Abstract
The rising population is increasing food demand, yet actual crop production is limited by the poor efficiency of classical fertilizers. In particular, only about 40–60% of fertilizer nitrogen, 15–20% of phosphorus and 50–60% of potassium are used by crop plants, the rest ending polluting the environment. Nanofertilizers are promising alternatives. Here, we review plant nutrients, synthesis of zinc oxide nanoparticles, encapsulation of nanoparticles in fertilizers, and effect on plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The earth population is increasing so fast that by 2050, it is expected to cross 9 billion. This may create a lot of problems, especially in the production of agricultural goods. The demand for food is expected to increase by 70–80% due to this upsurge (Bahar et al. 2020). Currently, the agricultural sector all around the world is severely affected by the poor yield of crops. This has occurred due to shortage of nutrients, soil organic matter, micronutrients, with additional factors including pest and insects attack and ineffective fertilizer (Prost 2021). The statistics gathered by the Food and Agriculture Organization of the United Nations (FAO) reports that the reduction in water resources and increase in soil salinity depleted the arable land for crop cultivation. This creates a serious challenge towards the growth of food and agricultural goods for meeting the demand and supply gap created by the increasing population (Rajam 2020).
Nanoscience and technology are the emerging field of science that has revolutionized many engineering branches such as the electronic industry, medicinal science, power sector, and automotive industries. This technology has a huge perspective in botanical and remedial fields like smart drugs release, biosensors, tissue synthesis, and nanofertilizers as shown in Fig. 1. Nanoparticles possess higher surface to volume fractions and distinctive features that build them very appropriate for use as compared to their bulk counterparts. The joint venture of nanotechnologies with agricultural and food science is emerging as a potential approach to increase plant growth and yield (Cheng et al. 2016; Moulick et al. 2020). With the help of this technology, farmers can utilize the nanoparticles effectively and precisely with other limited resources like water and costly synthetic fertilizers. Precision farming helps agriculture to become sustainable and greener by minimizing the usage of energy resources and waste (Duhan et al. 2017). Table 1 has categorized and tabulated different nanomaterial applications in the past decade.
Applications of nanomaterials in different areas of agriculture, i.e., in crop growth utilizing nanofertilizers that enhances the gemination, in crop improvement by producing transgenic plants, in soil enhancement, in nanopesticides using nanoparticles as nanocarriers, in stress tolerance in plants and precision forming using nanoparticles as sensors
The nanotechnology application in the form of nanofertilizer used both bulk and nanoscale materials. The nanoparticles normally range from 1 to 100 nm in at least one dimension (e.g., Zinc nanoparticles, and Iron nanoparticles) while the carriers of nanoparticles are at the bulk scale with more than 100 nm in size (Askary et al. 2017; García-Gómez et al. 2020). Therefore, the data presented in this review are related to nanomaterials and their utilization as a fertilizer. The major focus of this review is on the micronutrients utilized for the growth of plants. The most frequently used micronutrients are Zn and Fe; therefore, detailed literature regarding Zn nanoparticles is presented in this study. The novelty of this work is that up to our knowledge, no study has been conducted yet which highlights the data related to Zn nanoparticles and their usage as nanofertilizer. The literature discussed here allows the readers to understand the benefits of Zn nanoparticles or the development of crops through sustainable and precision agriculture.
Plant nutrients
Increasing population enforced farmers to enhance crops yield to meet the demand and supply gap of food in the developing world. Farmers used synthetic fertilizers in fields to promote the higher production of crops. Nutrient loss associated with the higher dissolution of fertilizers contributes towards financial loss and environmental pollution, along with lower crop productivity with poor dietary content for humans and domestic animals. Although fertilizers are well-known to increase crops yields; however, due to their higher solubility, the nutrient release from those particles are unable to match the sequential needs of crops (Meyer et al. 2018). For example, excessive nutrient nitrogen is wasted in the environment by ammonia emissions, leaching, runoff, and volatilization. On the other hand, extra phosphorus content remains present in the soil and get fixed by making a chemical bond with soil trace metals like calcium, magnesium, aluminium, iron and zinc. This bonding makes these essential micronutrients unavailable for plants’ uptake for their growth (Zhang et al. 2021).
This mismatch of nutrient release causes severe problems like fertilizer burns, ammonia volatilization, and leaching losses. The mismatch occurs due to lower nutrient requirements at different stages of plant growth, especially the initial stage. The plant uptake efficiency is very low due to the conversion of nutrients into unsolvable forms in the fields. Crops only utilize about 40–60% of nitrogen, 15–20% of phosphorus, and 50–60% of applied potassium products due to the above-mentioned problems (Chinta et al. 2020). The substances fixation, leaching, and volatilization cause both water and air contamination. These nutrients losses not only affect crop yield but also conversely misbalance the earth nutrient equilibrium and diminish the natural fertility of the top soil (Fertahi et al. 2021; Le et al. 2021). Therefore, it is need of the hour to develop a method that is sustainable and environmentally friendly for the delivery of nutrients to the soil with minimum use of resources and fertilizers. Whereas common synthetic fertilizers are used in bulk quantities in the agriculture fields generally ranging from 80 to 140 kg per hectare of land. On the other hand, nanoparticles as a fertilizer reduce this huge quantity to a greater extent.
Nanofertilizer possesses properties that stop nutrient loss which increases the nutrient use efficiency and optimal plant growth. In the present scenario, “smart drug delivery carriers” are gaining much interest in the delivery of nutrients to the fields for the replenishment of exhausted minerals. Nanoparticles of the desired micronutrients are loaded on the bulk carrier acted as a substrate. Nanoparticles under a smart drug delivery system will control the solubility of macronutrients and decrease the adsorption and fixation while promoting bioavailability in soil (Solanki et al. 2015; Polman et al. 2020). The nanoparticles are being loaded in macronutrients fertilizer with absorption, attachment via mediated ligands, encapsulation, or entrapment. After nanoparticles loading on fertilizers, nutrient(s) discharge could be controlled, which might enhance the nutrient use efficiency. The mass production of nanofertilizers for crop improvement is still debatable due to safety and economics-related issues with nanoparticles (Dubey and Mailapalli 2016).
Techniques to improve plant growth
Biotic and abiotic stresses are involved all around the world for the poor growth of crops causing 50% yield decline. The biotic stresses occur because of living organisms like bacteria, viruses, fungi, parasites, beneficial and harmful insects, whereas, include excessive heat, rain, winter, salinity, nutrients scarcity and chemical toxicity (Mareri et al. 2019; Ahmed et al. 2021). Both these factors adversely affect the growth and productivity of plants. In addition, they change the physical, chemical, and molecular nature of plants (Atkinson and Urwin 2012). Therefore, different strategies are used to remove these stresses. The most adopted technique includes foliar spray or application of some active ingredients via soil. Plants need CO2, H2O along with sunlight for their growth. CO2 and H2O are composed of carbon, hydrogen, and oxygen; therefore, not classified as mineral nutrients. Additionally, 16 other essential plant nutrients are required in small amounts (Fe, Cu, Zn, Mn, B, Mo, Ni, Na and Cl) and fewer are necessary for higher concentration (N, P, K, Mg, Ca, S and Si) for their growth. After the application of these nutrients, the effects of stresses can be minimized or even eliminated. After passing through roots, they move to various parts of plants where their transformation takes place (Klaic et al. 2021). Through fertilizer application, these micro- and macronutrients reach their designated positions for necessary chemical reactions for crop improvement as shown in Fig. 2.
Essential nutrients required by plants for their growth alongside the symptoms of scarcity of respective nutrient. The scarcity of iron causes new growing leaves with yellow or green veins, scarcity of potassium causes yellow edged leaves with dead spots and yellow patches on leaves and scarcity of manganese cause yellow spots elongated holes between veins. Likewise, magnesium lacked plant causes lower leaves turning yellow from outside, zinc shortage in the plant may cause pale and yellow leaves or short darker veins with black spots. Furthermore, shortage of Sulphur causes light green and pale green veins, phosphate shortage comes with darker leaves and loss of leaves while the shortage of calcium may cause the plant to stunt and mishappen new leaves
Due to the low efficiency of the applied fertilizers, most of these plant nutrients are lost to the surrounding environment which causes damages and loss in terms of productivity as explained in Table 1. For boosting the plant yield, these micro- and macronutrients, nanotechnology could be coupled with conventional fertilizers. These improved fertilizers might, therefore, be more efficient and meet the sequential need of plants. Nanofertilizers are supposed to be less harmful to crop plants as well as to the environment since their discharge rate for specific nutrients is slow (Monreal et al. 2016; Kottegoda et al. 2017). There are various technologies developed for the synthesis of nanofertilizers. Initially, nanoparticles of target nutrients are made and after that coupling of those nanoparticles are done with some substrates or base materials like urea or DAP fertilizer (Pohshna et al. 2020; Ramírez-Rodríguez et al. 2020). Nanofertilizers (encapsulated with nanoparticles and essential crop nutrients) can deliver the active ingredients due to their nanosize to the crops in an efficient way using slow-release techniques compared to bulk materials or ionic compounds of nutrients. These nanomaterials meet the plant need with higher target effective discharge in crop fields (Guo et al. 2018; Pirzadah et al. 2019).
Synthesis of nanoparticles
The outcome of nanomaterials on crop growth is dependent upon intrinsic and extrinsic interactions of the nanoparticles. The synthesis methods of nanomaterials also affect their properties greatly. The synthesis of nanomaterials is a very important task in the field of life sciences, medical, agricultural, environmental, and engineering applications. Two different strategies are used for the synthesis of nanoparticles from various sources as shown in Fig. 3. These sources include metallic and non-metallic substances (Subramanian et al. 2015). Under these strategies, further classification is done which includes two main classes, i.e., wet and dry synthesis methods. The wet method includes sol–gel, hydrothermal, precipitation, green synthesis using microorganisms and reversed micelles, whereas mechanical attrition and aerosol techniques come under the umbrella of the dry synthesis approach. (Kaul et al. 2012; Tarafdar and Raliya 2013; Dubey and Mailapalli 2016).
Methods of nanoparticles synthesis using top-down and bottom-up approaches. In the top-down approach, materials are formed from bulk level materials to nanoparticles whereas, in the bottom-up approach, nanoparticles are synthesized from atom level to nanoparticles. Different techniques of bottom-up and top-down approaches are listed as well
In the bottom-up approach, nanomaterials are built from smaller atoms and molecules which assemble themselves chemically by the principle of molecular recognition (Ariga et al. 2012). Nanomaterials are synthesized gradually atom by atom on a larger level like in the synthesis of nitrate salts metallic layer deposition in electronics industries. Each nanomaterial possesses distinctive properties as a result of different production and processing techniques under the bottom-up approach (Rajput 2015). The conventional adopted technique for the synthesis of nanoparticles is a wet-chemical method. The process takes start in a liquid media constitutes of different materials. For the prevention of agglomerates during the synthesis step, stabilizers are applied along with reactants (Ealias and Saravanakumar 2017; Nishida et al. 2018). In the bottom-up approach, a substantial number of nanomaterials are formed with minimal production cost. The major drawbacks associated with the bottom-up approach include dangerous by-products co-generation due to toxic solvents. Additionally, the final product gets contaminated due to starting materials (Jadoun et al. 2021).
The top-down approach yields nanomaterials that are synthesized from bigger molecules lacking atomic-level control (Khan 2020). Mechanical attrition is used widely to synthesize nanoparticles using a ball mill or planetary mill. The factors affecting final nanoparticle properties include the nature of the material, time of attrition, and attrition conditions. Another method other than attrition is pyrolysis in which an organic compound is enforced to pass through an orifice under high temperature, pressure conditions of 1000 °C and 10–15 bar, respectively (Rajput 2015). The major drawback with the top-down approach is non-uniformity of nanoparticles and surface imperfections due to mechanical operation (Ealias and Saravanakumar 2017). The nanoparticles utilized as fertilizer application must be produced on large scale with such adaptive technology which is economical and yield particles with superior physicochemical properties.
Synthesis of zinc-based nanoparticles
Zinc is the most essential micronutrient required by animals and plants for their optimum growth. It plays a vital role in many cell functions especially in protein and enzyme synthesis reactions (Khan 2020). Most of the places around the world possess very low levels of plant available zinc. This shortage in the soil spreads zinc deficiency among the growing crops (Alloway 2009; Ahmed et al. 2021). In addition to the above, approximately 33% of the world population has taken zinc-deficient diets due to large intakes of cereals (Rehman et al. 2018; Aziz et al. 2019). Therefore, continuous efforts are required to address the zinc deficiency and more focus is needed for increasing the zinc micronutrient in crops and humans (Gomez-Coronado et al. 2016; Paradisone et al. 2021). Different schemes are available for increasing the zinc content in growing crops as shown in Fig. 4 (Nakandalage et al. 2016). Biofortification (modifications in seeds) and the use of fertilizers with zinc have been identified as the most effective methods to remove zinc deficiency (Zaman et al. 2018; Du et al. 2019). Zinc fertilization is the fastest approach to meet the zinc demand among the crops in comparison to biological modification which is the time taking process.
On the other hand, zinc blended fertilizer does not always yield optimum results. The most important factor that affects fertilizer performance is the soil pH. High soil pH increases the negative charges on soil particles which increases the zinc exchange capacity of the soil and vice versa. Thus, the alkaline soil needs small zinc content for the removal of zinc deficiency continuously for greater fertility efficiency (Gomez-Coronado et al. 2016; Guo et al. 2018). Many research studies have been carried out using zinc sulphate as a zinc source either used directly to the soil or as a foliar spray. The soil application of fertilizer is the most effective technique, but ZnSO4 salt is unable to meet the requirement of plants and results in environmental pollution. So, there is a need to address the issue by identifying new smart fertilizers containing zinc as a micronutrient with high efficiency that has a lower risk factor of environmental pollution (Kopittke et al. 2019; Priyanka et al. 2019). Therefore, more research has been carried out to use nanotechnology for replacing conventional fertilizer with new nanomaterials containing Zn as nanoparticles in it (Kopittke et al. 2019).
Zinc oxide (ZnO) possesses very distinctive chemical and physical characteristics like higher physiochemical stability, high chemical coupling ability, a wide range of radiation absorptivity, and higher photo-stability (Shankar and Rhim 2019; Song et al. 2020). The rigidity and hardness of ZnO nanomaterials make them popular in ceramic engineering and manufacturing. At the same time, due to the negligible level of toxicity, biocompatibility, and biodegradability of ZnO are widely used for medicinal purposes. ZnO nanoparticles also show promising usage as micronutrient fertilizer and replacing conventional zinc salts fertilizers (Ludi and Niederberger 2013; Sabir et al. 2014; Panpatte et al. 2016). In this article, different preparation methods of zinc nanoparticles are reviewed. Zinc nanoparticles can be derived or synthesized from different zinc salts using different techniques including vapour deposition, water precipitation method, hydrothermal synthesis, the sol–gel method, and mechanical size reduction as shown in Fig. 5. Each salt possesses distinctive chemical and physical characteristics. Products obtained from different processes show a wide range of particles sizes, shapes, and structures. Additionally, the application of zinc nanoparticles in the agricultural sector has been discussed with its encapsulation in the polymer matrix as nanofertilizer.
Zinc nanoparticles synthesis exploits different techniques including different chemical synthesis methods, physical synthesis methods and biological synthesis methods. All basic methods are divided into sub-methods such as chemical methods that could be a gas phase or liquid phase and so on. Reprinted from Ref. (Król et al. 2017) with permission from Advances in Colloid and Interface Science.
Controlled precipitation
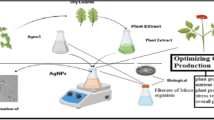
This technique is extensively applied for the synthesis of zinc nanoparticles from different raw materials with diverse properties as shown in Table 2. The process starts with a spontaneous reduction reaction of zinc precursor salt in the presence of a strong reducing agent with precipitation step as shown in Fig. 5. The development and properties of nanoparticles are controlled with the addition of a reducing agent. The impurities present in precursor salts are removed using high temperature, post-treatment steps with a milling operation. The precipitation of Zn nanoparticles is optimized by varying pH, temperature, reducing agent amount, and time. Lanje et al. (2013) adopted controlled precipitation for the preparation of zinc nanoparticles using zinc nitrate as precursor salt with sodium hydroxide. The starch molecules are used to eliminate agglomerates as hydroxyl functional groups hold the nanoparticles in the nucleating phase. The precipitation process requires surfactants to control the production of nanoparticles from precursor salts. These chemicals perform multiple functions and act as coagulants and flocculants. Suresh et al. (2018b) used the biocompatible precipitation method to develop ZnO nanoparticles using zinc nitrate and leaf extracts from Costus pictus plant. The schematic illustration of their method is shown in Fig. 6.
Sol–gel method
The sol–gel methodology for the synthesis of zinc nanoparticles possesses many advantages due to lower operational cost, process ease with reliability and reproducibility of a similar product. The reaction requires milder conditions for the production of nanoparticles using sol–gel route (Benhebal et al. 2013; Ismail et al. 2019). Yue et al. (2013) prepared zinc oxide nanoparticles using sol–gel technique with zinc 2-ethylhexanoate as precursor salt and Propan-2-ol as a solvent. Tetra Methyl Ammonium Hydroxide with a pH of ~ 14 was added by the authors, dropwise, in the solution of zinc salt and solvent. The reaction mixture was placed for 30 min, and then, washing was done with ethanol and water for removal of any impurities present in it. The transmission electron microscopy depicted the size of the nanoparticles in the range of 20–50 nm. The concentration of precursor salt in the solution did not affect the properties of nanoparticles. Likewise, Taghavi Fardood et al. (2019) fabricated ZnO nanoparticles using Arabic gum as stabilizing and reducing agent using a novel sol–gel method as depicted in Fig. 7. The properties and process parameters of sol–gel process are shown in Table 3.
Hydrothermal synthesis
The synthesis of nanoparticles via the hydrothermal route can easily be pursued in the absence of organic solvent with no post-treatment steps. Lack of organic solvent and calcination treatment makes this process greener, cleaner, and benign. The preparation of nanoparticles takes to starts with auto calving of the reaction mixture at a temperature ranging from 100 to 300 °C. This process continues for a few days. The reaction mixture cools after heating, which forms crystals due to nucleation phenomena. This process continues till the growth of nanocrystals. The hydrothermal process possesses several operational benefits due to the lower temperature and pressure of reaction media especially, with no post-treatment step as tabulated in Table 3 along with sol–gel method. Furthermore, a wide range of products are formed during the hydrothermal process in terms of shape and size (Tofa et al. 2019), Bulcha et al. (2021). Many studies have been carried out using microwave-assisted reactor for heating purposes in hydrothermal processes. Microwave-heated reactors make the system efficient with minimal loss. These efficient systems enhance the reaction rate with better product yield. The schematic diagram of the hydrothermal method is shown in Fig. 8.
Hydrothermal synthesis of zinc oxide nanoparticles (ZnO NPs) using calcium acetate (CA) as precursor. The hydrothermal synthesis methods take place at low temperature, do not involve organic solvents, and finish without any post-treatment processes which makes them green. Reprinted from Ref. (Basnet and Chatterjee 2020) with permission from Nano-Structures & Nano-Objects.
Physical methods
Zinc oxide nanoparticles can also be prepared using other techniques different from chemical synthesis processes. These techniques include mechanical milling operation, metallurgical processes, microwave-assisted thermal processes, etc. In the metallurgical process, zinc oxide nanoparticles are obtained by the roasting of zinc ore at high temperatures. International organization of standardization (Kołodziejczak-Radzimska and Jesionowski 2014) classifies zinc oxide from the metallurgical process into two types. Type A zinc oxide nanoparticles are formed via a direct process also known as the American process whereas indirect or French process yields Type B zinc oxide nanoparticles. In a direct process, the zinc ore reduces in the presence of coal within situ oxidation in the reactor. The bed in a reactor is divided into two separate layers. The top layer comprises of coal while the bottom layer constitutes a mixture of coal and zinc ore. The zinc ore is reduced with the help of carbon monoxide supplied with the hot air.
The only drawback associated with this process is low zinc oxide purity due to the presence of different metallic compounds. The presence of metal oxides like those of lead, iron and cadmium affect the white colour of zinc oxide nanoparticles. In the indirect synthesis technique, metal zinc is melted down in furnaces at around 910 °C. The vaporized zinc reacts with oxygen to form zinc (oxide) nanoparticles. The synthesized particles are cooled down in a cooling duct and are captured in the filter bag. Shi et al. (2014) prepared zinc oxide nanoparticles using a combination of chemical and mechanical processes to yield particles size of 21 nm. The milling process lasts for 6 h and produced zinc carbonate as a zinc oxide starting material. The post-treatment step of nanoparticles was carried out at 600 °C. The morphology of zinc oxide nanoparticles was affected by the duration of milling and temperature of heat treatment.
Green synthesis
Green synthesis is a cleaner and greener approach to produce zinc nanoparticles using renewable raw materials for example plants, microorganisms, algae, etc. as shown in Fig. 8. This synthesis mechanism is safer, environment friendly and economical in terms of raw materials and solvents requirements (Salam et al. 2014; Jamdagni et al. 2018). The biggest advantage of the green approach is the absence of harmful impurities with the synthesized nanoparticles (Agarwal et al. 2017). Green synthesis uses more benign chemicals which make particles more active and safer to use (Fakhari et al. 2019). Different green plants are used for the synthesis of nanoparticles (Baker et al. 2017). Mostly plant roots, shoots, leaves, barks and fruits are used for the synthesis process (Adelere and Lateef 2016; Saratale et al. 2018). Along with the wide range of applications, zinc oxide nanoparticles are safer and easier to produce with cheaper manufacturing processes (Jayaseelan et al. 2012). Different green sources are available for the synthesis of zinc oxide nanoparticles. These sources include plant extract, bacteria, algae, and other biological sources as illustrated in Fig. 9. A wider range of literature is available about different sources for the synthesis of zinc oxide nanoparticles. Few natural sources for zinc oxide nanoparticles are shown in Table 4.
Green synthesis of zinc nanoparticles utilizing neem leaf extract. ZnO NPs is the abbreviation of zinc nanoparticles. Reprinted from Ref. (Haque et al. 2020) with permission from Nano Express.
Encapsulation of zinc oxide nanoparticles in fertilizers
Encapsulation of nanoparticles is the biggest challenge due to their smaller size with higher surface area and energy. Generally, encapsulation is applied for coating food ingredients, nanoparticles, and biological substances on a micro-level. This technique is mostly used in pharmaceuticals to control the release of active agents (Morin-Crini et al. 2019; Rajan et al. 2020). Control release system offers many advantages including shielding effect from degradation, targeted delivery, and controlled release of active agents. The reason behind encapsulation is to isolate the core material from an external environment or to release the active agent from the core in a controlled manner (Anita and Ramach 2012; Tamanna et al. 2015). For example, the urea core is encapsulated using polymers for the slow release of nitrogen. The encapsulation also decreases the solubility of urea (Arjona et al. 2018; Ibrahim et al. 2019; Beig et al. 2020a).
Zinc nanoparticles are one of the most important and widely used metallic oxide nanoparticles. These nanoparticles are frequently applied due to their peculiar behaviour and chemical characteristics (Jiang et al. 2018). The only drawback of these metallic nanoparticles is their inherent thermodynamic instability due to reactive surfaces. The biggest challenge towards the synthesis of metallic nanoparticles is to kinetically stabilize their reactivity. This reactivity in the field of agriculture as a micronutrient source is because of their quick release (Dapkekar et al. 2018; Dimkpa et al. 2019; Phan and Haes 2019). The quick release of zinc nanoparticles might result in the wastage of micronutrients (zinc). The quick release of micronutrients would not match with the plant sequential needs and thus reduce the efficiency of nano-zinc-fertilizers (Madzokere et al. 2021). For minimization of nanoparticles reactivity, different techniques are available. The most used and applied method is an encapsulation of the zinc nanoparticle using a suitable material. These materials include polymers and mineral salts (Petchthanasombat et al. 2012; Zeng et al. 2019).
Polymeric encapsulation
Polymeric materials are widely applied for the controlled release of different substances subjected to a variety of intended applications (Kumar et al. 2021). These polymers have a wide range of applications in retarding the release of target elements for insecticides, pesticides, fungicides, germicides, and growth stimulants. The usage of these polymers is highly dependent on different other impactive parameters such as cost, climatic condition, biocompatibility, biodegradability, and ease of processing (Madzokere et al. 2021). Moreover, these polymers contribute towards the controlled release of the target material by self-degradation (Choudhary et al. 2019). The target material discharge rate is highly dependent on the dissolution of polymers in the environment (Roy et al. 2014; Beig et al. 2020b). Generally, the control release process is divided into two major steps: encapsulation of active material with the help of polymer matrix and complex molecular structure formation (comprised of polymeric back bones) with active material.
Many different polymers including natural, and synthetic like carboxymethyl cellulose (Davidson et al. 2013), cellulose acetate phthalate (Mukherjee and De 2016), gelatin (Wilson et al. 2018), gum Arabic (De Oliveira et al. 2018), polyvinyl alcohol (PVA), and polyacrylamide (Paradelo et al. 2019) are widely used for encapsulation. The controlled release systems are highly dependent on the molecular weight and cross-linking of polymeric materials (> 10,000) (Frizzo et al. 2019). The higher cross-linking and weight of polymers favour better results in terms of discharge. The encapsulated materials are termed as nanomaterials due to the nanosize of target materials. Mainly, polymeric materials are used to encapsulate metallic nanoparticles in the agriculture sector for controlling the release of particles (Prasad et al. 2017). The metallic nanoparticles usually used are copper, zinc, iron, and titanium for the healthy growth of crop plants (Aslani et al. 2014; Tan et al. 2018; Ponnamma et al. 2019). This encapsulation enhances the effectiveness by reducing the dissolution and protecting biologically active molecules against early degradation. In this way, the overall efficiency of the fertilizer system increases with very little dose applied in the field (Venkatachalam 2017). Additionally, these nanomaterials are expected to reduce the toxicity level associated with these metallic nanoparticles (Thunugunta et al. 2018).
Effects of Zn-based nanoparticles on plants
Zn regulates the functions of enzymes present in plants (Noulas et al. 2018). Different studies on zinc oxide nanoparticles have reported that these are imperative in boosting enzyme activities which would promote the growth and biomass yield in long term. Dhoke et al. (2013) checked the effect of zinc oxide nanoparticles on the mung bean (Vigna radiata) seeds. The application of nanoparticles on mung bean increases the biomass content of roots and plant tissues. Dimkpa et al. (2017) also evaluated the performance of bulk zinc and zinc oxide nanoparticles on the development of sorghum. Different methods of fertilizer application (soil and foliar spray) were also studied and compared to check its effect on yield, nutrient use efficiency and Zn content in grains. The foliar spray of both samples considerably improved the sorghum yield and Zn content in grains. Subbaiah et al. (2016) carried out experimentation on maize using zinc oxide nanoparticles with a particle size of 25 nm. The maize growth was checked on different concentrations of Zn ranging from 50 to 2000 ppm. The 1500 ppm ZnO nanoparticles gave the optimum results which increase the germination rate by 80% and the seedling index.
On the other hand, the dose of 2000 ppm of nanoparticles has no inhibition effect on seed germination whereas the same value of zinc sulphate bulk salt stops the germination of maize. Zn oxide nanoparticles also reduce the effect of stress on plants, e.g., effect of salinity, drought and cadmium stress (Taran et al. 2017; Dimkpa et al. 2019). Haripriya et al. (2018) reported the reduction in stress by the foliar spray of ZnO nanoparticles on the finger millet cultivated on saline land. Zhang et al. (2017) used ZnO nanoparticles and ZnSO4 to check their effect on tissue generation in wheat. The zinc concentration increases in the tissues of wheat. The grain zinc concentration is considerably higher in the case of nanoparticles. The results show that the nanoparticles were more efficient in comparison to bulk salt of zinc. Zhao et al. (2015) also presented the results using ZnO–NPs (400 or 800 mg/kg) which yield higher Zn concentration in roots of corn by approximately 30-fold in comparison to control. Du et al. (2019) reported the soil-based experimentation results of zinc nanoparticles. The results show the increased zinc content of wheat, but no zinc was reported in the plant tissues. The investigation results were more promising for acidic soils as compared to alkaline ones (Watson et al. 2015).
Conclusion
We reviewed the synthesis of zinc nanoparticles for plant application to increase growth and disease resistance. Surface modification can be done with metal oxide nanoparticles to alter the properties as per requirement for the desired application. In recent times, more focus is given to developing nanoparticles based on green methods. Few research has been carried out showing the benefits of metallic nanoparticles for sustainable agriculture, but the mechanism of delivery and usage are at their early stage.
Availability of data and materials
Not applicable.
References
Adelere IA, Lateef A (2016) A novel approach to the green synthesis of metallic nanoparticles: the use of agro-wastes, enzymes, and pigments. Nanotechnol Rev 5(6):567–587. https://doi.org/10.1515/ntrev-2016-0024
Agarwal H, Kumar SV, Rajeshkumar S (2017) A review on green synthesis of zinc oxide nanoparticles–an eco-friendly approach. Res-Effic Technol 3(4):406–413. https://doi.org/10.1016/j.reffit.2017.03.002
Ahmed AKA, Shi X, Hua L, Manzueta L, Qing W, Marhaba T, Zhang W (2018) Influences of air, oxygen, nitrogen, and carbon dioxide nanobubbles on seed germination and plant growth. J Agric Food Chem 66(20):5117–5124. https://doi.org/10.1021/acs.jafc.8b00333
Ahmed B, Rizvi A, Ali K, Lee J, Zaidi A, Khan MS, Musarrat J (2021) Nanoparticles in the soil–plant system: a review. Environ Chem Lett 19:1–65. https://doi.org/10.1007/s10311-020-01138-y
Aladpoosh R, Montazer M (2015) The role of cellulosic chains of cotton in biosynthesis of ZnO nanorods producing multifunctional properties: mechanism, characterizations and features. Carbohyd Polym 126:122–129. https://doi.org/10.1016/j.carbpol.2015.03.036
Ali K, Dwivedi S, Azam A, Saquib Q, Al-Said MS, Alkhedhairy AA, Musarrat J (2016) Aloe vera extract functionalized zinc oxide nanoparticles as nanoantibiotics against multi-drug resistant clinical bacterial isolates. J Colloid Interface Sci 472:145–156. https://doi.org/10.1016/j.jcis.2016.03.021
Alloway B (2009) Soil factors associated with zinc deficiency in crops and humans. Environ Geochem Health 31(5):537–548. https://doi.org/10.1007/s10653-009-9255-4
Anita S, Ramach T (2012) Preparation, characterization and functional analysis of zinc oxide nanoparticles coated single jersey cotton fabric. J Textile Sci Eng. https://doi.org/10.4172/2165-8064.1000114
Ariga K, Ito H, Hill JP, Tsukube H (2012) Molecular recognition: from solution science to nano/materials technology. Chem Soc Rev 41(17):5800–5835. https://doi.org/10.1039/C2CS35162E
Arjona J, Valenzuela-Diaz F, Wiebeck H, Wang S, Silva-Valenzuela M (2018) Evaluation of urea encapsulation by microcapsules of PHB/MMT and PHB/OMMT nanocomposites. TMS Annual Meet Exhib 2:365–372. https://doi.org/10.1007/978-3-319-72484-3
Askary M, Talebi SM, Amini F, Bangan ADB (2017) Effects of iron nanoparticles on Mentha piperita L. under salinity stress. Biologija. https://doi.org/10.6001/biologija.v63i1.3476
Aslani F, Bagheri S, Muhd Julkapli N, Juraimi AS, Hashemi FSG, Baghdadi A (2014) Effects of engineered nanomaterials on plants growth: an overview. Sci World J 2014:1–28. https://doi.org/10.1155/2014/641759
Atkinson NJ, Urwin PE (2012) The interaction of plant biotic and abiotic stresses: from genes to the field. J Exp Bot 63(10):3523–3543. https://doi.org/10.1093/jxb/ers100
Aziz Y, Shah GA, Rashid MI (2019) ZnO nanoparticles and zeolite influence soil nutrient availability but do not affect herbage nitrogen uptake from biogas slurry. Chemosphere 216:564–575. https://doi.org/10.1016/j.chemosphere.2018.10.119
Bahar NH, Lo M, Sanjaya M, Van Vianen J, Alexander P, Ickowitz A, Sunderland TJGEC (2020) Meeting the food security challenge for nine billion people in 2050: what impact on forests. Glob Environ Chang 62:102056. https://doi.org/10.1016/j.gloenvcha.2020.102056
Baker S, Volova T, Prudnikova SV, Satish S, Prasad N (2017) Nanoagroparticles emerging trends and future prospect in modern agriculture system. Environ Toxicol Pharmacol 53:10–17. https://doi.org/10.1016/j.etap.2017.04.012
Basnet P, Chatterjee S (2020) Structure-directing property and growth mechanism induced by capping agents in nanostructured ZnO during hydrothermal synthesis–a systematic review. Nano-Struct Nano-Objects 22:100426. https://doi.org/10.1016/j.nanoso.2020.100426
Beig B, Niazi MBK, Jahan Z, Kakar SJ, Shah GA, Shahid M, Zia M, Haq MU, Rashid MI (2020a) Biodegradable polymer coated granular urea slows down N release kinetics and improves spinach productivity. Polymers 12(11):2623. https://doi.org/10.3390/polym12112623
Beig B, Niazi MBK, Jahan Z, Pervaiz E, Abbas Shah G, Ul Haq M, Zafar MI, Zia M (2020b) Slow-release urea prills developed using organic and inorganic blends in fluidized bed coater and their effect on spinach productivity. Sustainability 12(15):5944. https://doi.org/10.3390/su12155944
Benhebal H, Chaib M, Salmon T, Geens J, Leonard A, Lambert SD, Crine M, Heinrichs B (2013) Photocatalytic degradation of phenol and benzoic acid using zinc oxide powders prepared by the sol–gel process. Alex Eng J 52(3):517–523. https://doi.org/10.1016/j.aej.2013.04.005
Bulcha B, Leta Tesfaye J, Anatol D, Shanmugam R, Dwarampudi LP, Nagaprasad N, Bhargavi V, Krishnaraj RJJoN (2021) Synthesis of zinc oxide nanoparticles by hydrothermal methods and spectroscopic investigation of ultraviolet radiation protective properties. J Nanomater, 2021, 1-10. https://doi.org/10.1155/2021/8617290
Cheng H, Klasson K, Asakura T, Wu, Q (2016) Nanotechnology in agriculture. Nanotechnol: Deliv Promise Vol, 2, 233–242. https://doi.org/10.1021/bk-2016-1224.ch012
Chinta YD, Uchida Y, Araki HJSH (2020) Availability of nitrogen supply from cover crops during residual decomposition by soil microorganisms and its utilization by lettuce (Lactuca sativa L.). Sci Hortic 270:109415. https://doi.org/10.1016/j.scienta.2020.109415
Choudhary RC, Kumaraswamy R, Kumari S, Sharma S, Pal A, Raliya R, Biswas P, Saharan V (2019) Zinc encapsulated chitosan nanoparticle to promote maize crop yield. Int J Biol Macromol 127:126–135. https://doi.org/10.1016/j.ijbiomac.2018.12.274
Dapkekar A, Deshpande P, Oak MD, Paknikar KM, Rajwade JM (2018) Zinc use efficiency is enhanced in wheat through nanofertilization. Sci Rep 8(1):1–7. https://doi.org/10.1038/s41598-018-25247-5
Davidson DW, Verma MS, Gu FX (2013) Controlled root targeted delivery of fertilizer using an ionically crosslinked carboxymethyl cellulose hydrogel matrix. Springerplus 2(1):1–9. https://doi.org/10.1186/2193-1801-2-318
De Oliveira JL, EnVR C, Pereira AE, Nunes LE, Da Silva CC, Pasquoto T, Lima R, Smaniotto G, Polanczyk RA, Fraceto LF (2018) Geraniol encapsulated in chitosan/gum arabic nanoparticles: a promising system for pest management in sustainable agriculture. J Agric Food Chem 66(21):5325–5334. https://doi.org/10.1021/acs.jafc.8b00331
Dhoke SK, Mahajan P, Kamble R, Khanna A (2013) Effect of nanoparticles suspension on the growth of mung (Vigna radiata) seedlings by foliar spray method. Nanotechnol Dev 3(1):e1–e1. https://doi.org/10.4081/nd.2013.e1
Dimkpa CO, White JC, Elmer WH, Gardea-Torresdey J (2017) Nanoparticle and ionic Zn promote nutrient loading of sorghum grain under low NPK fertilization. J Agric Food Chem 65(39):8552–8559. https://doi.org/10.1021/acs.jafc.7b02961
Dimkpa CO, Singh U, Bindraban PS, Elmer WH, Gardea-Torresdey JL, White JC (2019) Zinc oxide nanoparticles alleviate drought-induced alterations in sorghum performance, nutrient acquisition, and grain fortification. Sci Total Environ 688:926–934. https://doi.org/10.1016/j.scitotenv.2019.06.392
Dobrucka RD, J (2016) Biosynthesis and antibacterial activity of ZnO nanoparticles using Trifolium pratense flower extract. Saudi J Biol Sci. 23, 517–523. https://doi.org/10.1016/j.sjbs.2015.05.016
Du W, Yang J, Peng Q, Liang X, Mao H (2019) Comparison study of zinc nanoparticles and zinc sulphate on wheat growth: from toxicity and zinc biofortification. Chemosphere 227:109–116. https://doi.org/10.1016/j.chemosphere.2019.03.168
Dubey A, Mailapalli DR (2016) Nanofertilisers, nanopesticides, nanosensors of pest and nanotoxicity in agriculture. Sustain Agric Rev 19:307–330. https://doi.org/10.1007/978-3-319-26777-7_7
Duhan JS, Kumar R, Kumar N, Kaur P, Nehra K, Duhan S (2017) Nanotechnology: The new perspective in precision agriculture. Biotechnol Rep 15:11–23. https://doi.org/10.1016/j.btre.2017.03.002
Ealias AM, Saravanakumar M (2017) A review on the classification, characterisation, synthesis of nanoparticles and their application. IOP Conf Ser Mater Sci Eng 263:032019. https://doi.org/10.1088/1757-899X/263/3/032019
Elumalai K, Velmurugan S (2015) Applied surface science green synthesis, characterization and antimicrobial activities of zink oxide nanoparticles from the leaf extract of Azadirachta indica (L.). Appl Surf Sci 345:329–336. https://doi.org/10.1016/j.apsusc.2015.03.176
Emami S, Alikhani HA, Pourbabaei AA, Etesami H, Motashare Zadeh B, Sarmadian F (2018) Improved growth and nutrient acquisition of wheat genotypes in phosphorus deficient soils by plant growth-promoting rhizospheric and endophytic bacteria. Soil Sci Plant Nutr 64(6):719–727. https://doi.org/10.1080/00380768.2018.1510284
Fakhari S, Jamzad M, Kabiri Fard H (2019) Green synthesis of zinc oxide nanoparticles: a comparison. Green Chem Lett Rev 12(1):19–24. https://doi.org/10.1080/17518253.2018.1547925
Fatima F, Hashim A, Anees SJES, Research P (2021) Efficacy of nanoparticles as nanofertilizer production: a review. Environ Sci Pollut Res 28(2), 1292–1303. https://doi.org/10.1007/s11356-020-11218-9
Fertahi S, Ilsouk M, Zeroual Y, Oukarroum A, Barakat AJJoCR, (2021) Recent trends in organic coating based on biopolymers and biomass for controlled and slow release fertilizers. J Control Release 330:341–361. https://doi.org/10.1016/j.jconrel.2020.12.026
Frizzo MS, Feuser PE, Berres PH, Ricci-Júnior E, Campos CE, Costa C, de Araújo PHH, Sayer C (2019) Simultaneous encapsulation of zinc oxide and octocrylene in poly (methyl methacrylate-co-styrene) nanoparticles obtained by miniemulsion polymerization for use in sunscreen formulations. Colloids Surf, A 561:39–46. https://doi.org/10.1016/j.colsurfa.2018.10.062
Fu L, Fu Z (2015) Plectranthus amboinicus leaf extract–assisted biosynthesis of ZnO nanoparticles and their photocatalytic activity. Ceram Int 41(2):2492–2496. https://doi.org/10.1016/j.ceramint.2014.10.069
García-Gómez C, García-Gutiérrez S, Obrador A, Fernández MD (2020) Study of Zn availability, uptake, and effects on earthworms of zinc oxide nanoparticle versus bulk applied to two agricultural soils: acidic and calcareous. Chemosphere 239:124814. https://doi.org/10.1016/j.chemosphere.2019.124814
Geilfus C-MJPS (2018) Review on the significance of chlorine for crop yield and quality. Plant Sci 270:114–122. https://doi.org/10.1016/j.plantsci.2018.02.014
Gomez-Coronado F, Poblaciones MJ, Almeida AS, Cakmak I (2016) Zinc (Zn) concentration of bread wheat grown under Mediterranean conditions as affected by genotype and soil/foliar Zn application. Plant Soil 401(1–2):331–346. https://doi.org/10.1007/s11104-015-2758-0
Guo W, Nazim H, Liang Z, Yang D (2016) Magnesium deficiency in plants: an urgent problem. Crop J 4(2):83–91. https://doi.org/10.1016/j.cj.2015.11.003
Guo H, White JC, Wang Z, Xing B (2018) Nano-enabled fertilizers to control the release and use efficiency of nutrients. Curr Opin Environ Sci Health 6:77–83. https://doi.org/10.1016/j.coesh.2018.07.009
Hafsi C, Falleh H, Saada M, Ksouri R, Abdelly C (2017) Potassium deficiency alters growth, photosynthetic performance, secondary metabolites content, and related antioxidant capacity in Sulla carnosa grown under moderate salinity. Plant Physiol Biochem 118:609–617. https://doi.org/10.1016/j.plaphy.2017.08.002
Haque MJ, Bellah MM, Hassan MR, Rahman SJNE (2020) Synthesis of ZnO nanoparticles by two different methods & comparison of their structural, antibacterial, photocatalytic and optical properties. Nano Express 1(1):010007. https://doi.org/10.1088/2632-959X/ab7a43
Haripriya P, Stella P, Anusuya S (2018) Foliar spray of zinc oxide nanoparticles improves salt tolerance in finger millet crops under glass-house condition. SCIOL Biotechnol 1:20–29
Hassan MU, Aamer M, Chattha MU, Ullah MA, Sulaman S, Nawaz M, Zhiqiang W, Yanqin M, Guoqin H (2017) The role of potassium in plants under drought stress: mini review. J Basic Appl Sci 13:268–271. https://doi.org/10.6000/1927-5129.2017.13.44
Ibrahim KA, Naz MY, Shukrullah S, Sulaiman SA, Ghaffar A, AbdEl-Salam N (2019) Controlling nitrogen pollution via encapsulation of urea fertilizer in cross-linked corn starch. BioResources 14(4):7775–7789. https://doi.org/10.1537/biores.14.4.7775-7789
Ismail A, Menazea A, Kabary HA, El-Sherbiny A, Samy A (2019) The influence of calcination temperature on structural and antimicrobial characteristics of zinc oxide nanoparticles synthesized by Sol-Gel method. J Mol Struct 1196:332–337. https://doi.org/10.1016/j.molstruc.2019.06.084
Jadoun S, Arif R, Jangid NK, Meena RKJECL (2021) Green synthesis of nanoparticles using plant extracts:a review. Environ Chem Lett 19(1):355–374. https://doi.org/10.1007/s10311-020-01074-x
Jamdagni P, Khatri P, Rana J (2018) Green synthesis of zinc oxide nanoparticles using flower extract of Nyctanthes arbor-tristis and their antifungal activity. J King Saud Univ-Sci 30(2):168–175. https://doi.org/10.1016/j.jksus.2016.10.002
Jayaseelan C, Rahuman AA, Kirthi AV, Marimuthu S, Santhoshkumar T, Bagavan A, Gaurav K, Karthik L, Rao KB (2012) Novel microbial route to synthesize ZnO nanoparticles using Aeromonas hydrophila and their activity against pathogenic bacteria and fungi. Spectrochim Acta Part A Mol Biomol Spectrosc 90:78–84. https://doi.org/10.1016/j.saa.2012.01.006s
Jia W, Dang S, Liu H, Zhang Z, Yu C, Liu X, Xu B (2012) Evidence of the formation mechanism of ZnO in aqueous solution. Mater Lett 82:99–101. https://doi.org/10.1016/j.matlet.2012.05.013
Jiang J, Pi J, Cai J (2018) The advancing of zinc oxide nanoparticles for biomedical applications. Bioinorg Chem Appl 2018:1–18. https://doi.org/10.1155/2018/1062562
Kaul R, Kumar P, Burman U, Joshi P, Agrawal A, Raliya R, Tarafdar J (2012) Magnesium and iron nanoparticles production using microorganisms and various salts. Mater Sci-Pol 30(3):254–258. https://doi.org/10.2478/s13536-012-0028-x
Khan FA (2020) Synthesis of nanomaterials: methods & technology. Appl Nanomater Human Health 2:15–21. https://doi.org/10.1007/978-981-15-4802-4_2
Khoshhesab ZM, Sarfaraz M, Houshyar Z (2012) Influences of Urea on Preparation of Zinc Oxide Nanostructures Through Chemical Precipitation in Ammonium Hydrogencarbonate Solution. Synth React Inorg, Met-Org, Nano-Met Chem 42(10):1363–1368. https://doi.org/10.1080/15533174.2012.680119
Klaic R, Guimaraes GG, Giroto AS, Bernardi AC, Zangirolami TC, Ribeiro C, Farinas CSJCM (2021) Synergy of Aspergillus niger and Components in Biofertilizer Composites Increases the Availability of Nutrients to Plants. Curr Microbiol 78(4):1529–1542. https://doi.org/10.1007/s00284-021-02406-y
Kołodziejczak-Radzimska A, Jesionowski T (2014) Zinc oxide–from synthesis to application: a review. Materials 7(4):2833–2881. https://doi.org/10.3390/ma7042833
Kopittke PM, Lombi E, Wang P, Schjoerring JK, Husted S (2019) Nanomaterials as fertilizers for improving plant mineral nutrition and environmental outcomes. Environ Sci Nano 6(12):3513–3524. https://doi.org/10.1039/C9EN00971J
Kottegoda N, Sandaruwan C, Priyadarshana G, Siriwardhana A, Rathnayake UA, Berugoda Arachchige DM, Kumarasinghe AR, Dahanayake D, Karunaratne V, Amaratunga GA (2017) Urea-hydroxyapatite nanohybrids for slow release of nitrogen. ACS Nano 11(2):1214–1221. https://doi.org/10.1021/acsnano.6b07781
Król A, Pomastowski P, Rafińska K, Railean-Plugaru V, Buszewski B (2017) Zinc oxide nanoparticles: synthesis, antiseptic activity and toxicity mechanism. Adv Coll Interface Sci 249:37–52. https://doi.org/10.1016/j.cis.2017.07.033
Krupa AND, Vimala R (2016) Evaluation of tetraethoxysilane (TEOS) sol–gel coatings, modified with green synthesized zinc oxide nanoparticles for combating microfouling. Mater Sci Eng, C 61:728–735. https://doi.org/10.1016/j.msec.2016.01.013
Kumar A, Choudhary A, Kaur H, Mehta S, Husen AJNRL (2021) Smart nanomaterial and nanocomposite with advanced agrochemical activities. Nanoscale Res Lett 16(1):1–26. https://doi.org/10.1186/s11671-021-03612-0
Lanje AS, Sharma SJ, Ningthoujam RS, Ahn J-S, Pode RB (2013) Low temperature dielectric studies of zinc oxide (ZnO) nanoparticles prepared by precipitation method. Adv Powder Technol 24(1):331–335. https://doi.org/10.1016/j.apt.2012.08.005
Le VS, Herrmann L, Hudek L, Nguyen TB, Bräu L, Lesueur DJECL (2021) How application of agricultural waste can enhance soil health in soils acidified by tea cultivation: a review. Environ Chem Lett 2:1–27. https://doi.org/10.1007/s10311-021-01313-9
Ludi B, Niederberger M (2013) Zinc oxide nanoparticles: chemical mechanisms and classical and non-classical crystallization. Dalton Trans 42(35):12554–12568. https://doi.org/10.1039/C3DT50610J
Madan H, Sharma S, Suresh D, Vidya Y, Nagabhushana H, Rajanaik H, Anantharaju K, Prashantha S, Maiya PS (2016) Facile green fabrication of nanostructure ZnO plates, bullets, flower, prismatic tip, closed pine cone: their antibacterial, antioxidant, photoluminescent and photocatalytic properties. Spectrochim Acta Part A Mol Biomol Spectrosc 152:404–416. https://doi.org/10.1016/j.saa.2015.07.067
Madzokere T, Murombo L, Chiririwa HJMTP (2021) Nano-based slow releasing fertilizers for enhanced agricultural productivity. Mater Today: Proc 45:3709–3715. https://doi.org/10.1016/j.matpr.2020.12.674
Malhotra H, Sharma S, Pandey R (2018) Phosphorus nutrition: plant growth in response to deficiency and excess. Plant Nutr Abiotic Stress Toler 2:171–190. https://doi.org/10.1007/978-981-10-9044-8_7
Manikandan B, Endo T, Kaneko S, Murali K, John R (2018) Properties of sol gel synthesized ZnO nanoparticles. J Mater Sci: Mater Electron 29(11):9474–9485. https://doi.org/10.1007/s10854-018-8981-8
Manuel T-J, Alejandro C-A, Angel L, Aurora G, Emilio F (2018) Roles of molybdenum in plants and improvement of its acquisition and use efficiency. Plant Micronutr Use Effic 2:137–159. https://doi.org/10.1016/B978-0-12-812104-7.00009-5
Mareri L, Romi M, Cai GJPB-AIJDwaAoPB (2019) Arabinogalactan proteins: actors or spectators during abiotic and biotic stress in plants? Plant Biosyst-An Inter J Dealing Asp Plant Biol 153(1), 173-185. https://doi.org/10.1080/11263504.2018.1473525
Meyer G, Frossard E, Mäder P, Nanzer S, Randall DG, Udert KM, Oberson AJP, Soil (2018) Water soluble phosphate fertilizers for crops grown in calcareous soils–an outdated paradigm for recycled phosphorus fertilizers? Plant Soil 424 (1), 367-388. https://doi.org/10.1007/s11104-017-3545-x
Miri AH, SHAkIb ES, EbRAHIMI O, Sharifi-Rad J (2017) Impacts of nickel nanoparticles on grow characteristics, photosynthetic pigment content and antioxidant activity of Coriandrum Sativum L. Oriental J Chem 33 (3), 1297–1303. https://doi.org/10.13005/ojc/330329
Monreal C, DeRosa M, Mallubhotla S, Bindraban P, Dimkpa C (2016) Nanotechnologies for increasing the crop use efficiency of fertilizer-micronutrients. Biol Fertil Soils 52(3):423–437. https://doi.org/10.1007/s00374-015-1073-5
Morin-Crini N, Lichtfouse E, Torri G, Crini GJECL (2019) Applications of chitosan in food, pharmaceuticals, medicine, cosmetics, agriculture, textiles, pulp and paper, biotechnology, and environmental chemistry. Environ Chem Lett 17(4):1667–1692. https://doi.org/10.1007/s10311-019-00904-x
Moulick RG, Das S, Debnath N, Bandyopadhyay KJPBR (2020) Potential use of nanotechnology in sustainable and ‘smart’agriculture: advancements made in the last decade. Plant Biotechnol Rep 3:1–9. https://doi.org/10.1007/s11816-020-00636-3
Mukherjee R, De S (2016) Preparation, characterization and application of powdered activated carbon-cellulose acetate phthalate mixed matrix membrane for treatment of steel plant effluent. Polym Adv Technol 27(4):444–459. https://doi.org/10.1002/pat.3690
Mulaudzi T, Hendricks K, Mabiya T, Muthevhuli M, Ajayi RF, Mayedwa N, Gehring C, Iwuoha E (2020) Calcium improves germination and growth of sorghum bicolor seedlings under salt stress. Plants 9(6):730. https://doi.org/10.3390/plants9060730
Nakandalage N, Nicolas M, Norton RM, Hirotsu N, Milham PJ, Seneweera S (2016) Improving rice zinc biofortification success rates through genetic and crop management approaches in a changing environment. Front Plant Sci 7:764. https://doi.org/10.3389/fpls.2016.00764
Nieves-Cordones M, Al Shiblawi FR, Sentenac H (2016) Roles and transport of sodium and potassium in plants. The Alkali Metal Ions: Their Role Life 4:291–324. https://doi.org/10.1007/978-3-319-21756-7_9
Nishida N, Amagasa S, Kobayashi Y, Yamada YJJoNR (2018) Synthesis of Cu-doped δ-FeOOH nanoparticles by a wet chemical method. J Nanoparticle Res 20 (7), 1-8. https://doi.org/10.1007/s11051-018-4275-6
Noulas C, Tziouvalekas M, Karyotis T (2018) Zinc in soils, water and food crops. J Trace Elem Med Biol 49:252–260. https://doi.org/10.1016/j.jtemb.2018.02.009
Panpatte DG, Jhala YK, Shelat HN, Vyas RV (2016) Nanoparticles: the next generation technology for sustainable agriculture. Microbial Inoculants Sustain Agric Product 5:289–300. https://doi.org/10.1007/978-81-322-2644-4_18
Paradelo R, Basanta R, Barral M (2019) Water-holding capacity and plant growth in compost-based substrates modified with polyacrylamide, guar gum or bentonite. Sci Hortic 243:344–349. https://doi.org/10.1016/j.scienta.2018.08.046
Paradisone V, Navarro-León E, Ruiz JM, Esposito S, Blasco BJAPP (2021) Calcium silicate ameliorates zinc deficiency and toxicity symptoms in barley plants through improvements in nitrogen metabolism and photosynthesis. Acta Physiol Plant 43(12):1–11. https://doi.org/10.1007/s11738-021-03325-y
Paulkumar K, Gnanajobitha G, Vanaja M, Rajeshkumar S, Malarkodi C, Pandian K, Annadurai G (2014) Piper nigrum leaf and stem assisted green synthesis of silver nanoparticles and evaluation of its antibacterial activity against agricultural plant pathogens. Sci World J 2014:1–9. https://doi.org/10.1155/2014/829894
Petchthanasombat C, Tiensing T, Sunintaboon P (2012) Synthesis of zinc oxide-encapsulated poly (methyl methacrylate)–chitosan core–shell hybrid particles and their electrochemical property. J Colloid Interface Sci 369(1):52–57. https://doi.org/10.1016/j.jcis.2011.12.001
Phan HT, Haes AJ (2019) What does nanoparticle stability mean? J Phys Chem C 123(27):16495–16507. https://doi.org/10.1021/acs.jpcc.9b00913
Pirzadah TB, Malik B, Maqbool T, Rehman RU (2019) Development of nano-bioformulations of nutrients for sustainable agriculture. Nanobiotechnol Bioformulations 4:381–394. https://doi.org/10.1007/978-3-030-17061-5_16
Pohshna C, Mailapalli DR, Laha T (2020) Synthesis of nanofertilizers by planetary ball milling. Sustain Agric Rev 40(5):75–112. https://doi.org/10.1007/978-3-030-33281-5_3
Polman EM, Gruter G-JM, Parsons JR, Tietema AJSoTTE (2020) Comparison of the aerobic biodegradation of biopolymers and the corresponding bioplastics: a review. Sci Total Environ. 3, 141953. https://doi.org/10.1016/j.scitotenv.2020.141953
Ponnamma D, Cabibihan J-J, Rajan M, Pethaiah SS, Deshmukh K, Gogoi JP, Pasha SK, Ahamed MB, Krishnegowda J, Chandrashekar B (2019) Synthesis, optimization and applications of ZnO/polymer nanocomposites. Mater Sci Eng, C 98:1210–1240. https://doi.org/10.1016/j.msec.2019.01.081
Prabha S, Durgalakshmi D, Rajendran S, Lichtfouse EJECL (2021) Plant-derived silica nanoparticles and composites for biosensors, bioimaging, drug delivery and supercapacitors: a review. Environ Chem Lett 19(2):1667–1691. https://doi.org/10.1007/s10311-020-01123-5
Prasad R, Bhattacharyya A, Nguyen QD (2017) Nanotechnology in sustainable agriculture: recent developments, challenges, and perspectives. Front Microbiol 8:1014. https://doi.org/10.3389/fmicb.2017.01014
Priyanka N, Geetha N, Ghorbanpour M, Venkatachalam P (2019) Role of engineered zinc and copper oxide nanoparticles in promoting plant growth and yield: present status and future prospects. Adv Phytonanotechnol 2019:183–201. https://doi.org/10.1016/B978-0-12-815322-2.00007-9
Prost L (2021) Revitalizing agricultural sciences with design sciences. Agric Syst 193:103225. https://doi.org/10.1016/j.agsy.2021.103225
Qian Y, Yao J, Russel M, Chen K, Wang X (2015) Characterization of green synthesized nano-formulation (ZnO–A vera) and their antibacterial activity against pathogens. Environ Toxicol Pharmacol 39(2):736–746. https://doi.org/10.1016/j.etap.2015.01.015
Rajam MV (2020) RNA silencing technology: A boon for crop improvement. J Biosci 45(1):118. https://doi.org/10.1007/s12038-020-00082-x
Rajan M, Anthuvan AJ, Muniyandi K, Kalagatur NK, Shanmugam S, Sathyanarayanan S, Chinnuswamy V, Thangaraj P, Narain NJAJfS, Engineering (2020) Comparative study of biological (Phoenix loureiroi fruit) and chemical synthesis of chitosan-encapsulated zinc oxide nanoparticles and their biological properties. 45(1), 15–28. https://doi.org/10.1007/s13369-019-04174-1
Rajput N (2015) Methods of preparation of nanoparticles-a review. Int J Adv Eng Technol 7(6):1806. https://doi.org/10.4236/anp.2016.51004
Ramírez-Rodríguez GB, Dal Sasso G, Carmona FJ, Miguel-Rojas C, Pérez-de-Luque A, Masciocchi N, Guagliardi A, Delgado-López JM (2020) Engineering biomimetic calcium phosphate nanoparticles: a green synthesis of slow-release multinutrient (NPK) nanofertilizers. ACS Appl Bio Mater 3(3):1344–1353. https://doi.org/10.1021/acsabm.9b00937
Rana M, Bhantana P, Sun X, Imran M, Shaaban M, Moussa M, Hamzah Saleem M, Elyamine A, Binyamin R, Alam M (2020) Molybdenum as an essential element for crops: an overview. Int J Sci Res Growth 24:18535. https://doi.org/10.2671/BJSTR.2020.24.004104
Razaq M, Zhang P, Shen H-l (2017) Influence of nitrogen and phosphorous on the growth and root morphology of Acer mono. PLoS ONE 12(2):e0171321. https://doi.org/10.1371/journal.pone.0171321
Rehman A, Farooq M, Ozturk L, Asif M, Siddique KH (2018) Zinc nutrition in wheat-based cropping systems. Plant Soil 422(1):283–315. https://doi.org/10.1007/s11104-017-3507-3
Rochman NT, Akwalia PR (2017) Fabrication and characterization of Zinc Oxide (ZnO) nanoparticle by sol-gel method. J Phys: Conf Ser 853(1):012041. https://doi.org/10.1088/1742-6596/853/1/012041
Rout GR, Sahoo S (2015) Role of iron in plant growth and metabolism. Rev Agric Sci 3:1–24. https://doi.org/10.7831/ras.3.1
Roy A, Singh S, Bajpai J, Bajpai A (2014) Controlled pesticide release from biodegradable polymers. Open Chem 12(4):453–469. https://doi.org/10.2478/s11532-013-0405-2
Sabir S, Arshad M, Chaudhari SK (2014) Zinc oxide nanoparticles for revolutionizing agriculture: synthesis and applications. Sci World J. https://doi.org/10.1155/2014/925494
Salam HA, Sivaraj R, Venckatesh R (2014) Green synthesis and characterization of zinc oxide nanoparticles from Ocimum basilicum L. var. purpurascens Benth.-Lamiaceae leaf extract. Mater Lett 131:16–18. https://doi.org/10.1016/j.matlet.2014.05.033
Saratale RG, Saratale GD, Shin HS, Jacob JM, Pugazhendhi A, Bhaisare M, Kumar G (2018) New insights on the green synthesis of metallic nanoparticles using plant and waste biomaterials: current knowledge, their agricultural and environmental applications. Environ Sci Pollut Res 25(11):10164–10183. https://doi.org/10.1007/s11356-017-9912-6
Schmidt SB, Jensen PE, Husted S (2016) Manganese deficiency in plants: the impact on photosystem II. Trends Plant Sci 21(7):622–632. https://doi.org/10.1016/j.tplants.2016.03.001
Shankar S, Rhim J-WJECL (2019) Effect of Zn salts and hydrolyzing agents on the morphology and antibacterial activity of zinc oxide nanoparticles. Environ Chem Lett 17(2):1105–1109. https://doi.org/10.1007/s10311-018-00835-z
Shi L-E, Li Z-H, Zheng W, Zhao Y-F, Jin Y-F, Tang Z-X (2014) Synthesis, antibacterial activity, antibacterial mechanism and food applications of ZnO nanoparticles: a review. Food Addit Contam: Part A 31(2):173–186. https://doi.org/10.1080/19440049.2013.865147
Shireen F, Nawaz MA, Chen C, Zhang Q, Zheng Z, Sohail H, Sun J, Cao H, Huang Y, Bie Z (2018) Boron: functions and approaches to enhance its availability in plants for sustainable agriculture. Int J Mol Sci 19(7):1856. https://doi.org/10.3390/ijms19071856
Solanki P, Bhargava A, Chhipa H, Jain N, Panwar J (2015) Nano-fertilizers and their smart delivery system. Nanotechnol Food Agric 2015:81–101. https://doi.org/10.1007/978-3-319-14024-7_4
Song U, Kim JJH, Environment,, Biotechnology (2020) Zinc oxide nanoparticles: a potential micronutrient fertilizer for horticultural crops with little toxicity. 61(3), 625–631. https://doi.org/10.1007/s13580-020-00244-8
Souri Z, Khanna K, Karimi N, Ahmad P (2020) Silicon and plants: current knowledge and future prospects. J Plant Growth Regul 2:1–20. https://doi.org/10.1007/s00344-020-10172-7
Sturikova H, Krystofova O, Huska D, Adam V (2018) Zinc, zinc nanoparticles and plants. J Hazard Mater 349:101–110. https://doi.org/10.1016/j.jhazmat.2018.01.040
Subbaiah LV, Prasad TNVKV, Krishna TG, Sudhakar P, Reddy BR, Pradeep T (2016) Novel effects of nanoparticulate delivery of zinc on growth, productivity, and zinc biofortification in maize (Zea mays L.). J Agric Food Chem 64(19):3778–3788. https://doi.org/10.1021/acs.jafc.6b00838
Subramanian KS, Manikandan A, Thirunavukkarasu M, Rahale CS (2015) Nano-fertilizers for balanced crop nutrition. Nanotechnol Food Agric 2015:69–80. https://doi.org/10.1007/978-3-319-14024-7_3
Suresh J, Pradheesh G, Alexramani V, Sundrarajan M, Hong SIJAiNSN, Nanotechnology (2018a) Green synthesis and characterization of zinc oxide nanoparticle using insulin plant (Costus pictus D. Don) and investigation of its antimicrobial as well as anticancer activities. Adv Nat Sci: Nanosci 9(1), 015008. https://doi.org/10.1088/2043-6254/aaa6f1
Suresh J, Pradheesh G, Alexramani V, Sundrarajan M, Hong SIJAiNSN, Nanotechnology (2018b) Green synthesis and characterization of zinc oxide nanoparticle using insulin plant (Costus pictus D. Don) and investigation of its antimicrobial as well as anticancer activities. Adv Nat Sci: Nanosci Nanotechnol, 9(1), 015008. https://doi.org/10.1088/2043-6254/aaa6f1
Taghavi Fardood S, Ramazani A, Moradnia F, Afshari Z, Ganjkhanlu S, Yekke Zare FJCM (2019) Green synthesis of ZnO nanoparticles via Sol-gel method and investigation of its application in solvent-free synthesis of 12-aryl-tetrahydrobenzo [α] xanthene-11-one derivatives under microwave irradiation. Chem Methodol 3(6), 632–642. https://doi.org/10.33945/SAMI/CHEMM.2019.6.2
Tamanna T, Bulitta JB, Yu A (2015) Controlling antibiotic release from mesoporous silica nano drug carriers via self-assembled polyelectrolyte coating. J Mater Sci–mater Med 26(2):117. https://doi.org/10.1007/s10856-015-5444-0
Tan W, Peralta-Videa JR, Gardea-Torresdey JL (2018) Interaction of titanium dioxide nanoparticles with soil components and plants: current knowledge and future research needs–a critical review. Environ Sci Nano 5(2):257–278. https://doi.org/10.1039/C7EN00985B
Tarafdar JC, Raliya R (2013) Rapid, low-cost, and ecofriendly approach for iron nanoparticle synthesis using aspergillus oryzae TFR9. J Nanoparticles 2013:1–4. https://doi.org/10.1155/2013/141274
Taran N, Storozhenko V, Svietlova N, Batsmanova L, Shvartau V, Kovalenko M (2017) Effect of zinc and copper nanoparticles on drought resistance of wheat seedlings. Nanoscale Res Lett 12(1):1–6. https://doi.org/10.1186/s11671-017-1839-9
Thema F, Manikandan E, Dhlamini M, Maaza M (2015) Green synthesis of ZnO nanoparticles via agathosma betulina natural extract. Mater Lett 161:124–127. https://doi.org/10.1016/j.matlet.2015.08.052
Thor K (2019) Calcium—Nutrient and messenger. Front Plant Sci 10:440. https://doi.org/10.3389/fpls.2019.00440
Thunugunta T, Reddy AC, Seetharamaiah SK, Hunashikatti LR, Chandrappa SG, Kalathil NC, Reddy LRDCJIn (2018) Impact of zinc oxide nanoparticles on eggplant (S. melongena): studies on growth and the accumulation of nanoparticles. IET Nanobiotechnol 12(6), 706–713. https://doi.org/10.1049/iet-nbt.2017.0237
Tofa TS, Kunjali KL, Paul S, Dutta JJECL (2019) Visible light photocatalytic degradation of microplastic residues with zinc oxide nanorods. Environ Chem Lett 17(3):1341–1346. https://doi.org/10.1007/s10311-019-00859-z
Umair Hassan M, Aamer M, Umer Chattha M, Haiying T, Shahzad B, Barbanti L, Nawaz M, Rasheed A, Afzal A, Liu Y (2020) The critical role of zinc in plants facing the drought stress. Agriculture 10(9):396. https://doi.org/10.3390/agriculture10090396
Utrera-Barrios S, Verdejo R, López-Manchado MA, Santana MHJMH (2020) Evolution of self-healing elastomers, from extrinsic to combined intrinsic mechanisms: a review. Mater Horiz 7(11):2882–2902. https://doi.org/10.1039/D0MH00535E
Venkatachalam P (2017) Biofabricated zinc oxide nanoparticles coated with phycomolecules as novel micronutrient catalysts for stimulating plant growth of cotton. Adv Nat Sci: Nanosci Nanotechnol. https://doi.org/10.1088/2043-6262/7/4/045018
Watson J-L, Fang T, Dimkpa CO, Britt DW, McLean JE, Jacobson A, Anderson AJ (2015) The phytotoxicity of ZnO nanoparticles on wheat varies with soil properties. Biometals 28(1):101–112. https://doi.org/10.1007/s10534-014-9806-8
Wilson HT, Amirkhani M, Taylor AG (2018) Evaluation of gelatin as a biostimulant seed treatment to improve plant performance. Front Plant Sci 9:1006. https://doi.org/10.3389/fpls.2018.01006
Yan B, Hou Y (2018) Effect of soil magnesium on plants: a review. IOP Conference Series: Earth and Environmental Science 170 (2), 022168. https://doi.org/10.1088/1755-1315/170/2/022168
Yue S, Yan Z, Shi Y, Ran G (2013) Synthesis of zinc oxide nanotubes within ultrathin anodic aluminum oxide membrane by sol–gel method. Mater Lett 98:246–249. https://doi.org/10.1016/j.matlet.2013.02.037
Yue X, Wang H, Kong J, Li B, Yang J, Li Q, Zhang J (2020) A novel and green sulfur fertilizer from CS2 to promote reproductive growth of plants. Environ Pollut 263:114448. https://doi.org/10.1016/j.envpol.2020.114448
Zaman QU, Aslam Z, Yaseen M, Ihsan MZ, Khaliq A, Fahad S, Bashir S, Ramzani P, Naeem M (2018) Zinc biofortification in rice: leveraging agriculture to moderate hidden hunger in developing countries. Arch Agron Soil Sci 64(2):147–161. https://doi.org/10.1080/03650340.2017.1338343
Zeng W, Nyapete CO, Benziger AH, Jelliss PA, Buckner SW (2019) Encapsulation of reactive nanoparticles of aluminum, magnesium, zinc, titanium, or boron within polymers for energetic applications. Curr Appl Polym Sci 3:3–13. https://doi.org/10.2174/2452271602666180917095629
Zhang T, Sun H, Lv Z, Cui L, Mao H, Kopittke PM (2017) Using synchrotron-based approaches to examine the foliar application of ZnSO4 and ZnO nanoparticles for field-grown winter wheat. J Agric Food Chem 66(11):2572–2579. https://doi.org/10.1021/acs.jafc.7b04153
Zhang M, Wang W, Bai SH, Liu S, Chen C, Xu Z, Guo X (2021) Intensive management of phosphorus fertilization in Camellia oleifera Abel to minimize phosphorus losses to the environment. Industrial Crops Prod 170:113824. https://doi.org/10.1016/j.indcrop.2021.113824
Zhao L, Sun Y, Hernandez-Viezcas JA, Hong J, Majumdar S, Niu G, Duarte-Gardea M, Peralta-Videa JR, Gardea-Torresdey JL (2015) Monitoring the environmental effects of CeO2 and ZnO nanoparticles through the life cycle of corn (Zea mays) plants and in situ μ-XRF mapping of nutrients in kernels. Environ Sci Technol 49(5):2921–2928. https://doi.org/10.1021/es5060226
Acknowledgements
The authors are grateful for the financial support from the Engineering and Physical Sciences Research Council (EPSRC) UK, the Higher Education Commission of Pakistan and Brazil University for the Institutional Research Development and Support Grant to carry on this research.
Author information
Authors and Affiliations
Contributions
BB and MBKN involved in conceptualization, methodology, and software. FS, ZJ, and USM involved in data curation and writing—original draft preparation. MBKN, JHPA-P, MDK, and D-VNV involved in visualization, writing—original draft preparation, and investigation. FS and MBKN involved in supervision. BB, D-VNV and USM involved in software and validation. Farooq Sher involved in writing—reviewing, submission, and editing and funding.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Beig, B., Niazi, M.B.K., Sher, F. et al. Nanotechnology-based controlled release of sustainable fertilizers. A review. Environ Chem Lett 20, 2709–2726 (2022). https://doi.org/10.1007/s10311-022-01409-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10311-022-01409-w