Abstract
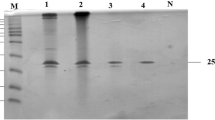
Two extracellular xylanases, denominated X2 and X3, were purified and characterized from the halotolerant bacterium Bacillus sp. Asc6BA isolated from “Salar de Ascotán” in the Atacama Desert. Xylanases were purified by anion exchange, cation exchange and size exclusion liquid chromatography. Xylanase X2 and X3 were purified ~ 690-fold and ~ 629-fold, respectively, compared to the concentrated extracellular fraction with a final specific activity of 169 and 154 u mg−1, respectively. Optimal conditions of pH and temperature of xylanolytic activity were 6.0 and 60 °C for X2 and 7.0 and 60 °C for X3. Half-life of X2 xylanase was 30 min at 50 °C, while X3 xylanase was remarkably more thermostable, retaining more than 70% of its activity after 32 h of incubation at 50 °C. X2 exhibited Km, Vmax and kcat values of 7.17 mg mL−1, 1.28 mM min−1 mg−1 and 425.33 s−1, respectively. X3 exhibited Km, Vmax and kcat values of 6.00 mg mL−1, 19.25 mM min−1 mg−1 and 82,515 s−1, respectively. In addition to their thermal stabilities, these enzymes were shown to be resistant to freeze-drying. These stability properties, in addition to the ability of these enzymes to be active in a wide range of temperatures and pHs, make these xylanases good candidates for industrial applications.






Similar content being viewed by others
References
Amenabar MJ, Blamey JM (2012) Purification and characterization of a thermostable glutamate dehydrogenase from a thermophilic bacterium isolated from a sterilization drying oven. BMB Rep 45:91–95. https://doi.org/10.5483/BMBRep.2012.45.2.91
Bajaj P, Mahajan R (2019) Cellulase and xylanase synergism in industrial biotechnology. Appl Microbiol Biotechnol 103:8711–8724
Banka AL, Guralp SA, Gulari E (2014) Secretory expression and characterization of two hemicellulases, xylanase, and β-xylosidase, isolated from Bacillus subtilis M015. Appl Biochem Biotechnol 174:2702–2710
Bastawde K (1992) Xylan structure, microbial xylanases, and their mode of action. World J Microbiol Biotechnol 8:353–368
Beg Q, Kapoor M, Mahajan L, Hoondal G (2001) Microbial xylanases and their industrial applications: a review. Appl Microbiol Biotechnol 56:326–338
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Buchert J, Tenkanen M, Kantelinen A, Viikari L (1994) Application of xylanases in the pulp and paper industry. BioresourTechnol 50:65–72
Cáceres-Moreno P, Muñoz-Ibacache SA, Monsalves MT, Amenabar MJ, Blamey JM (2019) Functional approach for the development and production of novel extreme biocatalysts. In: Next generation biomanufacturing technologies. ACS Publications, Washington, DC, pp 1–22
Chi W-J, Chang Y-K, Hong S-K (2012) A novel alkaliphilicxylanase from the newly isolated mesophilic Bacillus sp. MX47: production, purification, and characterization. Appl Biochem Biotechnol 168:899–909
Collins T, Gerday C, Feller G (2005) Xylanases, xylanase families and extremophilic xylanases. FEMS Microbiol Rev 29:3–23
Damiano VB, Ward R, Gomes E, Alves-Prado HF, Da Silva R (2006) Purification and characterization of two xylanases from alkalophilic and thermophilic Bacillus licheniformis 77–2. In: Twenty-seventh symposium on biotechnology for fuels and chemicals. Springer, New York, pp 289–302
de QueirozBrito Cunha CC, Gama AR, Cintra LC, Bataus LAM, Ulhoa CJ (2018) Improvement of bread making quality by supplementation with a recombinant xylanase produced by Pichia pastoris. PLoS ONE 13:e0192996
Dornez E, Verjans P, Arnaut F, Delcour JA, Courtin CM (2011) Use of psychrophilic xylanases provides insight into the xylanase functionality in bread making. J Agric Food Chem 59:9553–9562
Ermler U, Merckel M, Thauer R, Shima S (1997) Formylmethanofuran: tetrahydromethanopterin formyltransferase from Methanopyrus kandleri—new insights into salt-dependence and thermostability. Structure 5:635–646
Ferraz A, Guerra A, Mendonça R, Masarin F, Vicentim MP, Aguiar A, Pavan PC (2008) Technological advances and mechanistic basis for fungal biopulping. Enzyme MicrobTechnol 43:178–185
Garg S (2016) Xylanase: applications in biofuel production. Curr Metab 4:23–37
Giridhar PV, Chandra T (2010) Production of novel halo-alkali-thermo-stable xylanase by a newly isolated moderately halophilic and alkali-tolerant Gracilibacillussp. TSCPVG Process Biochem 45:1730–1737
Heo S-Y, Kwak J-Y, Oh H-W, Park D-S, Bae K-S, Shin D-H, Park H-Y (2006) Characterization of an extracellular xylanase in Paenibacillus sp. HY-8 isolated from an herbivorous longicorn beetle. J Microbiol Biotechnol 16:1753–1759
Juodeikiene G, Basinskiene L, Vidmantiene D, Makaravicius T, Bartkiene E (2012) Benefits of β-xylanase for wheat biomass conversion to bioethanol. J Sci Food Agric 92:84–91
Juturu V, Wu JC (2012) Microbial xylanases: engineering, production and industrial applications. Biotechnol Adv 30:1219–1227
Kamble RD, Jadhav AR (2012) Isolation, purification, and characterization of xylanase produced by a new species of Bacillus in solid state fermentation. Int J Microbiol 2012:1–8
Kenealy WR, Jeffries TW (2003) Enzyme processes for pulp and paper: a review of recent developments. ACS Publications, Washington, DC
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680
Lei X, Lin L, Li K (2008) Effect of xylanasepretreatment of wood chips on fiber separation in the CTMP refining process. BioResources 3:801–815
Lineweaver H, Burk D (1934) The determination of enzyme dissociation constants. J Am Chem Soc 56:658–666
Mc Alpine P, O’shea C, Varley P, O’Doherty J (2012) The effect of protease and xylanase enzymes on growth performance and nutrient digestibility in finisher pigs. J Anim Sci 90:375–377
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428
Monsalves MT, Ollivet-Besson GP, Amenabar MJ, Blamey JM (2020) Isolation of a psychrotolerant and UV-C-resistant bacterium from elephant island, Antarctica with a highly thermoactive and thermostable catalase. Microorganisms 8:95
Paës G, Berrin J-G, Beaugrand J (2012) GH11 xylanases: structure/function/properties relationships and applications. Biotechnol Adv 30:564–592
Park D-J, Lee Y-S, Chang J, Fang S-J, Choi Y-L (2013) An β-1, 4-xylanase with exo-enzyme activity produced by Paenibacillus xylanilyticus KJ-03 and its cloning and characterization. J Microbiol Biotechnol 23:397–404
Perkins DN, Pappin DJ, Creasy DM, Cottrell JS (1999) Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophor Int J 20:3551–3567
Pollet A, Schoepe J, Dornez E, Strelkov SV, Delcour JA, Courtin CM (2010) Functional analysis of glycoside hydrolase family 8 xylanases shows narrow but distinct substrate specificities and biotechnological potential. Appl Microbiol Biotechnol 87:2125–2135
Prakash B, Vidyasagar M, Jayalakshmi S, Sreeramulu K (2012) Purification and some properties of low-molecular-weight extreme halophilic xylanase from Chromohalobacter sp. TPSV 101. J MolCatal B Enzym 74:192–198
Sharma D, Chaudhary R, Kaur J, Arya SK (2020) Greener approach for pulp and paper industry by Xylanase and Laccase. Biocatal Agric Biotechnol 25:101604
Silva L, Terrasan CRF, Carmona EC (2015) Purification and characterization of xylanases from Trichoderma inhamatum. Electron J Biotechnol 18:307–313
Singh B (2019) Production, characteristics, and biotechnological applications of microbial xylanases. Appl Microbiol Biotechnol 103:8763–8784
Subramaniyan S (2012) Isolation, purification and characterisation of low molecular weight xylanase from Bacillus pumilus SSP-34. Appl Biochem Biotechnol 166:1831–1842
Subramaniyan S, Prema P (2002) Biotechnology of microbial xylanases: enzymology, molecular biology, and application. Crit Rev Biotechnol 22:33–64
Thite V, Nerurkar A (2015) Xylanases of Bacillus spp. isolated from ruminant dung as potential accessory enzymes for agro-waste saccharification. Lett Appl Microbiol 60:456–466
Thomas L, Joseph A, Singhania RR, Patel A, Pandey A (2017) Industrial enzymes: xylanases. In: Current developments in biotechnology and bioengineering. Elsevier, Amsterdam, pp 127–148
Torres C, Negro C, Fuente E, Blanco A (2012) Enzymatic approaches in paper industry for pulp refining and biofilm control. Appl Microbiol Biotechnol 96:327–344
Wejse PL, Ingvorsen K, Mortensen KK (2003) Purification and characterisation of two extremely halotolerant xylanases from a novel halophilic bacterium. Extremophiles 7:423–431
Yang G, Lucia LA, Chen J, Cao X, Liu Y (2011) Effects of enzyme pretreatment on the beatability of fast-growing poplar APMP pulp. BioResources 6:2568–2580
Acknowledgements
This research was funded by Fundación Biociencia.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Communicated by M. Moracci.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Contreras, F., Amenabar, M.J. & Blamey, J.M. Purification and characterization of two thermostable xylanases from a halotolerant Bacillus sp. Asc6BA isolated from Salar de Ascotán, Atacama Desert. Extremophiles 25, 51–59 (2021). https://doi.org/10.1007/s00792-020-01210-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-020-01210-z