Abstract
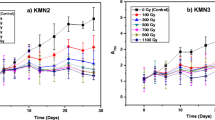
Microalgae are a promising alternative source of lipid for biodiesel production. One of the most important decisions is biomass productivity and the lipid content of microalgae. However, few studies have investigated the correlation between low-dose-rate (LDR) chronic irradiation and lipid production using microalgae to date. The present study was undertaken to investigate whether the LDR chronic irradiation increases cell growth and lipid content in Tetraselmis suecica, Dunaliella tertiolecta, Phaeodactylum tricornutum and Nannochloropsis oceanic. Exposure to LDR chronic irradiation increased cell density, specific growth rate and biomass in four microalgae strains. Furthermore, T. suecica, D. tertiolecta and P. tricornutum enhanced the lipid-specific BODIPY fluorescence intensity dependent on low-dose-rate irradiation. In particular, T. suecica showed the highest increase ratio of biomass and lipid content in 6 mGy h−1. These results suggest that LDR chronic irradiation induces enhancement of biomass and lipid content of marine microalgae.





Similar content being viewed by others
References
Becker EW (1994) Microalgae: biotechnology and microbiology. Cambridge University Press, New York
Bigogno C, Khozin-Goldberg I, Boussiba S, Vonshak A, Cohen Z (2002) Lipid and fatty acid composition of the green oleaginous alga Parietochloris incisa, the richest plant source of arachidonic acid. Phytochemistry 60(5):497–503
Carioca JO (2010) Biofuels: problems, challenges and perspectives. Biotechnol J 5(3):260–273
Cheng J, Feng J, Sun J, Huang Y, Zhou J, Cen K (2014) Enhancing the lipid content of the diatom Nitzschia sp. by 60Co-γ irradiation mutation and high-salinity domestication. Energy 78(15):9–15
Chisti Y (2008) Biodiesel from microalgae beats bioethanol. Trends Biotechnol 26(3):126–131
Chung BY, Lee YB, Baek MH, Kim JH, Wi SG, Kima JS (2006) Effects of low-dose gamma-irradiation on production of shikonin derivatives in callus cultures of Lithospermum erythrohizon S. Radiat Phys Chem 75(9):1018–1023
Conter A, Dupouy D, Planel H (1983) Demonstration of a biological effect of natural ionizing radiations. Radiat Biol Relat Stud Phys Chem Med 43(4):421–432
Conter A, Dupouy D, Planel H (1984) Influence of growth phase on radiation stimulation of proliferation in Synechococcus lividus in culture. Radiat Res 99(3):651–658
Conter A, Dupouy D, Delteil C, Planel H (1986) Influence of very low doses of ionizing radiation on Synechococcus lividus metabolism during the initial growth phase. Arch Microbiol 144(3):286–290
Cooper MS, Hardin WR, Petersen TW, Cattolico RA (2010) Visualizing” green oil” in live algal cells. J Biosci Bioeng 109(2):198–201
Croute F, Soleilhavoup J, Vidal S, Dupouy D, Planel H (1982) Paramecium tetraurelia growth stimulation under low-level chronic irradiation: investigations on a possible mechanism. Radiat Res 92(3):560–567
Elle IC, Olsen LCB, Pultz D, Rødkær SV, Færgeman NJ (2010) Something worth dyeing for: molecular tools for the dissection of lipid metabolism in Caenorhabditis elegans. FEBS Lett 584(11):2183–2193
Elsey D, Jameson D, Raleigh B, Cooney MJ (2007) Fluorescent measurement of microalgal neutral lipids. J Microbiol Methods 68(3):639–642
Esnault MA, Legue F, Chenal C (2010) Ionizing radiation: advances in plant response. Environ Exp Bot 68:231–237
Feng J, Cheng J, Cheng R, Zhang C, Zhou J, Cen K (2015) Screening the diatom Nitzschia sp. re-mutated by 137Cs-γ irradiation and optimizing growth conditions to increase lipid productivity. J Appl Phycol 27:661–672
Folch J, Lees M, Stanley GHS (1957) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226:497–509
Gordillo FJ, Goutx M, Figueroa FL, Niell FX (1998) Effects of light intensity, CO2 and nitrogen supply on lipid class composition of Dunaliella viridis. J Appl Phycol 10(2):135–144
Guzmán HM, de la Jara Valido A, Duarte LC, Presmanes KF (2010) Estimate by means of flow cytometry of variation in composition of fatty acids from Tetraselmis suecica in response to culture conditions. Aquacult Int 18(2):189–199
Hu Q, Sommerfeld M, Jarvis E, Ghirardi M, Posewitz M, Seibert M, Darzins A (2008) Microalgal triacylglycerols as feedstocks for biofuel production: perspectives and advances. Plant J 54(4):621–639
Katarzyna C, Facundo-Joaquin MR (2004) Kinetic and stoichiometric relationships of the energy and carbon metabolism in the culture of microalgae. Biotechnol 3(1):21–34
Khotimchenko SV, Yakovleva IM (2005) Lipid composition of the red alga Tichocarpus crinitus exposed to different levels of photon irradiance. Phytochemistry 66(1):73–79
Kim JH, Chung BY, Kim JS, Wi SG (2005) Effects of in Planta gamma-irradiation on growth, photosynthesis, and antioxidative capacity of red pepper (Capsicum annuum L.) plants. J Plant Biol 48(1):47–56
Kim JS, Son Y, Bae MJ, Lee SS, Park SH, Lee HJ, Lee SI, CG, Kim SD, Jo WS (2015) Continuous exposure to low-dose-rate gamma irradiation reduces airway inflammation in ovalbumin-induced asthma. PLoS ONE 10(11):e0143403
Kume T, Furuta M, Todoriki S, Uenoyama N, Kobayashi Y (2009) Status of food irradiation in the world. Radiat Phys Chem 78(3):222–226
Li Y, Qin JG (2005) Comparison of growth and lipid content in three Botryococcus braunii strains. J Appl Phycol 17(6):551–556
Li Y, Horsman M, Wang B, Wu N, Lan CQ (2008) Effects of nitrogen sources on cell growth and lipid accumulation of green alga Neochloris oleoabundans. Appl Microbiol Biotechnol 81(4):629–636
Liu ZY, Wang GC, Zhou BC (2008) Effect of iron on growth and lipid accumulation in Chlorella vulgaris. Bioresour Technol 99(11):4717–4722
Luckey TD, Lawrence KS (2006) Radiation Hormesis: the Good, the Bad, and the Ugly. Dose Response 4(3):169–190
Mata TM, Martins AA, Caetano NS (2010) Microalgae for biodiesel production and other applications: a review. Renew. Sustainable Energy Rev 14(1):217–232
Mutanda T, Ramesh D, Karthikeyan S, Kumari S, Anandraj A, Bux F (2011) Bioprospecting for hyper-lipid producing microalgal strains for sustainable biofuel production. Bioresour Technol 102(1):57–70
Renaud SM, Thinh LV, Parry DL (1999) The gross chemical composition and fatty acid composition of 18 species of tropical Australian microalgae for possible use in mariculture. Aquaculture 170:147–159
Renaud SM, Thinh L-V, Lambrinidis G, Parry DL (2002) Effect of temperature on growth, chemical composition and fatty acid composition of tropical Australian microalgae grown in batch cultures. Aquaculture 211(1):195–214
Rodolfi L, Chini Zittelli G, Bassi N, Padovani G, Biondi N, Bonini G, Tredici MR (2009) Microalgae for oil: strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotechnol Bioeng 102(1):100–112
Sheehan J, Dunahay T, Benemann J, Roessler P (1998) A look back at the US Department of Energy’s aquatic species program: biodiesel from algae. National Renewable Energy Lab, Department of Energy, Golden, Colorado, U.S.A. Report number NREL/TP-580-24190
Singh S, Kate BN, Banerjee UC (2005) Bioactive compounds from cyanobacteria and microalgae: an overview. Rev Biotechnol 25(3):73–95
Singh A, Nigam PS, Murphy JD (2011) Renewable fuels from algae: an answer to debatable land based fuels. Bioresour Technol 102(1):10–16
Takagi M, Yoshida T (2006) Effect of salt concentration on intracellular accumulation of lipids and triacylglyceride in marine microalgae Dunaliella cells. J Biosci Bioeng 101(3):223–226
Takagi M, Watanabe K, Yamaberi K, Yoshida T (2000) Limited feeding of potassium nitrate for intracellular lipid and triglyceride accumulation of Nannochloris sp. UTEX LB1999. Appl Microbiol Biotechnol 54(1):112–117
Tornabene T, Holzer G, Lien S, Burris N (1983) Lipid composition of the nitrogen starved green alga Neochloris oleoabundans. Enzyme Microb Technol 5:435–440
UNSCEAR (2000) The United Nations Scientific Committee on the effects of atomic radiation. Health Phys 79:314
Williams PJLB (2007) Biofuel: microalgae cut the social and ecological costs. Nature 450(7169):478
Wood AM, Everroad R, Wingard L (2005) Measuring growth rates in microalgal cultures. Algal culturing techniques. Elsevier Academic Press, San Diego, pp 269–285
Wu X, Ruan R, Du Z, Liu Y (2012) Current status and prospects of biodiesel production from microalgae energies 5:2667–2682
Xiong W, Li X, Xiang J, Wu Q (2008) High-density fermentation of microalga Chlorella protothecoides in bioreactor for microbio-diesel production. Appl Microbiol Biotechnol 78(1):29–36
Yamaguchi I (2001) Forty years of mutation breeding in Japan. Research and fruits. Gamma. Field Symp 40:1–14
Acknowledgments
This work was supported by Nuclear R&D Program of the Ministry of Education, Science and Technology, Korea [50493-2011].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Additional information
Dong Hyeok Jeong and Min Ho Jeong have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Jeong, D.H., Jeong, M.H., Jeong, S.K. et al. Effect of continuous exposure to low-dose-rate gamma irradiation on cell growth and lipid accumulation of marine microalgae. Aquacult Int 25, 589–601 (2017). https://doi.org/10.1007/s10499-016-0054-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-016-0054-5