Abstract
Biocontrol with hypocrealean entomopathogenic fungi (EF) is a key tool to develop Integrated Pest Management (IPM) programs for the progressive replacement of synthetic chemical insecticides with more environmentally friendly pest control measures. These fungi stand out among entomopathogens not only for their contact mechanism of infection through the arthropod integument, but also for developing close associations with plants including the endophytic lifestyle and rhizosphere competence that can enable them to make broader contributions to IPM and crop production. Anyhow, the interaction of EF with the plants incorporates multitrophic complexity at different levels including insect pests, plants, and their natural enemies. The aim of the present review was to gather and summarize all available data on multitrophic interactions of EF. These fungi can influence both the chemical ecology of host-plant selection by insect pests and the host or prey selection by parasitoid or predators, respectively. Moreover, EF treatments are compatible with natural enemies in terms of safety and effectiveness, which could allow biocontrol strategies for their synergistic application in IPM programs. A comprehensive understanding of the impact of these multitrophic interactions in longer term, farm-level real-life biocontrol implementation studies will provide new opportunities in plant protection and production.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hypocrealean entomopathogenic fungi (EF) stand out among microbial control agents not only because their mode of infection by direct penetration through the cuticle and relatively easy mass production, but also because the newly described EF plant-interacting lifestyles, which place them at the forefront of crop protection and production tools (Quesada-Moraga et al. 2020). The current knowledge of the primary mode of action of EF and the molecules involved in different steps of their infection pathway has been extensively reviewed (Vega et al. 2012; Mannino et al. 2019). Besides, newly described modes of action of EF have also been discovered such the accidental death caused by stress in the insects through oral infection (Butt et al. 2013; Garrido-Jurado et al. 2015) and indirect mortality related to EF associations with the plants (Akello et al. 2008; Butt et al. 2013; Vidal and Jaber 2015; Klieber and Reineke 2016; Garrido-Jurado et al. 2017, 2020; Resquín-Romero et al. 2016; Vega 2018; Zhu et al. 2018; Russo et al. 2019).
Particularly noteworthy are the associations of EF with plants as epiphytic (with the plant surface), endophytic (inside the plant), and rhizosphere competent microorganisms described mainly in the twenty-first century. As epiphytic microorganisms, EF propagules can become part of the phylloplane microbiota of different types of vegetation in natural and transformed habitats (Meyling and Eilenberg 2006; Ormond et al. 2010; Meyling et al. 2011; Garrido-Jurado et al. 2015). Recent studies on the adaptation and evolution of EF towards epiphytic, endophytic, or rhizosphere lifestyles reveal passive or active dispersal of EF soil conidia by wind or arthropods, respectively (Meyling and Eilenberg 2006; Garrido-Jurado et al. 2015; Fernández-Bravo et al. 2017; González-Mas et al. 2021b).
The endophytic behavior of EF with biocontrol potential, first described in corn in 1991 (Bing and Lewis 1991), has been thoroughly and widely reported in numerous cultivated and non-cultivated plant species, both naturally colonized and artificially inoculated (Quesada-Moraga et al. 2014; Vidal and Jaber 2015; Vega 2018; Quesada-Moraga 2020). Moreover, it has been proposed that entomopathogenic fungal endophytes may be important bodyguards having negative effect on polyphagous and sucking insect pests (Gange et al. 2019). Entomopathogenic fungi asymptomatically colonize plant tissues (Saikkonen et al. 2006; Arnold and Lutzoni 2007) and can even promote growth and protect the plant against biotic stresses, pests, and diseases, or abiotic ones such as water deficit, nutritional deficiencies, etc. (Quesada-Moraga, 2020). The degree of EF colonization of the different tissues and organs of the plant and fungal persistence over time vary according to the plant species and fungal strain, from local to systemic colonization of the plant tissues, with even vertical transmission detected (Landa et al. 2013; Quesada-Moraga et al. 2014; Garrido-Jurado et al. 2017; Quesada-Moraga, 2020).
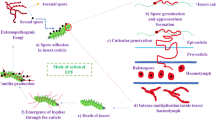
The persistence and biological activity of EF are also promoted in the rhizosphere (Hu and St Leger 2002; Pava-Ripoll et al. 2011; Wyrebek et al. 2011; Barelli et al. 2016; McKinnon et al. 2018) (Fig. 1). The rhizosphere is the narrow zone of soil that is influenced by root secretions that can contain an enormous diversity of microbes (Mendes et al. 2011). The rhizosphere is an important niche for soil-borne fungal entomopathogens in which EF may be developed both to soil dwelling pest control and to provide additional ecosystem services such as plant growth promotion and direct disease antagonism (Bruck 2010). Indeed, an adaptation mechanism as a rhizosphere competent organism has been reported for Metarhizium anisopliae (Mets.) Sorokin (Ascomycota: Hypocreales), which expresses a specific subset of genes induced by plant root exudates different from the one expressed during infection of the arthropod hosts (Bruck 2005; Hu and St Leger 2002; Pava-Ripoll et al. 2011).
Increasing complexity in the multitrophic relationships of entomopathogenic fungi (EF). a EF-mediated trophic interactions between insects and plants. b EF-mediated trophic interactions between insects and their natural enemies either predators or parasitoids. c Multitrophic interactions involving entomopathogenic fungi. Solid connecting arrows represent direct effect while stippled connecting arrows represent an indirect effect. In Fig. 1a, 1 and 2 represent respectively a chewing or a sap-sucking insect feeding on a plant challenged by an epiphyte, endophyte, or rhizosphere competent entomopathogenic fungus. In Fig. 1c, 1, 2 and 3 represent respectively aerial chewing, aerial sap-sucking and soil dwelling insects infected by entomopathogenic fungi. Numbers 4, 5 and 6 represent cadavers of the same insects with fungal outgrowth
The role of EF on plant-mediated effects, insect population dynamics in crop ecosystems and semi-natural habitats and communities and the ecological principles of community interactions have been reviewed (Cory and Ericsson 2010; Hesketh et al. 2010; Meyling and Hajek 2010). New and noteworthy works indicate that EF are entomopathogens to insects through direct infection and toxin production but also indirectly through metabolite production in plants or plant defense activation (Gange et al., 2019). However, it is now necessary to expand on this previous work to examine how the trophic complexity created by the close association of EF with plants might influence multitrophic insect-plant and insect-natural enemy relationships, to take advantage of this EF-plant association to develop new crop protection and crop production strategies (Fig. 1a, b, c).
The aim of this review was to update the relatively scant data available on EF-mediated trophic interactions of plants, insect pests, and their natural enemies (hereafter natural enemies are predators and parasitoids). Trophic interactions are considered as two or three trophic levels interactions between EF and plants or between EF-infected insects and predators or parasitoids while interactions of more than three trophic levels are defined as multitrophic, such as the one including EF-colonized plants, insect pests, and their natural enemies.
Entomopathogenic-fungi-mediated trophic interactions between insects and plants
It is known that various host plant species can modify the susceptibility of insect pests to EF (Santiago-Álvarez et al. 2006; Cory and Ericsson, 2010; Ocampo-Hernández et al. 2019). Even, it has been shown that the behavior of insects can be indirectly affected by both EF propagule infestation of the plant surface or endophytic colonization (Pell and Vandenberg 2002; Meyling and Pell 2006; Lam et al. 2010; Yanagawa et al. 2011; Davis et al. 2013; Mburu et al. 2013; Rashki and Shirvani 2013; Gange et al., 2019). However, this section aims to examine in greater depth the possible behavioral responses of insects to EF-colonized plants, which might be indirectly related to metabolite secretion in plants or plant defense activation (Gange et al., 2019) (Fig. 1a). Most of the behavioral responses in insects, such as foraging, mating, preference for an oviposition site, or interaction with natural enemies, are regulated by olfactory chemical signals produced by plants, insects and natural enemies (Dicke and Grostal 2001; Sigsgaard 2005; Bruce et al. 2005; Xu and Turlings 2018). In addition, the volatile profile emitted by plants can be altered by their colonization by microorganisms, which can modify the insect-plant and insect-natural enemy relationships (Yue et al. 2001; Hempel et al. 2009; Shikano et al. 2017; Contreras-Cornejo et al. 2018; Tasin et al. 2018) (Fig. 1a). Thus, it has been shown that Beauveria bassiana (Balsamo) Vuillemin (Ascomycota: Hypocreales) influences the choice of host plant by the cotton aphid Aphis gossypii Glover (Hemiptera: Aphididae), which selects non-colonized over B. bassiana-colonized plants (Rashki and Shirvani 2013). In this regard, the limited knowledge available on the ability of plant-associated EF to influence plant-feeding insects is not conclusive, with reports on repellency (Sword et al. 2017; Rondot and Reineke 2017) or attraction (Kepler and Bruck 2006). Anyhow, unraveling whether endophytic EF colonization can cause alterations in the chemical signals produced by plants, and therefore in insect-plant relationships, or even in those of phytophagous insects with their natural enemies, is a key research goal. Hence, Lygus hesperus Knight (Hemiptera: Miridae) and Nezara viridula (Linnaeus) (Hemiptera: Pentatomidae) bugs can detect and subsequently avoid flowers and fruits developed in plants whose tissues are endophytically colonized by B. bassiana and prefer control plants in selection experiments (Sword et al. 2017). In addition, B. bassiana endophytic colonization led to a deterrent effect in adults of the vine weevil Otiorhynchus sulcatus (Fabricius) (Coleoptera: Curculionidae), which preferred the control plants (Rondot and Reineke 2017). In contrast, the larvae of this weevil were shown to be attracted to pots containing plants with M. anisopliae (Kepler and Bruck 2006).
These studies reveal the ability of insects to detect EF endophytic colonization of plant tissues, a behavior that could be regulated by variations in the profile of plant volatile compounds (González-Mas et al. 2021a) (Fig. 1a). It has been noteworthy shown that endophytic colonization by B. bassiana influences volatile emissions by melon and cotton plants, either unharmed or after being damaged by sap-sucking aphids or leaf-chewing caterpillars (González-Mas et al. 2021a). Some of the emitted compounds have been previously reported to be released in response to herbivory and have been implicated in natural enemy attraction, or even to have antimicrobial properties. Hence, colonization by B. bassiana might help not only to directly control insect pests but also to increase the resistance of plants against agronomically important pests and phytopathogenic microorganisms (González-Mas et al. 2021a). By using an axenic consortium of B. bassiana and Trichoderma asperellum Samuels, Lieckf. & Nirenberg (Ascomycota: Hypocreales) against Ostrinia furnacalis (Guenée) (Lepidoptera: Crambidae), it has been demonstrated that colonization by EF may have a positive effect on increasing herbivory-induced defenses and restricting pest survival and growth (Batool et al. 2022). This effect on increasing herbivory-induced defenses and restricting pest survival and growth has also been observed by Cotes et al. (2020), who demonstrated that root-associated entomopathogenic fungi indirectly influence herbivorous insect performance by causing an increase in the production of jasmonic, ( +)-7-iso-jasmonoyl-l-isoleucine and salicylic acid in certain parts of the host plant. The above examples illustrate that EF can influence the chemical ecology of host-plant selection by insect and mite pests.
Entomopathogenic-fungi-mediated trophic interactions between insects and their natural enemies
Whilst the use of natural enemies and entomopathogenic microorganisms in biological control reduces the effects on the environment and non-target organisms compared to the use of conventional insecticides, it is necessary to evaluate the compatibility between them for developing IPM programs (Roy et al. 2010). In general, it has been found that EF treatments can be considered to be of low-risk for predators and parasitoids and therefore compatible with them in the light of the numerous investigations on the safety and effectiveness of the combined use of EF and other biocontrol agents (Roy and Pell 2000; Acevedo et al. 2007; Labbé et al. 2009; Ansari et al. 2010; Martins et al. 2014) (Fig. 1b). Indeed, infection of phytophagous insects by EF, initiated either by direct contact with the fungal inoculum or by the insects feeding or developing in EF endophytically colonized tissues, can affect their behavior, and therefore their intra- and interspecific relationships (Meyling and Pell 2006; Roy et al. 2006) (Fig. 1b). Table 1 summarizes the works done so far by different authors on EF-mediated tritrophic interactions. In this section, we highlight recent advances in the knowledge about direct effects of entomopathogenic fungi on predator/parasitoid survival and fitness and indirect effects on natural enemy behavior/capacity (Fig. 1a).
Direct effects of entomopathogenic fungi on natural enemy survival and fitness
In unlikely scenarios in real situations (worst-case scenarios), by spraying or immersion of high doses of different fungal strains (Castillo et al. 2009; Da Silva et al. 2016; Miranda-Fuentes et al. 2021), the direct application of EF suspensions to Hymenoptera parasitoid braconids and eulophids can decrease their longevity (Labbé et al. 2009; Tamayo-Mejía et al. 2015; Miranda-Fuentes et al. 2020) (Table 1). In general, the compatibility of EF with parasitoids and predators is influenced, among other factors, by the species involved, the application technique, the fungal dosage, the degree of prey/host infection, and the time interval between the fungal application and the release of the predators or parasitoids (Mesquita and Lacey 2001; Aqueel and Leather 2013; Ibarra-Cortés et al. 2018). Decreasing the doses and applying the natural enemy before EF inoculation minimize the possible negative effects on various groups of predators such as predatory coccinellids (James et al. 1995; Pingel and Lewis 1996; Todorova et al. 1996; Smith and Krischik 2000; Roy and Pell 2000; Pell and Vandenberg 2002; Roy et al. 2008), lacewings (Portilla et al. 2017), and several species of aphid parasitoids (Brodeur and Rosenheim 2000; Mesquita and Lacey 2001; Jeong et al. 2005; Aqueel and Leather 2013; Oreste et al. 2016; Shrestha et al. 2017) (Table 1).
Regarding predators, it has also been shown that B. bassiana and M. anisopliae are compatible with the generalist predator Coccinella septempunctata L. (Coleoptera: Coccinellidae) (Rizwan et al. 2021) (Table 1). Neither fungus induced any significant changes in the development time (egg-adult), fecundity rate, adult preoviposition period, total preoviposition period, or mean generation time as compared to control treatment (Rizwan et al. 2021). When evaluating B. bassiana and phytoseiid mites that can independently contribute to suppressing the two-spotted spider mite, Tetranychus urticae Koch (Acari: Tetranychidae), it was demonstrated that although several B. bassiana strains displayed a high virulence in T. urticae, there was no evident pathogenicity to phytoseiid mites (Wu et al. 2016) (Table 1). In worst-case scenarios, by direct spraying of Phytoseiulus persimilis Athias-Henriot (Acarina: Phytoseiidae) with B. bassiana conidia at high dosages, significant negative effects on fecundity and life table parameters (net reproductive rate, intrinsic rate of natural increase, mean generation time, finite rate of increase, and doubling time) were found when B. bassiana was applied to the adult stage (Ullah and Lim 2017). Indeed, laboratory and potted plant investigations on the predatory behavior of the predatory mite P. persimilis against T. urticae indicated that P. persimilis showed significant aversion behavior to the initial fungal spray, but gradually dispersed over the entire bean plants, with no significant differences between the treatments in the number of T. urticae consumed (Wu et al. 2018). Fungal spray did not affect the predation capability of P. persimilis and poses a negligible risk to its behavior (Wu et al. 2018) (Table 1).
Regarding parasitoids, some studies have shown a high level of compatibility between EF and parasitoids (Polanczyk et al. 2010; Rossoni et al. 2016; Shrestha et al. 2017; González-Mas et al. 2019a; Miranda-Fuentes et al. 2020), while others have shown antagonistic interactions (Oreste et al. 2015; Tamayo-Mejía et al. 2015) (Table 1). Despite this, most studies have demonstrated that combining EF and parasitoids in IPM programs is always beneficial when release times are adjusted appropriately, with emphasis on which agent is administered first and whether the treatments are timed correctly (Da Silva et al. 2016; Jarrahi and Safavi 2016; Shrestha et al. 2017). Emami et al. (2013) found that extending the release interval of the parasitoid Aphidius colemani Viereck (Hymenoptera: Braconidae) following B. bassiana application for control of the green peach aphid, Myzus persicae (Sulzer) (Hemiptera: Aphididae), decreased the quantity of parasitoid pupae growing and the percentage emerging as adults. It has even been reported that using commercial isolates of EF had no influence on the survival rates and enhanced parasitism rates of the parasitoid Encarsia formosa Gahan (Hymenoptera: Aphelinidae) (Labbé et al. 2009). Mohammed and Hatcher (2017) observed that, when M. persicae treated with the fungus Lecanicillium muscarium (Petch) Zare & W. Gams (Ascomycota: Hypocreales) were offered to the parasitoid A. colemani 3–4 days after fungal infection, they were less likely to be parasitized than when offered 1–2 days after fungal infection. In whiteflies, Labbé et al. (2009) discovered that applying B. bassiana after parasitism by E. formosa had no influence on parasitoid numbers or parasitism rates. Furthermore, (Mohammed and Hatcher 2017) found that applying the fungus L. muscarium to M. persicae 3–7 days after A. colemani parasitism had no effect on the proportion of aphids parasitized. It should be noted that the use of parasitoids as vectors of EF has recently been documented, showing that the presence of Habrobracon hebetor (Hymenoptera: Braconidae) females significantly (1.5–13 fold) increased the mycoses level in clusters of Galleria mellonella L. (Lepidoptera: Pyralidae) (Kryukov et al. 2018), revealing not only compatibility of EF with natural enemies but also a synergistic interaction (Table 1). Beauveria bassiana caused no negative effects either on the development of the immature stages of the parasitoid Coptera haywardi (Ogloblin) (Hymenoptera: Diapriidae) or on female fecundity during the first 18 days of adult life, and it is therefore possible to develop management strategies using these two natural enemies in biological control against Anastrepha obliqua (Macquart) (Diptera: Tephritidae) (Martínez-Barrera et al. 2020) (Table 1).
Hymenopteran eulophid Tamarixia triozae (Burks) adults may die prematurely if B. bassiana is used to suppress Bactericera cockerelli (Šulc) (Hemiptera: Triozidae), without affecting their overall reproductive potential (Tamayo-Mejía et al. 2015). Other researchers have found that previous inoculation with EF can impact fitness of the parasitoid wasp Trybliographa rapae (Westw.) (Hymenoptera: Eucoilidae), shortening its lifetime while raising oviposition rates as a response to fungal presence (Rännbäck et al. 2015). Under controlled conditions, Potrich et al. (2015) described negligible effects of M. anisopliae on the biological parameters of Trichogramma pretiosum Riley (Hymenoptera: Trichogrammatidae) on Anagasta kuehniella Zeller (Lepidoptera: Pyralidae). The potential of M. brunneum applied by direct contact and/or as an endophyte to control S. littoralis larvae alone or in combination with the solitary endoparasitoid Hyposoter didymator (Thunberg) (Hymenoptera: Ichneumonidae) in melon plants has also been investigated (Miranda-Fuentes et al. 2020, 2021). In contact treatments, when applied at high concentrations, the fungus significantly reduced the parasitoid’s longevity, but had no effect on the parasitoid female’s reproductive potential during the three days after treatment. Indeed, in several simultaneous use scenarios (inoculation of S. littoralis larvae with the fungus before being exposed to parasitoid females and vice versa), the combinations of the two agents to control S. littoralis were explored, with additive impact in all cases (Miranda-Fuentes et al. 2020). Martínez-Barrera et al. (2020) found similar results when they investigated several techniques for controlling Anastrepha obliqua (Macquart) (Diptera: Tephritidae) with B. bassiana and the parasitoid Coptera haywardi Loiácono (Hymenoptera: Diapriidae) (Table 1).
Effect of prey or host infection by entomopathogenic fungi on natural enemy behavior/capacity.
In the case of predators, it has been detected that lacewings quite frequently do not completely consume S. littoralis larvae when they are infected by the M. brunneum fungus, either to avoid mycosed areas of the body or because the fungal infection can reduce the nutritional quality of the prey (Ríos-Moreno et al. 2018) (Table 1). Other studies also indicate the ability of predators to discriminate between healthy and EF-infected prey (Pell and Vandenberg 2002; Meyling and Pell 2006; Ríos-Moreno et al. 2018). Indeed, it should be noted that several predators have been observed to prefer control prey over B. bassiana-infected one, such as Anthocoris nemorum (L.) (Hemiptera: Anthocoridae) (Meyling and Pell 2006) or C. septempunctata (Ormond et al. 2011), although the specific mechanisms that give rise to this behavior are as yet unknown (Table 1).
The number of A. gossypii females consumed by C. carnea, as well as the consumption time, were not significantly affected after direct exposure to a B. bassiana conidia suspension, compared to what was observed with the control aphids (González-Mas et al. 2019a). However, lacewings did not completely consume aphids that showed signs of fungal infection, as described when C. carnea consumed larvae of S. littoralis infected by M. brunneum (Ríos-Moreno et al. 2018), in what is presumably a lacewing safety mechanism (Table 1).
There are very few studies investigating the parasitoid’s influence on host susceptibility to the fungus. It has been reported that parasitism by H. didymator improved EF infection of S. littoralis larvae, with parasitization dramatically reducing the total hemocytes in S. littoralis hemolymph, encouraging fungal infection (Miranda-Fuentes et al. 2020). Therefore, the combined use of EF and predator or parasitoids can enhance the effect of the entomopathogen that might be relevant for biocontrol in terms of both the direct effect of the fungus on the target insect population and the dissemination and spread of the fungal inoculum to uninfected insect hosts.
Multitrophic interactions involving entomopathogenic fungi
Another question that arises is whether prey or host feeding on plants endophytically colonized by EF alters predator or parasitoid behavior/capacity in multitrophic systems with a crop plant colonized by an entomopathogenic fungus on which a pest is feeding and becomes a prey or a host for a predator or parasitoid, respectively (Fig. 1c). There are few studies investigating whether endophytic colonization of the plant by EF can influence natural enemies at the third trophic level, and the few that exist have focused on its effect on predators or parasitoids. Table 2 summarizes the works done so far by different authors on multitrophic interactions involving entomopathogenic fungi.
It has been shown that there is no effect on the predatory efficacy of C. carnea when feeding on A. gossypii aphids that had previously fed on melon plants endophytically colonized with B. bassiana, although a reduction in the consumption of prey was detected and an increase in consumption time compared to the control (González-Mas et al. 2019a) (Table 2). A significant preference of lacewings for A. gossypii aphids that feed on B. bassiana-colonized melon plants was observed, compared to the control plants. This could be related to compounds detected in the plants that were endophytically colonized affecting the behavior of the insects by acting as attractants (i.e., beta-ionone) (Obata et al. 1983; Flath et al. 1994; González-Mas et al., 2019b). In another choice assay, the number of aphids parasitized by A. colemani and their sex ratio were not influenced by whether or not the aphids had been feeding on B. bassiana-colonized plants (González-Mas et al. 2019a) (Table 2).
In a multitrophic system consisting of the endophytic fungus M. brunneum colonizing the melon plant offered to S. littoralis together with the parasitoid H. didymator, the presence of the parasitoid had a substantial impact on total mortality of S. littoralis larvae in all tests (Miranda-Fuentes et al. 2021). Treatments including the parasitoid had the highest death rates both in vitro and in planta. The total mortality of S. littoralis larvae was not significantly increased by simultaneous exposure to the fungus and the parasitoid when compared to the parasitoid alone (Miranda-Fuentes et al. 2021). Jaber and Araj (2018) also report that EF endophytic colonization of plants had no effect on A. colemani parasitism rates. Akutse et al. (2014) discovered that feeding EF-colonized plants to Liriomyza huidobrensis (Blanchard) (Diptera: Agromyzidae) larvae had no effect on the parasitoids Phaedrotoma scabriventris (Hymenoptera: Braconidae) and Diglyphus isaea (Walker) (Hymenoptera: Eulophidae) (Table 2).
Whilst in the M. brunneum–S. littoralis–melon–H. didymator system neither the application mode (contact or endophytic) nor the fungal exposure period had a significant effect on S. littoralis mortality (Miranda-Fuentes et al. 2020, 2021), other authors have reported that fungal exposure time was a significant factor affecting performance of the combined use of EF with the parasitoid A. colemani against M. persicae (Emami et al. 2013; Mohammed and Hatcher 2017). In the M. brunneum–S. littoralis–melon–H. didymator system, the parasitoid demonstrated a substantial preference for larvae fed on control plants compared to larvae fed on fungus-colonized plants (Miranda-Fuentes et al. 2021). This preference for untreated hosts is thought to be due to the parasitoid’s ability to recognize and avoid the fungus. Mesquita and Lacey (2001) found that the parasitoid Aphelinus asychis Walker (Hymenoptera: Aphelinidae) probed the ovipositors of infected aphid hosts for a shorter period, followed by rejection and absence of oviposition, due to strong internal cues. González-Mas et al. (2019a) discovered that offering aphids fed on EF-colonized plants had no effect on the oviposition preference of the parasitoid A. colemani. It is unknown what the preference outcomes would be in a similar scenario if EF and H. didymator were used together to control S. littoralis in the field. According to Mesquita and Lacey (2001), parasitoids will avoid possible hosts that have been exposed to fungus and will look for those that have not, which is good for parasitoid survival in the long term. Indeed, the histological investigation of S. littoralis larvae simultaneously parasitized by H. didymator and infected with M. brunneum revealed that both agents coexisted within the same host and even parasitoid larvae grew inside the host despite fungal invasion (Miranda-Fuentes et al. 2020). Although the fungus may outcompete immature parasitoids within the host, there have been no reports of the fungus invading parasitoid tissues when they are both attacking the same host (Furlong and Pell 2005; Miranda-Fuentes et al. 2020, 2021).
There are very few works investigating whether endophytic colonisation by EF can change secondary metabolites or trigger different plant defense pathways that could affect natural enemies. Jensen et al. (2020) investigated how the endophytic colonization of broad beans by B. bassiana influences the fitness and host-choice of the aphid parasitoid A. colemani, as well as differences in the plant defense responses to aphid infestation. Their study revealed that there are changes in the plants’ initial defense response to the aphids in the EF-treated plants compared to non-fungus treated control plants by measuring changes in the expression of the specific marker genes PR1 and PR2 involved in the salicylic acid pathway, as well as ERF-1, involved in the ethylene pathway (Table 2).
Conclusions and future perspectives
The potential uses of EF are going beyond their conventional function of controlling insect pests due to their plant-interacting lifestyles, mainly as plant endophytes and rhizosphere competent microorganisms. However, the close association of EF with plants incorporates trophic complexity because it can influence multitrophic relationships. Our comprehensive review of the scant data available on multitrophic relationships of EF shows that plant associated EF can influence the insect-plant interaction mainly by altering both the chemical ecology of host-plant selection by insect pests and insect pest selection by natural enemies, predators and parasitoids. Overall, EF treatments directly targeting the insect pest or indirectly via endophytism do not compromise predator and parasitoid fitness and behaviour, an important compatibility that should be further explored and utilized in biocontrol strategies for a synergistic application in IPM programs. Nonetheless, the fact that the available data summarized in the present work is mainly based upon short term and small-scale experiments makes necessary much more longer-term farm level real-life implementation research to fully understand the biocontrol impact of the multitrophic interactions of EF. Indeed, it remains unknown whether the newly described lifestyles of EF can also impact other key beneficial arthropods such as pollinators.
References
Acevedo JPM, Samuels RI, Machado IR, Dolinski C (2007) Interactions between isolates of the entomopathogenic fungus Metarhizium anisopliae and the entomopathogenic nematode Heterorhabditis bacteriophora JPM4 during infection of the sugar cane borer Diatraea saccharalis (Lepidoptera: Pyralidae). J Invertebr Pathol 96:187–192
Akello J, Dubois T, Coyne D, Kyamanywa S (2008) Effect of endophytic Beauveria bassiana on populations of the banana weevil, Cosmopolites sordidus, and their damage in tissue-cultured banana plants. Entomol Exp Appl 129:157–165
Akutse KS, Fiaboe KKM, Van Den Berg J, Ekesi S, Maniania NK (2014) Effects of endophyte colonization of Vicia faba (Fabaceae) plants on the life-history of leafminer parasitoids Phaedrotoma scabriventris (Hymenoptera: Braconidae) and Diglyphus isaea (Hymenoptera: Eulophidae). PLoS ONE 9(10):e109965
Ansari MA, Shah FA, Butt TM (2010) The entomopathogenic nematode Steinernema kraussei and Metarhizium anisopliae work synergistically in controlling overwintering larvae of the black vine weevil, Otiorhynchus sulcatus, in strawberry growbags. Biocontrol Sci Technol 20:99–105
Aqueel MA, Leather SR (2013) Virulence of Verticillium lecanii (Z.) against cereal aphids; does timing of infection affect the performance of parasitoids and predators? Pest Manag Sci 69:493–498
Arnold AE, Lutzoni F (2007) Diversity and host range of foliar fungal endophytes: are tropical leaves biodiversity hotspots? Ecology 88:541–549
Barelli L, Moonjely S, Behie SW, Bidochka MJ (2016) Fungi with multifunctional lifestyles: endophytic insect pathogenic fungi. Plant Mol Biol 90:657–664
Batool R, Umer MJ, Wang Y, He K, Shabbir MZ, Zhang T, Bai S, Chen J, Wang Z (2022) Myco-synergism boosts herbivory-induced maize defense by triggering antioxidants and phytohormone signaling. Front Plant Sci 13:790504
Bing LA, Lewis LC (1991) Suppression of Ostrinia nubilalis (Hübner) (Lepidoptera: Pyralidae) by Endophytic Beauveria bassiana (Balsamo) Vuillemin. Environ Entomol 20:1207–1211
Brodeur J, Rosenheim JA (2000) Intraguild interactions in aphid parasitoids. Entomol Exp Appl 97:93–108
Bruce TJA, Wadhams LJ, Woodcock CM (2005) Insect host location: a volatile situation. Trends Plant Sci 10:269–274
Bruck DJ (2005) Ecology of Metarhizium anisopliae in soilless potting media and the rhizosphere: implications for pest management. Biol Control 32:155–163
Bruck DJ (2010) Fungal entomopathogens in the rhizosphere. BioControl 55:103–112
Butt TM, Greenfield BPJ, Greig C, Maffeis TGG, Taylor JWD, Piasecka J, Dudley E, Abdulla A, Dubovskiy IM, Garrido-Jurado I, Quesada-Moraga E, Penny MW, Eastwood DC (2013) Metarhizium anisopliae pathogenesis of mosquito larvae: a verdict of accidental death. PLoS ONE 8(12): e81686
Castillo A, Gómez J, Infante F, Vega FE (2009) Susceptibilidad del parasitoide Phymastichus coffea LaSalle (Hymenoptera: Eulophidae) a Beauveria bassiana en condiciones de laboratorio. Neotrop Entomol 38:665–670
Contreras-Cornejo HA, Del-Val E, Macías-Rodríguez L, Alarcón A, González-Esquivel CE, Larsen J (2018) Trichoderma atroviride, a maize root associated fungus, increases the parasitism rate of the fall armyworm Spodoptera frugiperda by its natural enemy Campoletis sonorensis. Soil Biol Biochem 122:196–202
Cory JS, Ericsson JD (2010) Fungal entomopathogens in a tritrophic context. BioControl 55:75–88
Cotes B, Marie L, Maria R, Hans B, Norli R, Meyling NV, Rämert B, Anderson P (2015) Habitat selection of a parasitoid mediated by volatiles informing on host and intraguild predator densities. Oecologia 179:151–162
Cotes B, Thöming G, Amaya-Gómez CV, NováK O, Nansen C (2020) Root-associated entomopathogenic fungi manipulate host plants to attract herbivorous insects. Scien Rep 10:22424
Da Silva CCM, Marques EJ, De Oliveira JV, de Alburquerque AC (2016) Effects of entomopathogenic fungi on different developmental stages of Cotesia flavipes (Cam.) a parasitoid of Diatraea flavipennella (Box) (Lepidoptera: Crambidae). Semin Agrar 37:25–32
Davis TS, Crippen TL, Hofstetter RW, Tomberlin JK (2013) Microbial volatile emissions as insect semiochemicals. J Chem Ecol 39:840–859
Dicke M, Grostal P (2001) Chemical detection of natural enemies by arthropods: an ecological perspective. Annu Rev Ecol Syst 32:1–23
Emami F, Alichi M, Minaei K (2013) Interaction between the entomopathogenic fungus, Beauveria bassiana (Ascomycota: Hypocreales) and the parasitoid wasp, Aphidius colemani Viereck (Hymenoptera: Braconidae). J Entomol Acarol Res 45:e4
Fernández-Bravo M, Flores-León A, Calero-López S, Gutiérrez-Sánchez F, Valverde-García P, Quesada-Moraga E (2017) UV-B radiation-related effects on conidial inactivation and virulence against Ceratitis capitata (Wiedemann) (Diptera; Tephritidae) of phylloplane and soil Metarhizium sp. strains. J Invertebr Pathol 148:142–151
Flath RA, Cunningham RT, Liquido NJ, McGovern TP (1994) Alpha-ionol as attractant for trapping Bactrocera latifrons (Diptera: Tephritidae). J Econ Entomol 87:1470–1476
Furlong MJ, Pell JK (2005) Interactions between entomopathogenic fungi and arthropod natural enemies. In: Vega FE, Blackwell M (eds) Insect-fungal associations: ecology and evolution. Oxford University Press, New York, pp 51–73
Gange AC, Koricheva J, Currie AF, Jaber LR, Vidal S (2019) Meta-analysis of the role of entomopathogenic and unspecialized fungal endophytes as plant bodyguards. New Phytol 223:2002–2010
Garrido-Jurado I, Fernández-Bravo M, Campos C, Quesada-Moraga E (2015) Diversity of entomopathogenic Hypocreales in soil and phylloplanes of five Mediterranean cropping systems. J Invertebr Pathol 130:97–106
Garrido-Jurado I, Resquín-Romero G, Amarilla SP, Ríos-Moreno A, Carrasco L, Quesada-Moraga E (2017) Transient endophytic colonization of melon plants by entomopathogenic fungi after foliar application for the control of Bemisia tabaci Gennadius (Hemiptera: Aleyrodidae). J Pest Sci 90:319–330
Garrido-Jurado I, Montes-Moreno D, Sanz-Barrionuevo P, Quesada-Moraga E (2020) Delving into the causes and effects of entomopathogenic endophytic Metarhizium brunneum foliar application-related mortality in Spodoptera littoralis larvae. Insects 11:429
Gathage JW, Lagat ZO, Fiaboe KKM, Akutse KS, Ekesi S, Maniania NK (2016) Prospects of fungal endophytes in the control of Liriomyza leafminer flies in common bean Phaseolus vulgaris under field conditions. BioControl 61:741–753
González-Mas N, Cuenca-Medina M, Gutiérrez-Sánchez F, Quesada-Moraga E (2019a) Bottom-up effects of endophytic Beauveria bassiana on multitrophic interactions between the cotton aphid, Aphis gossypii, and its natural enemies in melon. J Pest Sci 92:1271–1281
González-Mas N, Sánchez-Ortiz A, Valverde-García P, Quesada-Moraga E (2019b) Effects of endophytic entomopathogenic ascomycetes on the life-history traits of Aphis gossypii Glover. Insects 10:165
González-Mas N, Gutiérrez-Sánchez F, Sánchez-Ortiz A, Grandi L, Turlings TCJ, Muñoz-Redondo JM, Moreno-Rojas JM, Quesada-Moraga E (2021a) Endophytic colonization by the entomopathogenic fungus Beauveria bassiana affects plant volatile emissions in the presence or absence of chewing and sap-sucking insects. Front Plant Sci 12:660460
González-Mas N, Valverde-García R, Gutiérrez-Sánchez F, Quesada-Moraga E (2021b) Effect of passage through the plant on virulence and endophytic behavioural adaptation in the entomopathogenic fungus Beauveria bassiana. Biol Control 160:104687
Hempel S, Stein C, Unsicker SB, Renker C, Auge H, Weisser WW, Buscot F (2009) Specific bottom-up effects of arbuscular mycorrhizal fungi across a plant-herbivore-parasitoid system. Oecologia 160:267–277
Hesketh H, Roy HE, Eilenberg J, Pell JK, Hails RS (2010) Challenges in modelling complexity of fungal entomopathogens in semi-natural populations of insects. BioControl 55:55–73
Hu G, St Leger RJ (2002) Field studies using a recombinant mycoinsecticide (Metarhizium anisopliae) reveal that it is rhizosphere competent. Appl Environ Microbiol 68:6383–6387
Ibarra-Cortés KH, González-Hernández H, Guzmán-Franco AW, Ortega-Arenas LD, Villanueva-Jiménez JA, Robles-Bermúdez A (2018) Interactions between entomopathogenic fungi and Tamarixia radiata (Hymenoptera: Eulophidae) in Diaphorina citri (Hemiptera: Liviidae) populations under laboratory conditions. J Pest Sci 91:373–384
Jaber LR, Araj SE (2018) Interactions among endophytic fungal entomopathogens (Ascomycota: Hypocreales), the green peach aphid Myzus persicae Sulzer (Homoptera: Aphididae), and the aphid endoparasitoid Aphidius colemani Viereck (Hymenoptera: Braconidae). Biol Control 116:53–61
James R, Shaffer BT, Croft B, Lighthart B (1995) Field evaluation of Beauveria bassiana: Its persistence and effects on the pea aphid and a non-target coccinellid in alfalfa. Biocontrol Sci Technol 5:425–438
Jarrahi A, Safavi SA (2016) Sublethal effects of Metarhizium anisopliae on life table parameters of Habrobracon hebetor parasitizing Helicoverpa armigera larvae at different time intervals. BioControl 61:167–175
Jensen RE, Cabral C, Enkegaard A, Steenberg T (2020) Influence of the plant interacting entomopathogenic fungus Beauveria bassiana on parasitoid host choice-behavior, development, and plant defense pathways. PLoS ONE 15(9):e0238943
Jeong JK, Kyu CK, Roberts DW (2005) Impact of the entomopathogenic fungus Verticillium lecanii on development of an aphid parasitoid, Aphidius colemani. J Invertebr Pathol 88:254–256
Kepler RM, Bruck DJ (2006) Examination of the interaction between the black vine weevil (Coleoptera: Curculionidae) and an entomopathogenic fungus reveals a new tritrophic interaction. Environ Entomol 35:1021–1029
King EG, Bell JV (1978) Interactions between a braconid, Microplitis croceipes, and a fungus, Nomuraea rileyi, in laboratory-reared bollworm larvae. J Invertebr Pathol 31:337–340
Klieber J, Reineke A (2016) The entomopathogen Beauveria bassiana has epiphytic and endophytic activity against the tomato leaf miner Tuta absoluta. J Appl Entomol 140:580–589
Kryukov VY, Kryukova NA, Tyurin MV, Yaroslavtseva ON, Glupov VV (2018) Passive vectoring of entomopathogenic fungus Beauveria bassiana among the wax moth Galleria mellonella larvae by the ectoparasitoid Habrobracon hebetor females. Insect Sci 25:643–654
Labbé RM, Gillespie DR, Cloutier C, Brodeur J (2009) Compatibility of an entomopathogenic fungus with a predator and a parasitoid in the biological control of greenhouse whitefly. Biocontrol Sci Technol 19:429–446
Lam K, Tsang M, Labrie A, Gries R, Gries G (2010) Semiochemical-mediated oviposition avoidance by female house flies, Musca domestica, on animal feces colonized with harmful fungi. J Chem Ecol 36:141–147
Landa BB, López-Díaz C, Jiménez-Fernández D, Montes-Borrego M, Muñoz-Ledesma A, Ortiz-Urquiza A, Quesada-Moraga E (2013) In-planta detection and monitorization of endophytic colonization by a Beauveria bassiana strain using a new-developed nested and quantitative PCR-based assay and confocal laser scanning microscopy. J Invertebr Pathol 114:128–138
Mannino MC, Huarte-Bonnet C, Davyt-Colo B, Pedrini N (2019) Is the insect cuticle the only entry gate for fungal infection? insights into alternative modes of action of entomopathogenic fungi. J Fungi 5:33
Martínez-Barrera OY, Toledo J, Cancino J, Liedo P, Gómez J, Valle-Mora J, Montoya P (2020) Interaction between Beauveria bassiana (Hypocreales: Cordycipitaceae) and Coptera haywardi (Hymenoptera: Diapriidae) for the Management of Anastrepha obliqua (Diptera: Tephritidae). J Insect Sci 20(2):6; 1–10
Martins ICF, Silva RJ, Alencar JRDCC, Silva KP, Cividanes FJ, Duarte RT, Agostini LT, Polanczyk RA (2014) Interactions between the entomopathogenic fungi Beauveria bassiana (Ascomycota: Hypocreales) and the aphid parasitoid Diaeretiella rapae (Hymenoptera: Braconidae) on Myzus persicae (Hemiptera: Aphididae). J Econ Entomol 107:933–938
Mburu DM, Maniania NK, Hassanali A (2013) Comparison of volatile blends and nucleotide sequences of two Beauveria bassiana isolates of different virulence and repellency towards the termite Macrotermes michealseni. J Chem Ecol 39:101–108
McKinnon AC, Glare TR, Ridgway HJ, Mendoza-Mendoza A, Holyoake A, Godsoe WK, Bufford JL (2018) Detection of the entomopathogenic fungus Beauveria bassiana in the rhizosphere of wound-stressed Zea mays plants. Front Microbiol 9:1161
Mendes R, Kruijt M, de Bruijn I, Dekkers E, van der Voort M, Schneider JH, Piceno YM, DeSantis TZ, Andersen GL, Bakker PA, Raaijmakers JM (2011) Deciphering the rhizosphere microbiome for disease-suppressive bacteria. Science 332:1097–1100
Mesquita ALM, Lacey LA (2001) Interactions among the entomopathogenic fungus, Paecilomyces fumosoroseus (Deuteromycotina: Hyphomycetes), the parasitoid, Aphelinus asychis (Hymenoptera: Aphelinidae), and their aphid host. Biol Control 22:51–59
Mesquita ALM, Lacey LA, Ceianu CS, Dabire R (1999) Predatory and parasitic activity of Aphelinus asychis (Hymenoptera: Aphelinidae) following exposure to the entomopathogenic fungus Paecilomyces fumosoroseus (Deuteromycotina: Hyphomycetes) under different humidity regimes. An Da Soc Entomológica Do Bras 28:661–673
Meyling NV, Eilenberg J (2006) Isolation and characterisation of Beauveria bassiana isolates from phylloplanes of hedgerow vegetation. Mycol Res 110:188–195
Meyling NV, Hajek AE (2010) Principles from community and metapopulation ecology: application to fungal entomopathogens. BioControl 55:39–54
Meyling NV, Pell JK (2006) Detection and avoidance of an entomopathogenic fungus by a generalist insect predator. Ecol Entomol 31:162–171
Meyling NV, Pell JK, Eilenberg J (2006) Dispersal of Beauveria bassiana by the activity of nettle insects. J Invertebr Pathol 93:121–126
Meyling NV, Thorup-Kristensen K, Eilenberg J (2011) Below- and aboveground abundance and distribution of fungal entomopathogens in experimental conventional and organic cropping systems. Biol Control 59:180–186
Miranda-Fuentes P, Quesada-Moraga E, Aldebis HK, Yousef-Naef M (2020) Compatibility between the endoparasitoid Hyposoter didymator and the entomopathogenic fungus Metarhizium brunneum: a laboratory simulation for the simultaneous use to control Spodoptera littoralis. Pest Manag Sci 76:1060–1070
Miranda-Fuentes P, Yousef-Yousef M, Valverde-García P, Rodríguez-Gómez I, Garrido-Jurado I, Quesada-Moraga E (2021) Entomopathogenic fungal endophyte-mediated tritrophic interactions between Spodoptera littoralis and its parasitoid Hyposoter didymator. J Pest Sci 94:933–945
Mohammed AA, Hatcher PE (2017) Combining entomopathogenic fungi and parasitoids to control the green peach aphid Myzus persicae. Biol Control 110:44–55
Obata T, Koh H-S, Kim M, Fukami H (1983) Constituents of planthopper attractant in rice plant. Appl Entomol Zool 18:161–169
Ocampo-Hernández JA, Tamayo-Mejía F, Tamez-Guerra P, Gao Y, Guzmán-Franco AW (2019) Different host plant species modifies the susceptibility of Bactericera cockerelli to the entomopathogenic fungus Beauveria bassiana. J Appl Entomol 143:984–991
Oreste M, Baser N, Bubici G, Tarasco E (2015) Effect of Beauveria bassiana strains on the Ceratitis capitata—Psyttalia concolor system. Bull Insectology 68:265–272
Oreste M, Bubici G, Poliseno M, Tarasco E (2016) Effects of entomopathogenic fungi on Encarsia formosa Gahan ( Hymenoptera : Aphelinidae ) activity and behavior. Biol Control 100:46–53
Ormond EL, Thomas APM, Pugh PJA, Pell JK, Roy HE (2010) A fungal pathogen in time and space: the population dynamics of Beauveria bassiana in a conifer forest. FEMS Microbiol Ecol 74:146–154
Ormond EL, Thomas APM, Pell JK, Freeman SN, Roy HE (2011) Avoidance of a generalist entomopathogenic fungus by the ladybird, Coccinella septempunctata. FEMS Microbiol Ecol 77:229–237
Pava-Ripoll M, Angelini C, Fang W, Wang S, Posada FJ, St Leger R (2011) The rhizosphere-competent entomopathogen Metarhizium anisopliae expresses a specific subset of genes in plant root exudates. Microbiology 157:47–55
Pell JK, Vandenberg JD (2002) Interactions among the aphid Diuraphis noxia, the entomopathogenic fungus Paecilomyces fumosoroseus and the Coccinellid hippodamia convergens. Biocontrol Sci Technol 12:217–224
Pingel RL, Lewis LC (1996) The fungus Beauveria bassiana (Balsamo) Vuillemin in a corn ecosystem: its effect on the insect predator Coleomegilla maculata de geer. Biol Control 6:137–141
Polanczyk RA, Pratissoli D, Dalvi LP, Grecco ED, Franco CR (2010) Effect of Beauveria bassiana (Bals.) Vuillemin and Metarhizium anisopliae (Metsch.) Sorokin on the biological parameters of Trichogramma atopovirilia Oatman & Platner (Hymenoptera: Trichogrammatidae). Cienc e Agrotecnologia 34:1412–1416
Potrich M, Alves LFA, Lozano E, Roman JC (2015) Interactions between Beauveria bassiana and Trichogramma pretiosum under laboratory conditions. Entomol Exp Appl 154:213–221
Portilla M, Snodgrass G, Luttrell R (2017) Lethal and sub-lethal effects of Beauveria bassiana (Cordycipitaceae) strain NI8 on Chrysoperla rufilabris (Neuroptera: Chrysopidae). Florida Entomol 100:627–633
Quesada-Moraga E (2020) Entomopathogenic fungi as endophytes: their broader contribution to IPM and crop production. Biocontrol Sci Technol 30:864–877
Quesada-Moraga E, López-Díaz C, Landa BB (2014) The hidden habit of the entomopathogenic fungus Beauveria bassiana: first demonstration of vertical plant transmission. PLoS ONE 9 (2): e89278
Quesada-Moraga E, Yousef M, Garrido-Jurado I (2020) Advances in the use of entomopathogenic fungi as biopesticides in suppressing crop pests. In: Birch N, Glare T (eds) Biopesticides for sustainable agriculture. Burleigh Dodds Science, Cambridge
Rännbäck LM, Cotes B, Anderson P, Rämert B, Meyling NV (2015) Mortality risk from entomopathogenic fungi affects oviposition behavior in the parasitoid wasp Trybliographa rapae. J Invertebr Pathol 124:78–86
Rashki M, Shirvani A (2013) The effect of entomopathogenic fungus, Beauveria bassiana on life table parameters and behavioural response of Aphis gossypii. Bull Insectology 66:85–91
Resquín-Romero G, Garrido-Jurado I, Delso C, Ríos-Moreno A, Quesada-Moraga E (2016) Transient endophytic colonizations of plants improve the outcome of foliar applications of mycoinsecticides against chewing insects. J Invertebr Pathol 136:23–31
Ríos-Moreno A, Quesada-Moraga E, Garrido-Jurado I (2018) Treatments with Metarhizium brunneum BIPESCO5 and EAMa 01/58-Su strains (Ascomycota: Hypocreales) are low risk for the generalist predator Chrysoperla carnea. J Pest Sci 91:385–394
Rizwan M, Atta B, Arshad M, Khan RR, Dageri A, Rizwan M, Ullah MI (2021) Nondetrimental impact of two concomitant entomopathogenic fungi on life history parameters of a generalist predator, Coccinella septempunctata (Coleoptera: Coccinellidae). Sci Rep 11:20699
Rondot Y, Reineke A (2017) Association of Beauveria bassiana with grapevine plants deters adult black vine weevils, Otiorhynchus sulcatus. Biocontrol Sci Technol 27:811–820
Rossoni C, Pereira FF, Kassab SO, Rodrigues A, Barbosa RH, Zanuncio JC (2016) Development of Eulophidae (Hymenoptera) parasitoids in Diatraea saccharalis (Lepidoptera: Crambidae) pupae exposed to entomopathogenic fungi. Can Entomol 148:716–723
Roy HE, Pell JK (2000) Interactions between entomopathogenic fungi and other natural enemies: implications for biological control. Biocontrol Sci Technol 10:737–752
Roy HE, Steinkraus DC, Eilenberg J, Hajek AE, Pell JK (2006) Bizarre interactions and endgames: entomopathogenic fungi and their arthropod hosts. Annu Rev Entomol 51:331–357
Roy HE, Brown PMJ, Rothery P, Ware RL, Majerus MEN (2008) Interactions between the fungal pathogen Beauveria bassiana and three species of coccinellid: Harmonia axyridis, Coccinella septempunctata and Adalia bipunctata. BioControl 53:265–276
Roy HE, Brodie EL, Chandler D, Goettel MS (2010) Deep space and hidden depths: understanding the evolution and ecology of fungal entomopathogens. BioControl 55:1–6
Russo ML, Scorsetti AC, Vianna MF, Allegrucci N, Ferreri NA, Cabello MN, Pelizza SA (2019) Effects of endophytic Beauveria bassiana (Ascomycota: Hypocreales) on biological, reproductive parameters and food preference of the soybean pest Helicoverpa gelotopoeon. J King Saud Univ - Sci 31:1077–1082
Saikkonen K, Lehtonen P, Helander M, Koricheva J, Faeth SH (2006) Model systems in ecology: dissecting the endophyte-grass literature. Trends Plant Sci 11:428–433
Santiago-Álvarez C, Maranha EA, Maranha E, Quesada-Moraga E (2006) Host plant influences pathogenicity of Beauveria bassiana to Bemisia tabaci and its sporulation on cadavers. BioControl 51:519–532
Shikano I, Rosa C, Tan CW, Felton GW (2017) Tritrophic interactions: microbe-mediated plant effects on insect herbivores. Annu Rev Phytopathol 55:313–331
Shrestha G, Enkegaard A, Reddy GVP, Skovgard H, Steenberg T (2017) Susceptibility of larvae and pupae of the aphid parasitoid Aphelinus abdominalis (hymenoptera: Aphelinidae) to the entomopathogenic fungus Beauveria bassiana. Ann Entomol Soc Am 110:121–127
Sigsgaard L (2005) Oviposition preference of Anthocoris nemoralis and A. nemorum (Heteroptera: Anthocoridae) on pear leaves affected by leaf damage, honeydew and prey. Biocontrol Sci Technol 15:139–151
Smith SF, Krischik VA (2000) Effects of biorational pesticides on four coccinellid species (Coleoptera: Coccinellidae) having potential as biological control agents in interiorscapes. J Econ Entomol 93:732–736
Sword GA, Tessnow A, Ek-Ramos MJ (2017) Endophytic fungi alter sucking bug responses to cotton reproductive structures. Insect Sci 24:1003–1014
Tamayo-Mejía F, Tamez-Guerra P, Guzmán-Franco AW, Gomez-Flores R (2015) Can Beauveria bassiana Bals. (Vuill) (Ascomycetes: Hypocreales) and Tamarixia triozae (Burks) (Hymenoptera: Eulophidae) be used together for improved biological control of Bactericera cockerelli (Hemiptera: Triozidae)? Biol Control 90:42–48
Tasin M, Larsson Herrera S, Knight AL, Barros-Parada W, Fuentes-Contreras E, Pertot I (2018) Volatiles of grape inoculated with microorganisms: modulation of grapevine moth oviposition and field attraction. Microb Ecol 76:751–761
Todorova SI, Côté JC, Coderre D (1996) Evaluation of the effects of two Beauveria bassiana (Balsamo) Vuillemin strains on the development of Coleomegilla maculata lengi Timberlake (Col., Coccinellidae). J Appl Entomol 120:159–163
Ullah MS, Lim UT (2017) Laboratory evaluation of the effect of Beauveria bassiana on the predatory mite Phytoseiulus persimilis (Acari: Phytoseiidae). J Invertebr Pathol 148:102–109
Vega FE (2018) The use of fungal entomopathogens as endophytes in biological control: a review. Mycologia 110:4–30
Vega FE, Meyling NV, Luangsa-ard JJ, Blackwell M (2012) Chapter 6—Fungal entomopathogens, 2nd edn. Academic Press, San Diego, pp 171–220
Vidal S, Jaber LR (2015) Entomopathogenic fungi as endophytes: plant-endophyte-herbivore interactions and prospects for use in biological control. Curr Sci 109:46–54
Wu S, Xie H, Li M, Xu X, Lei Z (2016) Highly virulent Beauveria bassiana strains against the two-spotted spider mite, Tetranychus urticae, show no pathogenicity against five phytoseiid mite species. Exp Appl Acarol 70:421–435
Wu S, Xing Z, Sun W, Xu X, Meng R, Lei Z (2018) Effects of Beauveria bassiana on predation and behavior of the predatory mite Phytoseiulus persimilis. J Invertebr Pathol 153:51–56
Wyrebek M, Huber C, Sasan RK, Bidochka MJ (2011) Three sympatrically occurring species of Metarhizium show plant rhizosphere specificity. Microbiology 157:2904–2911
Xu H, Turlings TCJ (2018) Plant volatiles as mate-finding cues for insects. Trends Plant Sci 23:100–111
Yanagawa A, Fujiwara-Tsujii N, Akino T, Yoshimura T, Yanagawa T, Shimizu S (2011) Behavioral changes in the termite, Coptotermes formosanus (Isoptera), inoculated with six fungal isolates. J Invertebr Pathol 107:100–106
Yue Q, Wang C, Gianfagna TJ, Meyer WA (2001) Volatile compounds of endophyte-free and infected tall fescue (Festuca arundinacea Schreb.). Phytochemistry 58:935–941
Zhu H, Kim JJ (2012) Target-oriented dissemination of Beauveria bassiana conidia by the predators, Harmonia axyridis (Coleoptera: Coccinellidae) and Chrysoperla carnea (Neuroptera: Chrysopidae) for BioControl of Myzus persicae. Biocontrol Sci Technol 22:393–406
Zhu F, Zhou YK, Ji ZL, Chen XR (2018) The plant ribosome-inactivating proteins play important roles in defense against pathogens and insect pest attacks. Front Plant Sci 9:146
Funding
This work was funded by the Spanish Ministry of Science and Innovation project PID2019-103844RB-I00. We sincerely thanks and tribute to the research group AGR 163 “Agricultural Entomology” of the University of Cordoba (Spain). Also, we acknowledge financial support from the Spanish Ministry of Science and Innovation, the Spanish State Research Agency, through the Severo Ochoa and María de Maeztu Program for Centers and Units of Excellence in R&D (Ref. CEX2019-000968-M).
Author information
Authors and Affiliations
Contributions
EQM conceived, wrote, and designed the review structure. NGM developed figures and table and revised the manuscript. IGJ and MY helped in the literature review and revised the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest associated with this publication.
Ethical approval
There are no ethical concerns regarding the organisms and the topic of this research.
Research involving animal rights
This article does not refer to any studies with human participants or animals (vertebrates) performed by any of the authors.
Additional information
Handling editor: Nicolai Meyling.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Quesada-Moraga, E., Garrido-Jurado, I., Yousef-Yousef, M. et al. Multitrophic interactions of entomopathogenic fungi in BioControl. BioControl 67, 457–472 (2022). https://doi.org/10.1007/s10526-022-10163-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-022-10163-5