et al. in press). Importantly, these latest studies (Dick et al. 2013; MacNeil et al. in press) show remarkable congruence of laboratory derived functional responses with actual field patterns of invader and resident predator impacts. Further demonstrations of such congruence will provide great confidence that the methodology has real value in predicting field patterns of impact (see “Challenges, future research and applications” section below).
A comparative functional response framework for invasion ecology
In Fig. 2a–l, we present a framework for comparative functional responses in invasion ecology, whereby the comparisons are between an invasive and a native species of functional response Types II and III (statistical methods available in e.g. R; frair package, Pritchard 2013); however, this could also be comparisons of multiple invasive species, multiple populations of the same invasive species, or an invasive species under differing environmental conditions (e.g. temperature) or states (e.g. parasitism; see Dick et al. 2010). Further, derivation of functional responses can include other context-dependencies, such as multiple predator effects and trait-mediated indirect interactions (see “Challenges, future research and applications” below). We use the maximum feeding rate asymptote on the Y axis to vary the magnitude of these hypothetical functional responses, while also varying their shape, that is, Type II and Type III functional responses, in all their potential combinations. In Fig. 2a–c, the invader can be judged as having a higher, lower or similar Type II functional response compared to the native (the same can be said of two invaders, two populations of invaders, or an invader under two environmental conditions, or with/without parasites, for example). In Fig. 2d–f, the same argument as above applies for Type III functional responses. Then, Fig. 2g–l show combinations of Type II and Type III functional responses; the benefits of using these schemes when applied to invader impacts are explored below. Our narrative, for simplicity, tends to refer to predator/prey functional responses, but we recognise that other trophic interactions, such as herbivore/plant, are also applicable (see “Challenges, future research and applications” section).
a–l A framework for comparative functional responses in invasion ecology, whereby Type II and Type III functional responses are compared between invader and native species to explain and predict invader impact. The scheme also applies to comparisons between two invaders, an invader under two differing environmental circumstances, or an invader with/without parasites, for example. Further multiple comparisons are of course possible but not drawn for simplicity. Arrows show the danger of point ‘snapshot’ comparisons of feeding rates (see text for details)
With respect to the feeding rates of invaders in comparison to natives, studies most often choose a prey density, or provide prey in excess, and measure prey consumed per unit time (e.g. Kelly et al. 2002; Fielding et al. 2003; Renai and Gherardi 2004; Rehage et al. 2005; Olden et al. 2009; Stoffels et al. 2011). The same is true with other feeding rate comparisons, such as between two invaders (Lohrer and Whitlatch 2002; DeGraaf and Tyrrell 2004; Tyrrell et al. 2006); between parasitized and unparasitized invaders (Fielding et al. 2003); investigations of individual invader species impacts (e.g. Bourdeau and O’Connor 2003; Brousseau and Baglivo 2005; Pintor et al. 2009; Pangle and Peacor 2009; but see Hooff and Bollens 2004); and native species predation of invaders and biotic resistance to invaders by natives (deRivera et al. 2005; Bishop and Peterson 2006; Veiga et al. 2011; but see Griswold and Lounibos 2005; Twardochleb et al. 2012). However, the problem with arbitrarily setting one particular level of prey availability is that, because of its ‘snapshot’ nature, any differences in predatory impact may be missed as no opportunity is given for functional response types and magnitudes to emerge and perhaps diverge. Figure 2a, for example, shows that, depending on an arbitrarily set resource density, an invader might be judged as having a similar (Arrow A) or higher (Arrow B) feeding rate. The scheme of Fig. 2 may also be applied in other contexts, such as comparisons of invaders that are either parasitized and unparasitised. Thus, for example, one study (Fielding et al. 2003) showed that parasitized and unparasitized male invasive amphipods were no different in their predation rates; however, this was because both predator groups effectively ran out of prey in the experimental trials, thus driving the non-significant difference (i.e. the prey density chosen was too low on the potential functional response curve; see Arrow A, Fig. 2a). On the other hand, when prey densities were increased in a functional response experimental design for the same invader and parasite system, the divergence of predation rates of those individuals parasitized and unparasitized was evident and significant (Dick et al. 2010; see Arrow B, Fig. 2a). This type of situation might also be evident with Type III functional responses (see Arrows A and B in Fig. 2d). Thus, invading and native species may have similar types of functional response (II or III), though with different or similar magnitudes (Fig. 2a–f); however, single prey density ‘snapshot’ experiments cannot reveal such differences, and indeed, different conclusions could be derived depending on the arbitrary densities chosen by the experimenter (see Fig. 2a,b,d, e). Whilst many studies ensure that prey are not totally depleted during experiments by supplying the prey in excess (e.g. Rehage et al. 2005; Veiga et al. 2011), functional response types can still not be revealed by such studies because, with only one prey density examined, the shape of the curve is not known. Thus, impacts on prey populations in terms of the functional response type are not discernible from ‘snapshot’ study designs.
If we now examine mixed Type II and III functional responses of invader and native species, we could find that an invader has a higher Type II functional response and a comparator native a lower Type III functional response (Fig. 2g), or vice versa (Fig. 2h). While the former might predict that the invader will impact native prey more so than the native, the latter predicts the opposite. In another scenario, the invader and native species could have similar maximum feeding rates (curve asymptotes; Fig. 2i, j), but either the invader displays a Type II and the native a Type III functional response (Fig. 2i) or vice versa (Fig. 2j). Thus, whilst maximum feeding rates are similar, the population-level outcomes could be quite different, as the former scenario predicts a de-stabilising effect of the invader and stabilising effect of the native, whereas the latter scenario predicts the opposite. Note also that an experiment that simply provides sufficient prey density such that prey depletion is not a problem could still be misleading, as the functional response types can be very different even when maximum feeding rates are similar (Fig. 2i, j). Finally, as in Fig. 2k, an invader could have a higher maximum feeding rate than a native, but the invader has a Type III functional response and the native a Type II functional response; or, as in Fig. 2l, the invader could have a lower maximum feeding rate than the native, but the invader has a Type II functional response and the native a Type III functional response. In the former scenario (Fig. 2k), the higher maximum feeding rate of the invader may be misleading with respect to impact on the prey, as the invader functional response is in theory (and in the absence of other mitigating factors) more stabilising and the native more de-stabilising (see Murdoch and Oaten 1975); in the latter scenario (Fig. 2l), the lower maximum feeding rate of the invader may be misleading with respect to impact on prey, as the invader functional response is more de-stabilising and the native more stabilising. It is particularly clear from Fig. 2k & l that the use of a single prey density could have very misleading conclusions. For example, at point A on Fig. 2k, the native species has a higher feeding rate than the invader, whereas at point B the opposite is apparent (and feeding rates would be judged equal where the curves cross over); however, if the curves depicted were real data, we would predict strong prey population regulation by the invader but potential prey extinction by the native. In Fig. 2l, points A and B again illustrate the problem of choosing just one prey density, with the invader having a higher feeding rate at point A and the opposite at point B (and equal where the curves cross over). However, in this case if the curves depicted real data, we would predict the native would regulate but the invader could drive extinction.
A major issue with regards to deriving functional response curve shapes and parameters is the design of studies where prey (or other resources) are replaced or not replaced as they are consumed (see Alexander et al. 2012). There are statistical measures to account for non-prey replacement that can allow better estimates of curve parameters (see Alexander et al. 2012), but this does not help distinguish between two predators which may have been differentially constrained in their prey consumption. This is outlined in Fig. 3a, whereby in a non-replacement design, at low prey densities (see Arrow), most if not all prey are consumed and, since prey are not replaced, the slope of the curve is necessarily constrained in its early phase (dotted line, Fig. 3a). On the other hand, if prey are replaced, the same predator can potentially consume more prey and the early part of the curve rises more steeply (solid line, Fig. 3a). The asymptote, or maximum feeding rate, may be the same, but important information on the predator’s impact may be missed, especially since prey population viability is increasingly sensitive to predator effects as prey densities fall. We illustrate in Figs. 3b-g how such replacement designs might be more able to discriminate between invaders and natives (and other combinations outlined above) with respect to functional response shapes, parameters and hence predictions of impact. Taking Fig. 2c as a potential outcome of the comparison of an invader and a native where prey are not replaced, a replacement design (Fig. 3b) might show that in fact the invader, whilst having a similar maximum feeding rate to the native comparator, reaches that asymptote with a much steeper initial slope (Fig. 3b). Figure 3c–g illustrate a range of outcomes where invader and native are compared within a replacement design: Fig. 3c illustrates an invader exhibiting a higher maximum feeding rate and steeper initial slope, with the latter unlikely to be revealed in a non-replacement design (cf Fig. 2a); Fig. 3d illustrates a native exhibiting a higher maximum feeding rate but an invader a steeper initial slope, again not revealed in a non-replacement design (cf Fig. 2b); Fig. 3e illustrates invader and native having similar maximum feeding rates but the native a steeper initial slope (cf Fig. 2c); Fig. 3f illustrates a native with both a higher maximum feeding rate and a steeper initial slope (cf Fig. 2b); Fig. 3g illustrates an invader with a higher maximum feeding rate but a native with a steeper initial slope (cf Fig. 2a). Similar arguments would hold if replacement designs were applied within Type III functional response comparisons (see Fig. 3h). The choice of replacement versus non-replacement designs is often due to practicalities, as the former are more labour intensive and the latter a pragmatic solution. However, we encourage replacement designs where feasible (see, for example, Alexander et al. 2012) but, certainly, where non-replacement designs are used and no difference in functional responses are found, any conclusions should be caveated.
Challenges, future research and applications
While functional responses could theoretically be derived for any consumer of any resource, since we are interested here primarily in the population level outcomes for that resource, we have assumed resources are living. However, where non-living resources are concerned, such as nutrients as resources for plants, invader/native comparisons of functional responses could still be useful in determining reasons for, and perhaps predictions of, the success and ecological damage associated with invasive plants. Such resource use is a challenge to measure directly and, in any case, resource use efficiency, as measured indirectly (e.g. photosynthetic rate), has been shown to associate with invasiveness in plants (Funk and Vitousek 2007). Further, while our narrative tends to stress the functional responses of predators towards prey, our intention is not to limit the framework to this trophic interaction; for example, herbivores clearly show functional responses to vegetation (Farnsworth et al. 2002). In addition, there needs to be more imagination in the ways in which functional responses are derived; although many would view this as a laboratory procedure, many functional responses can be derived from field data (e.g. Moustahfid et al. 2010), and techniques such as stable isotope analyses and perhaps even qPCR (see Dick et al. 2013 for further discussion).
Our framework requires extensive and varied empirical testing. We could, for example, ask: When invaders show greater magnitudes of functional responses, that is, higher maximum feeding rates within a functional response type (e.g. Fig. 2a,d), does this concur with field patterns of high invader impact? Which of the individual parameters of functional responses (classically attack rate ‘a’, handling time ‘h’ and maximum feeding rate ‘T/h’) are the best predictors of invader impacts? We already have evidence that high maximum feeding rates and values of ‘a’, plus lower values of ‘h’, can all predict invader impact in one invasion scenario, that of the invasive Ponto-Caspian ‘bloody red’ shrimp Hemimysis anomala, which shows higher Type II functional responses than native mysid species. Remarkably, the magnitude of difference in maximum feeding rates is tightly correlated with degree of known field impact (Dick et al. 2013; Fig. 1b–d). Further demonstrations of such congruence will provide greater confidence that the methodology has real value in predicting field patterns of invader impacts, but we need sufficient case studies for a formal meta-analysis.
What is also challenging is designing studies that not only make relevant comparisons among invaders and natives, but detect and discriminate between functional response Types II and III and relate these to field patterns. This is challenging because functional response type can be sensitive to environmental variables (Jeschke et al. 2004), such as substrate type (Alexander et al. 2012). However, we stress that it is the comparison of invader with native (or other comparisons outlined above) that are important, and that the use of comparative functional responses as outlined here is phenomenological rather than strictly mechanistic (c.f. Jeschke et al. 2002). This is reflected in the use of functional responses as tools rather than as true reflections of the processes generating their shape (i.e. Types II and III). An interesting avenue for future research will be to extend the current framework such that it is connected to mechanistic functional response models (see e.g. Jeschke et al. 2002). Also, we must caveat conclusions about functional response types and population consequences (e.g. not all Type II responses will lead to extinction of prey; Twardochleb et al. 2012), but at the same time ask if real invasions and their impacts are explicable, and thus potentially predictable, from functional response comparisons as outlined in the framework of Figs. 2 & 3. Further, as mentioned earlier, a full assessment of consumer-resource dynamics (such as predator–prey) requires assessment of the demographic and/or aggregative numerical response (and hence total response). However, this may be impractical and moves away from our intention of providing a framework for the rapid assessment of invaders through comparative functional responses, which might be overwhelmingly able to provide ecological impact prediction. A number of proxies of the numerical response might, however, be available to improve the assessment of overall impact of invaders/natives, such as abundance and density data that are a reflection of the numerical response. This also helps with derivation of total ecological impact of invaders as proposed by Parker et al. (1999), that is, the per capita effect multiplied by abundance and range. Thus, for example, functional responses could be combined with abundance data to provide an overall score of impact (actual or predicted) as this describes the product of per capita effects with the number of individuals acting as consumers.
The largest impediment to prediction in invasion ecology is arguably the context-dependency of the success and impact of introduced species (Parker et al. 1999; Ricciardi 2003; Thomsen et al. 2011; Ricciardi et al. 2013). Much of this contingency is driven by organismal responses to variation in abiotic and biotic conditions across space and time (Ricciardi 2003; Strayer et al. 2006; Branch et al. 2010). Thus, we would expect that, despite the consistency of functional responses demonstrated for a trans-Atlantic invader (Hemimysis anomala; Dick et al. 2013), functional responses of invaders will vary across environmental gradients. Indeed, we tested the Environmental Matching Hypothesis of Ricciardi et al. (2013) and showed that the optimal maximum feeding rates as derived from functional responses are close to optimal growth or preferred temperatures of invasive species (Iacarella et al. unpubl. data). Further, rather than viewing environmental influences on the shape and magnitude of functional responses as a nuisance (see above), relevant environmental variables can be incorporated into study designs and explored for their main and interactive effects, hence perhaps refining predictions of invasive species impacts under global change. To more fully develop this predictive approach, we must also account for inter- and intra-population variation among individuals, and, especially, differences between populations in native versus invasive ranges (see van Kleunen et al. 2010). Extensions of the concept may include determining temporal changes in functional responses of individuals over time since invasion (as a result of adaptation by the invader and native prey, e.g. Carthey and Banks 2012; see also Wright et al. 2010); and examining phylogenetic variation in functional responses to determine the extent to which the functional responses and impacts of invaders can be predicted from the functional responses of closely-related species.
Further context-dependencies that require inclusion in comparative functional response assessments are the effects on individuals of the wider community within which they are embedded. Hence, functional responses of individuals might be influenced by the density of conspecifics (Pintor et al. 2009) and emergent multiple predator effects or MPEs (see Griffen 2006), whereby the presence of other individuals (be they conspecifics or other predator species) might lead to interference or facilitation (see Medoc et al. 2013). Also, intermediate consumers might be influenced by higher trophic-level predators through trait-mediated indirect interactions (TMIIs); for example, functional responses can both decrease and increase due to TMIIs and their interaction with habitat heterogeneity (e.g. in a fish-amphipod-isopod system; Alexander et al. in press). Indeed, in the most recent study, the difference in functional responses between the invasive Hemimysis anomala and native Mysis salemaai was exacerbated by the presence of a higher trophic-level predator (Barrios-O’Neill et al. in press). Derivation of functional responses thus require attention to the myriad effects of threats to the individual, the so-called ‘landscape of fear’ (see Laundré et al. 2010) or ‘ecology of fear’ (see Clinchy et al. 2013). Finally, whilst not essential in the overall goal of comparing functional responses of invaders and natives towards prey, disentangling the relative roles of predator ‘novelty’ and prey ‘naievete’ (see Sih et al. 2010) would provide insight into reasons for the higher functional responses of invaders and hence insight into ecological impact.
A measure of the utility of the comparative functional response methodology, or any of its derivatives, will be its adoption into tests of major hypotheses in invasion ecology. We have discussed above that tests of the ‘enemy release’ hypothesis with functional responses revealed that parasites, rather than decreasing the feeding rate of hosts (and thus perhaps decreasing competitive ability/ecological impact) were shown to actually increase host feeding rates and potential impact of the invader (Dick et al. 2010). Also, functional responses have been successfully adopted as a method of revealing and predicting ‘biotic resistance’ (Twardochleb et al. 2012; MacNeil et al. in press). With 28 of the 29 hypotheses described by Catford et al. (2009) involving some element of resource use, and hence the potential to measure functional responses, we are confident that functional responses can help better formulate and test such hypotheses. For example, such hypotheses involve elements of how ‘competitive’ invaders are compared to natives, the ability of invaders to dominate resources in communities, their growth and reproductive potentials, whether invaders are specialists or generalists, their role as ecosystem engineers and the effect of disturbance in altering invader as compared to native resource use (see Catford et al. 2009).
Finally, there are challenges in the incorporation of any theoretical or empirical advances in invasion ecology into applied methodologies that can reduce the risk of future harmful invasions. Refining risk assessment (RA) protocols is one such major challenge for the management of invasive species (Ricciardi and Rasmussen 1998; Parker et al. 1999; Byers et al. 2002; Andersen et al. 2004; Kumschick et al. 2012; Leung et al. 2012; Kumschick and Richardson 2013), and impact is usually not satisfactorily included in RAs (Kumschick et al. 2012). Including comparative functional responses in risk assessments for invasive species could be a useful way of improving the prediction of ecological consequences, namely impact (measures of per capita effects; Parker et al. 1999) of species introductions and therefore increase the predictive power of RA. This would require studies conducted prior to the introduction of a species, similar to those performed for putative biological control agents. However, in contrast to the discipline of biocontrol, the colonization and impact potential of probable future invaders are rarely assessed (see Ricciardi and Rasmussen 1998), a difficult task, considering the enormous number of plant and animal taxa transported around the globe. However, for some groups of species, such as those used in aquaculture and those commonly found in ballast water, comparative functional responses would be a valuable additional framework to consider for RA, especially given that (1) they can be derived from a variety of laboratory and field methods (see Dick et al. 2013) and (2) there is evidence that differentials in functional responses are conserved across the geographical range of invasive species. The application of this approach might also serve as an early warning method for identifying potentially problematic invaders residing in donor region ‘hotspots’ (e.g. the Ponto-Caspian; Ricciardi and MacIsaac 2000), or among those predicted to exploit emerging vectors and pathways.
References
Alexander ME, Dick JTA, O’Connor N (in press) Trait-mediated indirect interactions in a marine intertidal system as quantified by functional responses. Oikos
Alexander ME, Dick JTA, O’Connor NE, Haddaway NR, Farnsworth KD (2012) Functional responses of the intertidal amphipod Echinogammarus marinus: effects of prey supply, model selection and habitat complexity. Mar Ecol Prog Ser 468:191–202
Andersen MC, Adams H, Hope B, Powell M (2004) Risk analysis for invasive species: general framework and research notes. Risk Anal 24:893–900
Barbeau MA, Scheibling RE, Hatcher BG (1998) Behavioural responses of predatory crabs and sea stars to varying density of juvenile sea scallops. Aquaculture 169:87–98
Barnhisel DR, Kerfoot WC (2004) Fitting into food webs: behavioural and functional response of young lake trout (Salvelinus namaycush) to an introduced prey, the spiny cladoceran (Bythotrephes cederstroemi). J Gt Lake Res 30:300–314
Barrios-O’Neill D, Dick JTA, Emmerson M, Ricciardi A, MacIsaac HJ, Alexander ME, Bovy H (in press) Fortune favours the bold: a higher predator reduces the impact of a native but not an invasive intermediate predator. J Anim Ecol
Bayoumy MH (2011) Foraging behaviour of the coccinellid Nephus includes (Coleoptera: Coccinellidae) in response to Aphis gossypii (Hemiptera: Aphididae) with particular emphasis on larval parasitism. Envir Entomol 40:835–843
Bishop MJ, Peterson CH (2006) When r-selection may not predict introduced-species proliferation: predation of a non-native oyster. Ecol Appl 16:718–730
Blackburn TM, Cassey P, Lockwood JL (2009) The role of species traits in the establishment success of exotic birds. Glob Change Biol 15:2852–2860
Bollache L, Dick JTA, Farnsworth KD, Montgomery WI (2008) Comparison of the functional responses of invasive and native amphipods. Biol Lett 4:166–169
Bourdeau PE, O’Connor JN (2003) Predation by the non-indigenous Asian shore crab Hemigrapsus sanguineus on macroalgae and molluscs. North Nat 10:319–334
Branch GM, Steffani CN (2004) Can we predict the effects of alien species? A case-history of the invasion of South Africa by Mytilus galloprovincialis (Lamerck). J Exp Mar Biol Ecol 300:189–215
Branch GM, Odendaal F, Robinson TB (2010) Competition and facilitation between the alien mussel Mytilus galloprovincialis and indigenous species: moderation by wave action. J Exp Mar Biol Ecol 383:65–78
Brooks M, D’Antonio CM, Richardson DM, Grace JB, Keeley J, Di Tomaso JM, Hobbs RJ, Pellant M, Pyke D (2004) Effects of invasive alien plants on fire regimes. BioScience 54:677–688
Brousseau DJ, Baglivo JA (2005) Laboratory investigations of food selection by the Asian shore crab, Hemigrapsus sanguineus: algal versus animal preference. J Crust Biol 25:130–134
Buhle ER, Ruesink JL (2009) Impacts of invasive oyster drills on Olympia oyster (Ostrea lurida Carptenter 1864) recovery in Willapa Bay, Washington, United States. J Shell Res 28:87–96
Byers JE, Reichard S, Randall JM, Parker IM, Smith CS, Lonsdale WM, Atkinson IAE, Seastedt TR, Williamson M, Chornesky E, Hayes D (2002) Directing research to reduce the impacts of nonindigenous species. Conserv Biol 16:630–640
Carrillo D, Pena JE (2012) Prey-stage preferences and functional and numerical responses of Amblyseius largoensis (Acari: Phytoseiidae) to Raoiella indica (Acari: Tenuipalpidae). Exp App Acar 57:361–372
Carthey AJR, Banks PB (2012) When does an alien become a native species? A vulnerable native mammal recognizes and responds to its long-term predator. PLoS ONE 7(2):e31804
Catford JA, Jansson R, Nilsson C (2009) Reducing redundancy in invasion ecology by integrating hypotheses into a single theoretical framework. Divers Distrib 15:22–40
Chapin FS, Reynolds H, D’Antonio CM, Eckhart V (1996) The functional role of species in terrestrial ecosystems. In: Walker B, Steffen W (eds) Global change in terrestrial ecosystems. Cambridge University Press, Cambridge, pp 403–428
Chapple DG, Simmonds SM, Wong BBN (2012) Can behavioural and personality traits influence the success of unintentional species introductions? Trends Ecol Evol 27:57–64
Clavero M, Garcia-Berthou E (2005) Invasive species are a leading cause of animal extinctions. Trends Ecol Evol 20:110
Clinchy M, Sheriff MJ, Zanette LY (2013) Predator-induced stress and the ecology of fear. Funct Ecol 27:56–65
Cucherousset J, Olden JD (2011) Ecological impacts of non-native freshwater fishes. Fisheries 36:215–230
Davis MA (2011) Researching invasive species 50 years after Elton: a cautionary tale. In Richardson DM (ed) Fifty Years of Invasion Ecology: The Legacy of Charles Elton Wiley-Blackwell, Oxford, pp 269–276
DeGraaf JD, Tyrrell MC (2004) Comparison of the feeding rates of two introduced crab species, Carcinus maenas and Hemigrapsus sanguineus, on blue mussel, Mytilus edulis. North Nat 11:163–167
deRivera CE, Ruiz GM, Hines AH, Jivoff P (2005) Biotic resistance to invasion: native predator limits abundance and distribution of an introduced crab. Ecology 86:3364–3376
Dick JTA, Armstrong M, Clarke HC, Farnsworth KD, Hatcher MJ, Ennis M, Kelly A, Dunn AM (2010) Parasitism may enhance rather than reduce the predatory impact of an invader. Biol Lett 6:636–638
Dick JTA, Gallagher K, Avlijas S, Clarke HC, Lewis SE, Leung S, Minchin D, Caffrey J, Alexander ME, Maguire C, Harrod C, Reid N, Haddaway NR, Farnsworth KD, Penk M, Ricciardi A (2013) Ecological impacts of an invasive predator explained and predicted by comparative functional responses. Biol Inv 15:837–846
Dubs DOL, Corkum LD (1996) Behavioural interactions between round gobies (Neogobius melanostomus) and mottled sculpins (Cottus bairdi). J Gr Lakes Res 22:838–844
Eggleston DB (1990) Behavioural mechanisms underlying variable functional responses of blue crabs, Callinectes sapidus feeding on juvenile oysters, Crassostrea virginica. J Anim Ecol 59:615–630
Farnsworth KD, Focardi S, Beecham JA (2002) Grassland-herbivore interactions: how do grazers coexist? Am Nat 159:24–39
Farrokhi S, Ashouri A, Shirazi J, Allahyari H, Huigens ME (2010) A comparative study on the functional response of Wolbachia-infected and uninfected forms of the parasitoid wasp Trichogramma brassicae. J Ins Sci 10:1–11
Fielding NJ, MacNeil C, Dick JTA, Elwood RW, Riddell GE, Dunn AM (2003) Effects of the acanthocephalan parasite Echinorhynchus truttae on the feeding ecology of Gammarus pulex (Crustacea: Amphipoda). J Zool Lond 261:321–325
Funk JL, Vitousek PM (2007) Resource-use efficiency and plant invasion in low resource systems. Nature 446:1079–1081
Greenberg SM, Legaspi BC, Jones WA (2001) Comparison of functional response and mutual interference between two aphelinid parasitiods of Bemisia argentifolii (Homoptera: Alveyrodidae). J Ent Sci 36:1–8
Griffen BD (2006) Detecting emergent effects of multiple predator species. Oecologia 148:702–709
Griffen BD, Delaney DG (2007) Species invasion shifts the importance of predator dependence. Ecology 88:3012–3021
Griswold MW, Lounibos LP (2005) Does differential predation permit invasive and native mosquito larvae to coexist in Florida? Ecol Ent 30:122–127
Haddaway NR, Wilcox RH, Heptonstall REA, Griffiths HM, Mortimer RJG, Christmas M, Dunn AM (2012) Predatory functional response and prey choice identify predation differences between native/invasive and parasitized/unparasitised crayfish. PLoS ONE 7(2):e32229
Hassell MP (1978) Functional responses. In: Hassell MP (ed) The dynamics of arthropod predator-prey systems. Princeton University Press, Princeton, pp 28–49
Hayes KR, Barry SC (2008) Are there any consistent predictors of invasion success? Biol Inv 10:483–506
Heger T, Pahl AT, Botta-Dukat Z, Gherardi F, Hoppe C, Hoste I, Jax K, Lindstrom L, Boets P, Haider S, Kollmann J, Wittmann MJ, Jeschke JM (2013) Conceptual frameworks and methods for advancing invasion ecology. Ambio 42:527–540
Holling CS (1959a) Some characteristics of simple types of predation and parasitism. Can Entomol 91:38–398
Holling CS (1959b) The components of predation as revealed by a study of small-mammal predation of the European pine sawfly. Can Entomol 91:293–320
Hooff RC, Bollens SM (2004) Functional response and potential predatory impact of Tortanus dextrilobatus, a carnivorous copepod recently introduced to the San Francisco Estuary. Mar Ecol Prog Ser 277:167–179
Jeschke JM, Strayer DL (2006) Determinants of vertebrate invasion success in Europe and North America. Glob Change Biol 12:1608–1619
Jeschke JM, Kopp M, Tollrian R (2002) Predator functional responses: discriminating between handling and digesting prey. Ecol Monogr 72:95–112
Jeschke JM, Kopp M, Tollrian R (2004) Consumer-food systems: why type I functional responses are exclusive to filter feeders. Biol Rev 79:337–349
Jeschke JM, Gómez Aparicio L, Haider S, Heger T, Lortie CJ, Pyšek P, Strayer DL (2012) Support for major hypotheses in invasion biology is uneven and declining. NeoBiota 14:1–20
Johnson BM, Martinez PJ, Hawkins JA, Bestgen KR (2008) Ranking predatory threats by non-native fishes in the Yampa River, Colorado via bioenergetics modeling. N Am J Fish Manage 28:1941–1953
Jones DB, Giles KL, Berberet RC, Royer TA, Elliot NC, Payton ME (2003) Functional responses of an introduced parasitoid and an indigenous parasitoid on greenbug at four temperatures. Environ Entomol 32:425–432
Jones C, Pech R, Forrester G, King CM, Murphy EC (2011) Functional responses of an invasive top predator Mustela erminea to invasive meso-predators Rattus rattus and Mus musculus, in New Zealand forests. Wildl Res 38:131–140
Juliano SA (2001) Nonlinear curve fitting: predation and functional response curves. In: Scheiner SM, Gurevitch J (eds) Design and analysis of ecological experiments. Oxford University Press, Oxford, pp 178–196
Kelly DW, Dick JTA, Montgomery WI (2002) Predation on mayfly nymph, Baetis rhodani, by native and introduced Gammarus: direct effects and the facilitation of salmonid predation. Freshw Biol 47:1257–1268
Kestrup AM, Dick JTA, Ricciardi A (2011) Interactions between invasive and native crustaceans: differential functional responses of intraguild predators towards juvenile hetero-specifics. Biol Inv 13:731–737
Koski ML, Johnson BM (2002) Functional response of kokanee salmon (Oncorhynchus nerka) to Daphnia at different light levels. Can J Fish Aq Sci 59:707–716
Kulhanek SA, Ricciardi A, Leung B (2011) Is invasion history a useful tool for predicting the impacts of the world’s worst aquatic invasive species? Ecol Appl 21:189–202
Kumschick S, Richardson DM (2013) Species-based risk assessments for biological invasions: advances and challenges. Divers Distrib 19:1095–1105
Kumschick S, Bacher S, Dawson W, Heikkilä J, Sendek A, Pluess T, Robinson TB, Kühn I (2012) A conceptual framework for prioritization of invasive alien species for management according to their impact. NeoBiota 15:69–100
Kumschick S, Bacher S, Blackburn TM (2013) What determines the impact of alien birds and mammals in Europe? Biol Inv 15:785–797
Kushner RB, Hovel KA (2006) Effects of native predators and eelgrass habitat structure on the introduced Asian mussel Musculista senhousia (Benson in Cantor). J Exp Mar Biol Ecol 332:166–177
Laundré JW, Hernández L, Ripple WR (2010) The landscape of fear: ecological implications of being afraid. Open Ecol J 3:1–7
Lester PJ, Harmsen R (2002) Functional and numerical responses do not always indicate the most effective predator for biological control: an analysis of two predators in a two-prey system. J Appl Ecol 39:455–468
Leung B, Roura-Pascual N, Bacher S, Jaakko H, Brotons L, Burgman MA, Dehnen-Schmutz K, Essl F, Hulme PE, Richardson DM, Sol D, Vila M (2012) TEASIng apart alien species risk assessments: a framework for best practices. Ecol Lett 15:1475–1493
Lipcius RN, Hines AH (1986) Variable functional responses of a marine predator in dissimilar homogeneous microhabitats. Ecology 67:1361–1371
Lohrer AM, Whitlatch RB (2002) Relative impacts of two exotic brachyuran species on blue mussel populations in Long Island Sound. Mar Ecol Prog Ser 227:135–144
Lohrer AM, Whitlatch RB, Wada K, Yasuo F (2000) Home and away: comparison of resource utilization by a marine species in native and invaded habitats. Biol Inv 2:41–57
Mack RN (1996) Predicting the identity and fate of plant invaders: emergent and emerging approaches. Biol Conserv 78:107–121
MacNeil C, Dick JTA, Alexander ME, Dodd JA, Ricciardi A (in press) Predators vs. alien: differential biotic resistance to an invasive species by two resident predators. NeoBiota
Marchetti MP, Moyle PB, Levine R (2004a) Alien fishes in California watersheds: characteristics of successful and failed invaders. Ecol Appl 14:587–596
Marchetti MP, Moyle PB, Levine R (2004b) Invasive species profiling? Exploring the characteristics of non-native fishes across invasions stages in California. Freshw Biol 49:646–661
Medoc V, Spataro T, Arditi R (2013) Prey: predator ratio dependence in the functional response of a freshwater amphipod. Freshw Biol 58:858–865
Mistri M (2004) Predatory behaviour and preference of a successful invader, the mud crab Dyspanopeus sayi (Panopeidae), on its bivalve prey. J Exp Mar Biol Ecol 312:385–398
Monserrat AL, Funes MC, Novaro AJ (2005) Dietary response of three raptor species to an introduced prey in Patagonia. Rev Chil Hist Nat 78:425–439
Morrison WE, Hay ME (2011) Feeding and growth of native, invasive and non-invasive alien apple snails (Ampullariidae) in the United States: invasives eat more and grow more. Biol Inv 13:945–955
Moustahfid H, Tyrrell MC, Link JS, Nye JA, Smith BE, Gamble RJ (2010) Functional feeding responses of piscivorous fishes from the northeast US continental shelf. Oecologia 163:1059–1067
Murdoch WW, Oaten A (1975) Predation and population stability. Adv Ecol Res 9:1–131
Nentwig W, Kuhnel E, Bacher S (2009) A generic impact-scoring system applied to alien mammals in Europe. Conserv Biol 24:302–311
O’Neil RJ (1990) Functional response of arthropod predators and its role in the biological control of insect pests in agricultural systems. In: Dunn PE, Baker RR (eds) New directions in biological control: alternatives for suppressing agricultural pests and diseases. Alan R. Liss, Inc., New York, pp 83–96
Olden JD, Poff LR, Bestgen KR (2006) Life-history strategies predict fish invasions and extirpations in the Colorado River basin. Ecol Monogr 76:25–40
Olden JD, Larson ER, Mims MC (2009) Home-field advantage: native signal crayfish (Pacifastacus leniusculus) out consume newly introduced crayfishes for invasive Chinese mystery snail (Bellamya chinensis). Aquat Ecol 43:1073–1084
Palacio-Lopez K, Gianoli E (2011) Invasive plants do not display greater phenotypic plasticity than their native or non-invasive counterparts: a meta-analysis. Oikos 120:1393–1401
Pangle KL, Peacor SD (2009) Light-dependent predation by the invertebrate planktivore Bythotrephes longimanus. Can J Fish Aquat Sci 66:1748–1757
Parker IM, Simberloff D, Lonsdale WM, Goodell K, Wonham M, Kareiva PM, Williamson MH, Von Holle B, Moyle PB, Byers JE, Goldwasser L (1999) Impact: toward a framework for understanding the ecological effects of invaders. Biol Inv 1:3–19
Parker JD, Torchin ME, Hufbauer RA, Lemoine NP, Alba C, Blumenthal DM, Bossdorf O, Byers JE, Dunn AM, Heckman RW, Hejda M, Jarosik V, Kanarek AR, Martin LB, Perkins SE, Pysek P, Schierenbeck K, Schloder C, van Klinken R, Vaughn KJ, Williams W, Wolfe LM (2013) Do invasive species perform better in their new ranges? Ecology 94:985–994
Pintor LM, Sih A (2009) Differences in growth and foraging behaviour of native and introduced populations of an invasive crayfish. Biol Inv 11:1895–1902
Pintor LM, Sih A, Kerby JL (2009) Behavioral correlations provide a mechanism for explaining high invader densities and increased impacts on native prey. Ecology 90:581–587
Pritchard DW (2013) Frair: functional response analysis in R. https://github.com/dpritchard/frair
Pyšek P, Krivanek M, Jarosik V (2009) Planting intensity, residence time, and species traits determine invasion success of alien woody species. Ecology 90:2734–2744
Rehage JS, Barnett BK, Sih A (2005) Foraging behaviour and invasiveness: do invasive Gambusia exhibit higher feeding rates and broader diets than their noninvasive relatives? Ecol Freshw Fish 14:352–360
Remanjek M, Richardson DM (1996) What attributes make some plant species more invasive? Ecology 77:1655–1661
Renai B, Gherardi F (2004) Predatory efficiency of crayfish: comparison between indigenous and non-indigenous species. Biol Inv 6:89–99
Ricciardi A (2003) Predicting the impacts of an introduced species from its invasion history: an empirical approach applied to zebra mussel invasions. Freshw Biol 48:972–981
Ricciardi A, Atkinson SK (2004) Distinctiveness magnifies the impact of biological invaders in aquatic ecosystems. Ecol Lett 7:781–784
Ricciardi A, MacIsaac HJ (2000) Recent mass invasion of the North American Great Lakes by Ponto-Caspian species. Trends Ecol Evol 15:62–65
Ricciardi A, Rasmussen JB (1998) Predicting the identity and impact of future biological invaders: a priority for aquatic resource management. Can J Fish Aquat Sci 55:1759–1765
Ricciardi A, Hoopes MF, Marchetti MP, Lockwood JL (2013) Progress toward understanding the ecological impacts of nonnative species. Ecol Monogr 83:263–282
Richman SE, Lovvorn JR (2004) Relative foraging value to lesser scaup ducks of native and exotic clams from San Francisco Bay. Ecol Appl 14:1217–1231
Rindone RR, Eggleston DB (2011) Predator-prey dynamics between recently established stone crabs (Menippe spp.) and oyster prey (Crassostrea virginica). J Exp Mar Biol Ecol 407:216–225
Rossong MA, Quijon PA, Snelgrove PVR, Barrett TJ, McKenzie CH, Locke A (2012) Regional differences in foraging behaviour of invasive green crab (Carcinus maenas) populations in Atlantic Canada. Biol Inv 14:659–669
Roy HE, Adriaens T, Isaac NJB, Kenis M, Onkelinx T, San Martin G, Brown PMJ, Hautier L, Poland R, Roy DB, Comont R, Eschen R, Frost R, Zindel R, Van Vlaenderen J, Nedved O, Ravn HP, Gregoire JC, de Biseau JC, Maes D (2012) Invasive alien predator causes rapid declines of native European ladybirds. Divers Distrib 18:717–725
Ruscoe WA, Elkinton JS, Choquenot D, Allen RB (2005) Predation of beech seed by mice: effects of numerical and functional responses. J Anim Ecol 74:1005–1019
Sabelis MW (1992) Arthropod predators. In: Crawley MJ (ed) Natural enemies, the population biology of predators, parasites and diseases. Blackwell, Oxford, pp 225–264
Sakai AK, Allendorf FW, Holt JS, Lodge DM, Molofsky J, With KA, Baughman S, Cabin RJ, Choen JE, Ellstrand NC, McCauley DE, O’Neill P, Parker IM, Thompson JN, Weller SG (2001) The population biology of invasive species. Annu Rev Ecol Syst 32:305–332
Salo P, Korpimaki E, Banks PB, Nordstrom M, Dickman CR (2007) Alien predators are more dangerous than native predators to prey populations. Proc R Soc Lond B 274:1237–1243
Sih A, Bolnick DI, Luttbeg B, Orrock JL, Peacor SD, Pintor LM, Preisser E, Rehage JS, Vonesh JR (2010) Predator-prey naivete, antipredator behavior, and the ecology of predator invasions. Oikos 119:610–621
Simberloff D, Martin J-L, Genovesi P, Maris V, Wardle DA, Aronson J, Courchamp F, Galil B, Garcıa-Berthou E, Pascal M, Pyšek P, Sousa R, Tabacchi E, Vila M (2013) Impacts of biological invasions: what’s what and the way forward. Trends Ecol Evol 28:58–66
Snyder WE, Evans EW (2006) Ecological effects of invasive arthropod generalist predators. Annu Rev Ecol Evol Syst 37:95–122
Sol D, Timmermans S, Lefebvre L (2002) Behavioural flexibility and invasion success in birds. Anim Behav 63:495–502
Solomon ME (1949) The natural control of animal populations. J Anim Ecol 18:1–35
Soluk DA (1993) Multiple predator effects: predicting combined functional response of stream fish and invertebrate predators. Ecology 74:219–225
Stoffels BEMW, Tummers JS, Van der Velde G, Platvoet D, Hendriks HWM, Leuven RSEW (2011) Assessment of predatory ability of native and non-native freshwater gammaridean species: a rapid test with water fleas as prey. Curr Zool 57:836–843
Strayer DL, Caraco NF, Cole JJ, Findlay S, Pace ML (1999) Transformation of freshwater ecosystems by bivalves: a case study of zebra mussels in the Hudson River. BioScience 49:19–27
Strayer DL, Eviner VT, Jeschke JM, Pace ML (2006) Understanding the long-term effects of species invasions. Trends Ecol Evol 21:645–651
Taylor DL, Collie JS (2003) Effect of temperature on the functional response and foraging behaviour of the sand shrimp Crangon septemspinosa preying on juvenile winter flounder Pseudopleuronetes americanus. Mar Ecol Prog Ser 263:217–234
Taylor CM, Duggan IC (2012) Can biotic resistance be utilized to reduce establishment rates of non-indigenous species in constructed waters? Biol Inv 14:307–322
Thomsen MS, Wernberg T, Olden JD, Griffin JN, Silliman BR (2011) A framework to study the context-dependent impacts of marine invasions. J Exp Mar Biol Ecol 400:322–327
Twardochleb LA, Novak M, Moore JW (2012) Using the functional response of a consumer to predict biotic resistance to invasive prey. Ecol Appl 22:1162–1171
Tyrrell MC, Guarino PA, Harris LG (2006) Predatory impacts of two introduced crab species: inferences from mesocosms. North Nat 13:375–390
Van Drische R, Bellows TS (2011) Biological control. Springer, Berlin
Van Kleunen M, Dawson W, Schlaepfer D, Jeschke JM, Fischer M (2010) Are invaders different? A conceptual framework of comparative approaches for assessing determinants of invasiveness. Ecol Lett 13:947–958
Van Leeuwen E, Jansen VAA, Bright PW (2007) How population dynamics shape the functional response in a one-predator-two-prey system. Ecology 88:1571–1581
Veiga P, Rubal M, Arenas F, Incera M, Olabarria C, Sousa-Pinto I (2011) Does Carcinus maenas facilitate the invasion of Xenostrobus secures? J Exp Mar Biol Ecol 406:14–20
Vitousek PM (1990) Biological invasions and ecosystem processes: towards an integration of population biology and ecosystem studies. Oikos 57:7–13
Walther GR, Roques A, Hulme PE, Sykes MT, Pyšek P, Kühn I, Zobel M, Bacher S, Botta-Dukát Z, Bugmann H, Czúcz B, Dauber J, Hickler T, Jarosík V, Kenis M, Klotz S, Minchin D, Moora M, Nentwig W, Ott J, Panov VE, Reineking B, Robinet C, Semenchenko V, Solarz W, Thuiller W, Vilà M, Vohland K, Settele J (2009) Alien species in a warmer world: risks and opportunities. Trends Ecol Evol 24:686–693
Ward DM, Nislow KH, Folt CL (2008) Predators reverse the direction of density dependence for juvenile salmon mortality. Oecologia 156:515–522
Weis JS (2010) The role of behavior in the success of invasive crustaceans. Mar Freshw Behav Physiol 43:83–98
Wright TF, Eberhard JR, Hobson EA, Avery ML, Russello MA (2010) Behavioral flexibility and species invasions: the adaptive flexibility hypothesis. Ethol Ecol Evol 22:393–404
Zuharah WF, Lester PJ (2011) Are exotic invaders less susceptible to native predators? A test using native and exotic mosquito species in New Zealand. Pop Ecol 53:307–317
Acknowledgments
We thank the Leverhulme Trust, Natural Environment Research Council UK (grant NE/G01521X/1), the DST-NRF Centre of Excellence for Invasion Biology (Stellenbosch University, South Africa), the National Research Foundation (South Africa, grant 85417), Natural Sciences and Engineering Research Council (Canada) and the Deutsche Forschungsgemeinschaft (grant JE 288/4-1) for funding. SK acknowledges financial support from the Swiss National Science Foundation and the Drakenstein Trust. We appreciate comments from reviewers and editors, particularly Joel Trexler, that greatly improved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Dick, J.T.A., Alexander, M.E., Jeschke, J.M. et al. Advancing impact prediction and hypothesis testing in invasion ecology using a comparative functional response approach. Biol Invasions 16, 735–753 (2014). https://doi.org/10.1007/s10530-013-0550-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-013-0550-8