Abstract
Bark beetles (Curculionidae: Scolytinae) spend most of their life in tissues of host plants, with several species representing economically relevant pests. Their behaviour is largely guided by complex olfactory cues. The compound verbenone was discovered early in the history of bark beetle pheromone research and is now sometimes referred to as a ‘universal bark beetle repellent’. However, some studies aiming to protect trees with verbenone have failed. In fact, most research effort has gone into applied studies, leaving many questions regarding the ecological functions of verbenone for various species unanswered. Here, we review and analyse the scientific literature from more than 50 years. Behavioural responses to verbenone are common among pest bark beetles (< 1% of scolytine species studied so far). Indeed, attraction is inhibited in 38 species from 16 genera, while some secondary species are unaffected or even attracted to verbenone. It is not clear whether the beetles can control the biosynthesis of verbenone; its release may not be an active signal by the beetles, but a passive cue resulting from microorganisms during host colonisation. In this context, we advocate to recognise a bark beetle and its microbiome as an entity (‘holobiont’), to better understand temporal release patterns and deduce the specific function of verbenone for a given species. Surprisingly, natural enemies are not commonly attracted by verbenone, but more taxa need to be studied. A better understanding of the ecological functions of verbenone will help to make verbenone-based tools more effective and improve integrated pest management strategies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Key message
-
Verbenone is long known and now sometimes referred to as a ‘universal bark beetle repellent’.
-
Research focus has mostly gone into applied studies, leaving many ecological questions unanswered.
-
Here, we review the scientific literature from more than 50 years to interpret present knowledge.
-
The function of verbenone as an active signal or passive cue may vary across bark beetle species.
-
Filling identified gaps of knowledge will improve verbenone-based pest management approaches.
Introduction
Bark and ambrosia beetles of the weevil subfamily Scolytinae are exceptional as they spend almost their entire life inside the protective tissues of various plants. Thus, some of them are, or have the potential, to become economically relevant pests in agricultural and forest ecosystems, even though the great majority are harmless to living plants (Gregoiré and Evans 2004; Raffa et al. 2015). During the short phase of adult dispersal, they have to find and colonise a host tree, and encounter mating partners. This critical period of their life cycles is characterised by high mortality rates due to natural enemies and abiotic stressors (Lindgren and Raffa 2013). Thus, there is a strong selection on efficient recognition for both suitable host plants and mates. Host and mate searching behaviours, as well as host choice, are regulated by complex interactions of different environmental cues, which may be visual, gustatory, and olfactory, originating from conspecifics and heterospecifics of various trophic levels (Byers 1989, 2004; Borden 1997; Strom et al. 1999). Semiochemicals are olfactory cues that can be grouped by their origin and their implications for the sender and the receiver (Nordlund and Lewis 1976). Bark beetles rely on a broad range of semiochemicals (Borden 1997), including pheromones that are released and received by individuals from the same species, and allelochemicals that mediate communication between species (Nordlund and Lewis 1976). The latter are further divided into kairomones that are released by one species (e.g. host trees) and are to the benefit of the receiver of another species (e.g. bark beetles), allomones that are beneficial for the emitter of another species, and synomones that are to the benefit of both the sender and the receiver species (Nordlund and Lewis 1976).
Bark beetles deploy different strategies to interact with their hosts. They can be classified according to whether they colonise dead or dying trees (hereon referred to as ‘secondary’ species), they live in vital trees as parasites or their colonisation results in the death of their host trees (hereon referred to as ‘primary’ species) (Lindgren and Raffa 2013). Additionally, bark beetles can be gregarious—i.e. many individuals aggregate to colonise a tree by the attraction of male and female conspecifics—or solitary (Raffa et al. 2015). Following host acceptance, gregarious bark beetle species start to release aggregation pheromones to attract conspecifics for mating and to collectively overcome tree defence (Silverstein et al. 1966; Raffa and Berryman 1983; Berryman et al. 1985). There is a large variety of mating systems in bark beetles, e.g. in respect to the sex that initiates the gallery or the number of females per male in polygynous mating systems (Kirkendall 1983).
Gregarious bark beetle species risk to suffer intraspecific competition from too many galleries inside a tree that will reduce the survival odds of the offspring and thus decrease fitness (Anderbrant et al. 1985; Sallé and Raffa 2007). Two non-mutually exclusive mechanisms have been proposed to terminate the colonisation of a tree: the cessation of the synthesis of aggregation pheromone components, and the release of anti-aggregation pheromones (Byers and Wood 1980; Byers et al. 1984; Vité and Francke 1985; Pureswaran et al. 2000). Most scolytine species are non-gregarious, including solitary bark beetles without known aggregation pheromones, as well as most of the paraphyletic group of ambrosia beetles, cultivating fungi in the wood as a food source (Lindgren and Raffa 2013; Hulcr and Stelinski 2017). In non-gregarious and secondary species, the effect of interaction with conspecifics is even more disadvantageous because there is no gain from collective colonisation, instead all conspecifics impose immediate competition for limited resources (Raffa 2001), which means that also these species presumably have an advantage in detecting and reacting to non-suitable, possibly already colonised, host trees.
In the context of avoidance of non-suitable hosts, verbenone has been discovered in bark beetles more than half a century ago (Renwick 1967). It was early proposed as an anti-aggregation component (Renwick and Vité 1969; Rudinsky 1973). Libbey et al. (1974) and Rudinsky et al. (1974) were the first to use the term ‘anti-aggregative pheromone’ with the purpose of terminating attacks (Byers et al. 1984; Leufvén et al. 1984; Byers 1989; Hunt and Borden 1990) and shift new attacks to adjacent trees (Bakke 1981). Because verbenone is also associated with the decay of wood infested by bark beetles (Byers et al. 1989), it is also referred to as an indicator of old and thus unsuitable host material (Lindgren and Miller 2002b; Byers 2004). Indeed, much of the verbenone biosynthesis may actually be derived from microbes inside or outside the beetles’ bodies and the respective contributions of insect vs. microbes to verbenone biosynthesis are still elusive (Byers et al. 1984, 1989; Leufvén and Birgersson 1987; Hunt and Borden 1990). In some bark species, verbenone may thus be an actual ‘signal’, that is purposefully released by the sender–and would thus be called a pheromone—while in other species, it may be a passive ‘cue’ emitted by microorganisms (Leonhardt et al. 2016).
Today, the concept of verbenone as a ‘universal bark beetle repellent’ (Audley et al. 2020; Huber et al. 2020) or a ‘general sign of host unsuitability’ (Byers 2004) is well established and is, besides non-host or green leaf volatiles (Byers et al. 1998, 2004; Zhang and Schlyter 2004), one of the most prominent bark beetle aggregation inhibitors (Mafra-Neto et al. 2022). Numerous studies focussed on the potential of verbenone in pest population management as a repellent tree protectant (e.g. Richerson and Payne 1979; Bedard et al. 1980; Livingston 1983; Borden et al. 1997). However, this has impeded the further investigation of its ecological function and underlying response mechanisms (Borden 1997). Some studies also failed to demonstrate the inhibitory effect of verbenone (e.g. Jakuš et al. 2003), leading some authors to speak of ‘a history of unpredictability’ (Progar 2003). The major reason is that we still know little about the reasons why verbenone seems to be a ubiquitous cue in many bark beetle species, while applied studies observe so much uncertainty. There are still many open questions on the ecological and evolutionary roles of verbenone for the highly diverse group of bark beetles with various life histories. Is it an actual anti-aggregation pheromone synthesised by the beetles themselves or rather an indicator of poor host quality (degradation or competition)? Does it only inhibit attraction under the presence of aggregation pheromones to mask a fully colonised host or is verbenone repellent itself also in the absence of attractive compounds? Does it mediate intraspecific and also interspecific competition for breeding habitat and food resources? And finally, if verbenone is so commonly associated with bark beetles, are higher trophic levels such as predators and parasitoids also able to rely on verbenone to detect their prey?
Here, we summarise and synthesise a broad body of literature from more than 50 years of basic and applied research on the effects of verbenone on bark and ambrosia beetles. We focus on pheromone biosynthesis and perception and infer its ecological implications in the communication of these insects. A simple quantitative meta-analysis was conducted on a few species for which the response to verbenone has been studied, to relate a species’ response to verbenone with its mating system and its host use behaviour (primary vs. secondary species).
In the following, we first focus on the emission of verbenone in space and time. Q1.1.: Who and where?—Which organisms produce verbenone? Which anatomical structures are involved in biosynthesis? What are the relevant pathways? Q1.2.: When and how much?—In which phases of host colonisation is verbenone produced? Which quantities are ecologically relevant? The second part then focusses on the receivers of verbenone as a behaviourally relevant cue. Q2.1: Olfaction and neurophysiology—How is verbenone detected? Q2.2: Behaviour—What is the behavioural response of bark beetles to verbenone, and which factors influence this response? Finally, the role of verbenone is set into a larger ecological and evolutionary context discussing its implications on intra- and interspecific interactions, as well as trophic relationships.
The roles of verbenone in the natural history of bark beetles—an overview
Verbenone emittance—Who is producing it?
Verbenone, i.e. 4,6,6-trimethyl-bicyclo[3.1.1]hept-3-en-2-one or 2-pinen-4-one (CAS 80–57-9, Fig. 1), is a monoterpene ketone with two enantiomers: (–)-S- and ( +)-R-verbenone. It was first identified as an oxidation product in coniferous turpentine oil (Blumann and Zeitschel 1913). Accordingly, it can be found in various gymnosperm tree species (Shepherd et al. 2007), including common bark beetle host species (Flechtmann et al. 1999; Dvořáková et al. 2007; Liazid et al. 2007; Szmigielski et al. 2012), but also in various angiosperm species (e.g. Shepherd and Sullivan 2013; Guo et al. 2017; Pedrali et al. 2019; Çetin and Güdek 2020).
Q1.1: Who and where?—Which organisms produce verbenone?
Chemical synthesis
Verbenone synthesis almost inevitably occurs under an aerobic environment when one or both of the two precursors α-pinene, a major component of conifer resin (Lieutier et al. 1989; Nagel et al. 2022) or verbenol, an oxidation product of the former, are present (Blomquist et al. 2010; Ramakrishnan et al. 2022; Fig. 1). The conversion of verbenol to verbenone can occur spontaneously by autoxidation (Bhattacharyya et al. 1960), but this only seems to matter after long time spans: the percentage of verbenone in turpentine oil from Picea abies increased from initially 0.2% to 50% after 20 days (Dvořáková et al. 2007), even though this can (Flechtmann et al. 1999), but does not necessarily lead to an increase in verbenone release from cut logs after a comparable time span (Pettersson and Boland 2003). The oxidation of verbenol into verbenone is irreversible (Leufvén et al. 1984; Lindmark-Henriksson et al. 2003) and without by-products (Brand et al. 1976).
Apart from autoxidation, the beetles themselves or associated microorganisms enhance verbenone production. These include the beetle-associated ascomycete blue stain fungi (Cale et al. 2019; Kandasamy et al. 2023), yeasts (Brand et al. 1976; Leufvén et al. 1984; Hunt and Borden 1990) and bacteria (Xu et al. 2015). Also, wood-degrading basidiomycetes have been shown to biotransform α-pinene to verbenol (Busmann and Berger 1994; Trapp et al. 2018), as well as saprophytes (Bhattacharyya et al. 1960; Agrawal et al. 1999). The capability to convert verbenol to verbenone seems to be common among bark beetle-associated microorganisms: Leufvén et al. (1984) found six yeast species from the guts of Ips typographus, capable of converting cis-verbenol to verbenone. In fact, microorganisms might not only be capable of biosynthesising verbenone, they might even be the main responsible agents. For example, in Dendroctonus valens fed with antibiotics, the verbenone amounts in guts and frass were considerably lower in both sexes compared to the antibiotic-free control group (Xu et al. 2015), but it is not clear whether the remaining amounts were due to autoxidation or the beetles’ metabolism. Also, numerous bacterial isolates were capable of converting cis-verbenol to verbenone, including Pseudomonas spp., Serratia sp. and Rahnella aquatilis, which also made a high proportion of the bacterial community in the gut and frass (Xu et al. 2015). Similarly, in Dendroctonus ponderosae suppression of associated microorganisms even completely inhibited the synthesis of verbenone (Hunt and Borden 1989), suggesting that the beetles alone do not biosynthesise verbenone. These examples demonstrate the potential importance of microorganisms in verbenone biosynthesis; however, it is still less clear, to what extent they contribute under natural conditions (Hunt and Borden 1990) and whether autoxidation could also be relevant. In conclusion, it seems that bark beetles accelerate the inevitable decay of host tissue by introducing microbes, resulting in the release of verbenone. However, to our knowledge, no enzymes that mediate the conversion of verbenol to verbenone have been characterised so far, so that the underlying biochemical processes are not known.
Stereochemistry
Verbenone, its direct precursors verbenol and its pre-precursor α-pinene are chiral compounds (Fig. 1). The intermediate product verbenol also possesses two geometric isomers (cis- and trans-verbenol). In many studies, the optical isomers of verbenone originating from bark beetles are not further differentiated (e.g. Shepherd et al. 2007; Zhang et al. 2007a, b; Shi and Sun 2010; Liu et al. 2019; Ramakrishnan et al. 2022) and are thus comparatively poorly assessed.
It seems that (–)-verbenone might be the more relevant enantiomer in bark beetle chemical ecology. It was predominant in hindgut extracts from male Pseudips orientalis (Zhang et al. 2011), Ips nitidus (Zhang et al. 2009) as well as Ips pini (Pureswaran et al. 2000), and it was the only conversion product from trans-verbenol of two ophiostomatoid fungi associated with D. ponderosae (Cale et al. 2019). The enantiomeric configuration of verbenone is determined by its pre-precursor α-pinene (R-, S-, or racemic) (Lindmark-Henriksson et al. 2003; Fang et al. 2020). Concordantly, verbenone biosynthesis may not be enantioselective within a species but depend on the host substrate. As a consequence, also the resulting ratios of both verbenone enantiomers are variable within a species. For example, in D. frontalis ratios of (+)- and (–)-verbenone varied strongly (between 20 and 80%) among sexes and across geographic regions for males (Grosman et al. 1997), probably due to variation in enantiomeric ratios of α-pinene (Lindström et al. 1989; Taft et al. 2015). A rather strong intraspecific variation in the ratio of both enantiomers is consistent with the finding that typically both enantiomers elicit a behavioural response to some degree (e.g. Byers et al. 2018, see Q2.1; but see Dickens 1979; Raffa 2001; Zhang et al. 2006).
The geometric configuration of the intermediate substrate verbenol also seems to be important. Both enantiomers of verbenol can lead to the synthesis of verbenone. cis-Verbenol can also be converted to trans-verbenol (Leufvén et al. 1984; Hunt and Borden 1990; Lindmark-Henriksson et al. 2003), but the reverse reaction has not been reported. This one-way reaction might represent a ‘dead end’ and impede verbenone synthesis, e.g. only three of six yeast strains extracted from the guts of I. typographus were able to produce verbenone from trans-verbenol, but all six were able to do so when cis-verbenol was the substrate (Leufvén et al. 1984). In two Dendroctonus species trans-verbenol was the predominant isomer: both sexes of D. brevicomis were capable of converting either enantiomer of α-pinene to the respective enantiomer of trans-verbenol and myrtenol with only trace amounts of cis-verbenol (Byers 1983a, b; Byers et al. 1984). Similarly, two ophiostomatoid fungi typically associated with D. ponderosae exclusively produced (–)-verbenone from trans-verbenol (Cale et al. 2019). The fate of cis-verbenol to be converted directly to verbenone or to trans-verbenol instead also depended on the optical configuration of cis-verbenol for at least some of the yeasts extracted from I. typographus (Leufvén et al. 1984). Probably due to these differences in the synthesis, typically one of the two isomers of verbenol predominates in a given species. Interestingly, the respective other enantiomer can typically be found to a smaller extent, as is the case in I. typographus (Birgersson et al. 1984; Leufvén and Birgersson 1987; Schlyter et al. 1987; Birgersson and Bergström 1989; Ramakrishnan et al. 2022), D. frontalis, and D. ponderosae (Grosman et al. 1997; Pureswaran et al. 2000).
Site of verbenone biosynthesis
Unlike other insect species where pheromones are biosynthesised in specific cells or glands located in the abdomen and/or epidermal cells (Tillman et al. 1999), bark beetles do not have specific organs for pheromone biosynthesis (Tittiger and Blomquist 2016). Instead, evidence from many species suggests that the major organ of verbenone synthesis is the hindgut (Table 1), as is also described for most bark beetle pheromones (Byers 1989), particularly those derived from host monoterpenes (Tittiger and Blomquist 2016). This is coherent with the finding above that verbenone biosynthesis is mostly mediated by microorganisms, which are located in the gut.
The relative contribution of the gut to verbenone emission from bark beetle infestations, however, is not yet clear. Verbenone amounts increased in headspace samples of Scots pine logs infested with Tomicus piniperda over 5 days, while non-infested control logs released little verbenone at a constant rate (Byers et al. 1989), stressing the importance of bark beetle colonisation for verbenone emission. Other studies likewise detected verbenone outside the beetles’ bodies. For instance, bacteria associated with D. valens, like Pantoea conspicua, Enterobacter xiangfangensis, Staphylococcus warneri and at least six other species, were responsible for the conversion of verbenol to verbenone in adult frass (Xu et al. 2015, 2016; Cao et al. 2018). Leufvén and Birgersson (1987) analysed the chemical composition of the phloem around galleries of I. typographus at different attack phases on Norway spruce. They proposed four possible pathways explaining the occurrence of oxygenated monoterpenes—including verbenone—in the phloem, which could (i) either be released by the beetles and thereafter absorbed by the phloem, (ii) synthesised by associated microorganisms, (iii) oxygenated via host-derived enzymes, or (iv) via autoxidation (Leufvén and Birgersson 1987). Thus, a substantial amount of verbenone could be produced in close proximity outside the beetles; however, the relative importance of each source is still not clear.
Sex-specific differences
Across many species with different mating systems, males seem to produce more verbenone compared to females. In Dendroctonus species, females are the gallery-initiating sex. In female D. brevicomis, no verbenone was detected, while considerable amounts were found in males (Byers 1983a). This was also the case for D. ponderosae, where a switch in verbenone synthesis from females to males was proposed upon mating (Pureswaran and Borden 2003). The same was proposed for D. frontalis (Rudinsky 1973), which is in conflict with the finding of another study that found that hindgut extracts from male beetles had 68-fold greater amounts of verbenone compared to females already right after emergence (Grosman et al. 1997). In agreement, solvent extraction and chemical analysis of male and female D. brevicomis that had recently landed on a ponderosa pine showed that females lacked verbenone and males had a high amount of verbenone, which declined after joining a female in her gallery (Byers and Wood 1980; Byers et al. 1984). Regardless of the time point, males in Dendroctonus seem to have greater amounts of verbenone. Interestingly, in Grosman et al. (1997) the females were found to accumulate trans-verbenol, which might be further oxidised to verbenone later on. An accumulation of trans-verbenol in females was also reported for the monogynous Conophthorus coniperda, while males contained verbenone (Birgersson et al. 1995). It is not clear whether the verbenol-to-verbenone ratio also changes in the females of these species during colonisation or if trans-verbenol is directly released without further oxygenation. Also in the monogynous species T. piniperda, males contained more verbenone than females (Lanne et al. 1987), while this was the opposite in Tomicus minor (Wu et al. 2019). In Ips spp. and other polygynous species, males initiate the galleries and are typically the main aggregation pheromone producers (Byers 1989). Accordingly, almost no aggregation pheromone components were found in female I. typographus (Birgersson et al. 1984), I. nitidus (Zhang et al. 2009), Ips duplicatus (Zhang et al. 2007b) and P. orientalis, including also verbenone (Zhang et al. 2011).
As described above, the biosynthesis of verbenone is linked to the host substrate and the detoxification of α-pinene. However, since both sexes usually feed on the same substrate, differences in the amounts of verbenone must be derived from differences in the downstream pathways. When the microbial conversion of cis-verbenol to verbenone was inhibited by antibiotics, the resulting verbenone concentration in the guts and in the frass was considerably more reduced in female D. valens, compared to males (Xu et al. 2015). This indicates that the relative importance of specific biosynthesis pathways may differ between the sexes, possibly by sex-specific microbial communities (see Xu et al. 2016; Whittle et al. 2021). In the guts of I. typographus performing maturation feeding, high amounts of verbenone were initially found in both sexes (Ramakrishnan et al. 2022). This changed after the end of the maturation feeding and the emergence from brood trees, when verbenone was only found in males. Apparently, something in the metabolism must have changed (but this is not discussed further in the study). Similarly, male D. brevicomis exposed to vapours of the precursor α-pinene contained verbenone in detectable amounts while females did not (Byers 1983b). For other pheromone components, it has been shown that sex-specific differences are also due to differences in gene expression (reviewed in Blomquist et al. 2010). Sex-specific differences in the amounts of pheromone components are common in bark beetles (Birgersson et al. 1995; Byers 1989). It is noteworthy that in most cases this sex bias in verbenone content is of quantitative, not qualitative nature. It remains unclear though, whether the male bias in the synthesis of verbenone in many bark beetles is due to differences in the microbiome, differences in the feeding behaviour, a mixture of both, and/or whether active regulation is possible.
Q1.2: When and how much? In which phases of host colonisation is verbenone produced?
Concentrations and release rates
The composition of pheromone bouquets in bark beetles is typically studied from gut extracts or by headspace sampling. Only few studies analysed the content in the phloem or frass. Relative to its precursors, the amounts of verbenone are typically low. The amount of verbenone was only around 20% of the amount of verbenol in gut extracts of P. orientalis (Zhang et al. 2011), only 4% in Dendroctonus banksiana (Cale et al. 2015), but 50% in recently mated D. valens, whereas this was only 6% compared to the α-pinene content (Zhang and Sun 2006). These ratios from tissue extracts also seem to be reflected in the actual emission patterns. The maximum release rate of verbenone compared to the respective verbenol precursor was three times lower in D. frontalis and I. typographus (Birgersson and Bergström 1989; Pureswaran and Sullivan 2012). Most pheromones would be assumed to require short half-life spans because the information they convey is only valid for a short period of time (Shiota and Sakurai 2020). This may not be the case for verbenone as an indicator of poor habitat quality, because these conditions—once attained—are unlikely to improve again. Instead, the low availability of verbenone may be explained by its high sensitivity to sunlight under which it is photoisomerised to (+)-chrysanthenone (Kostyk et al. 1993), filifolone (Fettig et al. 2009a) and other transformation products (Erman 1967).
Absolute amounts of verbenone vary within a species depending on the host tree quality and feeding substrate (Byers 1983a; Raffa 2001; Taft et al. 2015), but also among individuals within a tree (Birgersson and Bergström 1989). Most individuals contain rather low levels, while only few individuals produce large amounts (Grosman et al. 1997; Zhang et al. 2000a, b). Absolute verbenone amounts commonly range from 0 to 100 ng per individual in a broad range of bark beetle species (Table 1). It is remarkable that in I. typographus maximum amounts of verbenone were less than 5 ng/beetle for both sexes across multiple life stages before and after mating (Birgersson et al. 1984; Ramakrishnan et al. 2022)—and another study even failed to detect verbenone at all (Birgersson et al. 1988)—even though verbenone is thought to be a relevant component in the pheromone communication of that species (Schlyter et al. 1989). Only low amounts of verbenone were also found in I. amitinus (Zuber 1994, Table 1) and I. cembrae (Zhang et al. 2000a, b). Contrarily, considerably larger amounts of verbenone (600–8,000 ng/beetle) were detected in male Dendroctonus brevicomis (Byers 1983a, b; Byers et al. 1984) and D. frontalis (Grosman et al. 1997). It is not clear why these two species contain up to tenfold more verbenone than other species, even from the same genus. Larger or heavier beetles may also produce larger amounts of pheromones (Anderbrant et al. 1985; Pureswaran and Borden 2003). However, body size cannot be the main factor here because D. frontalis is the smallest Dendroctonus species (Six and Bracewell 2015), and also D. brevicomis has an average body length of only 3.9 mm (Valerio-Mendoza et al. 2019). In all three studies cited above, beetles have been dissected right after emergence, so that the much larger amounts of verbenone compared to other species could be in parts due to an exhaustive maturation feeding and thus be a matter of timing. In fact, samples of callow adult males and females taken from under the bark, and emerged males and females of D. brevicomis showed that only emerged males contained large amounts of verbenone (Byers 1983b). At least in D. frontalis, also the relative amount of verbenone was high: it made up to 96% of the oxygenated monoterpenes (Grosman et al. 1997), which indicates that verbenone may be of great relevance for this species. The remarkably high amounts of verbenone may possibly be derived from differences in the metabolism of D. brevicomis and D. frontalis from that of other bark beetles and could indicate an active control of the synthesis, which requires further investigation.
Data on atmospheric verbenone concentrations, both natural background concentrations and in the proximity of trees colonised by bark beetles, are scarce. Verbenone has been identified from conifer needle litter (Isidorov et al. 2003), and atmospheric concentrations of around 0.17–1.32 ng/m3 have been found in urban, agricultural and also coniferous forest landscapes (Shimmo et al. 2004). While such concentrations are presumably below behaviourally relevant thresholds, these results can serve as a baseline to understand the natural dynamics in the release of verbenone from bark beetle infestations. The only estimation of verbenone release from an infested tree was reported in Salom et al. (1992) as unpublished data assuming that 0.45 mg/h of verbenone would be released from a tree infested with 1,400 pairs of D. frontalis. Headspace sampling around 50 artificial boreholes from female T. piniperda revealed release rates of up to 240 ng verbenone per hour (Byers et al. 1989). Assuming the same number of 1400 pairs per tree, this would correspond to a release of 0.01 mg/h. Further studies from other bark beetle species and host trees are needed to provide information on ecologically relevant concentrations that are perceived by the beetles under natural conditions. In that regard, it is not yet clear how the concentrations measured in the gut relate to actual verbenone release rates. For I. typographus, it has recently been found that the aggregation pheromone component cis-verbenol can be stored as verbenyl oleate in the fat body (Ramakrishnan et al. 2022). This finding suggests that there might be an active regulation of pheromone emission by a controlled release of these stored compounds. Future studies should link the amounts of pheromone components in the gut to actual release rates and extrapolate these values to the landscape scale. Finally, these findings should then be related to the bark beetles’ perception by their olfactory system and resulting behaviour (Q2).
Phenology of verbenone emission
If verbenone was a ‘perfect’ anti-aggregation pheromone, it should be expected not to occur at the early stages of an infestation by gregarious species (Fig. 2, solid blue line), because at this point mates are still needed to collectively overcome tree defence (Raffa and Berryman 1983). The amount of verbenone is then expected to increase immediately upon mating and further with an ongoing infestation in accordance with its putative function as an indicator of high colonisation densities and in order to shift an attack to a neighbouring tree (Renwick and Vité 1969; Rudinsky et al. 1974; Vité and Francke 1985; Byers 2004). But even in solitary bark beetle species, as well as ambrosia beetles, an attraction inhibitor like verbenone should not be released before mating was successful. In accordance, verbenone concentrations released from unmated females of the gregarious species D. frontalis were low and not significantly different from galleries with unsuccessful attacks, while the amount doubled once males joined the gallery (Pureswaran and Sullivan 2012). Also in the guts of freshly mated D. valens verbenone occurred in a similar high amount as cis- and trans-verbenol (Zhang and Sun 2006).
Schematic overview on the temporal verbenone release patterns from monogamous and polygynous bark beetles, (i) trajectory as expected for an ideal anti-aggregation pheromone to avoid overpopulation (‘active’ pathway, blue solid line), (ii) trajectory as expected if verbenone synthesis is a passive, unavoidable consequence of feeding activity (‘passive’ pathway, green solid line) or (iii) trajectories reported in the literature obtained from gut extracts (thin solid lines), phloem extracts (short-dashed lines) and atmospheric concentrations (long-dashed lines). Note that the developmental stages on the x-axis are evenly spaced for the sake of simplicity. In reality, these relative time spans would be species-dependent and strongly vary with ambient temperature. Amplitudes are relative within a study and not standardised among studies. All species presented here are gregarious except D. rhizophagus6. References: 1Cale et al. (2015), 2Pureswaran and Sullivan (2012), 3Birgersson and Bergström (1989), 4Shi and Sun (2010), 5Xie and Lv (2012), 6Cano-Ramírez et al. (2012), 7Liu et al. (2019), 8Zhang et al. (2009), 9Birgersson et al. (1984), 10Ramakrishnan et al. (2022), 11Zhang et al. (2000), 12Byers et al. (1984), 13Xie and Lv (2013), 14Leufvén and Birgersson (1987), 15Taft et al. (2015)
Several other studies, however, contradict this expected pattern by either showing that (i) verbenone amounts decline after construction of the nuptial chamber and/or mating, and that (ii) significant amounts of verbenone can already occur before mating (Fig. 2, thin solid lines). For instance, the amount of verbenone was reported to decrease after mating and during oviposition in both sexes of D. rhizophagus (Cano-Ramírez et al. 2012), I. nitidus (Zhang et al. 2009), I. typographus (Birgersson et al. 1984) and female D. armandi (Xie and Lv 2012).
Furthermore, verbenone has often been found at the early stages of the life cycle. For example, after maturation feeding but before emergence as in I. typographus (Ramakrishnan et al. 2022) or right after emergence as in D. frontalis (Grosman et al. 1997), D. brevicomis (Byers 1983a; Byers et al. 1984), D. ponderosae, and I. pini (Pureswaran et al. 2000). Some studies show that verbenone is also present some time after feeding has occurred, e.g. when landing on a tree (Byers et al. 1984) and during construction of the nuptial chamber (Zhang et al. 2007b, 2009; Xie and Lv 2013; Taft et al. 2015), which might be explained by feeding residues in the guts. However, callow beetles (during maturation feeding) of D. brevicomis of either sex did not contain verbenone in detectable amounts at this stage (Byers 1983b), but after dispersal when landing on a host pine tree (Byers et al. 1984) so that maturation feeding must have occurred in between or the production of verbenone from ingested host precursors occurred with some delay.
Instead of a steady increase in verbenone concentration over time, as observed in D. ponderosae during the first five days of colonisation (Cale et al. 2015), T. piniperda (Byers et al. 1989), T. yunnanensis, and T. minor (Wu et al. 2019), several studies suggest an early initial increase in verbenone, followed by a decline and then a steady increase in at least one sex (Birgersson and Bergström 1989; Taft et al. 2015; Liu et al. 2019; Ramakrishnan et al. 2022). These two resulting peaks might correlate with the production of ‘primary’ and ‘secondary resin’ as tree defence (Leufvén and Birgersson 1987). Moreover, the later increase may correspond to what would be expected in decaying host tissue even without bark beetles and/or their associated microorganisms in the form of autoxidation (Flechtmann et al. 1999; Dvořáková et al. 2007).
The fact that verbenone can often be found in bark beetles before mating challenges its role as a ‘perfect’ anti-aggregation pheromone. Instead, the occurrence of verbenone rather seems to be a passive consequence during phases of intense feeding activity instead of a controlled release of an ideal anti-aggregation pheromone (Fig. 2, solid green line; Pureswaran and Borden 2003; Shi and Sun 2010; Cano-Ramírez et al. 2012). This is in line with the strong linkage of verbenone to the host tissue quality and availability of the precursor compounds α-pinene and verbenol (Shi and Sun 2010; Cano-Ramírez et al. 2012; Cale et al. 2015). Also from an energetic perspective, it might be favourable for the beetles to rely on host compounds as cues instead of de novo synthesis (Byers 1983a, 1989). According to the ‘sender-precursor hypothesis’, what we observe could be the transition phase of a semiochemical—with an original ecological meaning (‘elevated bark beetle activity’)—to an actual pheromone. This hypothesis postulates that the presence of a compound associated with a specific state (here: the intense feeding of conspecifics) causes the evolution of the receiver’s sensory capacities necessary to detect that cue and the employment as a pheromone (Stökl and Steiger 2017). Assuming an ongoing evolution regarding the employment of verbenone, it is therefore possible that some beetle species can actively regulate biosynthesis (i.e. produce a signal), while others cannot (i.e. rely on a cue).
The finding that verbenone is synthesised to a large extent by associated microorganisms might further explain why verbenone is a semiochemical used by many scolytine species. At least some of the verbenone-synthesising yeasts, including Candida nitratophila, Hansenula capsulate and Pichia pinus were detected in the two geographically separated species D. ponderosae and I. typographus (Hunt and Borden 1990). In case microbial species are commonly shared among phylogenetically distant scolytine species, the ability of verbenone biosynthesis could be gained relatively quickly by incorporating the respective microbial species by means of horizontal transfer. The detection capability and response to verbenone could then be interpreted as an adaptation allowing to detect and respond to a ‘universal’ bark beetle microbiome.
Studies on the temporal occurrence of verbenone based only on extracts from adult beetles also may thus underestimate the amounts that occur in proximity to an infestation, particularly during advanced stages of an infestation, because they ignore verbenone originating from other sources outside the beetles, e.g. the gallery walls (Leufvén and Birgersson 1987). This issue should not arise from headspace samplings that inherently include volatiles emitted from any source. It must therefore be assumed that volatile samplings, contrary to extracts, more accurately reflect the verbenone concentrations that are relevant for newly arriving mates. Verbenone amounts in headspace samples indeed increased for D. ponderosae from host acceptance to oviposition (Cale et al. 2015) and in I. typographus after mating (Birgersson and Bergström 1989), but not in D. frontalis where the concentration increased before mating and declined again afterwards (Pureswaran and Sullivan 2012). In I. typographus, also the verbenone concentration inside the phloem showed a strong increase with the onset of oviposition (Leufvén and Birgersson 1987). Byers et al. (1989) observed a continuous increase in verbenone released from artificially inoculated windthrown pines with T. piniperda during the first five days and commented that this does not match with observations from gut extracts. It therefore seems that the expected increase in verbenone with later stages of bark beetle colonisation to indicate unsuitable habitat rather originates from sources outside the beetles, suggesting once more the importance of associated microorganisms.
So far, only one study investigated verbenone concentrations associated with larval frass. In the laboratory, phloem extracts had significantly higher proportions of verbenone when D. valens larvae were also present and larval frass extracts from Dendroctonus micans and D. valens collected in the field both contained verbenone (Grégoire et al. 1991). Thus, also larval activity may contribute to the release of verbenone and is another potential source not captured by gut analysis of adult beetles, possibly leading to an underestimation of relevant verbenone amounts. However, most studies ended before substantial larval activity could occur (i.e. they did not observe later than early oviposition) so that this bias is of low relevance for the literature comparison that is presented here. If the release of verbenone from larval frass occurs in the host tree, this may serve to prevent colonisation of the same tree by newly arriving beetles and also trigger the dispersal of the emerging beetles away from the maternal tree to prevent inbreeding.
According to the strict definition, pheromones are chemical signals that act within a species (Wyatt 2010). The findings here suggest the importance of associated microorganisms in the verbenone synthesis and sources outside the beetles. This obviously contradicts the concept of a pheromone sensu stricto requiring the sender and receiver to be from the same species. According to the classification of semiochemicals, verbenone must therefore rather be classified as an allelochemical if produced by microorganisms and perceived by bark beetles (Nordlund and Lewis 1976). Six (2013) advocates the concept of bark beetles as a ‘holobiont’, i.e. the entity of a bark beetle and its associated microorganisms, which may be more useful in this context than the taxonomic unit ‘species’ in case a more accurate functional understanding of the ecology of bark beetles is needed. This concept also seems useful to be applied in regard to verbenone, because even if the origin of this compound lies outside the beetles’ bodies, the relevant biological agents are clearly associated with bark beetle colonisation.
Interestingly, verbenone is not the only example for compound pairs of which the terpene alcohol is an attractant and the corresponding ketone a repellent. For example, seudenol (3-methyl-2-cyclohexen-1-ol) is part of the aggregation pheromone of D. pseudotsugae and seudenone (3-methyl-2-cyclohexen-1-one) is inhibitory (Brand et al. 1976; Foote et al. 2020). Quercivorol ((1S,4R)-4-isopropyl-1-methyl-2-cyclohexen-1-ol) is an attractant for the species complex Euwallacea fornicatus and piperitone (6-isopropyl-3-methyl-1-cyclohex-2-enone), the ketone of a constitutional isomer of quercivorol, and significantly lowered the attractiveness in a baited trap experiment (Dodge et al. 2017; Byers et al. 2018, 2020). This phenomenon might be explained by a parsimonious use of chemical compounds on one hand, and a well-preserved chain of behaviours within the scolytine beetles that includes attraction at the early stages of colonisation and anti-aggregation at later stages.
Verbenone reception and behavioural response
Q2.1: Olfaction and neurophysiology—How is verbenone detected?
Antennal and neuronal response
The antennal response to verbenone, as measured via electroantennograms (EAG) or gas chromatographic–electroantennographic detection (GC-EAD), is well established across several genera (Whitehead 1986; Pureswaran et al. 2000; Schlyter et al. 2000; Zhang et al. 2006; Shepherd et al. 2007; Zhang et al. 2007a, b; Andersson 2012; López et al. 2013; Ranger et al. 2014; Kandasamy et al. 2023). On the neurological level, both sexes tend to respond in a similar way (Ascoli-Christensen et al. 1993; Zhang et al. 2006; Cano-Ramírez et al. 2012; Shepherd and Sullivan 2013; Zhao et al. 2017), even though D. armandi showed a sex-specific response to different verbenone amounts (Zhao et al. 2017).
Unfortunately, the comparison of actual response threshold concentrations among species is nearly impossible for several reasons. Enantiomers, if not differentiated in a study—as is often the case—can function as different compounds and may possess completely different antennal detection thresholds (Dickens 1979). Comparison is further impeded since studies differ in test concentrations, the time span in between two stimuli, and the order in which different dosages are presented. Different solvents are used, which can affect the electrophysiological responses that are presented as absolute or often as normalised output relative to controls of different quality. Thus, it is unclear what the actual concentration of a compound is that reaches the antenna in a given study, and how this concentration compares to relevant concentrations in nature, making comparisons among studies difficult (Andersson et al. 2012).
Signal processing and interaction with other stimuli
Even though it is well established that verbenone elicits a neuronal response in bark beetle antenna, it is not fully clear how the signal is further processed and how it interacts with other incoming stimuli, e.g. attractive host cues or aggregation pheromones. Odour detection by olfactory receptor neurons (ORNs) in bark beetles is summarised in Andersson (2012). In brief, the responsible organs are the antennae (Fig. 3) and, to a lesser extent, the maxillary palps. They contain olfactory sensilla, which are hair-like structures housing the ORNs. These neurons express the odorant receptors (ORs) that bind the odour molecules to trigger neuronal responses. It is further thought that odorant-binding proteins (OBP) (Dickens 1997), also called pheromone binding proteins when a pheromone is the target compound (Breer 1997), may play an important role for a compound to solubilise and get access to the OR. Recently, MaltOBP1 has been identified in the longhorn beetle Monochamus alternatus with a high binding affinity to ( +)-α-pinene and (–)-verbenone (Zhang et al. 2020). Interestingly, its expression was not limited to the antennae, but also occurred in the legs, wings and to some degree also in other parts of the body. To our knowledge, it is still an open question whether OBPs that bind verbenone exist also in bark beetles and whether the detection of verbenone is limited to the antenna.
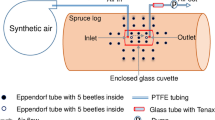
Odour detection at the example of Ips typographus. The bark beetle antenna a, b contains olfactory sensilla which are organised c in two distinct undulating bands A, B and one distal area C. The sensilla containing the olfactory receptor neurons (ORN) that respond to verbenone have been found primarily in B, to a lower extent in A and they appear to be completely missing in C. One sensillum generally contains two ORNs which house the odorant receptor (OR) membrane proteins in the dendrites of these neurons d. An OR that primarily responds to verbenone has so far not been identified and it is not yet clear whether odorant binding proteins (OBPs) play a role in the detection of verbenone. The inhibitory effect of verbenone on the attraction to the aggregation pheromone is most likely caused by integration of the incoming stimuli in the central nervous system. (Figure modified from Jacquin-Joly and Merlin (2004), Andersson et al. (2009) and Kandasamy et al. (2023), figure credit to Fenris Mäling)
To act as an inhibitor (see Q2.2), verbenone must somehow interfere with an attracting stimulus. This can either occur inside the olfactory organs i) as ‘peripheral inhibition’, e.g. by direct interference with ORs for attractive stimuli or by the interactions between ORNs housed inside the same sensilla (Andersson et al. 2010; Su et al. 2012), or ii) in the central nervous system by central integration of attractive and aversive signals (Visser and de Jong 1988; Byers 1989). In I. typographus, an ORN class responding specifically to verbenone was originally reported by Tømmerås (1985) and later by Andersson et al. (2009). However, in more recent single sensillum recordings by Kandasamy et al. (2023) including additional monoterpene ketones, the same ORN class also showed similar responses to α-and β-isophorone (the latter reported in low amounts in the gut of mated females; Birgersson et al. 1984), suggesting that verbenone detection is less specific than previously thought. The verbenone-responsive neurons are not co-localised in the same sensilla as neurons detecting the aggregation pheromone components cis-verbenol or 2-methyl-3-methyl-2-ol, respectively; several of the cis-verbenol neurons are instead co-localised with ORNs detecting the inhibitory spruce defence compound 1,8-cineole (Andersson et al. 2010). This in combination with the fact that verbenone does not inhibit the ORN classes that detect aggregation pheromone components suggests that the verbenone stimulus is most likely integrated centrally in the nervous system and thus activates neuronal circuits responsible for an aversive behavioural response. In addition to the verbenone-responsive ORN class thoroughly characterised in I. typographus, neurons primarily responding to this compound have been reported also in other species such as I. pini, D. pseudotsugae, and D. frontalis (summarised in Andersson 2012). Thus far, an OR that is responsible for the recorded ORN responses to verbenone remains to be identified. Certain compounds (i.e. C6 green leaf volatiles and 2-phenylethanol) that are ecologically relevant for several species within the Curculionidae were recently shown to activate evolutionarily conserved ORs with highly similar response specificities in different species (Roberts et al. 2022). The widespread use of verbenone among scolytine beetles begs the question of whether this compound also is detected by an OR that has been conserved across this beetle subfamily.
Understanding the neurological signal transduction in response to an olfactory stimulus is only the first step. But how does the change in neuro-activity affect the beetle’s physiology? This question has been even less addressed so far and remains speculative. Dickens and Payne (1978) found a reduced muscle potential in the antenna of freshly emerged D. frontalis as an immediate response to trans-verbenol and verbenone. However, it is unlikely that a single change in muscle potential will affect the complex host search behaviour. Byers et al. (2004) observed flying P. bidentatus actively avoiding baited traps with additional non-host tissue by changing the direction of flight when approaching the traps by 0.5–1 m. An active flight response away from a non-attractive cue also seems more logical than an inhibition of the flight muscles, which would not allow the beetles to actually avoid non-suitable hosts. It therefore seems that the behavioural response to verbenone involves a complex—and largely unknown—interplay of a multitude of distinct factors, probably also including the physiological and nutritional state of an individual.
Q2.2: What is the behavioural response of bark beetles to verbenone and which factors influence this response?
Behavioural response to verbenone
For at least 45 scolytine species (45/53 species that were studied) from 18 different genera, a behavioural response to verbenone was described (Table 2). Most reports refer to coniferophagous bark beetles, but also some angiosperm-feeding species (e.g. Kohnle et al. 1992; Audley et al. 2020) and ambrosia beetles (e.g. Ranger et al. 2014; Byers et al. 2020, 2021; Martini et al. 2020) respond to verbenone.
Most studies tested for, and also proved, ‘inhibition’ by verbenone (Table 2, Fig. 4), which is defined here as the reduced or completely suppressed attraction response to an otherwise attractive source (Table 3). Experimental release rates of verbenone point sources range from only ~ 1 mg/d (e.g. Erbilgin et al. 2008; Burbano et al. 2012; Byers et al. 2018, 2020) up to ~ 100 mg/d (Bertram and Paine 1994a; Hayes et al. 1994; Gillette et al. 2014), but most of the times intermediate release rates around 20–50 mg/d were used (e.g. Borden et al. 1992; Fettig et al. 2013; Strom et al. 2013; Agnello et al. 2017; Audley et al. 2020). Inhibition was most prominently studied in the economically important species D. ponderosae, D. frontalis, D. brevicomis and I. typographus (Table 2). While most work confirms the inhibitory effect of verbenone, some studies did not find an inhibitory effect (e.g. Niemeyer et al. 1995; Jakuš et al. 2003; Shepherd and Sullivan 2013). From 53 species for which we found relevant studies, 38 (≈ 72%) were inhibited by verbenone (Table 2). The proportion was slightly larger in primary (20/24 ≈ 83%) compared to secondary species (16/23 ≈ 70%). Verbenone never completely inhibits the response to attractants, and only in some studies the attraction of baited traps was reduced to a level similar to that of blank controls (e.g. Ranger et al. 2013; Audley et al. 2020) or more than 90% (e.g. Tilden and Bedard 1988; Hayes and Strom 1994). In other cases, the reduction was around or below 50% (e.g. Strom et al. 1999; Zhang et al. 1999, 2006; Fettig et al. 2009b; Zhao et al. 2017). Accordingly, also attacks on host trees treated with verbenone are typically not completely suppressed (e.g. Lindgren et al. 1989; Bertram and Paine 1994a; Gillette et al. 2012a). The fact that some beetle individuals ‘ignore’ the verbenone signal (Fig. 5) is also supported by the finding from several Dendroctonus species that trees protected with pure verbenone or verbenone-containing blends often show a greater number of unsuccessful attacks: the number of attacking beetles is reduced to a level so that tree defence cannot be overcome and comparatively more beetles—namely those ignoring verbenone–die in the resin (Shea et al. 1992; Lindgren and Borden 1993; Fettig et al. 2009a).
Interaction of the mating system (left) and host use strategy (right) of selected scolytine beetles (53 species for which relevant studies were available) with their behavioural response to verbenone as summarised from the literature (see Table 2). Qualitative classification of bark beetle species for all three parameters (response to verbenone, mating system, and host use strategy) was made according to the information presented in Table 2; if multiple response types were reported for a species, the most common one was considered. Chi-square test was used for testing statistical significance among and within groups and Cramér’s V for estimation of the effect size (R package: ggstatsplot, Patil (2021))
Simplified host finding process at the example of the Eurasian spruce bark beetle (Ips typographus). 1. In a first step, male pioneer beetles find suitable hosts by chance or guided by primary attraction. 2. Once they establish, they produce aggregation pheromones to attract conspecifics of both sexes. 3. Under the presence of verbenone (at later stages of the colonisation) further individuals are either repelled a or their response to the aggregation pheromone is inhibited b, both resulting in a decrease in landing and attacks on the tree. Some individuals, however, ignore or do not manage to avoid the verbenone and continue landing on the tree c. (Modified according to Vité and Francke (1985), figure credits to Fenris Mäling)
‘Attraction’ to pure verbenone or ‘repellence’ (see Table 3 for definitions) have been studied considerably less, requiring an approach that includes non-baited (passive) traps with verbenone as the only compound tested. Pure verbenone was neither attractive to several Conophthorus species in the field (de Groot and DeBarr 2000; Rappaport et al. 2000), nor to I. paraconfusus in a walking assay (McPheron et al. 1997). However, verbenone was shown to synergise attraction in two scenarios, i) for some secondary species and ii) for gregarious species at specific release rates or specific phases within the population dynamics. For example, verbenone only showed a weak effect in preventing infestation and brood development on pine stumps of the mostly secondary T. piniperda (McCullough et al. 1998, but see Schlyter et al. 1988), and Hylurgops palliatus (Byers 1992a). Livingston et al. (1983) found more Hylurgops planirostris in traps around pine bolts naturally infested with D. adjunctus when treated with verbenone, but only 19 beetles were caught altogether. Attraction of H. palliatus to synthetic pine kairomones was increased in one of four similar experiments when also verbenone was present and significantly different from non-baited control traps (Schlyter et al. 2000). Similarly, verbenone also increased the attractiveness of baited traps to Trypodendron lineatum (Lindgren and Miller 2002a). Lindgren and Miller (2002b) studied the response to verbenone of five bark beetle species with different levels of aggressiveness and found that aggressive species were inhibited, while trap catches of secondary species were not affected by verbenone. They concluded that verbenone acts as a signal for decaying wood for primary species that depend on fresh host material, while secondary species are indifferent to verbenone. A quantitative analysis of the collected literature supports this hypothesis by showing a slightly greater proportion of non-responding species and species attracted to verbenone among secondary species (6/23 ≈ 26% of species tested) compared to primary species (4/24 ≈ 17%, Fig. 4). A high proportion of species that are indifferent to verbenone was also found among six cone and seed feeding species (Fig. 4, but only for six species the response has been tested), suggesting that the host use behaviour (i.e. primary, secondary, cone/seed feeding) is to some extent related to the behavioural response to verbenone.
Furthermore, verbenone synergizes attraction to aggregation pheromones for primary species at specific release rates or specific phases within the population dynamics. The synergistic attraction was observed for C. ponderosae (Rappaport et al. 2000), but was not consistent across various years. The authors hypothesised that the attractive effect of verbenone only occurs at low population densities and disappears at higher population densities (Rappaport et al. 2000). Orthotomicus erosus was attracted by high amounts of verbenone (40 mg/d), and I. sexdentatus might be attracted to moderate amounts of verbenone (release rate of 2 mg/d) (Etxebeste et al. 2013), similar to what has been proposed for D. frontalis (Rudinsky 1973). The attraction of Ips grandicollis to baited traps was more than doubled by verbenone with a release rate of 40 mg/d, but not to verbenone-containing traps stapled to host trees without bait (Dodds and Miller 2010). Also, I. typographus was attracted to high release rates of verbenone (93 mg/d) in the presence of host kairomones, even though the overall number of attacks per tree was low (Niemeyer et al. 1995).
Actual ‘repellence’ has been reported even less, but this is presumably also because beetle catch numbers are typically extremely low when passive traps are used without any bait (Reeve and Strom 2004): the addition of a repellent cannot decrease the trap catches any further, unless an extremely large sample size is used that allows to statistically differentiate the low trap catches of blank traps to even lower catches in traps with repellents. For example, no repellent effect was observed in T. yunnanensis and T. minor using an olfactory walking assay in the laboratory (Wu et al. 2019) and in D. brevicomis and I. sexdentatus in the field (Etxebeste and Pajares 2011; Fettig et al. 2012a). VanDerLaan and Ginzel (2013) tested verbenone without additional compounds and thus caught fewer individuals of the target species X. germanus and X. crassiusculus than in non-baited control traps. This is so far the only study providing evidence for a repellent effect of verbenone that is not attraction inhibition in the field. Similarly, in walking assays, the coffee berry borer Hypothenemus hampei had a significant preference for the solvent-only treatment when exposed to verbenone (Jaramillo et al. 2013). Technically, in studies where only inhibition is tested (i.e. verbenone tested against an attractive blend), repellence—if present—cannot be detected since it would be covered by the inhibitory effect. Therefore, for many species it is not known whether verbenone may also be a repellent that acts as an inhibitor when presented together with attractants (see Fig. 5-3 options a, b). This difference, however, is important if we want to understand whether verbenone also has an effect in the absence of attractive compounds as assumed to be the case at later stages of an attack, when the release of aggregation pheromones expires (Bakke 1981; Zhang et al. 2000a, b; Pureswaran and Sullivan 2012). It has also direct implications for semiochemical-based management approaches because this knowledge helps to identify scenarios during which the application of the inhibitory verbenone is efficacious and when not.
The majority of behavioural studies addressed flight response (conducted with baited traps), and comparatively few investigated walking behaviour or host colonisation (including host finding, host acceptance, oviposition and feeding; Table 2) under the influence of verbenone. In case more than one behaviour was studied for a species, the response is typically coherent in the sense that the inhibitory effect of verbenone affects the behaviour at different levels (e.g. Byers and Wood 1980, 1981; Ryker and Yandell 1983; Byers et al. 1989; Hayes et al. 1994; Zhang et al. 1999; Zhang et al. 2006; López et al. 2013; Zhao et al. 2017; Wu et al. 2019; and also see Table 2). Concordantly, verbenone did not affect the ratio of landing vs. attacking D. brevicomis (Bertram and Paine 1994a). Other effects of verbenone have rarely been tested, but point in a similar direction. Walking D. ponderosae showed arresting behaviour in proximity to their aggregation pheromones less frequently in the presence of verbenone (Ryker and Yandell 1983). In laboratory assays with walking I. paraconfusus, increasing release rates of (+)-verbenone over four orders of magnitude caused declining proportions of both sexes to reach the source of their aggregation pheromone components (Byers and Wood 1981). Inhibition of colonisation might also be due to a feeding deterrence by verbenone in at least some species: verbenone had no effect on the frequency of incomplete galleries of I. sexdentatus and O. erosus (Etxebeste et al. 2013), but it seemed to slow down the boring activity of male I. pini (Sallé and Raffa 2007). Feeding performance and acceptance of artificial diet were also reduced in I. typographus even to a higher degree than was observed for α-pinene (Faccoli et al. 2005), a major compound of tree defence in conifers. In fact, also the toxicity of verbenone to bark beetles was higher than that of α-pinene (Sallé and Raffa 2007). This is surprising given the fact that the conversion of α-pinene is commonly regarded as detoxification to improve the quality of the brood habitat (Hunt and Borden 1989; Lieutier 2004). Nevertheless, the toxicity of verbenone is still considerably lower compared to neurotoxins used as conventional insecticides (Rivera-Davila et al. 2021).
The beetle response to their pheromones depends on the context, e.g. it can vary between generations and throughout the season (Heber et al. 2021) and also depends on the nutritional status of an individual (Bennett and Borden 1971; Němec et al. 1993). While it is obvious that verbenone conveys relevant information to dispersing beetles, it is, however, easy to imagine that fatigued individuals may be obliged to land on a tree that is rather unattractive due to high verbenone emissions and accept a low chance of reproduction if the only alternative is incipient exhaustion and death. Analysis of the nutritional state of this proportion of individuals that land in verbenone-baited traps could shed some light on the importance of bark beetle physiology. Altogether, we still know very little about why some individuals within a population are indifferent to verbenone (Fig. 5-3c) and why these proportions vary strongly among different studies, years, and sites. Identification of these drivers will enhance our understanding of the fascinating ecology of scolytine beetles, but will also help to make the practical application of verbenone as a semiochemical for pest management more effective.
Dose dependency and synergistic inhibition
Many of the behavioural studies on the response of bark beetles to verbenone are driven by a strongly applied background. Therefore, they often address the questions on effective doses or relevant concentrations needed to affect beetle behaviour. The inhibitory effect of verbenone on baited traps in the field seems to be positively correlated with the verbenone dosage in general (but see Paine and Hanlon 1991; Erbilgin et al. 2008; López et al. 2013; Byers et al. 2018, 2020). This relation can be linear (Schlyter et al. 1989) but also log-linear (Lindgren and Miller 2002b), log–log like (Tilden and Bedard 1988; Miller et al. 1995; Lindgren and Miller 2002b) or a kinetic decay function of second order (Byers et al. 2020). Most frequently, response thresholds were described (Ryker and Yandell 1983; Schlyter et al. 1989; Bertram and Paine 1994b; Devlin and Borden 1994; Rappaport et al. 2000; Lindgren and Miller 2002a; Fettig et al. 2007; López et al. 2013; Audley et al. 2020), even though this could in some cases be an artefact of sample size and/or unlucky choice of verbenone release rates that lead to the observation of an ‘all-or-northing’ response threshold. The results from field trapping experiments agree with what was found on the colonisation behaviour, i.e. stronger inhibition with higher amounts of verbenone (Salom et al. 1997; Borden et al. 2003; Bentz et al. 2005; Schiebe et al. 2011; Etxebeste et al. 2013; Byers et al. 2020; Fettig and Munson 2020), but with some exceptions that show a consistent inhibitory effect for various dosages that were tested (Bakke 1987; Safranyik et al. 1992; Devlin and Borden 1994; Fettig et al. 2020).
Some volatiles from other sources including non-host species (and therefore generally referred to as ‘non-host volatiles’, NHV) can also inhibit bark beetle aggregation (Byers et al. 1998, 2004; Zhang and Schlyter 2004; Huber et al. 2020). There is ample evidence across many bark beetle genera, that blends containing various NHVs and verbenone elicit stronger inhibition than each compound alone (e.g. Zhang et al. 2001; Fettig et al. 2005, 2009b; Shepherd et al. 2007; Shepherd and Sullivan 2013; Unelius et al. 2014), suggesting that there is some kind of synergism in attraction inhibition. Even if verbenone and volatiles emitted from plants have different origins and thus ecological meanings, the integration of different stimuli can sometimes lead to a modified behavioural response compared to the stimulus of a single compound (Andersson et al. 2010). For this literature analysis, verbenone was considered a synergistic inhibitor (Table 2), if a blend containing verbenone was inhibitory compared to bait-only controls, regardless of the effect of each individual blend component. From a practical point of view, verbenone and other volatile compounds are often used together in blends to optimise attraction inhibition for tree protection (e.g. Zhang et al. 2000a, b; Zhang and Schlyter 2004; Unelius et al. 2014; Huber et al. 2020).
Ratios of inhibitors vs. attractants might be more relevant than absolute amounts (Bentz et al. 2005). Byers et al. (2020) investigated the effect of relative verbenone concentration compared to the attractant quercivorol in E. fornicatus and found a sigmoidal positive relationship between verbenone release rate and reduction in attraction. This might explain why verbenone occurs at the early stages of colonisation (see Q. 1.2), but without inhibiting attraction at this stage. However, also in studies in which variable absolute amounts of verbenone were tested, the concentrations of the attractants were typically not adapted to the varying verbenone concentrations. Thus, verbenone-to-attractant ratios are not stable, and in fact relative amounts are tested. Bertram and Paine (1994b) conducted the only study to disentangle absolute and relative release rates and found that for D. brevicomis, absolute release rates were more important than ratios. They hypothesised that this could be due to different compound-specific detection thresholds and supports their independent perception and additive effects. Finally, since natural variation in levels of attractive compounds may underlie lower variation compared to inhibitory signals (J.A. Byers, pers. comm.), ratios of attractant-to-inhibitor may matter for some lower concentrations, while extremely high amounts of an inhibitor may be effective regardless of the level of attraction.
Ecological implications
Intraspecific signalling and sex-specific differences
Like most of the aggregation pheromone components that attract both sexes of gregarious bark beetle species to mediate the attack on hosts, there is ample evidence that also the inhibitory effect of verbenone affects both sexes, for example in D. brevicomis (Bedard et al. 1980; Hayes and Strom 1994; Strom et al. 2001; Fettig et al. 2005, 2009a; Erbilgin et al. 2007, 2008), D. ponderosae (Miller et al. 1995; Borden et al. 1998; Fettig et al. 2012b) and D. valens (Zhang et al. 2006; Fettig et al. 2007), but possibly not in D. frontalis (Salom et al. 1992; Hayes et al. 1994) and D. rhizophagous (Cano-Ramírez et al. 2012) in which males showed a greater response. The latter case also seems to be typical for some Ips species. Male I. typographus generally seem to react more strongly to inhibitory compounds (Byers 1993), including verbenone in the context of baited traps (Schlyter et al. 1989; Zhang and Schlyter 2003; Unelius et al. 2014; but contrary to our pers. obs. in the field). Male response to verbenone-baited traps was also more strongly inhibited in I. duplicatus (Zhang et al. 2001). The opposite was the case in a multi-year field study on trap logs (Bakke 1981). Also in I. paraconfusus, I. latidens and I. pini females reacted somewhat more strongly to verbenone or no difference between the sexes was observed (Byers and Wood 1980, 1981; Miller et al. 1995). Furthermore, the frequencies of ORN classes in I. typographus do not appear to differ between sexes (Andersson et al. 2009) and this is also valid for the ORN class that detects verbenone (M.N. Andersson, pers. obs.). Furthermore, no sex difference was found in response to the spatial separation of the aggregation pheromone and verbenone (Binyameen et al. 2014). These findings suggest that a stronger inhibition of males in some Ips species in response to verbenone is likely not due to a higher olfactory sensitivity to the compound.
Altogether, one of the differences between the monogamous genus Dendroctonus and the polygynous genus Ips may be, that in the latter males react somewhat stronger to inhibitory compounds. One might expect that it is more crucial for the colonising sex to discern suitable breeding sites. In many other insects where females choose the oviposition site, they generally show a stronger response than males to host plant volatiles (Szendrei and Rodriguez-Saona 2010). On the other hand, as verbenone biosynthesis is rather associated with male bark beetles (Q1.1) verbenone could represent a general signal for male rivalry (Rudinsky et al. 1974) to avoid competition among male conspecifics. According to Kirkendall (1983), female-initiated monogyny is the ancient mating system of bark and ambrosia beetles. The later joining male has an evolutionary benefit by preventing the mating of the female with a second male (‘guarding’, Kirkendall 1983) and habitat competition by further colonising females. Nevertheless, also females should avoid competition from other egg-laying individuals, as their offspring compete for space and food (Byers and Wood 1980; Byers et al. 1984; Anderbrant et al. 1985). The quantitative analysis of life history traits likewise suggests that the mating system of a species is not strongly associated with its response to verbenone (Fig. 4, e.g. inhibition occurred in 17/26 ≈ 65% of monogamous, 9/12 ≈ 75% of polygamous and 12/15 ≈ 80% of inbreeding species; p = 0.14). In fact, the mating system seems to be of low relevance to predict a species’ response to verbenone compared to its host use strategy (Cramér’s V: 0.19 vs. 0.24, Fig. 4; but, for example, only for six cone/seed feeding species a response to verbenone was described), at least for the pool of species for which a behavioural response to verbenone has been described so far (N = 53). The common response to verbenone may also be conserved even in genera like Ips that has evolved polygynous mating systems. To test this hypothesis, the evolutionary origins of verbenone receptors in various species of bark and ambrosia beetles need to be investigated. Ultimately, sex-specific density regulation may also be caused by other compounds that guide female site selection, e.g. trans-verbenol in D. brevicomis (Byers 1983a, 1984).
Nevertheless, it is noteworthy that verbenone amounts associated with I. typographus and I. paraconfusus are relatively low, while for D. brevicomis and D. frontalis enormously large amounts were found (Q1.2). Ips typographus is assumed to require a comparatively high infestation density when colonising living trees—more than twice the necessary colonisation density as needed for D. brevicomis (Raffa 2001). It could be the reason why I. typographus does not release large amounts of verbenone and suggests that a species-specific interpretation is needed, despite the commonly shared inhibitory effect of verbenone. The differences between sexes in the response to verbenone observed for some scolytine species can also be dose-dependent (López et al. 2013; Zhao et al. 2017; Audley et al. 2020) and are generally never pronounced: there is no report that one sex remained completely unaffected by inhibitory compounds. In conclusion, it therefore seems that verbenone is a more general indicator of high feeding activity of Scolytinae affecting both sexes to some degree to reduce intraspecific competition. At this point, a species-specific perspective is required to characterise the function of verbenone for intraspecific communication and define whether verbenone is a passive cue or an actual signal in a given species.
To assess the ecological function of verbenone, also the spatial range of its effect needs to be considered. It has often been observed that verbenone treatments reduce both the risk of infestation for single trees and the colonisation density within a tree at the same time (Lindgren et al. 1989; Safranyik et al. 1992, and pers. obs.; but see Devlin and Borden 1994). Verbenone did not affect the ratio of landing vs. successfully colonising D. brevicomis, but caused a reduced colonisation density (Bertram and Paine 1994a), suggesting its function as a wide-range inhibitor, i.e. to mediate the colonisation among trees, but not within a tree. For the same species, it was observed that their resting behaviour next to the aggregation pheromone was less frequent in the presence of verbenone (Ryker and Yandell 1983), underlining its function as a short-range inhibitor. The ‘active inhibitory range’ (AIR) can be defined as the maximum distance at which a significant change in beetle behaviour can still be observed compared to untreated controls (Zhang and Schlyter 2003). Most field studies suggest an AIR of verbenone of only a few metres (Shore et al. 1992; Niemeyer et al. 1995; Huber and Borden 2001; Jakuš et al. 2003; Zhang and Schlyter 2003; Ranger et al. 2013; Fettig et al. 2015; Byers et al. 2018, 2020), only a few report inhibition between 10 and 15 m (Mafra-Neto et al. 2014). Concordantly, grid distances of verbenone point sources typically lie between 5 and 9 m in studies where verbenone is tested to inhibit aggregation within a whole area (Borden et al. 1992; Devlin and Borden 1994; Fettig and Munson 2020; Fettig et al. 2020) or 10–15 m (Amman et al. 1989; Lindgren et al. 1989; Devlin and Borden 1994; Borden et al. 2003, 2006; Progar 2005). For some ambrosia beetle species, however, the AIR might be below 1 m, depending on the rate of verbenone release (Byers et al. 2020; Rivera et al. 2020). This might be due to their low tendency to show a gregarious behaviour (Hulcr and Stelinski 2017) and suggests that verbenone might have a greater importance for bark beetles as a short-range inhibitor to mediate spacing within a tree and thereby limit intraspecific competition (Byers et al. 1984). Given that the applied verbenone dosages are often unnaturally high (Bertram and Paine 1994a; McPheron et al. 1997; Pureswaran and Sullivan 2012), the observed AIR could be overestimated compared to natural conditions. This would at least not necessarily contradict the interpretation of verbenone as a short-range inhibitor with an AIR of 1 m or less. Bentz et al. (1997) did not measure pheromone contents, but investigated the attack dynamics of D. ponderosae around a focal tree that was baited for 24 h. They found that attacks continued on an already infested tree, while neighbouring trees were already under attack before the focal tree was saturated. They concluded that the inhibitory effect of verbenone rather lies in the very short range, below one metre, and an attack shift to an adjacent tree in this species is most likely caused by a spread of the aggregation pheromone plume increasing the attractiveness of not yet attacked trees. However, actual verbenone concentrations were not measured in this study. Byers (1984, 1992b) reported a species-specific, minimum distance of spacing apart attack holes for several primary bark beetle species. He likewise suggested that in some species aggregation pheromones and verbenone may play a role in spacing colonisation sites within a tree and allow the reduction in intraspecific competition in the phloem layer.
Interspecific signalling and trophic relationships
Verbenone seems to be ubiquitous among Scolytinae. Thus, the question arises whether verbenone might also help to avoid competition between sympatric bark and ambrosia beetle species. Most studies indicate that this is to the benefit of all competing species, rather than imposing an advantage for only a single species. For example, the commonly co-occurring species I. typographus and Pityogenes chalcographus are both inhibited by verbenone (Bakke 1987; Byers 1993), which is consistent with the fact that their interaction is not strictly negative despite an overlap in habitat preference (Göthlin et al. 2000; Schebeck et al. 2023, and pers. obs. on windfelled Norway spruce). Furthermore, three sympatric pine-feeding species I. latidens, I. pini and D. ponderosae were equally inhibited by verbenone (Miller et al. 1995), and likewise D. ponderosae and D. brevicomis (Hayes and Strom 1994). Inhibition by verbenone seems also common among sympatric ambrosia beetle species (Werle et al. 2019; Rivera et al. 2020). This common response is not surprising, since also attractive pheromone components are sometimes shared among species (Pureswaran et al. 2008).
The hypothesis that verbenone is a general cue to avoid interspecific competition is also supported by the finding that the enantiomeric composition of verbenone usually does not have a great effect on the species’ response (Bakke 1981; Paine and Hanlon 1991; Zhang et al. 2006; Byers et al. 2018) and thus is generic. In addition, the enantiomeric composition of verbenone within a species is rather variable (Q1.1), corroborating its general nature. In a complex olfactory landscape with high levels of background odours (Byers et al. 1984, 2000; Byers 2004; Raffa 2001), this flexibility can help to reliably avoid competition among numerous bark and ambrosia beetles using the same habitat, but also allows secondary species to detect suitable habitats.
Even if verbenone is informative for many sympatric scolytine species and causes the same behavioural response, it may still be possible that some species can exploit this signal to their advantage. Wu et al. (2019) found that both sexes of the sympatric species T. minor and T. yunnanensis are inhibited by verbenone. However, they observed that verbenone biosynthesis was highest in female T. minor and concluded that interspecific competition was regulated by the colonising sex of the less aggressive species. Finally, slight differences in concentration and release rate, probably related to differences in detection thresholds, may further affect intraspecific competition. For example, O. erosus was attracted to high release rates of verbenone, while verbenone at the same release rate caused inhibition in I. sexdentatus, and this was the opposite for low release rates (Etxebeste et al. 2013). Given that in many studies with a focus on practical application, rather unnaturally high release rates of verbenone were used (Bertram and Paine 1994a; McPheron et al. 1997; Pureswaran and Sullivan 2012), thus, fine-tuned and species-specific differences in the response to verbenone to regulate interspecific competition might have remained undetected.
As verbenone is common among Scolytinae, also bark beetle-associated organisms might be able to detect and respond to that cue. Predators, like Thanasimus spp., can detect (Hansen 1983) and respond to aggregation pheromone components of their bark beetle prey (Schlyter et al. 1989; Pureswaran et al. 2008; Heber et al. 2021). At least Thanasimus formicarius possesses neurons specific to (–)-verbenone (Tømmerås 1985), which elicits strong antennal responses with low thresholds (Hansen 1983). Surprisingly, bark beetle predators are not generally attracted by verbenone, even though this interaction has been intensely studied, particularly for the two genera Thanasimus (Col., Cleridae) and Temnochila (Col., Trogossitidae). Even contrary to the expectations, verbenone inhibits the attraction of T. formicarius to bark beetle kairomones (Etxebeste and Pajares 2011; Etxebeste et al. 2013). Also Thanasiumus dubius is either inhibited by (Strom et al. 1999; de Groot and DeBarr 2000) or indifferent to (Payne et al. 1992; Hayes et al. 1994) the addition of verbenone to bark beetle pheromone baited traps. The response of Temnochila chlorodia is rather variable (Gillette et al. 2009) and might depend on the release rate. A relatively high release rate of 50 mg/d increased the attraction for that species to host volatiles with and without bark beetle kairomones (Fettig et al. 2007) and a low release rate of only 2.5 mg/d was inhibitory (Erbilgin et al. 2007), as well as intermediate release rates of 8–12 mg/d (Hayes and Strom 1994; Strom et al. 2001). Also, Temnochila caerulae showed a negative response to verbenone (Etxebeste and Pajares 2011). In a field trapping assay on multiple target species, Thanasiums undulates, Enoclerus sphegeus, Enoclerus lecontei and Lasconotus complex were inhibited, while Lasconotus subcostulatus was not affected (Lindgren and Miller 2002a). The attraction of another bark beetle predator, Corticeus praetermissus, was not consistent across different experiments (Lindgren and Miller 2002a). The authors suggested that predators and parasitoids, that either depend on the presence of adult bark beetles or larvae at an early stage of development, may also avoid brood systems of a certain age and thus negatively react to verbenone. An eight-component blend containing verbenone was an oviposition stimulant for Rhizophagus grandis in a no-choice experiment on larvae from D. valens and D. micans in an artificial phloem medium (Grégoire et al. 1991).
Altogether, the evidence that predators of bark and ambrosia beetles are attracted to verbenone seems rather weak. This might be explained by the fact that the predators that are considered in these studies (mostly Thanasimus spp. and Temnochila spp.) are typically caught in low numbers compared to their bark beetle prey, making it difficult to detect significant trends with the same sample size otherwise used for gregarious bark beetles. Second, verbenone alone might not be attractive for these natural enemies, but require the presence of additional yet unknown cues. It would be possible that natural enemies only have specific periods during which they are susceptible to prey kairomones, while at other times they are indifferent, or exhibit seasonal fluctuations in abundance, making it difficult to study attraction (e.g. see Hayes and Strom 1994; Lindgren and Miller 2002a). There are a large number of natural enemies that prey on adult bark beetles or their larvae from diverse taxonomic groups including parasitoids from Hymenoptera, Diptera, or Acari (Wegensteiner et al. 2015), but these groups have so far received no attention regarding their response to verbenone. This, however, would be an interesting and important aspect to assess the efficacy of verbenone treatments to prevent bark beetle attacks in forest protection.
Conclusions
The antennal detection of and the response to verbenone seems to be well conserved in the highly evolved pheromone system of bark and ambrosia beetles and may thus represent an ancestral state. Verbenone is an attraction inhibitor for at least 38 scolytine species from 16 genera, i.e. 73% of the species for which the behavioural response has been tested, representing < 1% of the existing bark and ambrosia beetle species (Hulcr et al. 2015). An exception to this inhibitory effect may be found in strongly secondary species, which are more often either indifferent to or even attracted by verbenone. Attraction was also reported for a few primary species for specific release rates or during specific phases in the population dynamics. The host usage strategy (i.e. primary vs. secondary or cone/seed feeding) was more strongly related to a species’ response to verbenone than the mating system of a species. While many sympatric bark beetle species are commonly inhibited by verbenone, it is surprising that natural enemies are not consistently attracted by verbenone. More research should be invested in studying the effect of verbenone on other groups of natural enemies, such as parasitoid wasps or predatory flies.
Even if inhibition by verbenone seems most common among bark beetles, one should keep in mind that the most frequently studied taxa are not necessarily good representatives for the extraordinarily high diversity of lifestyles within the Scolytinae and so the description of verbenone as a ‘universal bark beetle repellent’ can be misleading. The term ‘inhibitor’ should be preferred over the term ‘repellent’ because that is what has been tested and proved in most field trapping studies, even though in pest management applications the latter is traditionally more common. Even if verbenone is an inhibitor to numerous species, its precise function may differ among species and vary according to the actual source, the timing of synthesis, sex-specific detection and response thresholds and the resulting spatial range of inhibition, which are not well studied for most species. Most importantly, we lack studies on atmospheric verbenone concentrations around infested trees as most knowledge about pheromone amounts stems from gut extracts, but we do not know how these concentrations relate to actual emissions.
The analysis of more than 200 studies suggests that verbenone can be interpreted as an intra- and interspecific inhibitor of sympatric Scolytinae, most likely to prevent overcrowding on densely colonised hosts. However, results from studies on the temporal patterns of verbenone synthesis often contradict the assumption that verbenone is a compound that is released from adult beetles at later stages to terminate colonisation for the avoidance of intra- and interspecific competition. Instead, relatively large amounts can be found much earlier, for example, in newly emerged beetles, too early to deter mates that are still needed to overwhelm tree defence and mating at this stage. There is a discrepancy, however, between studies on extracts vs. headspace measurements, stressing the importance of verbenone sources outside the beetles.
An increase in verbenone amounts at later stages of colonisation may primarily be caused by microbial processes outside the beetles’ bodies. At least for many species, instead of a pheromone mediating anti-aggregation, verbenone may rather be seen as a passive by-product of intense feeding activity on α-pinene-rich substrate. Bark beetles and their associated microorganisms help to accelerate the oxidation to the final product verbenone. The fact that probably a substantial proportion of this microbial biotransformation of verbenol to verbenone occurs outside the beetles’ body also contradicts the classification of verbenone as an actual pheromone, or requires the perception of beetles as a holobiont (Six 2013) as a more explanatory ecological unit. Nevertheless, a bark beetle species-specific interpretation is necessary to decide whether verbenone can be considered a pheromone of the bark beetle holobiont or whether it is rather described as an allelochemical. Altogether, it is likely that verbenone is a passive cue rather than an active signal in most species, but it remains open whether the beetles can actively control the amounts and the time point of verbenone release in response to intraspecific competition.
Filling these gaps in a more than 50-year-old field of research (Table 4) will on one hand improve our ecological understanding of how tiny bark beetles manage to organise their attacks to mass aggregate on suitable hosts—something, still not possible to predict—and can have effects so large that they become visible on a landscape scale. On the other hand, making use of inhibitory substances is an interesting, environmentally friendly approach to manage bark beetle populations in economically used forests and ambrosia beetles in horticultural tree crops (Table 4). While aggregation pheromones are routinely used for monitoring purposes, inhibition of mass aggregation with semiochemicals still comes with some inherent uncertainty. Despite available technologies, semiochemical-based tools are comparatively rarely used in agriculture and forestry so far, even though conventional insecticides as the historically only alternative decrease in acceptance due to their environmental, social and human health impact, so that more sustainable alternatives are urgently needed (Gillette and Fettig 2021; Mafra-Neto et al. 2022). A better understanding of the species-specific roles of verbenone, including knowledge about actual verbenone release rates from infested trees, the active or non-active participation of the beetles in verbenone release, intrinsic and extrinsic factors that cause beetle individuals to not respond to verbenone and the effects on natural enemies of bark beetles can help to optimise sustainable verbenone-based semiochemical management tools within the integrated management of pest populations.
Author contributions
TF developed the research question, conducted the literature search, summarised the literature, drafted, wrote, and revised the manuscript. MS, TB, and PHWB contributed to the overall structure of the manuscript and especially to the ecological interpretations. MNA, GH, and JK contributed to the content and its interpretation. PHWB helped to develop the idea and direction of this review at the very beginning. HD and TB initiated the project and together with PHWB provided guidance throughout the study. All authors critically revised the manuscript and approved the final version.
Availability of data and materials
The data set and R code generated and analysed during the current study are available from the corresponding author upon reasonable request.
References
Agnello AM et al (2017) Xylosandrus germanus (Coleoptera: Curculionidae: Scolytinae) occurrence, fungal associations, and management trials in New York apple orchards. J Econ Entomol 110:2149–2164. https://doi.org/10.1093/jee/tox189
Agrawal R, Deepika NU, Joseph R (1999) Strain improvement of Aspergillus sp. and Penicillium sp. by induced mutation for biotransformation of α-pinene to verbenol. Biotechnol Bioeng 63:249–252. https://doi.org/10.1002/(SICI)1097-0290(19990420)63:2%3c249::AID-BIT14%3e3.0.CO;2-D
Amman GD, Thier RW, McGregor MD, Schmitz RF (1989) Efficacy of verbenone in reducing lodgepole pine infestation by mountain pine beetles in Idaho. Can J for Res 19:60–64. https://doi.org/10.1139/x89-008
Amman GD, Thier RW, Weatherby JC, Rasmussen LA, Munson AS (1991) Optimum dosage of verbenone to reduce infestation of mountain pine-beetle in lodgepole pine stands of central Idaho. USDA Forest Service Intermountain Research Station Research Paper pp 1–6
Anderbrant O, Schlyter F, Birgersson G (1985) Intraspecific competition affecting parents and offspring in the bark beetle Ips typographus. Oikos 45:89–98. https://doi.org/10.2307/3565226
Andersson MN (2012) Mechanisms of odor coding in coniferous bark beetles: from neuron to behavior and application. Psyche 2012:149572. https://doi.org/10.1155/2012/149572
Andersson MN, Larsson MC, Schlyter F (2009) Specificity and redundancy in the olfactory system of the bark beetle Ips typographus: Single-cell responses to ecologically relevant odors. J Insect Physiol 55:556–567. https://doi.org/10.1016/j.jinsphys.2009.01.018
Andersson MN, Larsson MC, Blaženec M, Jakuš R, Zhang QH, Schlyter F (2010) Peripheral modulation of pheromone response by inhibitory host compound in a beetle. J Exp Biol 213:3332–3339. https://doi.org/10.1242/jeb.044396
Andersson MN, Schlyter F, Hill SR, Dekker T (2012) What reaches the antenna? How to calibrate odor flux and ligand–receptor affinities. Chem Sens 37:403–420. https://doi.org/10.1093/chemse/bjs009
Ascoli-Christensen A, Salom SM, Payne TL (1993) Olfactory receptor cell responses of Ips grandicollis (Eichhoff) (Coleoptera: Scolytidae) to intra- and interspecific behavioral chemicals. J Chem Ecol 19:699–712. https://doi.org/10.1007/bf00985002
Audley JP, Bostock RM, Seybold SJ (2020) Trap assays of the walnut twig beetle, Pityophthorus juglandis Blackman (Coleoptera: Curculionidae: Scolytinae), reveal an effective semiochemical repellent combination. J Chem Ecol 46:1047–1058. https://doi.org/10.1007/s10886-020-01228-9
Bakke A (1981) Inhibition of the response in Ips typographus to the aggregation pheromone; field evaluation of verbenone and ipsenol. J Appl Entomol 92:172–177. https://doi.org/10.1111/j.1439-0418.1981.tb01666.x
Bakke A (1987) Repression of Ips typographus infestations in stored logs by semiochemicals. Scand J for Res 2:179–185. https://doi.org/10.1080/02827588709382456
Bedard WD, Tilden PE, Lindahl KQ, Wood DL, Rauch PA (1980) Effects of verbenone and trans-verbenol on the response of Dendroctonus brevicomis to natural and synthetic attractant in the field. J Chem Ecol 6:997–1013. https://doi.org/10.1007/bf00994657
Bennett RB, Borden JH (1971) Flight arrestment of tethered Dendroctonus pseudotsugae and Trypodendron lineatum (Coleoptera: Scolytidae) in response to olfactory stimuli. Ann Entomol Soc Am 64:1273–1286. https://doi.org/10.1093/aesa/64.6.1273
Bentz BJ, Kegley S, Gibson K, Thier R (2005) A test of high-dose verbenone for stand-level protection of lodgepole and whitebark pine from mountain pine beetle (Coleoptera : Curculionidae: Scolytinae) attacks. J Econ Entomol 98:1614–1621. https://doi.org/10.1093/jee/98.5.1614
Bentz BJ, Logan JA, Powell JA (1997) Spatial and temporal attack dynamics of the mountain pine beetle: implications for management. In: Gregoire JC, Liebhold AM, Stephen FM, Day KR, Salom SM (eds) Integrating cultural tactics into the management of bark beetle and reforestation pests, Proceedings, vol 236. U.S. Department of Agriculture, Forest Service, Northeastern Forest Experiment Station, Radnor, pp 153-162. https://doi.org/10.2737/NE-GTR-236
Berryman AA, Dennis B, Raffa KF, Stenseth NC (1985) Evolution of optimal group attack, with particular reference to bark beetles (Coleoptera: Scolytidae). Ecology 66:898–903. https://doi.org/10.2307/1940552
Bertram SL, Paine TD (1994a) Influence of aggregation inhibitors (verbenone and ipsidenol) on landing and attack behavior of Dendroctonus brevicomis (Coleoptera: Scolytidae). J Chem Ecol 20:1617–1629. https://doi.org/10.1007/BF02059884
Bertram SL, Paine TD (1994b) Response of Dendroctonus brevicomis Le Conte (Coleoptera: Scolytidae) to different release rates and ratios of aggregation semiochemicals and the inhibitors verbenone and ipsdienol. J Chem Ecol 20:2931–2941. https://doi.org/10.1007/BF02098399
Bhattacharyya PK, Prema BR, Kulkarni BD, Pradhan SK (1960) Microbiological transformation of terpenes: hydroxylation of α-pinene. Nature 187:689–690. https://doi.org/10.1038/187689b0
Binyameen M, Jánkuvová J, Blazenec M, Jakus R, Song LW, Schlyter F, Andersson MN (2014) Co-localization of insect olfactory sensory cells improves the discrimination of closely separated odour sources. Funct Ecol 28:1216–1223. https://doi.org/10.1111/1365-2435.12252
Birgersson G, Bergström G (1989) Volatiles released from individual spruce bark beetle entrance holes: quantitative variations during the first week of attack. J Chem Ecol 15:2465–2483. https://doi.org/10.1007/BF01020377
Birgersson G, Schlyter F, Lofqvist J, Bergström G (1984) Quantitative variation of pheromone components in the spruce bark beetle Ips typographus from different attack phases. J Chem Ecol 10:1029–1055. https://doi.org/10.1007/BF00987511
Birgersson G, Schlyter F, Bergström G, Löfqvist J (1988) Individual variation in aggregation pheromone content of the bark beetle, Ips typographus. J Chem Ecol 14:1737–1761. https://doi.org/10.1007/BF01014641
Birgersson G et al (1995) Pheromones in white pine cone beetle, Conophthorus coniperda (Schwarz) (Coleoptera: Scolytidae). J Chem Ecol 21:143–167. https://doi.org/10.1007/bf02036648
Blomquist GJ et al (2010) Pheromone production in bark beetles. Insect Biochem Mol Biol 40:699–712. https://doi.org/10.1016/j.ibmb.2010.07.013
Blumann A, Zeitschel O (1913) Ein Beitrag zur Autoxydation des Terpentinöls. Ber Dtsch Chem Ges 46:1178–1198. https://doi.org/10.1002/cber.191304601154
Borden JH (1997) Disruption of semiochemical-mediated aggregation in bark beetles. In: Cardé RT, Minks AK (eds) Insect pheromone research. Springer, New york, pp 421–438
Borden JH, Devlin DR, Miller DR (1992) Synomones of two sympatric species deter attack by the pine engraver, Ips pini (Coleoptera: Scolytidae). Can J for Res 22:381–387. https://doi.org/10.1139/x92-050
Borden JH, Wilson IM, Gries R, Chong LJ, Pierce HD, Gries G (1998) Volatiles from the bark of trembling aspen, Populus tremuloides Michx. (Salicaceae) disrupt secondary attraction by the mountain pine beetle, Dendroctonus ponderosae Hopkins (Coleoptera: Scolytidae). Chemoecology 8:69–75. https://doi.org/10.1007/pl00001806
Borden JH, Chong LJ, Earle TJ, Huber DPW (2003) Protection of lodgepole pine from attack by the mountain pine beetle, Dendroctonus ponderosae (Coleoptera: Scolytidae) using high doses of verbenone in combination with nonhost bark volatiles. For Chron 79:685–691. https://doi.org/10.5558/tfc79685-3
Borden JH, Birmingham AL, Burleigh JS (2006) Evaluation of the push-pull tactic against the mountain pine beetle using verbenone and non-host volatiles in combination with pheromone-baited trees. For Chron 82:579–590. https://doi.org/10.5558/tfc82579-4
Brand JM, Bracke JW, Britton LN, Markovetz AJ, Barras SJ (1976) Bark beetle pheromones: Production of verbenone by a mycangial fungus of Dendroctonus frontalis. J Chem Ecol 2:195–199. https://doi.org/10.1007/bf00987742
Breer H (1997) Molecular mechanisms of pheromone reception in insect antennae. In: Cardé RT, Minks AK (eds) Insect pheromone research. Springer, New york, pp 115–130
Burbano EG, Wright MG, Gillette NE, Mori S, Dudley N, Jones T, Kaufmann M (2012) Efficacy of traps, lures, and repellents for Xylosandrus compactus (Coleoptera: Curculionidae) and other ambrosia beetles on Coffea arabica plantations and Acacia koa nurseries in Hawaii. Environ Entomol 41:133–140. https://doi.org/10.1603/en11112
Busmann D, Berger RG (1994) Bioconversion of terpenoid hydrocarbons by basidiomycetes. Dev Food Sci 35:503–507. https://www.sciencedirect.com/bookseries/developments-in-foodscience/volumes
Byers JA (1983a) Bark beetle conversion of a plant compound to a sex-specific inhibitor of pheromone attraction. Science 220:624–626. https://doi.org/10.1126/science.220.4597.624
Byers JA (1983b) Influence of sex, maturity and host substances on pheromones in the guts of the bark beetles, Ips paraconfusus and Dendroctonus brevicomis. J Insect Physiol 29:5–13. https://doi.org/10.1016/0022-1910(83)90100-2
Byers JA (1984) Nearest neighbor analysis and simulation of distribution patterns indicates an attack spacing mechanism in the bark beetle, Ips typographus (Coleoptera: Scolytidae). Environ Entomol 13:1191–1290. https://doi.org/10.1093/ee/13.5.1191
Byers JA (1989) Chemical ecology of bark beetles. Experientia 45:271–283. https://doi.org/10.1007/BF01951813
Byers JA (1992a) Attraction of bark beetles, Tomicus piniperda, Hylurgops palliatus, and Trypodendron domesticum and other insects to short-chain alcohols and monoterpenes. J Chem Ecol 18:2385–2402. https://doi.org/10.1007/BF00984957
Byers JA (1992b) Dirichlet tessellation of bark beetle spatial attack points. J Anim Ecol 61:759–768. https://doi.org/10.2307/5629
Byers JA (1993) Avoidance of competition by spruce bark beetles, Ips typographus and Pityogenes chalcographus. Experientia 49:272–275. https://doi.org/10.1007/BF01923539
Byers J (2004) Chemical ecology of bark beetles in a complex olfactory landscape. In: Lieutier F, Day KR, Battisti A, Gregoiré J-C, Evans H (eds) Bark and wood boring insects in living trees in Europe, a synthesis, 2nd edn. Springer, Dordrecht, pp 89–134
Byers JA, Wood DL (1980) Interspecific inhibition of the response of the bark beetles, Dendroctonus brevicomis and Ips paraconfusus, to their pheromones in the field. J Chem Ecol 6:149–164. https://doi.org/10.1007/BF00987534
Byers JA, Wood DL (1981) Interspecific effects of pheromones on the attraction of the bark beetles, Dendroctonus brevicomis and Ips paraconfusus in the laboratory. J Chem Ecol 7:9–18. https://doi.org/10.1007/BF00988631
Byers JA, Wood DL, Craig J, Hendry LB (1984) Attractive and inhibitory pheromones produced in the bark beetle, Dendroctonus brevicomis, during host colonization: Regulation of inter- and intraspecific competition. J Chem Ecol 10:861–877. https://doi.org/10.1007/BF00987969
Byers JA, Lanne BS, Löfqvist J (1989) Host tree unsuitability recognized by pine shoot beetles in flight. Experientia 45:489–492. https://doi.org/10.1007/BF01952042
Byers JA, Zhang Q-H, Schlyter F, Birgersson G (1998) Volatiles from nonhost birch trees inhibit pheromone response in spruce bark beetles. Sci Nat 85:557–561. https://doi.org/10.1007/s001140050551
Byers JA, Zhang Q-H, Birgersson G (2000) Strategies of a bark beetle, Pityogenes bidentatus, in an olfactory landscape. Naturwissenschaften 87:503–507. https://doi.org/10.1007/s001140050768
Byers JA, Zhang Q-H, Birgersson G (2004) Avoidance of nonhost plants by a bark beetle, Pityogenes bidentatus, in a forest of odors. Naturwissenschaften 91:215–219. https://doi.org/10.1007/s00114-004-0520-1
Byers JA, Maoz Y, Wakarchuk D, Fefer D, Levi-Zada A (2018) Inhibitory effects of semiochemicals on the attraction of an ambrosia beetle Euwallacea nr. fornicatus to quercivorol. J Chem Ecol 44:565–575. https://doi.org/10.1007/s10886-018-0959-8
Byers JA, Maoz Y, Fefer D, Levi-Zada A (2020) Semiochemicals affecting attraction of ambrosia beetle Euwallacea fornicatus (Coleoptera: Curculionidae: Scolytinae) to quercivorol: developing push-pull control. J Econ Entomol 113:2120–2127. https://doi.org/10.1093/jee/toaa127
Byers JA, Maoz Y, Cohen B, Golani M, Fefer D, Levi-Zada A (2021) Protecting avocado trees from ambrosia beetles by repellents and mass trapping (push–pull): Experiments and simulations. J Pest Sci. https://doi.org/10.1007/s10340-020-01310-x
Cale JA, Taft S, Najar A, Klutsch JG, Hughes CC, Sweeney JD, Erbilgin N (2015) Mountain pine beetle (Dendroctonus ponderosae) can produce its aggregation pheromone and complete brood development in naïve red pine (Pinus resinosa) under laboratory conditions. Can J for Res 45:1873–1877. https://doi.org/10.1139/cjfr-2015-0277
Cale JA, Ding RS, Wang FA, Rajabzadeh R, Erbilgin N (2019) Ophiostomatoid fungi can emit the bark beetle pheromone verbenone and other semiochemicals in media amended with various pine chemicals and beetle-released compounds. Fungal Ecol 39:285–295. https://doi.org/10.1016/j.funeco.2019.01.003
Cano-Ramírez C, Armendáriz-Toledano F, Macías-Sámano JE, Sullivan BT, Zúñiga G (2012) Electrophysiological and behavioral responses of the bark beetle Dendroctonus rhizophagus to volatiles from host pines and conspecifics. J Chem Ecol 38:512–524. https://doi.org/10.1007/s10886-012-0112-z
Cao QJ, Wickham JD, Chen L, Ahmad F, Lu M, Sun JH (2018) Effect of oxygen on verbenone conversion from cis-verbenol by gut facultative anaerobes of Dendroctonus valens. Front Microbiol. https://doi.org/10.3389/fmicb.2018.00464
Çetin H, Güdek M (2020) Effect of essential oil from the leaves of rosemary used in the control of Callosobruchus maculatus (F.) on the hydration coefficient, cookability, taste and color of the edible chickpea. J Essent Oil Bear Plants 23:301–310. https://doi.org/10.1080/0972060x.2020.1748522
Clarke SR, Salom SM, Billings RF, Berisford CW, Upton WW, McClellan QC, Dalusky MJ (1999) A scentsible approach to controlling southern pine beetles-two new tactics using verbenone. J For 97:26–31. https://doi.org/10.1093/jof/97.7.26
de Groot P, DeBarr GL (2000) Response of cone and twig beetles (Coleoptera: Scolytidae) and a predator (Coleoptera: Cleridae) to pityol, conophthorin, and verbenone. Can Entomol 132:843–851. https://doi.org/10.4039/Ent132843-6
Dethier VG, Browne BL, Smith CN (1960) The designation of chemicals in terms of the responses they elicit from insects. J Econ Entomol 53:134–136. https://doi.org/10.1093/jee/53.1.134
Devlin DR, Borden JH (1994) Efficacy of antiaggregants for the pine engraver, Ips pini (Coleoptera: Scolytidae). Can J for Res 24:2469–2476. https://doi.org/10.1139/x94-318
Díaz-Núñez V, Sanchez-Martinez G, Gillette NE (2006) Response of Dendroctonus mexicanus (Hopkins) to two optical isomers of verbenone. Agrociencia 40:349–354. https://agrociencia-colpos.org/index.php/agrociencia/article/view/468
Dickens JC (1979) Electrophysiological investigations of olfaction in bark beetles. J Swiss Entomol Soc 52:203–216
Dickens JC (1997) Neurobiology of pheromonal signal processing in insects. In: Cardé RT, Minks AK (eds) Insect pheromone research. Springer, New york, pp 210–217
Dickens JC, Payne TL (1978) Olfactory-induced muscle potentials in Dendroctonus frontalis: effects of trans-verbenol and verbenone. Experientia 34:463–464. https://doi.org/10.1007/BF01935928
Dickens JC, Billings RF, Payne TL (1992) Green leaf volatiles interrupt aggregation pheromone response in bark beetles infesting southern pines. Experientia 48:523–524. https://doi.org/10.1007/bf01928180
Dodds KJ, Miller DR (2010) Test of nonhost angiosperm volatiles and verbenone to protect trap trees for Sirex noctilio (Hymenoptera: Siricidae) from attacks by bark beetles (Coleoptera: Scolytidae) in the Northeastern United States. J Econ Entomol 103:2094–2099. https://doi.org/10.1603/ec10225
Dodge C, Coolidge J, Cooperband M, Cosse A, Carrillo D, Stouthamer R (2017) Quercivorol as a lure for the polyphagous and Kuroshio shot hole borers, Euwallacea spp. nr. fornicatus (Coleoptera: Scolytinae), vectors of Fusarium dieback. PeerJ. https://doi.org/10.7717/peerj.3656
Dvořáková M, Valterová I, Vaněk T (2007) Biotransformation of a monoterpene mixture by in vitro cultures of selected conifer species. Nat Prod Commun 2:233–238. https://doi.org/10.1177/1934578X0700200302
Erbilgin N, Gillette NE, Mori SR, Stein JD, Owen DR, Wood DL (2007) Acetophenone as an anti-attractant for the western pine beetle, Dendroctonus brevicomis LeConte (Coleoptera : Scolytidae). J Chem Ecol 33:817–823. https://doi.org/10.1007/s10886-007-9267-4
Erbilgin N, Gillette NE, Owen DR, Mori SR, Nelson AS, Uzoh F, Wood DL (2008) Acetophenone superior to verbenone for reducing attraction of western pine beetle Dendroctonus brevicomis to its aggregation pheromone. Agric for Entomol 10:433–441. https://doi.org/10.1111/j.1461-9563.2008.00407.x
Erman WF (1967) Photochemical transformations of unsaturated bicyclic ketones Verbenone and Its photodynamic products of ultraviolet irradiation. J Am Chem Soc 89:3828–3841. https://doi.org/10.1021/ja00991a026
Etxebeste I, Pajares JA (2011) Verbenone protects pine trees from colonization by the six-toothed pine bark beetle, Ips sexdentatus Boern. (Col.: Scolytinae). J Appl Entomol 135:258–268. https://doi.org/10.1111/j.1439-0418.2010.01531.x
Etxebeste I, Alvarez G, Pajares J (2013) Log colonization by Ips sexdentatus prevented by increasing host unsuitability signaled by verbenone. Entomol Exp Appl 147:231–240. https://doi.org/10.1111/eea.12061
Faccoli M, Blaženec M, Schlyter F (2005) Feeding response to host and nonhost compounds by males and females of the spruce bark beetle Ips typographus in a tunneling microassay. J Chem Ecol 31:745–759. https://doi.org/10.1007/s10886-005-3542-z
Fang JX, Zhang SF, Liu F, Cheng B, Zhang Z, Zhang QH, Kong XB (2020) Functional investigation of monoterpenes for improved understanding of the relationship between hosts and bark beetles. J Appl Entomol. https://doi.org/10.1111/jen.12850
Fettig CJ, Munson AS (2020) Efficacy of verbenone and a blend of verbenone and nonhost volatiles for protecting lodgepole pine from mountain pine beetle (Coleoptera: Curculionidae). Agric for Entomol 22:373–378. https://doi.org/10.1111/afe.12392
Fettig CJ, McKelvey SR, Huber DPW (2005) Nonhost angiosperm volatiles and verbenone disrupt response of western pine beetle, Dendroctonus brevicomis (Coleoptera: Scolytidae), to attractant-baited traps. J Econ Entomol 98:2041–2048. https://doi.org/10.1093/jee/98.6.2041
Fettig CJ, McKelvey SR, Dabney CP, Borys RR (2007) The response of Dendroctonus valens and Temnochila chlorodia to Ips paraconfusus pheromone components and verbenone. Can Entomol 139:141–145. https://doi.org/10.4039/n06-013
Fettig CJ, Dabney CP, McKelvey SR, Huber DPW (2008) Nonhost angiosperm volatiles and verbenone protect individual ponderosa pines from attack by western pine beetle and red turpentine beetle (Coleoptera: Curculionidae, Scolytinae). West J Appl For 23:40–45. https://doi.org/10.1093/wjaf/23.1.40
Fettig CJ, McKelvey SR, Borys RR, Dabney CP, Hamud SM, Nelson LJ, Seybold SJ (2009a) Efficacy of verbenone for protecting ponderosa pine stands from western pine beetle (Coleoptera: Curculionidae: Scolytinae) attack in California. J Econ Entomol 102:1846–1858
Fettig CJ, McKelvey SR, Dabney CP, Borys RR, Huber DPW (2009b) Response of Dendroctonus brevicomis to different release rates of nonhost angiosperm volatiles and verbenone in trapping and tree protection studies. J Appl Entomol 133:143–154. https://doi.org/10.1111/j.1439-0418.2008.01317.x
Fettig CJ, McKelvey SR, Dabney CP, Huber DPW (2012a) Responses of Dendroctonus brevicomis (Coleoptera: Curculionidae) in behavioral assays: implications to development of a semiochemical-based tool for tree protection. J Econ Entomol 105:149–160. https://doi.org/10.1603/ec11121
Fettig CJ, McKelvey SR, Dabney CP, Huber DPW, Lait CG, Fowler DL, Borden JH (2012b) Efficacy of Verbenone plus for protecting ponderosa pine trees and stands from Dendroctonus brevicomis (Coleoptera: Curculionidae) attack in British Columbia and California. J Econ Entomol 105:1668–1680. https://doi.org/10.1603/ec12184
Fettig CJ et al (2013) Factors influencing northern spruce engraver colonization of white spruce slash in interior Alaska. For Ecol Manag 289:58–68. https://doi.org/10.1016/j.foreco.2012.09.040
Fettig CJ, Munson AS, Reinke M, Mafra-Neto A (2015) A novel semiochemical tool for protecting Pinus contorta from mortality attributed to Dendroctonus ponderosae (Coleoptera: Curculionidae). J Econ Entomol 108:173–182. https://doi.org/10.1093/jee/tou038
Fettig CJ, Steed BE, Munson AS, Progar RA, Mafra-Neto A (2020) Evaluating doses of SPLAT® verb to protect lodgepole pine trees and stands from mountain pine beetle. Crop Prot. https://doi.org/10.1016/j.cropro.2020.105228
Flechtmann CAH, Dalusky MJ, Berisford CW (1999) Bark and ambrosia beetle (Coleoptera: Scolytidae) responses to volatiles from aging loblolly pine billets. Environ Entomol 28:638–648. https://doi.org/10.1093/ee/28.4.638
Foote GG et al (2020) A biodegradable formulation of MCH (3-methylcyclohex-2-en-1-one) for protecting Pseudotsuga menziesii from Dendroctonus pseudotsugae (Coleoptera: Curculionidae) colonization. J Econ Entomol. https://doi.org/10.1093/jee/toaa061
Gaylord ML, McKelvey SR, Fettig CJ, McMillin JD (2020) Verbenone inhibits attraction of Ips pini (Coleoptera: Curculionidae) to pheromone-baited traps in Northern Arizona. J Econ Entomol 113:3017–3020. https://doi.org/10.1093/jee/toaa192
Gaylord ML, Audley JP, McMillin JD, Fettig CJ (2023) Acetophenone and green leaf volatiles do not enhance the efficacy of verbenone for inhibiting attraction of Ips pini (Coleoptera: Curculionidae) to pheromone-baited traps in Northern Arizona. J Econ Entomol. https://doi.org/10.1093/jee/toad016
Gibson KE, Schmitz RF, Amman GD, Oakes RD (1991) Mountain pine-beetle response to different verbenone dosages in pine stands of western Montana. USDA Forest Service Intermountain Research Station Research Paper. pp 1–11
Gillette NE, Fettig CJ (2021) Semiochemicals for bark beetle (Coleoptera: Curculionidae) management in western North America: where do we go from here? Can Entomol 153:121–135. https://doi.org/10.4039/tce.2020.61
Gillette NE, Stein JD, Owen DA, Webster JN, Fiddler GO, Mori SR, Wood DL (2006) Verbenone-releasing flakes protect individual Pinus contorta trees from attack by Dendroctonus ponderosae and Dendroctonus valens (Coleoptera: Curculionidae, Scolytinae). Agric for Entomol 8:243–251. https://doi.org/10.1111/j.1461-9563.2006.00303.x
Gillette NE et al (2009) Aerially applied verbenone-releasing laminated flakes protect Pinus contorta stands from attack by Dendroctonus ponderosae in California and Idaho. For Ecol Manag 257:1405–1412. https://doi.org/10.1016/j.foreco.2008.12.017
Gillette NE, Hansen EM, Mehmel CJ, Mori SR, Webster JN, Erbilgin N, Wood DL (2012a) Area-wide application of verbenone-releasing flakes reduces mortality of whitebark pine Pinus albicaulis caused by the mountain pine beetle Dendroctonus ponderosae. Agric for Entomol 14:367–375. https://doi.org/10.1111/j.1461-9563.2012.00577.x
Gillette NE, Mehmel CJ, Mori SR, Webster JN, Wood DL, Erbilgin N, Owen DR (2012b) The push-pull tactic for mitigation of mountain pine beetle (Coleoptera: Curculionidae) damage in lodgepole and whitebark pines. Environ Entomol 41:1575–1586. https://doi.org/10.1603/en11315
Gillette NE, Kegley SJ, Costello SL, Mori SR, Webster JN, Mehmel CJ, Wood DL (2014) Efficacy of verbenone and green leaf volatiles for protecting whitebark and limber pines from attack by mountain pine beetle (Coleoptera: Curculionidae: Scolytinae). Environ Entomol 43:1019–1026. https://doi.org/10.1603/en12330
Göthlin E, Schroeder LM, Lindelöw A (2000) Attacks by Ips typographus and Pityogenes chalcographus on windthrown spruces (Picea abies) during the two years following a storm felling. Scand J for Res 15:542–549. https://doi.org/10.1080/028275800750173492
Graves AD et al (2008) Protection of spruce from colonization by the bark beetle, Ips perturbatus, in Alaska. For Ecol Manag 256:1825–1839. https://doi.org/10.1016/j.foreco.2008.07.008
Grégoire JC, Baisier M, Drumont A, Dahlsten DL, Meyer H, Francke W (1991) Volatile compounds in the larval frass of Dendroctonus valens and Dendroctonus micans (Coleoptera: Scolytidae) in relation to oviposition by the predator, Rhizophagus grandis (Coleoptera: Rhizophagidae). J Chem Ecol 17:2003–2019. https://doi.org/10.1007/BF00992584
Gregoiré J-C, Evans HF (2004) Damage and control of BAWBILT organisms an overview. In: Lieutier F, Day KR, Battisti A, Gregoiré J-C, Evans HF (eds) Bark and wood boring insects in living trees in Europe, a synthesis, 2nd edn. Springer, Dordrecht, pp 19–37
Grosman DM, Salom SM, Ravlin FW, Young RW (1997) Geographic and gender differences in semiochemicals in emerging adult southern pine beetle (Coleoptera: Scolytidae). Ann Entomol Soc Am 90:438–446. https://doi.org/10.1093/aesa/90.4.438
Guo SS et al (2017) Repellence of the main components from the essential oil of Glycosmis lucida Wall. ex Huang against two stored product insects. Nat Prod Res 31:1201–1204. https://doi.org/10.1080/14786419.2016.1226825
Hansen K (1983) Reception of bark beetle pheromone in the predaceous clerid beetle, Thanasimus formicarius (Coleoptera: Cleridae). J Comp Physiol 150:371–378. https://doi.org/10.1007/BF00605026
Hayes JL, Strom BL (1994) 4-Allylanisole as an inhibitor of bark beetle (Coleoptera: Scolytidae) aggregation. J Econ Entomol 87:1586–1594. https://doi.org/10.1093/jee/87.6.1586
Hayes JL, Strom BL, Roton LM, Ingram LL Jr (1994) Repellent properties of the host compound 4-allylanisole to the southern pine beetle. J Chem Ecol 20:1595–1615. https://doi.org/10.1007/BF02059883
Heber T, Helbig CE, Osmers S, Müller MG (2021) Evaluation of attractant composition application rate, and trap type for potential mass trapping of Ips typographus (L.). Forests 12:1727. https://doi.org/10.3390/f12121727
Huber DPW, Borden JH (2001) Protection of lodgepole pines from mass attack by mountain pine beetle, Dendroctonus ponderosae, with nonhost angiosperm volatiles and verbenone. Entomol Exp Appl 99:131–141. https://doi.org/10.1046/j.1570-7458.2001.00811.x
Huber DPW, Fettig CJ, Borden JH (2020) Disruption of coniferophagous bark beetle (Coleoptera: Curculionidae: Scolytinae) mass attack using angiosperm nonhost volatiles: from concept to operational use. Can Entomol. https://doi.org/10.4039/tce.2020.63
Hughes MA, Martini X, Kuhns E, Colee J, Mafra-Neto A, Stelinski LL, Smith JA (2017) Evaluation of repellents for the redbay ambrosia beetle, Xyleborus glabratus, vector of the laurel wilt pathogen. J Appl Entomol 141:653–664. https://doi.org/10.1111/jen.12387
Hulcr J, Stelinski LL (2017) The ambrosia symbiosis: from evolutionary ecology to practical management. Annu Rev Entomol 62:285–303. https://doi.org/10.1146/annurev-ento-031616-035105
Hulcr J, Atkinson TH, Cognato AI, Jordal BH, McKenna DD (2015) Morphology, taxonomy, and phylogenetics of bark beetles. In: Vega FE, Hofstetter RW (eds) bark beetles. Elsevier, Amsterdam, Netherlands, pp 41–84
Hunt DWA, Borden JH (1989) Terpene alcohol pheromone production by Dendroctonus ponderosae and Ips paraconfusus (Coleoptera: Scolytidae) in the absence of readily culturable microorganisms. J Chem Ecol 15:1433–1463. https://doi.org/10.1007/BF01012375
Hunt DWA, Borden JH (1990) Conversion of verbenols to verbenone by yeasts isolated from Dendroctonus ponderosae (Coleoptera: Scolytidae). J Chem Ecol 16:1385–1397. https://doi.org/10.1007/BF01021034
Isidorov VA, Vinogorova VT, Rafałowski K (2003) HS-SPME analysis of volatile organic compounds of coniferous needle litter. Atmos Environ 37:4645–4650. https://doi.org/10.1016/j.atmosenv.2003.07.005
Jacquin-Joly E, Merlin C (2004) Insect olfactory receptors: contributions of molecular biology to chemical ecology. J Chem Ecol 30:2359–2397. https://doi.org/10.1007/s10886-004-7941-3
Jakuš R et al (2003) Overview of development of an anti-attractant based technology for spruce protection against Ips typographus: from past failures to future success. J Pest Sci 76:89–99. https://doi.org/10.1046/j.1439-0280.2003.03020.x
Jaramillo J, Torto B, Mwenda D, Troeger A, Borgemeister C, Poehling HM, Francke W (2013) Coffee berry borer joins bark beetles in coffee klatch. PLoS One. https://doi.org/10.1371/journal.pone.0074277
Kandasamy D et al (2023) Conifer-killing bark beetles locate fungal symbionts by detecting volatile fungal metabolites of host tree resin monoterpenes. PLoS Biol 21:e3001887. https://doi.org/10.1371/journal.pbio.3001887
Kirkendall LR (1983) The evolution of mating systems in bark and ambrosia beetles (Coleoptera: Scolytidae and Platypodidae). Zool J Linn Soc 77:293–352. https://doi.org/10.1111/j.1096-3642.1983.tb00858.x
Kohnle U, Densborn S, Duhme D, Vité JP (1992) Bark beetle attack on host logs reduced by spraying with repellents. J Appl Entomol 114:83–90
Kostyk BC, Borden JH, Gries G (1993) Photoisomerization of antiaggregation pheromone verbenone: Biological and practical implications with respect to the mountain pine beetle, Dendroctonus ponderosae Hopkins (Coleoptera: Scolytidae). J Chem Ecol 19:1749–1759. https://doi.org/10.1007/BF00982305
Lanne BS et al (1987) Differences in attraction to semiochemicals present in sympatric pine shoot beetles, Tomicus minor and T. piniperda. J Chem Ecol 13:1045–1067. https://doi.org/10.1007/BF01020537
Leonhardt Sara D, Menzel F, Nehring V, Schmitt T (2016) Ecology and evolution of communication in social insects. Cell 164:1277–1287. https://doi.org/10.1016/j.cell.2016.01.035
Leufvén A, Birgersson G (1987) Quantitative variation of different monoterpenes around galleries of Ips typographus (Colleoptera: Scolytidae) attacking Norway spruce. Can J Bot 65:1038–1044. https://doi.org/10.1139/b87-144
Leufvén A, Bergström G, Falsen E (1984) Interconversion of verbenols and verbenone by identified yeasts isolated from the spruce bark beetle Ips typographus. J Chem Ecol 10:1349–1361. https://doi.org/10.1007/BF00988116
Liazid A, Barbero GF, Palma M, Brigui J, Barroso CG (2007) Optimization of a new extraction technique for analysis of verbenone and cis-verbenol in pine seeds. Chromatographia 66:571–575. https://doi.org/10.1365/s10337-007-0354-z
Libbey LM, Morgan ME, Putnam TB, Rudinsky JA (1974) Pheromones released during inter-and intra-sex response of the scolytid beetle Dendroctonus brevicomis. J Insect Physiol 20:1667–1671. https://doi.org/10.1016/0022-1910(74)90095-X
Lieutier F (2004) Host resistance to bark beetles and its variations. In: Lieutier F, Day ER, Battisti A, Grégoire J-C, Evans H (eds) Bark and wood boring insects in living trees in Europe-a synthesis, 2nd edn. Springer, Dordecht, pp 135–180
Lieutier F, Cheniclet C, Garcia J (1989) Comparison of the defense reactions of Pinus pinaster and Pinus sylvestris to attacks by two bark beetles (Coleoptera: Scolytidae) and their associated fungi. Environ Entomol 18:228–234. https://doi.org/10.1093/ee/18.2.228
Lindgren BS, Borden JH (1993) Displacement and aggregation of mountain pine beetles, Dendroctonus ponderosae (Coleoptera: Scolytidae), in response to their antiaggregation and aggregation pheromones. Can J for Res 23:286–290. https://doi.org/10.1139/x93-038
Lindgren BS, Miller DR (2002a) Effect of verbenone on attraction of predatory and woodboring beetles (Coleoptera) to kairomones in lodgepole pine forests. Environ Entomol 31:766–773
Lindgren BS, Miller DR (2002b) Effect of verbenone on five species of bark beetles (Coleoptera: Scolytidae) in lodgepole pine forests. Environ Entomol 31:759–765. https://doi.org/10.1603/0046-225X-31.5.759
Lindgren BS, Raffa KF (2013) Evolution of tree killing in bark beetles (Coleoptera: Curculionidae): trade-offs between the maddening crowds and a sticky situation. Can Entomol 145:471–495. https://doi.org/10.4039/tce.2013.27
Lindgren BS, Borden JH, Cushon GH, Chong LJ, Higgins CJ (1989) Reduction of mountain pine beetle (Coleoptera: Scolytidae) attacks by verbenone in lodgepole pine stands in British Columbia. Can J For Res 19:65–68. https://doi.org/10.1139/x89-009
Lindmark-Henriksson M, Isaksson D, Sjödin K, Högberg HE, Vaněk T, Valterová I (2003) Transformation of α-pinene using Picea abies suspension culture. J Nat Prod 66:337–343. https://doi.org/10.1021/np020426m
Lindström M, Norin T, Birgersson G, Schlyter F (1989) Variation of enantiomeric composition of α-pinene in Norway spruce, Picea abies, and its influence on production of verbenol isomers by Ips typographus in the field. J Chem Ecol 15:541–548. https://doi.org/10.1007/bf01014699
Liu F, Wu CX, Zhang SF, Kong XB, Zhang Z, Wang PY (2019) Initial location preference together with aggregation pheromones regulate the attack pattern of Tomicus brevipilosus (Coleoptera: Curculionidae) on Pinus kesiya. Forests. https://doi.org/10.3390/f10020156
Livingston WH, Bedard WD, Mangini AC, Kinzer HG (1983) Verbenone interrupts attraction of roundheaded pine beetle, Dendroctonus adjunctus (Coleoptera: Scolytidae), to sources of its natural attractant. J Econ Entomol 76:1041–1043. https://doi.org/10.1093/jee/76.5.1041
López S, Quero C, Iturrondobeitia JC, Guerrero A, Goldarazena A (2013) Electrophysiological and behavioural responses of Pityophthorus pubescens (Coleoptera: Scolytinae) to (E, E)-α-farnesene, (R)-(+)-limonene and (S)-(-)-verbenone in Pinus radiata (Pinaceae) stands in northern Spain. Pest Manag Sci 69:40–47. https://doi.org/10.1002/ps.3359
Mafra-Neto A, Fettig CJ, Munson AS, Stelinski LL (2014) Use of repellents formulated in specialized pheromone and lure application technology for effective insect pest management. In: Debboun M, Frances SP, Strickman D (eds) Insect repellents handbook, 2nd edn. CRC Press, Boca Raton, Florida, pp 291–313
Mafra-Neto A et al (2022) CHAPTER 15-Repellent semiochemical solutions to mitigate the impacts of global climate change on arthropod pests. In: Corona C, Debboun M, Coats J (eds) Advances in arthropod repellents. Academic Press, Cambridge, Massachusetts, pp 279–322. https://doi.org/10.1016/B978-0-323-85411-5.00010-8
Martini X, Sobel L, Conover D, Mafra-Neto A, Smith J (2020) Verbenone reduces landing of the redbay ambrosia beetle, vector of the laurel wilt pathogen, on live standing redbay trees. Agric For Entomol 22:83–91. https://doi.org/10.1111/afe.12364
McCullough DG, Haack RA, McLane WH (1998) Control of Tomicus piniperda (Coleoptera : Scolytidae) in pine stumps and logs. J Econ Entomol 91:492–499. https://doi.org/10.1093/jee/91.2.492
McPheron LJ, Seybold SJ, Storer AJ, Wood DL, Ohtsuka T, Kubo I (1997) Effects of enantiomeric blend of verbenone on response of Ips paraconfusus to naturally produced aggregation pheromone in the laboratory. J Chem Ecol 23:2825–2839. https://doi.org/10.1023/A:1022571212416
Miller DR, Borden JH, Lindgren BS (1995) Verbenone: Dose-dependent interruption of pheromone-based attraction of three sympatric species of pine bark beetles (Coleoptera: Scolytidae). Environ Entomol 24:692–696. https://doi.org/10.1093/ee/24.3.692
Nagel R, Hammerbacher A, Kunert G, Phillips MA, Gershenzon J, Schmidt A (2022) Bark beetle attack history does not influence the induction of terpene and phenolic defenses in mature Norway spruce (Picea abies) trees by the bark beetle-associated fungus Endoconidiophora polonica. Front Plant Sci. https://doi.org/10.3389/fpls.2022.892907
Němec V, Zumr V, Starý P (1993) Studies on the nutritional state and the response to aggregation pheromones in the bark beetle, Ips typographus (L.)(Col., Scolytidae). J Appl Entomol 116:358–363. https://doi.org/10.1111/j.1439-0418.1993.tb01208
Niemeyer H, Lenarduzzi M, Watzek G (1995) Effects of verbenone on the spruce bark beetle Ips typographus L (Col, Scolytidae). Anz Schädlkd Pflanzenschutz Umweltschutz 68:182–186. https://doi.org/10.1007/bf01927743
Nordlund DA, Lewis WJ (1976) Terminology of chemical releasing stimuli in intraspecific and interspecific interactions. J Chem Ecol 2:211–220. https://doi.org/10.1007/BF00987744
Paine TD, Hanlon CC (1991) Response of Dendroctonus brevicomis and Ips paraconfusus (Coleoptera: Scolytidae) to combinations of synthetic pheromone attractants and inhibitors verbenone and ipsdienol. J Chem Ecol 17:2163–2176. https://doi.org/10.1007/BF00987999
Patil I (2021) Visualizations with statistical details: the ggstatsplot approach. J Open Source Softwm. https://doi.org/10.21105/joss.03167
Payne TL, Billings RF (1989) Evaluation of (s)-verbenone applications for suppressing southern pine beetle (Coleoptera: Scolytidae) infestations. J Econ Entomol 82:1702–1708. https://doi.org/10.1093/jee/82.6.1702
Payne TL, Billings RF, Berisford CW, Salom SM, Grosman DM, Dalusky MJ, Upton WW (1992) Disruption of Dendroctonus frontalis (Col., Scolytidae) infestations with an inhibitor pheromone. J Appl Entomol 114:341–347. https://doi.org/10.1111/j.1439-0418.1992.tb01137.x
Pedrali A, Robustelli Della Cuna FS, Grisoli P, Corti M, Brusotti G (2019) Chemical composition and antimicrobial activity of the essential oil from the bark of Xylopia hypolampra. Nat Prod Commun. https://doi.org/10.1177/1934578x19857022
Perkins DL, Jorgensen CL, Rinella MJ (2015) Verbenone decreases whitebark pine mortality throughout a mountain pine beetle outbreak. For Sci 61:747–752. https://doi.org/10.5849/forsci.14-052
Perkins DL, Jorgensen CL, Rinella M (2011) Protecting whitebark pines through a mountain pine beetle epidemic with verbenone - is it working? In: Keane RE, Tomback DF, Murray MP, Smith CM (eds) Future of high-elevation, five-needle white pines in Western North America: proceedings of the high five symposium, 2010, vol 63. USDA Forest Service Rocky Mountain Research Station Proceedings. 94–95.
Pettersson EM, Boland W (2003) Potential parasitoid attractants, volatile composition throughout a bark beetle attack. Chemoecology 13:27–37. https://doi.org/10.1007/s000490300003
Poland TM, De Groot P, Burke S, Wakarchuk D, Haack RA, Nott R (2004) Semiochemical disruption of the pine shoot beetle, Tomicus piniperda (Coleoptera: Scolytidae). Environ Entomol 33:221–226. https://doi.org/10.1603/0046-225x-33.2.221
Progar RA (2003) Verbenone reduces mountain pine beetle attack in lodgepole pine. West J Appl For 18:229–232. https://doi.org/10.1093/wjaf/18.4.229
Progar RA (2005) Five-year operational trial of verbenone to deter mountain pine beetle (Dendroctonus ponderosae; Coleoptera : Scolytidae) attack of lodgepole pine (Pinus contorta). Environ Entomol 34:1402–1407. https://doi.org/10.1603/0046-225x-34.6.1402
Progar RA et al (2013) Population densities and tree diameter effects associated with verbenone treatments to reduce mountain pine beetle-caused mortality of lodgepole pine. J Econ Entomol 106:221–228. https://doi.org/10.1603/ec12292
Progar RA et al (2021) Comparisons of efficiency of two formulations of verbenone (4, 6, 6-trimethylbicyclo [3.1.1] hept-3-en-2-one) for protecting whitebark pine, Pinus albicaulis (Pinales: Pinaceae) from mountain pine beetle (Colopetera: Curculionidae). J Econ Entomol 114:209–214. https://doi.org/10.1093/jee/toaa289
Pureswaran DS, Borden JH (2003) Is bigger better? Size and pheromone production in the mountain pine beetle, Dendroctonus ponderosae Hopkins (Coleoptera: Scolytidae). J Insect Behav 16:765–782. https://doi.org/10.1023/B:JOIR.0000018319.37649.c4
Pureswaran DS, Sullivan BT (2012) Semiochemical emission from individual galleries of the southern pine beetle, (Coleoptera: Curculionidae: Scolytinae), attacking standing trees. J Econ Entomol 105:140–148. https://doi.org/10.1603/ec11222
Pureswaran DS, Gries R, Borden JH, Pierce HD (2000) Dynamics of pheromone production and communication in the mountain pine beetle, Dendroctonus ponderosae Hopkins, and the pine engraver, Ips pini (Sag) (Coleoptera : Scolytidae). Chemoecology 10:153–168. https://doi.org/10.1007/pl00001818
Pureswaran DS, Hofstetter RW, Sullivan BT (2008) Attraction of the southern pine beetle, Dendroctonus frontalis, to pheromone components of the western pine beetle, Dendroctonus brevicomis (Coleoptera: Curculionidae: Scolytinae), in an allopatric zone. Environ Entomol 37:70–78. https://doi.org/10.1603/0046-225x(2008)37[70:aotspb]2.0.co;2
Raffa KF (2001) Mixed messages across multiple trophic levels: the ecology of bark beetle chemical communication systems. Chemoecology 11:49–65. https://doi.org/10.1007/PL00001833
Raffa K, Berryman AA (1983) The role of host plant resistance in the colonization behavior and ecology of bark beetles (Coleoptera: Scolytidae). Ecological monographs. https://doi.org/10.2307/1942586
Raffa KF, Grégoire JC, Lindgren BS (2015) Natural history and ecology of bark beetles. In: Vega FE, Hofstetter RW (eds) Bark beetles. Academic Press, Cambridge, Massachusetts, pp 1–40
Ramakrishnan R et al (2022) Metabolomics and transcriptomics of pheromone biosynthesis in an aggressive forest pest Ips typographus. Insect Biochem Mol Biol 140:103680. https://doi.org/10.1016/j.ibmb.2021.103680
Ranger CM et al (2013) Interruption of the semiochemical-based attraction of ambrosia beetles to ethanol-baited traps and ethanol-injected trap trees by verbenone. Environ Entomol 42:539–547. https://doi.org/10.1603/en13016
Ranger CM, Gorzlancyk AM, Addesso KM, Oliver JB, Reding ME, Schultz PB, Held DW (2014) Conophthorin enhances the electroantennogram and field behavioural response of Xylosandrus germanus (Coleoptera: Curculionidae) to ethanol. Agric For Entomol 16:327–334. https://doi.org/10.1111/afe.12062
Rappaport NG, Stein JD, Mora AAD, DeBarr G, de Groot P, Mori S (2000) Responses of Conophthorus spp. (Coleoptera: Scolytidae) to behavioral chemicals in field trials: a transcontinental perspective. Can Entomol 132:925–937. https://doi.org/10.4039/Ent132925-6
Rappaport NG, Owen DR, Stein JD (2001) Interruption of semiochemical-mediated attraction of Dendroctonus valens (Coleoptera : Scolytidae) and selected nontarget insects by verbenone. Environ Entomol 30:837–841. https://doi.org/10.1603/0046-225x-30.5.837
Reeve JD, Strom BL (2004) Statistical problems encountered in trapping studies of scolytids and associated insects. J Chem Ecol 30:1575–1590. https://doi.org/10.1023/B:JOEC.0000042069.17533.3c
Renwick J (1967) Identification of two oxygenated terpenes from the bark beetles Dendroctonus frontalis and Dendroctonus brevicomis. Contrib Boyce Thompson Inst 23:355–360
Renwick JAA, Vité JP (1969) Bark beetle attractants: mechanism of colonization by Dendroctonus frontalis. Nature 224:1222–1223. https://doi.org/10.1038/2241222a0
Richerson JV, Payne TL (1979) Effects of bark beetle inhibitors on landing and attack behavior of the southern pine beetle and beetle associates. Environ Entomol 8:360–364
Rivera MJ, Martini X, Conover D, Mafra-Neto A, Carrillo D, Stelinski LL (2020) Evaluation of semiochemical based push-pull strategy for population suppression of ambrosia beetle vectors of laurel wilt disease in avocado. Sci Rep. https://doi.org/10.1038/s41598-020-59569-0
Rivera-Davila OL, Sanchez-Martinez G, Rico-Martinez R (2021) Ecotoxicity of pesticides and semiochemicals used for control and prevention of conifer bark beetle (Dendroctonus spp.) outbreaks. Chemosphere. https://doi.org/10.1016/j.chemosphere.2020.128375
Roberts RE et al (2022) Odorant receptor orthologues in conifer-feeding beetles display conserved responses to ecologically relevant odours. Mol Ecol n/a. https://doi.org/10.1111/mec.16494
Romón P, Iturrondobeitia JC, Gibson K, Lindgren BS, Goldarazena A (2007) Quantitative association of bark beetles with pitch canker fungus and effects of verbenone on their semiochemical communication in monterey pine forests in Northern Spain. Environ Entomol 36:743–750. https://doi.org/10.1603/0046-225x(2007)36[743:qaobbw]2.0.co;2
Rudinsky JA (1973) Multiple functions of the southern pine beetle pheromone verbenone. Environ Entomol 2:511–514. https://doi.org/10.1093/ee/2.4.511
Rudinsky JA, Morgan ME, Libbey LM, Putnam TB (1974) Antiaggregative-rivalry pheromone of the mountain pine beetle, and a new arrestant of the southern pine beetle. Environ Entomol 3:90–98. https://doi.org/10.1093/ee/3.1.90
Ryker LC, Yandell KL (1983) Effect of verbenone on aggregation of Dendroctonus ponderosae Hopkins (Coleoptera, Scolytidae) to synthetic attractant. J Appl Entomol 96:452–459
Safranyik L, Shore TL, Linton DA, Lindgren BS (1992) The effect of verbenone on dispersal and attack of the mountain pine beetle, Dendroctonus ponderosae Hopk. (Col., Scolytidae) in a lodgepole pine stand. J Appl Entomol 113:391–397. https://doi.org/10.1111/j.1439-0418.1992.tb00679.x
Sallé A, Raffa KF (2007) Interactions among intraspecific competition, emergence patterns, and host selection behaviour in Ips pini (Coleoptera: Scolytinae). Ecol Entomol 32:162–171. https://doi.org/10.1111/j.1365-2311.2006.00833.x
Salom SM et al (1992) Effect of verbenone enantiomers and racemic endo-brevicomin on response of Dendroctonus frontalis (Coleoptera: Scolytidae) to attractant-baited traps. Can J For Res 22:925–931. https://doi.org/10.1139/x92-123
Salom SM, Grosman DM, McClellan QC, Payne TL (1995) Effect of an inhibitor-based suppression tactic on abundance and distribution of the southern pine beetle (Coleoptera: Scolytidae) and its natural enemies. J Econ Entomol 88:1703–1716. https://doi.org/10.1093/jee/88.6.1703
Salom SM, Billings RF, Berisford CW, Clarke SR, McClellan QC, Upton WW, Dalusky MJ (1997) Integrating tree felling with application of an inhibitor pheromone for suppressing southern pine beetle infestations. vol 236. Integrating cultural tactics into the management of bark beetle and reforestation pests, Proceedings. U.S. Department of Agriculture, Forest Service, Northeastern Forest Experiment Station, Radnor
Schebeck M, Schopf A, Ragland GJ, Stauffer C, Biedermann PH (2023) Evolutionary ecology of the bark beetles Ips typographus and Pityogenes chalcographus. Bull Entomol Res 113:1–10. https://doi.org/10.1017/S0007485321000353
Schiebe C, Blaženec M, Jakuš R, Unelius CR, Schlyter F (2011) Semiochemical diversity diverts bark beetle attacks from Norway spruce edges. J Appl Entomol 135:726–737. https://doi.org/10.1111/j.1439-0418.2011.01624.x
Schlyter F, Birgersson G, Byers JA, Löfqvist J, Bergström G (1987) Field response of spruce bark beetle, Ips typographus, to aggregation pheromone candidates. J Chem Ecol 13:701–716. https://doi.org/10.1007/BF01020153
Schlyter F, Byers JA, Löfqvist J, Leufvén A, Birgersson G (1988) Reduction of attack density of the bark beetles Ips typographus and Tomicus piniperda on host bark by verbenone inhibition of attraction to pheromone and host kairomone. In: Payne TL, Saarenmaa H (eds) Integrated control of scolytid bark beetles. Virginia Tech Press, Blacksburg, pp 53–68
Schlyter F, Birgersson G, Leufven A (1989) Inhibition of attraction to aggregation pheromone by verbenone and ipsenol: density regulation mechanisms in bark beetle Ips typographus. J Chem Ecol 15:2263–2277. https://doi.org/10.1007/BF01014114
Schlyter F, Zhang Q-H, Anderson P, Byers JA, Wadhams LJ, Löfqvist J, Birgersson G (2000) Electrophysiological and behavioural responses of Tomicus piniperda and Tomicus minor (Coleoptera: Scolytidae) to non-host leaf and bark volatiles. Can Entomol 132:965–981. https://doi.org/10.4039/Ent132965-6
Schmitz RF, McGregor MD (1990) Antiaggregative effect of verbenone on response of the mountain pine beetle to baited traps. USDA Forest Service Intermountain Research Station Research Paper 1–8
Shea PJ, McGregor MD, Daterman GE (1992) Aerial application of verbenone reduces attack of lodgepole pine by mountain pine beetle. Can J For Res 22:436–441. https://doi.org/10.1139/x92-057
Shepherd WP, Sullivan BT (2013) Southern pine beetle, Dendroctonus frontalis, antennal and behavioral responses to nonhost leaf and bark volatiles. J Chem Ecol 39:481–493. https://doi.org/10.1007/s10886-013-0265-4
Shepherd WP, Huber DPW, Seybold SJ, Fettig CJ (2007) Antennal responses of the western pine beetle, Dendroctonus brevicomis (Coleoptera : Curculionidae), to stem volatiles of its primary host, Pinus ponderosa, and nine sympatric nonhost angiosperms and conifers. Chemoecology 17:209–221. https://doi.org/10.1007/s00049-007-0378-8
Shi ZH, Sun JH (2010) Quantitative variation and biosynthesis of hindgut volatiles associated with the red turpentine beetle, Dendroctonus valens LeConte, at different attack phases. Bull Entomol Res 100:273–277. https://doi.org/10.1017/s0007485309990228
Shimmo M, Jäntti J, Aalto P, Hartonen K, Hyötyläinen T, Kulmala M, Riekkola M-L (2004) Characterisation of organic compounds in aerosol particles from a Finnish forest by on-line coupled supercritical fluid extraction–liquid chromatography–gas chromatography–mass spectrometry. Anal Bioanal Chem 378:1982–1990. https://doi.org/10.1007/s00216-003-2424-x
Shiota Y, Sakurai T (2020) Molecular mechanisms of sex pheromone reception in moths. In: Ishikawa Y (ed) Insect sex pheromone research and beyond. Springer, Singapore, pp 185–206
Shore TL, Safranyak L, Lindgren BS (1992) The response of mountain pine beetle (Dendroctonus ponderosae) to lodgepole pine trees baited with verbenone and exo-brevicomin. J Chem Ecol 18:533–541. https://doi.org/10.1007/BF00987817
Silverstein RM, Rodin JO, Wood DL (1966) Sex attractants in frass produced by male Ips confusus in ponderosa pine. Science 154:509–510. https://doi.org/10.1126/science.154.3748.509
Six DL (2013) The bark beetle holobiont: why microbes matter. J Chem Ecol 39:989–1002. https://doi.org/10.1007/s10886-013-0318-8
Six DL, Bracewell R (2015) Dendroctonus. In: Vega FE, Hofstetter RW (eds) Bark beetles. Academic Press, Cambridge, Massachusetts, pp 305–351
Stökl J, Steiger S (2017) Evolutionary origin of insect pheromones. Curr Opin Insect Sci 24:36–42. https://doi.org/10.1016/j.cois.2017.09.004
Strom BL, Roton LM, Goyer RA, Meeker JR (1999) Visual and semiochemical disruption of host finding in the southern pine beetle. Ecol Appl 9:1028–1038. https://doi.org/10.2307/2641348
Strom BL, Goyer RA, Shea PJ (2001) Visual and olfactory disruption of orientation by the western pine beetle to attractant-baited traps. Entomol Exp Appl 100:63–67. https://doi.org/10.1046/j.1570-7458.2001.00848.x
Strom BL, Smith SL, Brownie C (2013) Attractant and disruptant semiochemicals for Dendroctonus jeffreyi (Coleoptera: Curculionidae: Scolytinae). Environ Entomol 42:323–332. https://doi.org/10.1603/en12300
Su C-Y, Menuz K, Reisert J, Carlson JR (2012) Non-synaptic inhibition between grouped neurons in an olfactory circuit. Nature 492:66–71. https://doi.org/10.1038/nature11712
Sullivan BT, Dalusky MJ, Wakarchuk D, Berisford CW (2007) Field evaluations of potential aggregation inhibitors for the southern pine beetle, Dendroctonus frontalis (Coleoptera: Curculionidae). J Entomol Sci 42:139–149. https://doi.org/10.18474/0749-8004-42.2.139
Sun JH, Gillette NE, Miao ZW, Kang L, Zhang ZN, Owen DR, Stein JD (2003) Verbenone interrupts attraction to host volatiles and reduces attack on Pinus tabuliformis (Pinaceae) by Dendroctonus valens (Coleoptera: Scolytidae) in the People’s Republic of China. Can Entomol 135:721–732. https://doi.org/10.4039/n03-021
Szendrei Z, Rodriguez-Saona C (2010) A meta-analysis of insect pest behavioral manipulation with plant volatiles. Entomol Exp Appl 134:201–210. https://doi.org/10.1111/j.1570-7458.2009.00954.x
Szmigielski R, Cieslak M, Rudziński KJ, Maciejewska B (2012) Identification of volatiles from Pinus silvestris attractive for Monochamus galloprovincialis using a SPME-GC/MS platform. Environ Sci Pollut Res 19:2860–2869. https://doi.org/10.1007/s11356-012-0792-5
Taft S, Najar A, Erbilgin N (2015) Pheromone production by an invasive bark beetle varies with monoterpene composition of its naïve host. J Chem Ecol 41:540–549. https://doi.org/10.1007/s10886-015-0590-x
Tilden PE, Bedard WD (1988) Effect of verbenone on response of Dendroctonus brevicomis to exo-brevicomin, frontalin, and myrcene. J Chem Ecol 14:113–122. https://doi.org/10.1007/BF01022535
Tillman JA, Seybold SJ, Jurenka RA, Blomquist GJ (1999) Insect pheromones—an overview of biosynthesis and endocrine regulation. Insect Biochem Mol Biol 29:481–514. https://doi.org/10.1016/S0965-1748(99)00016-8
Tittiger C, Blomquist GJ (2016) Pheromone production in pine bark beetles. In: Tittiger C, Blomquist GJ (eds) Pine bark beetles, vol 50. Advances in insect physiology. Academic Press Ltd-Elsevier Science Ltd, London, pp 235–263. https://doi.org/10.1016/bs.aiip.2016.02.002
Tobin KN, Ginzel MD (2022) The ambrosia beetle Anisandrus maiche (Stark) is repelled by conophthorin and verbenone and attracted to ethanol in a dose-dependent manner. Agric For Entomol. https://doi.org/10.1111/afe.12534
Tømmerås BÅ (1985) Specialization of the olfactory receptor cells in the bark beetle Ips typographus and its predator Thanasimus formicarius to bark beetle pheromones and host tree volatiles. J Comp Physiol 157:335–341. https://doi.org/10.1007/BF00618123
Trapp T, Zajul M, Ahlborn J, Stephan A, Zorn H, Fraatz MA (2018) Submerged cultivation of Pleurotus sapidus with molasses: aroma dilution analyses by means of solid phase microextraction and stir bar sorptive extraction. J Agric Food Chem 66:2393–2402. https://doi.org/10.1021/acs.jafc.6b05292
Unelius CR, Schiebe C, Bohman B, Andersson MN, Schlyter F (2014) Non-host volatile blend optimization for forest protection against the European spruce bark beetle, Ips typographus. PLoS One. https://doi.org/10.1371/journal.pone.0085381
Valerio-Mendoza O et al (2019) Cryptic species discrimination in Western Pine Beetle, Dendroctonus brevicomis LeConte (Curculionidae: Scolytinae), based on morphological characters and geometric morphometrics. InSects 10:377. https://doi.org/10.3390/insects10110377
VanDerLaan NR, Ginzel MD (2013) The capacity of conophthorin to enhance the attraction of two Xylosandrus species (Coleoptera: Curculionidae: Scolytinae) to ethanol and the efficacy of verbenone as a deterrent. Agric For Entomol 15:391–397. https://doi.org/10.1111/afe.12026
Visser JH, de Jong R (1988) Olfactory coding in the perception of semiochemicals. J Chem Ecol 14:2005–2018. https://doi.org/10.1007/BF01014246
Vité JP, Baader E (1990) Present and future use of semiochemicals in pest management of bark beetles. J Chem Ecol 16:3031–3041. https://doi.org/10.1007/BF00979610
Vité JP, Francke W (1985) Waldschutz gegen Borkenkäfer: Vom Fangbaum zur Falle. Chem Unserer Zeit 19:11–21. https://doi.org/10.1002/ciuz.19850190103
Wegensteiner R, Wermelinger B, Herrmann M (2015) Natural enemies of bark beetles: predators, parasitoids, pathogens, and nematodes. In: Vega FE, Hofstetter RW (eds) Bark beetles. Elsevier, Amsterdam, Netherlands, pp 247–304
Werle CT, Ranger CM, Schultz PB, Reding ME, Addesso KM, Oliver JB, Sampson BJ (2019) Integrating repellent and attractant semiochemicals into a push-pull strategy for ambrosia beetles (Coleoptera: Curculionidae). J Appl Entomol 143:333–343. https://doi.org/10.1111/jen.12594
Whitehead AT (1986) Electroantennogram responses by mountain pine beetles, Dendroctonus ponderosae Hopkins, exposed to selected semiochemicals. J Chem Ecol 12:1603–1621. https://doi.org/10.1007/BF01020267
Whittle M, Barreaux AMG, Bonsall MB, Ponton F, English S (2021) Insect-host control of obligate, intracellular symbiont density. Proc Biol Sci 288:20211993. https://doi.org/10.1098/rspb.2021.1993
Wu CX, Liu F, Zhang SF, Kong XB, Zhang Z (2019) Semiochemical regulation of the intraspecific and interspecific behavior of Tomicus yunnanensis and Tomicus minor during the shoot-feeding phase. J Chem Ecol 45:227–240. https://doi.org/10.1007/s10886-019-01048-6
Wyatt TD (2010) Pheromones and signature mixtures: defining species-wide signals and variable cues for identity in both invertebrates and vertebrates. J Comp Physiol 196:685–700. https://doi.org/10.1007/s00359-010-0564-y
Xie SA, Lv SJ (2012) An improved lure for trapping the bark beetle Dendroctonus armandi (Coleoptera: Scolytinae). Eur J Entomol 109:569–577. https://doi.org/10.14411/eje.2012.071
Xie SA, Lv SJ (2013) Effect of different semiochemicals blends on spruce bark beetle, Ips typographus (Coleoptera: Scolytidae). Entomol Sci 16:179–190. https://doi.org/10.1111/j.1479-8298.2012.00555.x
Xu LT, Lou QZ, Cheng CH, Lu M, Sun JH (2015) Gut-associated bacteria of Dendroctonus valens and their involvement in verbenone production. Microb Ecol 70:1012–1023. https://doi.org/10.1007/s00248-015-0625-4
Xu L, Lu M, Xu D, Chen L, Sun J (2016) Sexual variation of bacterial microbiota of Dendroctonus valens guts and frass in relation to verbenone production. J Insect Physiol 95:110–117. https://doi.org/10.1016/j.jinsphys.2016.09.014
Zhang QH, Schlyter F (2003) Redundancy synergism and active inhibitory range of non-host volatiles in reducing pheromone attraction in European spruce bark beetle Ips typographus. Oikos 101:299–310. https://doi.org/10.1034/j.1600-0706.2003.111595.x
Zhang QH, Schlyter F (2004) Olfactory recognition and behavioural avoidance of angiosperm nonhost volatiles by conifer-inhabiting bark beetles. Agric For Entomol 6:1–19. https://doi.org/10.1111/j.1461-9555.2004.00202.x
Zhang LW, Sun J (2006) Electrophysiological and behavioral responses of Dendroctonus valens (Coleoptera: Curculionidae: Scolytinae) to candidate pheromone components identified in hindgut extracts. Environ Entomol 35:1232–1237. https://doi.org/10.1603/0046-225x(2006)35[1232:eabrod]2.0.co;2
Zhang QH, Schlyter F, Anderson P (1999) Green leaf volatiles interrupt pheromone response of spruce bark beetle, Ips typographus. J Chem Ecol 25:2847–2861. https://doi.org/10.1023/a:1020816011131
Zhang Q-H, Birgersson G, Schlyter F, Chen G-F (2000a) Pheromone components in the larch bark beetle, Ips cembrae, from China: Quantitative variation among attack phases and individuals. J Chem Ecol 26:841–858. https://doi.org/10.1023/a:1005447922939
Zhang QH, Schlyter F, Birgersson G (2000b) Bark volatiles from nonhost angiosperm trees of spruce bark beetle, Ips typographus (L.)(Coleoptera: Scolytidae): Chemical and electrophysiological analysis. Chemoecology 10:69–80. https://doi.org/10.1007/s000490050010
Zhang QH, Liu GT, Schlyter F, Birgersson G, Anderson P, Valeur P (2001) Olfactory responses of Ips duplicatus from inner Mongolia, China to nonhost leaf and bark volatiles. J Chem Ecol 27:995–1009. https://doi.org/10.1023/a:1010395221953
Zhang LW, Sun JH, Clarke SR (2006) Effects of verbenone dose and enantiomer on the interruption of response of the red turpentine beetle, Dendroctonus valens LeConte (Coleoptera : Scolytidae), to its kariomones. Environ Entomol 35:655–660. https://doi.org/10.1603/0046-225x-35.3.655
Zhang Q-H, Schlyter F, Chen G, Wang Y (2007a) Electrophysiological and behavioral responses of Ips subelongatus to semiochemicals from its hosts, non-hosts, and conspecifics in China. J Chem Ecol 33:391–404. https://doi.org/10.1007/s10886-006-9231-8
Zhang Q-H, Schlyter F, Liu G-T, Sheng M-L, Birgersson G (2007b) Electrophysiological and behavioral responses of Ips duplicatus to aggregation pheromone in inner Mongolia, China: amitinol as a potential pheromone component. J Chem Ecol 33:1303–1315. https://doi.org/10.1007/s10886-007-9320-3
Zhang Q-H, Ma J-H, Zhao F-Y, Song L-W, Sun J-H (2009) Aggregation pheromone of the qinghai spruce bark beetle, Ips nitidus Eggers. J Chem Ecol 35:610–617. https://doi.org/10.1007/s10886-009-9634-4
Zhang Q-H, Ma J-H, Zhao F-Y, Song L-W, Sun J-H, Cognato AI (2011) Aggregation pheromone of the oriental spruce engraver Pseudips orientalis. Agric for Entomol 13:67–75. https://doi.org/10.1111/j.1461-9563.2010.00500.x
Zhang F et al (2020) Characterization of MaltOBP1, a minus-C odorant-binding protein, from the Japanese pine sawyer beetle Monochamus alternatus Hope (Coleoptera: Cerambycidae). Front Physiol. https://doi.org/10.3389/fphys.2020.00212
Zhao M, Dai L, Sun Y, Fu D, Chen H (2017) The pheromone verbenone and its function in Dendroctonus armandi (Coleoptera: Curculionidae: Scolytinae). Eur J Entomol 114:53–60. https://doi.org/10.14411/eje.2017.008
Zuber M (1994) Racemat and enantiomeres of ipsdienol for attracting Ips amitinus (Eich.) (Col., Scolytidae). Anz Schädlkd Pflanzenschutz Umweltschutz 67:92–93. https://doi.org/10.1007/bf01904696
Acknowledgements
We want to thank Sebastian Pollok and Anna-Mareike Meier for their help in literature research. We thank Anita Kiesel for providing the picture of a spruce bark beetle and Fenris Mäling for drafting, realisation, and assembly of several figures. We also thank two anonymous reviewers for their constructive feedback that helped to improve the manuscript. The project is funded by the German Federal Ministry of Food and Agriculture (BMEL) through the Agency of Renewable Resources (FNR) within the funding program ‘Renewable Resources’ and according to a decision of the German Parliament (FKZ 22017117 and 22012918). MNA was funded by the Swedish Research Council FORMAS (grant #2018-01444) and the Foundation in Memory of Oscar and Lili Lamm. PHWB acknowledges funding by the German Research Foundation (DFG) (Emmy Noether grant number BI 1956/1-1). MS was financially supported by the Austrian Federal Ministry for Agriculture, Forestry, Regions and Water Management, Waldfonds projects ‘NewIPS’ (project number: 101686) and ‘IpsEMAN’ (project number: 101687).
Funding
Open Access funding enabled and organized by Projekt DEAL. The project is funded by the German Federal Ministry of Food and Agriculture (BMEL) through the Agency of Renewable Resources (FNR) within the funding program ‘Renewable Resources’ and according to a decision of the German Parliament (FKZ 22017117 and 22012918). MNA was funded by the Swedish Research Council FORMAS (grant #2018–01444) and the Foundation in Memory of Oscar and Lili Lamm and PHWB was funded by the German Research Foundation (DFG) (Emmy Noether grant number BI 1956/1–1). MS was financially supported by the Austrian Federal Ministry for Agriculture, Forestry, Regions and Water Management, Waldfonds projects ‘NewIPS’ (project number: 101686) and ‘IpsEMAN’ (project number: 101687).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare no financial or non-financial conflict of interest.
Ethical approval
This statement is not applicable.
Additional information
Communicated by Antonio Biondi.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Frühbrodt, T., Schebeck, M., Andersson, M.N. et al. Verbenone—the universal bark beetle repellent? Its origin, effects, and ecological roles. J Pest Sci 97, 35–71 (2024). https://doi.org/10.1007/s10340-023-01635-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-023-01635-3