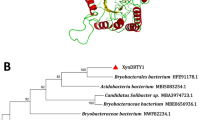
Abstract
A xylanase gene (xyn10A) was cloned from Bacillus sp. SN5 and expressed in Escherichia coli. It encoded a 348-residue polypeptide of ~45 kDa. The deduced amino acid sequence had 68 % identity with the endo-1,4-beta-xylanase from Paenibacillus lactis 154 that belonged to family 10 of the glycoside hydrolases. Purified recombinant Xyn10A had maximum activity at 40 °C and pH 7.0, with the specific activity of 105 U/mg and a Km of 0.6 mg/ml for beechwood xylan. Xyn10A retained more than 80 % activity between 25 and 45 °C and 29 % activity at 5 °C. It exhibited the highest activity (134 %) in 0.5 M NaCl and still retained 90 % activity in 2.5 M NaCl. It retained about 87 % activity after incubation in 2 M NaCl for 24 h. The cold-active and halo-tolerant properties of Xyn10A make it promising for application in the food industry, especially in the processing of saline food and sea food.



Similar content being viewed by others
References
Berrin JG, Juge N (2008) Factors affecting xylanase functionality in the degradation of arabinoxylans. Biotechnol Lett 30:1139–1150
Collins T, Gerday C, Feller G (2005) Xylanases, xylanase families and extremophilic xylanases. FEMS Microbiol Rev 29:3–23
Guo B, Chen XL, Sun CY et al (2009) Gene cloning, expression and characterization of a new cold-active and salt-tolerant endo-β-1,4-xylanase from marine Glaciecola mesophila KMM 241. Appl Microbiol Biotechnol 84:1107–1115
Henrissat B (1991) A classification of glycosyl hydrolases based on amino acid sequence similarities. Biochem J 280(Pt 2):309–316
Horikoshi K (1971) Production of alkaline enzymes by alkalophilic microorganisms. Part I. Alkaline protease produced by Bacillus no. 221. Agric Biol Chem 35:1407–1414
Hu Y, Zhang GM, Li AY et al (2008) Cloning and enzymatic characterization of a xylanase gene from a soil-derived metagenomic library with an efficient approach. Appl Microbiol Biotechnol 80:823–830
Hung KS, Liu SM, Fang TY et al (2011) Characterization of a salt-tolerant xylanase from Thermoanaerobacterium saccharolyticum NTOU1. Biotechnol Lett 33:1441–1447
Hwang IT, Lim HK, Song HY et al (2010) Cloning and characterization of a xylanase, KRICT PX1 from the strain Paenibacillus sp. HPL-001. Biotechnol Adv 28(5):594–601
Menon G, Mody K, Keshri J et al (2010) Isolation, purification, and characterization of haloalkaline xylanase from a marine Bacillus pumilus strain, GESF-1. Biotechnol Bioprocess Eng 6(15):998–1005
Setati ME (2010) Diversity and industrial potential of hydrolase-producing halophilic/halotolerant eubacteria. Afr J Biotechnol 9:1555–1560
Shi PJ, Tian J, Yuan TZ et al (2010) Paenibacillus sp. strain E18 bifunctional xylanase-glucanase with a single catalytic domain. Appl Environ Microbiol 76(11):3620–3624
Solomon V, Teplitsky A, Shulami S et al (2007) Structure-specificity relationships of an intracellular xylanase from Geobacillus stearothermophilus. Acta Crystallogr D Biol Crystallogr 63:845–859
Zhang G, Mao L, Zhao Y et al (2010) Characterization of a thermostable xylanase from an alkaliphilic Bacillus sp. Biotechnol Lett 32(12):1915–1920
Zhang F, Shi PJ, Bai YG et al (2011) An acid and highly thermostable xylanase from Phialophora sp. G5. Appl Microbiol Biotechnol 89:1851–1858
Acknowledgments
This study was supported by the National Basic Research Program of China (2011CBA00805 and 2009CB724700), Chinese National Programs for High Technology Research and Development (2011AA02A206).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bai, W., Xue, Y., Zhou, C. et al. Cloning, expression and characterization of a novel salt-tolerant xylanase from Bacillus sp. SN5. Biotechnol Lett 34, 2093–2099 (2012). https://doi.org/10.1007/s10529-012-1011-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-012-1011-7