Abstract
The process-based evaluation of adsorbents is considered the most foolproof method with respect to a particular application, as it provides data about the separation effectiveness in authentic operating conditions. This paper presents empirically obtained performance results of the kinetically-controlled air separation on multiple carbon molecular sieves carried out in a twin-bed pressure swing adsorption unit. The effect of adsorbent pellet size on nitrogen productivity and air demand is studied at different product purity levels (10–10,000 ppm O2 of the residual oxygen concentration), operating temperatures (25–45 °C), and half-cycle times (35–70 s). The selected process conditions correspond to the majority of practical applications. Guidelines for the suitable particle size depending on the desired nitrogen purity are given.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Even though modern instrumental analytics allow advanced methods of adsorbent characterisation, the proper selection of adsorbents for a particular industrial application remains a challenge [1,2,3]. An accurate prediction of the process performance exclusively based on standard material efficiency data, derived from single-component isotherms or breakthrough curves, often fails due to the employment of simplified mathematical models and/or inauthentic experimental conditions at the laboratory scale [4, 5]. For this reason, the process-based evaluation of adsorbents continues to provide the most reliable information for the industrial sector.
The design of separation processes carried out in pressure swing adsorption (PSA) plants requires not only information about capacity, selectivity, regenerability, kinetics, or costs of the adsorbent itself, but also about other factors which influence the overall system performance, i.a. the size of adsorbent particle, the pressure drop along the packed-bed as well as along the piping system, or the cycle organisation strategy [6, 7]. Therefore, after a preliminary selection of a particular adsorbent based on its standard properties, pilot-plant tests at conditions corresponding to the industrial application followed by process simulations is a usual practice. Especially with respect to kinetically-controlled separations conducted on carbon molecular sieves (CMS) it is impossible to accurately predict the dynamic behaviour of the adsorber without an experimental verification of the complete PSA system [8].
This paper presents the evaluation of different CMS grades for the production of high-purity nitrogen performed in a twin-bed PSA plant. As demonstrated in Fig. 1a, the effective separation of air is not accomplished by means of the equilibrium effect, as the difference in oxygen and nitrogen isotherms on CMS is not large enough. Therefore, as shown in Fig. 1b, a highly selective kinetic separation of air is realised due to the significantly faster diffusion of oxygen over nitrogen through intentionally narrowed micropore mouths of the adsorbent [9]. Moreover, as presented in Fig. 2, the equilibrium adsorbent loading is reached quicker in the system at elevated pressure and temperature conditions, which demonstrates the surface diffusion mechanism in microporous adsorbents exhibiting a strong influence of surface concentration on mass transfer rate as an effect of isotherm nonlinearity [9]. Bea and Lee employed the supercritical-structural-Langmuir model to study the effect of pressure and temperature on the sorption rate of individual components in CMS material [10]. It was found that the increase of temperature from 20 to 40 °C results in 41 % and 92 % enlargemnt of the apparent time constant for oxygen and nitrogen adsorption, respectively. Whereas it is anticipated that various commercial CMS grades would exhibit comparable uptake tendencies, the distinct mass transfer rates of gas mixture components can differ significantly depending on the applied material manufacturing strategy as well as experimental conditions [11].
(a) Equilibrium isotherms of oxygen and nitrogen on CMS; (b) Fractional uptake rates of oxygen and nitrogen in CMS [14]
Kinetic curves of relative uptake calculated from adsorption isotherm data of synthetic air (20 vol% O2 + 80 vol% N2) on Shirasagi MSC CT-350: (a) at 0.92 bar abs, (b) at 7.94 bar abs [15]
The N2-PSA technology is commercially established at product flow rates up to several thousand m3n/h (at 0 °C, 1 bar abs) and purity levels up to 10 ppm of the residual oxygen concentration. Due to multiple process variables and cycle design solutions, the process performance can be optimised according to specific requirements of customers. One parameter playing a vital role regarding the plant optimisation is the adsorbent particle diameter, however receiving less attention so far. Specifically, the selection of adsorbent particle size influences the separation effectiveness, as it affects (1) the pressure drop along the adsorber, (2) the interstitial gas velocity, (3) the progression of mass axial dispersion, as well as (4) the overall mass transfer efficiency owing to an altered packed-bed porosity [12, 13]. Thus, the effect of CMS pellet diameter on process performance of high-purity N2-PSA at different operating temperatures and cycle times is experimentally studied in a product purity range of 10–10,000 ppm O2 of the residual oxygen concentration. Additionally, recommendations of the adequate adsorbent particle size depending on the desired product purity level are given.
2 Experimental
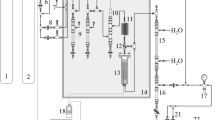
Experimental data are collected from a 2 × 2 L twin-bed PSA pilot plant, which scheme is presented in Fig. 3. The system structure resembles industrial-scale units. The plant is fed with dried ambient air, having a composition of about 78.1 vol.% of N2, 20.9 vol.% of O2, and 0.9 vol.% of Ar, as well as traces of other components present in the atmosphere. In each experiment, the inlet air pressure is carefully regulated, thereby the adsorption process is performed invariably at a working pressure of 8 bar abs. To control the product purity level, the product stream flow rate is adjusted. This control strategy represents the common operation mode of commercial plants. In the N2-PSA technology it is accepted that the product purity comprises the content of both nitrogen and argon since many industrial applications do not require an additional separation of inert gas mixtures. Consequently, the determination of the product purity is performed by assuming a binary gas mixture, where the difference to the oxygen concentration results in the nitrogen purity. Details of the unit, adsorber columns, and operating conditions are shown in Table 1.
As presented in Fig. 4, the process comprises a six-step PSA cycle, which consists of (1) co- and counter-current bed pressure equalisation; (2) co-current pressurisation by feed with (3) counter-current backflow of the product; (4) production; (5) counter-current blow-down; and (6) counter-current purge by the product gas. The cycle corresponds to the common operation regime of commercial PSA units. The flow rate of the counter-current purge equals in every experiment 40 % of the adsorber volume within the selected purge time. Details of the process organisation are described elsewhere [16]. In this work, the cycle strategy was unaltered during the entire experimental program. The PSA performance indicators, productivity [m3n/h N2 / m3 CMS] and air demand [m3n/h air / m3n/h N2], are defined, determined, and verified according to the strategy described elsewhere [16]. Results are collected at cycle-steady-state. The detailed analysis of measurement accuracy is presented elsewhere [16]. Alternatively, the productivity values can also be presented in reference to the adsorbent mass for a quick evaluation of investment costs. In practice, however, as the column volume represents a limiting factor for packing, the concern of obtained product flow rate with respect to the CMS bed volume proves to be a more realistic approach.
(a) Scheme of the PSA cycle design, here the light-grey bars indicate that the step duration depends on the existing pressure difference among absorbers and buffer vessels/ambient; (b) Valves operation scheme, here blue colour indicates that the valve is opened; (c) Graphical representation of streams during the PSA cycle
Four commercially available CMS grades of different origins are exploited to pack adsorbers by the snowstorm filling method. All utilised adsorbents are cylindrically shaped. The diameter of a single cylindrical pellet and obtained packed-bed density are shown in Table 2. The utilised CMS pellets are anonymised and named as CMS-A, CMS-B, CMS-C, and CMS-D, according to their growing particle size from 1.0 to 1.8 mm. The packed-bed density is in the range of 650–700 g/L. Any significant dissimilarities among packed-bed densities indicate (1) a unique structure of random particle packings, (2) a distinct pore volume and/or pore size distribution of CMS, and/or (3) alternative raw materials utilised for adsorbents production.
3 Results and discussion
3.1 Effect of the CMS pellet size at different half-cycle times
The optimisation of the cycle time is one of the most common methods of PSA performance intensification, i.e. increasing the product purity at a given productivity level or increasing the productivity at a given purity, and/or reducing the feed demand or improving the recovery. In industrial practice, short cycle times are favoured in order to increase the number of production cycles per operation time of the plant for boosting-up the nitrogen productivity. However, performing high-purity nitrogen generation at too short cycle times might be associated with limited fixed-bed utilisation and overconsumption of compressed air [7]. Therefore, the selection of the most suitable PSA cycle time for particular product requirements and operating conditions depends on the shape and the progress of the mass transfer zone (MTZ) along the adsorber, which characteristics are directly related to the adsorbent type and size. For that background, the influence of CMS pellet diameter on PSA performance at different half-cycle times is investigated in the range of 35–70 s, which corresponds to the majority of practical applications of N2-PSA systems. Results are presented in Figs. 5 and 6. Explicit data of experimental flow rates of PSA streams are reported in Tables 3 and 4.
Independent of the product purity level, Fig. 5 demonstrates that at an operating temperature of 25 °C a higher productivity is achieved when smaller CMS pellets are utilised. At low product purity levels (≥ 1000 ppm O2), all exploited adsorbents exhibit increased productivity values at reduced cycle times. This tactic prevents a premature column breakthrough caused by elongated mass transfer zone promoted by a relatively large gas superficial velocity and therefore a short contact time of phases. On the other hand, as the required product purity increases (≤ 100 ppm O2), the adjustment of short PSA cycles might lead to reduced productivity when large pellets are employed. This finding confirms that short cycles provide an insufficient adsorption/desorption time to encounter oxygen diffusion limitations. Thus, a slight extension of gas residence time in the adsorber column packed with relatively large CMS pellets is favoured. However, a further increase of the cycle time is not beneficial either as the system tends to approach a thermodynamic equilibrium at a relatively small gas superficial velocity, magnifying the adsorption of nitrogen and reducing the separation selectivity.
The air demand remains rather insensitive to the change of adsorbent size at low product purity levels (≥ 1000 ppm O2), whereas significant energy savings are feasible by applying small CMS particles at high product purities (≤ 100 ppm O2). The employment of CMS-B, CMS-C, and CMS-D results in lower air demand values at prolonged PSA cycles at all investigated product purity levels, pointing out enhanced bed utilisation as the self-sharpening MTZ progresses further along the adsorber. As the required product purity increases, the significant overconsumption of compressed air at short PSA cycles clearly demonstrates the downgraded adsorber recovery. In such a case, the oxygen MTZ is situated mostly in the bottom part of the packed-bed, whereas the top part is occupied almost exclusively by purified nitrogen. Therefore, masses of the product are vented out together with the tail-gas stream during the adsorber regeneration, which effect magnifies as larger pellets are utilised due to further reduced productivity. On the other hand, employment of CMS-A results in low air demand values at shortened PSA cycles at all investigated product purity levels. That distinctive outcome, together with notably higher productivity slopes in the function of the PSA half-cycle, indicates a significantly improved separation effectiveness of CMS-A in relation to other investigated adsorbents at 25 °C. An enhanced nitrogen recovery at short cycles seems to be attainable due to accelerated mass transfer of oxygen in CMS-A pellet during adsorption/desorption, while the rate of nitrogen transport remains marginal. Therefore, a proper regeneration is achieved at a short blow-down time, and a further increase of the desorption duration causes a slight product loss, most likely due to nitrogen evacuation from interparticle voids and/or ongoing purge flow. Additionally, in case the selectivity of the adsorbent is not satisfactory, the prolonged desorption time also favours nitrogen desorption, causing further product dissipation.
In contrast, Fig. 6 displays productivity and air demand results at an operating temperature level of 45 °C. Independent of the product purity level, Fig. 6 shows that all investigated CMS grades exhibit the highest productivity values at short PSA cycles. It confirms that a shorter gas residence time in the column prevents a premature adsorber breakthrough caused by extended MTZ due to a significantly reduced adsorption capacity and an accelerated nitrogen diffusivity at high temperatures [17]. At low product purity levels (> 1000 ppm O2), all tested adsorbents also allow the lowest air demand values at short PSA cycles. Since an increased system temperature favours the desorption process, a short blow-down step provides an adequate amount of time for a suitable packed-bed regeneration. Therefore, prolonged cycles result in extended nitrogen evacuation from the system, diminishing the recovery. The same behaviour is detected at higher product purities when CMS-A and CMS-D are employed. However, intermediate half-cycle time of 45 s is preferred to achieve the lowest air demand by utilising CMS-B and CMS-C. That finding suggests that at short cycles the amount of compressed air devoted to bringing the adsorber column up to the operating pressure level becomes dominant over the amount used for high-pressure nitrogen production. The adjustment of short half-cycle times makes the pressurisation step more significant in relation to the production step, which effect magnifies at lower product flow rates.
Independently from the required product purity level or the selected cycle time, CMS-B exhibits the highest productivity and the lowest air demand among other examined adsorbents at 45 °C. Thus, an intermediate pellet size becomes preferable as the PSA operating temperature elevates, pointing out its superior selectivity and/or regenerability at applied process conditions. However, taking the significantly higher productivity and air demand slopes in the function of the half-cycle of CMS-A in relation to other pellets into account, the operation of the PSA equipped with CMS-A at short cycles becomes advantageous as the required product purity increases. The poorest process performance is achieved by utilisation of CMS-D. As presented in Fig. 6d, the separation effectiveness of CMS-D does not allow nitrogen generation at a product purity level of 10 ppm O2 as a result of its reduced adsorption capacity at high operating temperature, thus limited mass transfer rate.
3.2 Effect of the CMS pellet size at different operating temperatures
N2-PSA systems are commonly operated in various climate zones. The ambient temperature of the unit affects the adsorption capacity as well as the selectivity of adsorbents in kinetically-controlled separations, as seen in the graphs before. Hence, the influence of the CMS pellet diameters on the PSA performance at different operating temperatures is investigated in more details in the range of 25–45 °C, which corresponds to the majority of practical applications of N2-PSA systems. Results are presented in Figs. 7 and 8. Additional experimental flow rates of streams are reported in Table 5 to expand the data presented in Tables 3 and 4.
Mostly at all investigated product purity levels, Fig. 7 demonstrates that at the half-cycle time of 45 s the investigated CMS grades exhibit the highest productivity numbers at a moderate ambient temperature of 25 °C, indicating their elevated adsorption capacity at low operating temperatures. The lowest air demand is also achieved at ambient temperature of 25 °C, representing an improved nitrogen recovery. The substantial overconsumption of compressed air at high PSA operating temperatures and high product purity levels demonstrates clearly that the very narrow oxygen MTZ is situated mostly at the bottom of the packed-bed, which causes a waste of purified nitrogen during the counter-current depressurisation. The smallest investigated particle CMS-A proved to provide the highest PSA performance at 25 °C among other investigated CMS grades, as it seems to exhibit a relatively high adsorption capacity and/or improved rate of mass transfer, resulting in the uppermost separation selectivity at applied operating conditions. The similar outcome is noticed at the half-cycle time of 60 s, being presented in Fig. 8.
As the PSA ambient temperature increases, CMS-B provides a superior PSA performance. Small adsorbent particles exhibit a larger volumetric heat-transfer coefficient in relation to large pellets [18], in addition to a generally magnified heat transfer between phases due to their enlarged external surface area [19]. Those effects might be a cause of the more extensive rise of the local gas temperature during the adsorption exhibited by small CMS with reference to large CMS, leading to a reduction in adsorption capacity and selectivity within the production step. Consequently, to maintain the target product purity level, CMS-A results in lower productivity values in relation to CMS-B at 45 °C. Whereas gas-solid heat transfer coefficients become larger at high operating temperatures and high gas superficial velocities [20], the separation effectiveness of CMS-C approaches CMS-B at prolonged PSA cycles, as shown in Fig. 8a. In this case, heat effects are magnified due to extended gas residence time in the adsorber and expanded oxygen MTZ. Consequently, as required product purity decreases, medium-large CMS becomes preferable at prolonged PSA cycles. On the other hand, medium-small particles become advantageous at shortened PSA cycles as the demanded product purity increases. Additionally, as manifested in Figs. 7d and 8d, the separation effectiveness of CMS-D at 45 °C is not yet sufficient to generate product at the purity level of 10 ppm O2, as the adsorption capacity is overly low at applied process conditions.
4 Conclusions
Various commercial CMS grades of different pellet size were exploited to experimentally determine the process performance of high-purity twin-bed N2-PSA plant at different product purity levels, operating temperatures, and half-cycle times. Tables 6 and 7 summarises the obtained productivity and air demand data, expressed as a relative change with respect to the reference pellet size. The following conclusions can be stated:
-
(1)
Independent of system operating conditions or applied cycle organisation strategies, the major effect on the PSA performance, i.e. productivity and air demand, originates in the desired nitrogen purity level.
-
(2)
Independent of the operating temperature or half-cycle time, the effect of CMS diameter on PSA performance becomes more significant as the required product purity increases. The finding demonstrates that at low gas superficial velocities, resulting in a long exposure time of gas to the adsorbent, the shape of oxygen MTZ becomes more sensitive to the overall separation selectivity of the material. Consequently, a prominent difference between mass transfer rates of considered components is crucial, which remains a function of equilibrium loadings at specific partial pressures and diffusion coefficients [8].
-
(3)
Independent of the required product purity level or half-cycle time, the selection of small CMS pellets is recommended when the PSA plant operates at moderate ambient temperatures of about 25 °C.
-
(4)
Independent of required product purity level or half-cycle time, the selection of intermediate CMS pellets is recommended when the PSA plant operates at high ambient temperatures of about 45 °C.
-
(5)
Since the reduction of particle size elevates the pressure drop along the packed-bed, the utilisation of small CMS pellets is associated with (1) an inhibited speed of pressurisation/depressurisation within the adsorber, as shown in Fig. 9, especially as the magnitude of the PSA unit approaches the industrial-scale, as well as (2) more pronounced pressure gradients along the packed-bed resulting in larger gas pressure at the bottom-section in relation to the top-section of the column. On one hand, those effects might lead to the unavoidable enlargement of inlet gas pressure for the purpose of efficient utilisation of the entire packed-bed, which would result in higher operating costs. Additionally, an insufficient adsorber regeneration during the counter-current blow-down step could occur, causing the overall process performance to drop. On the other hand, the increased gas pressure in the adsorber bottom-section could result in superior separation performance in terms of adsorption capacity and mass transfer rate, unless the heat release concerning adsorption enthalpy as well as gas compression becomes a limiting factor. Moreover, prolonged contract time between phases due to slower adsorber pressurisation during the production step can significantly increase the heavy component uptake, resulting in steeper MTZ and more efficient bed utilisation. Nevertheless, looking at industrial practice, medium pellet diameters exhibit an acceptable pressure drop, delivering the most advantageous separation performance. Therefore, the PSA process can be economically realised on those materials even in adsorbers having a height of a few meters.
-
(6)
In practice, the abrasion of the adsorbent becomes a parameter of high relevance. The mechanical resistance of pellets reduces drastically as their diameter becomes smaller. According to industrial experience, the life span of small CMS grades reaches about 2 years, whereas the medium/big grades can be utilised for nearly 15 years. Moreover, the limited durability of small pellets often leads to the fracture of beads at multiple adsorbent manufacturing stages. Therefore, as presented in Table 8, the smaller the pellet diameter the wider the particle size distribution, i.e. the fraction below the nominal pellet diameter becomes more prominent. Consequently, the adsorber filled with pellets of smaller diameter could become more non-uniformly packed in relation to a column filled with bigger pellets; thus, a denser packing would transpire as fractured particles fill voids between the intact ones [21]. This effect could also generally support the finding that elevated PSA performance is achieved when smallest pellets are utilised, being an outcome of a lower packed-bed porosity [22].
-
(7)
As samples utilised in this work are commercially available CMS adsorbents, it is expected that their basic material properties, i.a. pore size distribution or volume of micro- and macropores, might not be comparable due to almost certainly very distinct production strategies at multiple stages of the complex manufacturing process. For this reason, a systematic investigation of the PSA performance using commercial materials remains a challenge, as the observed effects always originate in (1) adsorbent properties, (2) cycle organisation, and (3) plant construction [8], which all depend on numerous parameters. On the top of that, it is expected that the optimal material-cycle-setup configuration exist.
References
Wiersum, A.D., Chang, J.-S., Serre, C., Llewellyn, P.L.: An adsorbent performance indicator as a first step evaluation of novel sorbents for gas separations: Application to metal-organic frameworks. Langmuir the ACS Journal of Surfaces and Colloids. 29, 3301–3309 (2013)
Burhan, M., Akhtar, F.H., Chen, Q., Shahzad, M.W., Ybyraiymkul, D., Ng, K.C.: A Universal Mathematical Methodology in characterization of materials for tailored design of porous surfaces. Front. Chem. 8, 601132, 1–11 (2021)
Park, J.-H., Yang, R.T.: Simple Criterion for Adsorbent Selection for Gas purification by pressure swing adsorption processes. Ind. Eng. Chem. Res. 44, 1914–1921 (2005)
Monpezat, A., Topin, S., Deliere, L., Farrusseng, D., Coasne, B.: Evaluation methods of adsorbents for air purification and gas separation at low concentration: Case Studies on Xenon and Krypton. Ind. Eng. Chem. Res. 58, 4560–4571 (2019)
Ackley, M.W., Rege, S.U., Saxena, H.: Application of natural zeolites in the purification and separation of gases. Microporous Mesoporous Mater. 61, 25–42 (2003)
K. S. Knaebel, Adsorbent selection, (2002)
Marcinek, A., Guderian, J., Bathen, D.: Process intensification of the high-purity nitrogen production in twin-bed pressure swing adsorption plants. Adsorption. 27, 937–952 (2021)
Marcinek, A., Möller, A., Guderian, J., Bathen, D.: Dynamic simulation of high-purity twin-bed N2-PSA plants. Adsorption. 27, 1149–1173 (2021)
Reid, C.R., Thomas, K.M.: Adsorption of gases on a Carbon Molecular Sieve used for air separation: Linear Adsorptives as Probes for Kinetic selectivity. Langmuir. 15, 3206–3218 (1999)
Bae, Y.-S., Lee, C.-H.: Sorption kinetics of eight gases on a carbon molecular sieve at elevated pressure. Carbon. 43, 95–107 (2005)
Chen, Y.D., Yang, R.T., Uawithya, P.: Diffusion of oxygen, nitrogen and their mixtures in carbon molecular sieve. AIChE J. 40, 577–585 (1994)
Papurello, D., Gandiglio, M., Lanzini, A.: Experimental analysis and model validation on the performance of impregnated activated Carbons for the removal of Hydrogen Sulfide (H2S) from Sewage Biogas. Processes. 7, 548, 1–26 (2019)
Jain, S., Moharir, A.S., Li, P., Wozny, G.: Heuristic design of pressure swing adsorption: A preliminary study. Sep. Purif. Technol. 33, 25–43 (2003)
Thomas, W.J., Crittenden, B.: Adsorption Technology & Design, (1998)
Marcinek, A.: Modelling and Simulation of Twin-Bed Pressure Swing Adsorption Plants for the Generation of High-Purity Nitrogen: (2021). https://doi.org/10.17185/duepublico/74937, PhD Dissertation,
Marcinek, A., Guderian, J., Bathen, D.: Performance determination of high-purity N2-PSA-plants. Adsorption. 26, 1215–1226 (2020)
Möller, A., Guderian, J., Möllmer, J., Lange, M., Hofmann, J., Gläser, R.: Kinetische Untersuchungen der adsorptiven Luftzerlegung an Kohlenstoffmolekularsieben. Chem. Ing. Tech. 85, 1680–1685 (2013)
Gouvalias, G.S., Markatos, N.C.: Mathematical modeling of heat and mass transfer in packed-bed adsorbers/regenerators. AIChE J. 39, 1799–1809 (1993)
Mitra, S., Muttakin, M., Thu, K., Saha, B.B.: Study on the influence of adsorbent particle size and heat exchanger aspect ratio on dynamic adsorption characteristics. Appl. Therm. Eng. 133, 764–773 (2018)
Singhal, A., Cloete, S., Radl, S., Quinta-Ferreira, R., Amini, S.: Heat transfer to a gas from densely packed beds of monodisperse spherical particles. Chem. Eng. J. 314, 27–37 (2017)
Mota, M., Teixeira, J.A., Yelshin, A.: Image analysis of packed beds of spherical particles of different sizes. Sep. Purif. Technol. 15, 59–68 (1999)
Marcinek, A., Bárcia, P., Guderian, J.: Scale-up analysis of a twin-bed PSA pilot plant. Adsorption. 29, 125–139 (2023)
Acknowledgements
The authors thank teams of production and technical applications of CarboTech AC GmbH, Essen, Germany, especially Mr Robert Elpers and Mr Carsten Schledorn, for fruitful discussions and valuable remarks concerning manufacturing strategies of activated carbons.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors certify that there is no actual or potential conflict of interest in relation to this article. JG recurrently consults CarboTech AC GmbH in Germany, which is a company acting – inter alia – as a distributor of CMS materials. AM joins the company with effect of 2023.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Marcinek, A., Guderian, J. Process-based evaluation of adsorbents: effect of CMS pellet size on N2-PSA performance. Adsorption 29, 363–375 (2023). https://doi.org/10.1007/s10450-023-00410-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10450-023-00410-1