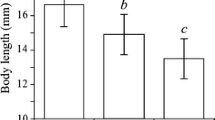
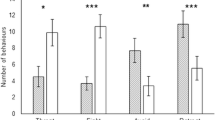
Abstract
The Ponto-Caspian freshwater amphipod Dikerogammarus villosus has colonized most of the water bodies of continental Europe where it causes strong structural alterations in recipient communities that can lead to changes in ecosystem-level processes, mainly because of a strong predatory behaviour. Most of the D. villosus populations from the invaded range have been found infected with the co-introduced microsporidian parasite Cucumispora dikerogammari, known to decrease the predation rate of its host. Infection might thus mitigate the ecological impact of D. villosus and we wanted to test this assumption using the comparative functional response approach. We compared the relationship between resource use and resource availability (i.e. the functional response, FR) of D. villosus, either with infected individuals or not, to that of two non-invasive gammarids: Gammarus pulex and Echinogammarus berilloni. With infected individuals included, D. villosus displayed a higher FR than the two non-invasive gammarids. Although this effect was not significant, C. dikerogammari infection tended to alter the FR of D. villosus with a slight decrease in attack rate and handling time, resulting in a less steep initial slope and a higher asymptote, respectively. Removing infected D. villosus from the dataset did not affect the FR comparison with G. pulex but suppressed the difference in FR with E. berilloni. Although we cannot exclude the role of sample size reduction in this effect, this suggests that C. dikerogammari infection might increase the predation pressure on local prey populations in case of species replacement between D. villosus and E. berilloni. From a more general perspective, our study illustrates how parasites may alter our capacity to predict invasive species impacts from FR comparisons.




Similar content being viewed by others
References
Bacela-Spychalska K, van Der Velde G (2013) There is more than one “killer shrimp”: trophic positions and predatory abilities of invasive amphipods of Ponto-Caspian origin. Freshw Biol 58:730–741. doi:10.1111/fwb.12078
Bacela-Spychalska K, Wattier RA, Genton C, Rigaud T (2012) Microsporidian disease of the invasive amphipod Dikerogammarus villosus and the potential for its transfer to local invertebrate fauna. Biol Inv 14:1831–1842. doi:10.1007/s10530-012-0193-1
Bacela-Spychalska K, Rigaud T, Wattier RA (2014) A co-invasive microsporidian parasite that reduces the predatory behaviour of its host Dikerogammarus villosus (Crustacea, Amphipoda). Parasitology 141:254–258. doi:10.1017/S0031182013001510
Barrios-O’Neill D, Dick JTA, Emmerson MC, Ricciardi A, MacIsaac HJ (2015) Predator-free space, functional responses and biological invasions. Funct Ecol 29:377–384. doi:10.1111/1365-2435.12347
Bollache L, Dick JTA, Farnsworth KD, Montgomery WI (2008) Comparison of the functional responses of invasive and native amphipods. Biol Lett 4:166–169. doi:10.1098/rsbl.2007.0554
Bovy HC, Barrios-O’Neill D, Emmerson MC, Aldridge DC, Dick JTA (2015) Predicting the predatory impacts of the “demon shrimp” Dikerogammarus haemobaphes, on native and previously introduced species. Biol Inv 17:597–607. doi:10.1007/s10530-014-0751-9
Buřič M, Kočí L, Petrusek A, Kouba A, Kozák P (2009) Invaders eating invaders: potential trophic interactions between the amphipod Dikerogammarus villosus and juvenile crayfish Orconectes limosus. Knowl Manag Aquat Ecosyst. doi:10.1051/kmae/2009015
Byers JE, Wright JT, Gribben PE (2010) Variable direct and indirect effects of a habitat—modifying invasive species on mortality of native fauna. Ecology 91:1787–1798. doi:10.1890/09-0712.1
Carpenter SR, Stanley EH, Vander Zanden MJ (2011) State of the world’s freshwater ecosystems: physical, chemical, and biological changes. Annu Rev Environ Resour 36:75–99. doi:10.1146/annurev-environ-021810-094524
Casellato S, Visentin A, La Piana G (2007) The predatory impact of Dikerogammarus on fish. In: Gherardi F (ed) Biological invaders in inland waters: profiles, distribution, and threats. Springer, Dordrecht, pp 495–506. 1:732. doi:10.1017/CBO9781107415324.004
Dick JT, Platvoet D (2000) Invading predatory crustacean Dikerogammarus villosus eliminates both native and exotic species. Proc R Soc Lond 267:977–983. doi:10.1098/rspb.2000.1099
Dick JTA, Platvoet D, Kelly DW (2002) Predatory impact of the freshwater invader Dikerogammarus villosus (Crustacea: Amphipoda). Can J Fish Aquat Sci 59:1078–1084. doi:10.1139/F02-074
Dick JTA, Armstrong M, Clarke HC, Farnsworth KD, Hatcher MJ, Ennis M, Kelly A, Dunn AM (2010) Parasitism may enhance rather than reduce the predatory impact of an invader. Biol Lett 6:636–638. doi:10.1098/rsbl.2010.0171
Dick JTA, Alexander ME, Jeschke JM, Ricciardi A, MacIsaac HJ, Robinson TB, Kumschick S, Weyl OLF, Dunn AM, Hatcher MJ, Paterson RA, Farnsworth KD, Richardson DM (2014) Advancing impact prediction and hypothesis testing in invasion ecology using a comparative functional response approach. Biol Inv 16:735–753. doi:10.1007/s10530-013-0550-8
Dodd JA, Dick JTA, Alexander ME, MacNeil C, Dunn AM, Aldridge DC (2014) Predicting the ecological impacts of a new freshwater invader: functional responses and prey selectivity of the “killer shrimp”, Dikerogammarus villosus, compared to the native Gammarus pulex. Freshw Biol 59:337–352. doi:10.1111/fwb.12268
Dunn AM (2009) Parasites and biological invasions. Adv Parasitol 68:161–184. doi:10.1016/S0065-308X(08)00607-6
Dunn AM, Hatcher MJ (2015) Parasites and biological invasions: parallels, interactions, and control. Trends Parasitol 31:189–199. doi:10.1016/j.pt.2014.12.003
Dunn AM, Smith JE (2001) Microsporidian life cycles and diversity: the relationship between virulence and transmission. Microbes Infect 3:381–388. doi:10.1016/S1286-4579(01)01394-6
Gentleman WC, Neuheimer AB (2008) Functional responses and ecosystem dynamics: how clearance rates explain the influence of satiation, food-limitation and acclimation. J Plankton Res 30:1215–1231. doi:10.1093/plankt/fbn078
Grabner DS, Weigand AM, Leese F, Winking C, Hering D, Tollrian R, Sures B (2015) Invaders, natives and their enemies: distribution patterns of amphipods and their microsporidian parasites in the Ruhr Metropolis, Germany. Parasit Vectors 8:419. doi:10.1186/s13071-015-1036-6
Hassell MP, Lawton JH, Beddington JR (1977) Sigmoid functional responses by invertebrate predators and parasitoids. J Anim Ecol 46:249–262
Hellmann C, Worischka S, Mehler E, Becker J, Gergs R, Winkelmann C (2015) The trophic function of Dikerogammarus villosus (Sowinsky, 1894) in invaded rivers: a case study in the Elbe and Rhine. Aquat Inv 10:385–397. doi:10.3391/ai.2015.10.4.03
Holling CS (1959a) Some characteristics of simple types of predation and parasitism. Can Entomol 91:385–398. doi:10.4039/Ent91385-7
Holling CS (1959b) The components of predation as revealed by a study of small-mammal predation of the european pine sawfly. Can Entomol 91:293–320. doi:10.4039/Ent91293-5
Juliano SA (2001) Non-linear curve fitting: predation and functional response curves. In: Scheiner SM, Gurevitch J (eds) Design and analysis of ecological experiments. Oxford University Press, Oxford, pp 178–196
Kalinkat G, Schneider FD, Digel C, Guill C, Rall BC, Brose U (2013) Body masses, functional responses and predator–prey stability. Ecol Lett 16:1126–1134. doi:10.1111/ele.12147
Keane RM, Crawley MJ (2002) Exotic plant invasions and the enemy release hypothesis. Trends Ecol Evol 17:164–170. doi:10.1016/S0169-5347(02)02499-0
Kelly ADW, Paterson RA, Townsend CR, Poulin R, Tompkins DM (2009) Parasite spillback: a neglected concept in invasion ecology? Ecology 90:2047–2056. doi:10.1890/08-1085.1
Kestrup ÅM, Dick JTA, Ricciardi A (2011) Interactions between invasive and native crustaceans: differential functional responses of intraguild predators towards juvenile hetero-specifics. Biol Inv 13:731–737. doi:10.1007/s10530-010-9863-z
Koester M, Bayer B, Gergs R (2016) Is Dikerogammarus villosus (Crustacea, Gammaridae) a “killer shrimp” in the River Rhine system? Hydrobiologia 768:299–313. doi:10.1007/s10750-015-2558-9
Kolar CS, Lodge DM (2001) Progress in invasion biology: predicting invaders. Trends Ecol Evol 16:199–204. doi:10.1016/S0169-5347(01)02101-2
Krisp H, Maier G (2005) Consumption of macroinvertebrates by invasive and native gammarids: a comparison. J Limnol 64:55–59. doi:10.4081/jlimnol.2005.55
Laverty C, Brenner D, McIlwaine C, Lennon JJ, Dick JTA, Lucy FE, Christian KA (2017) Temperature rise and parasitic infection interact to increase the impact of an invasive species. Int J Parasitol. doi:10.1016/j.ijpara.2016.12.004
Lymbery AJ, Morine M, Kanani HG, Beatty SJ, Morgan DL (2014) Co-invaders: the effects of alien parasites on native hosts. Int J Parasitol Parasites Wildl 3:171–177. doi:10.1016/j.ijppaw.2014.04.002
MacNeil C, Platvoet D (2005) The predatory impact of the freshwater invader Dikerogammarus villosus on native Gammarus pulex (Crustacea: Amphipoda); influences of differential microdistribution and food resources. J Zool 267:31. doi:10.1017/S0952836905007351
MacNeil C, Platvoet D, Dick JTA, Fielding N, Constable A, Hall N, Aldridge D, Renals T, Diamond M (2010) The Ponto-Caspian “killer shrimp”, Dikerogammarus villosus (Sowinsky, 1894), invades the British Isles. Aquat Inv 5:441–445. doi:10.3391/ai.2010.5.4.15
MacNeil C, Dick JTA, Platvoet D, Briffa M (2011) Direct and indirect effects of species displacements: an invading freshwater amphipod can disrupt leaf-litter processing and shredder efficiency. J N Am Benthol Soc 30:38–48. doi:10.1899/10-056.1
MacNeil C, Boets P, Lock K, Goethals PLM (2013) Potential effects of the invasive “killer shrimp” (Dikerogammarus villosus) on macroinvertebrate assemblages and biomonitoring indices. Freshw Biol 58:171–182. doi:10.1111/fwb.12048
Mattos KJ, Orrock JL (2010) Behavioral consequences of plant invasion: an invasive plant alters rodent antipredator behavior. Behav Ecol 21:556–561. doi:10.1093/beheco/arq020
Mayer G, Maier G, Maas A, Waloszek D (2008) Mouthparts of the ponto-caspian invader Dikerogammarus villosus (Amphipoda: Pontogammaridae). J Crustac Biol 28:1–15. doi:10.1651/07-2867R.1
Médoc V, Spataro T (2015) Predicting the impact of invasive species: a look forward on the comparative functional response approach. Rev Ecol (Terre Vie) 70(supp. 12):114–136
Médoc V, Spataro T, Arditi R (2013) Prey:predator ratio dependence in the functional response of a freshwater amphipod. Freshw Biol 58:858–865. doi:10.1111/fwb.12091
Médoc V, Albert H, Spataro T (2015) Functional response comparisons among freshwater amphipods: ratio-dependence and higher predation for Gammarus pulex compared to the non-natives Dikerogammarus villosus and Echinogammarus berilloni. Biol Inv 17:3625–3637. doi:10.1007/s10530-015-0984-2
Médoc V, Firmat C, Sheath DJ, Pegg J, Andreou D, Britton JR (2017) Parasites and biological invasions: predicting ecological alterations at levels from individual hosts to whole networks. Adv Ecol Res. doi:10.1016/bs.aecr.2016.10.003
Murdoch WW, Oaten A (1975) Predation and population stability. Adv Ecol Res 9:1–131
Nakaya T, Fotheringham AS, Brunsdon C, Charlton M (2005) Geographically weighted Poisson regression for disease association mapping. Stat Med 24:2695–2717. doi:10.1002/sim.2129
Norman R, Bowers RG, Begon M, Hudson PJ (1999) Persistence of tick-borne virus in the presence of multiple host species: tick reservoirs and parasite mediated competition. J Theor Biol 200:111–118. doi:10.1006/jtbi.1999.0982
Ojaveer H, Leppäkoski E, Olenin S, Ricciardi a (2002) Ecological impact of Ponto-Caspian invaders in the Baltic Sea, European inland waters and the Great Lakes: an inter-ecosystem comparison. In: Invasive aquat species of Europe. Distribution, impacts and management. Springer, Netherlands, pp 412–425
Ostfeld RS, Keesing F (2000) Biodiversity and disease risk: the case of Lyme disease. Conserv Biol 14:722–728. doi:10.1046/j.1523-1739.2000.99014.x
Ovcharenko MO, Bącela K, Wilkinson T, Ironside JE, Rigaud T, Wattier RA (2010) Cucumispora dikerogammari n. gen. (Fungi: Microsporidia) infecting the invasive amphipod Dikerogammarus villosus: a potential emerging disease in European rivers. Parasitology 137:191–204. doi:10.1017/S0031182009991119
Paolucci EM, Macisaac HJ, Ricciardi A (2013) Origin matters: Alien consumers inflict greater damage on prey populations than do native consumers. Divers Distrib 19:988–995. doi:10.1111/ddi.12073
Piscart C, Mermillod-Blondin F, Maazouzi C, Merigoux S, Marmonier P (2011) Potential impact of invasive amphipods on leaf litter recycling in aquatic ecosystems. Biol Inv 13:2861–2868. doi:10.1007/s10530-011-9969-y
Platvoet D, van der Velde G, Dick JTA, Li SQ (2009) Flexible omnivory in Dikerogammarus villosus (Sowinsky, 1894) (Amphipoda). Crustaceana 82:703–720. doi:10.1163/156854009x423201
Power AG, Mitchell CE (2004) Pathogen spillover in disease epidemics. Am Nat 164(S5):S79–S89. doi:10.1086/424610
Prenter J, MacNeil C, Dick JTA, Dunn AM (2004) Roles of parasites in animal invasions. Trends Ecol Evol 19:385–390. doi:10.1016/j.tree.2004.05.002
Rewicz T, Grabowski M, MacNeil C, Bącela-Spychalska K (2014) The profile of a ‘perfect’ invader the case of killer shrimp, Dikerogammarus villosus. Aquat Inv 9:267–288. doi:10.3391/ai.2014.9.3.04
Riley LA, Dybdahl MF (2015) The roles of resource availability and competition in mediating growth rates of invasive and native freshwater snails. Freshw Biol 60:1308–1315. doi:10.1111/fwb.12566
Sala OE, Chapin FS, Armesto JJ, Berlow E, Bloomfield J, Dirzo R, Huber-Sanwald E, Huenneke LF, Jackson RB, Kinzig A, Leemans R, Lodge DM, Mooney HA, Oesterheld M, LeRoy Poff N, Sykes MT, Walker BH, Walker M, Wall DH (2000) Global biodiversity scenarios for the year 2100. Science 287:1770–1774. doi:10.1126/science.287.5459.1770
Solomon ME (1949) The natural control of animal populations. J Anim Ecol 18:1–35
Strauss A, White A, Boots M (2012) Invading with biological weapons: the importance of disease-mediated invasions. Funct Ecol 26:1249–1261. doi:10.1111/1365-2435.12011
Terry RS, Smith JE, Sharpe RG, Rigaud T, Littlewood DTJ, Ironside JE, Rollinson D, Bouchon D, MacNeil C, Dick JTA, Dunn AM (2004) Widespread vertical transmission and associated host sex-ratio distortion within the eukaryotic phylum Microspora. Proc R Soc B Biol Sci 271:1783–1789. doi:10.1098/rspb.2004.2793
Tompkins DM, White AR, Boots M (2003) Ecological replacement of native red squirrels by invasive greys driven by disease. Ecol Lett 6:189–196. doi:10.1046/j.1461-0248.2003.00417.x
Torchin ME, Lafferty KD, Dobson AP, McKenzie VJ, Kuris AM (2003) Introduced species and their missing parasites. Nature 421:628–630. doi:10.1038/nature01346
Truhlar AM, Dodd JA, Aldridge DC (2014) Differential leaf-litter processing by native (Gammarus pulex) and invasive (Dikerogammarus villosus) freshwater crustaceans under environmental extremes. Aquat Conserv Mar Freshw Ecosyst 24:56–65. doi:10.1002/aqc.2375
US EPA (Environmental Protection Agency) (2008) Predicting future introductions of nonindigenous species to the Great Lakes, National Center for Environmental Assessment, Washington, DC; EPA/600/R-08/066F. Available from the National Technical Information Service, Spring Field, VA, and http://www.epa.gov/ncea
van der Velde G, Rajagopal S, Kelleher B, Muskó IB, Bij de Vaate A (2000) Ecological impact of crustacean invaders: general considerations and examples from the Rhine River. Crustac Issues 12:3–33
van der Velde G, Leuven RSEW, Platvoet D et al (2009) Environmental and morphological factors influencing predatory behaviour by invasive non-indigenous gammaridean species. Biol Invasions 11:2043–2054. doi:10.1007/s10530-009-9500-x
van Riel MC, van der Velde G, Rajagopal S, Marguillier S, Dehairs F, Bij de Vaate A (2006) Trophic relationships in the Rhine food web during invasion and after establishment of the Ponto-Caspian invader Dikerogammarus villosus. Hydrobiologia 565:39–58. doi:10.1007/s10750-005-1904-8
Wallentinus I, Nyberg CD (2007) Introduced marine organisms as habitat modifiers. Mar Pollut Bull 55:323–332. doi:10.1016/j.marpolbul.2006.11.010
Wattier RA, Haine ER, Beguet J, Martin G, Bollache L, Muskó IB, Platvoet D, Rigaud T (2007) No genetic bottleneck or associated microparasite loss in invasive populations of a freshwater amphipod. Oikos 116:1941–1953. doi:10.1111/j.2007.0030-1299.15921.x
Acknowledgements
We thank the Foljuif biological station for providing excellent working conditions and the three anonymous referees for their constructive comments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Iltis, C., Spataro, T., Wattier, R. et al. Parasitism may alter functional response comparisons: a case study on the killer shrimp Dikerogammarus villosus and two non-invasive gammarids. Biol Invasions 20, 619–632 (2018). https://doi.org/10.1007/s10530-017-1563-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-017-1563-5