Abstract
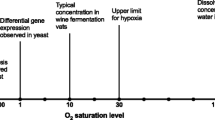
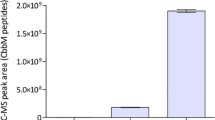
The yeast Arxula adeninivorans is considered to be a promising producer of recombinant proteins. However, growth characteristics are poorly investigated and no industrial process has been established yet. Though of vital interest for strain screening and production processes, rationally defined culture conditions remain to be developed. A cultivation system was evolved based on targeted sampling and mathematical analysis of rationally designed small-scale cultivations in shake flasks. The oxygen and carbon dioxide transfer rates were analyzed as conclusive online parameters. Oxygen limitation extended cultivation and led to ethanol formation in cultures supplied with glucose. Cultures were inhibited at pH-values below 2.8. The phosphorus demand was determined as 1.55 g phosphorus per 100 g cell dry weight. Synthetic SYN6 medium with 20 g glucose l−1 was optimized for cultivation in shake flasks by buffering at pH 6.4 with 140 mmol MES l−1. Optimized SYN6 medium and operating conditions provided non-limited cultivations without by-product formation. A maximal specific growth rate of 0.32 h−1 and short fermentations of 15 h were achieved. A pH optimum curve was derived from the oxygen transfer rates of differently buffered cultures, showing maximal growth between pH 2.8 and 6.5. Furthermore, it was shown that the applied medium and cultivation conditions were also suitable for non-limiting growth and product formation of a genetically modified A. adeninivorans strain expressing a heterologous phytase.






Similar content being viewed by others
References
Anderlei T, Büchs J (2001) Device for sterile online measurement of the oxygen transfer rate in shaking flasks. Biochem Eng J 7(2):157–162. doi:10.1016/S1369-703x(00)00116-9
Anderlei T, Zang W, Papaspyrou M, Büchs J (2004) Online respiration activity measurement (OTR, CTR, RQ) in shake flasks. Biochem Eng J 17(3):187–194. doi:10.1016/S1369-703x(03)00181-5
Atkinson B, Mavituna F (1983) Biochemical engineering and biotechnology handbook. Macmillan Publishers, Surrey
Beudeker RF, van Dam HW, van der Plaat JB, Vellenga K (1990) Yeast—biotechnology and biocatalysis. In: Developments in Baker′s yeast production. Marcel Dekker, New York
Blanchard JS (1984) Buffers for enzymes. Method Enzymol 104:404–414
Böer E, Breuer FS, Weniger M, Denter S, Piontek M, Kunze G (2011) Large-scale production of tannase using the yeast Arxula adeninivorans. Appl Microbiol Biotechnol 92(1):105–114. doi:10.1007/s00253-011-3320-5
Böer E, Gellissen G, Kunze G (2005) Production of recombinant proteins—novel microbial and eukaryotic expression systems. In: Arxula adeninivorans. Wiley-VCH, Weinheim
Böer E, Steinborn G, Florschütz K, Körner M, Gellissen G, Kunze G (2009) Yeast biotechnology: diversity and application. In: Arxula adeninivorans (Blastobotrys adeninivorans)—a dimorphic yeast of great biotechnological potential. Springer, Berlin Heidelberg New York
Brown DE (1988) Physiology of industrial fungi. In: The submerged culture of filamentous fungi. Blackwell Scientific Publications, Oxford
Büchs J, Maier U, Lotter S, Peter CP (2007) Calculating liquid distribution in shake flasks on rotary shakers at waterlike viscosities. Biochem Eng J 34(3):200–208. doi:10.1016/j.bej.2006.12.005
Dedyukhina EG, Eroshin VK (1991) Essential metal-ions in the control of microbial metabolism. Process Biochem 26(1):31–37. doi:10.1016/0032-9592(91)80005-A
Degelmann A (2002) Hansenula polymorpha-biology and applications. Methods. Wiley-VCH, Weinheim
Denison SH (2000) pH regulation of gene expression in fungi. Fungal Genet Biol 29(2):61–71. doi:10.1006/fgbi.2000.1188
Duboc P, Schill N, Menoud L, Vangulik W, Vonstockar U (1995) Measurements of sulfur, phosphorus and other ions in microbial biomass-influence on correct determination of elemental composition and degree of reduction. J Biotechnol 43(2):145–158. doi:10.1016/0168-1656(95)00135-0
Ferrer-Miralles N, Domingo-Espin J, Corchero JL, Vazquez E, Villaverde A (2009) Microbial factories for recombinant pharmaceuticals. Microb Cell Fact 8. doi:10.1186/1475-2859-8-17
Flikweert MT, Kuyper M, van Maris AJA, Kotter P, van Dijken JP, Pronk JT (1999) Steady-state and transient-state analysis of growth and metabolite production in a Saccharomyces cerevisiae strain with reduced pyruvate-decarboxylase activity. Biotechnol Bioeng 66(1):42–50. doi:10.1002/(Sici)1097-0290(1999)66:1<42:Aid-Bit4>3.0.Co;2-L
Gellissen G, Kunze G, Gaillardin C, Cregg JM, Berardi E, Veenhuis M, van der Klei I (2005) New yeast expression platforms based on methylotrophic Hansenula polymorpha and Pichia pastoris and on dimorphic Arxula adeninivorans and Yarrowia lipolytica-a comparison. FEMS Yeast Res 5(11):1079–1096. doi:10.1016/j.femsyr.2005.06.004
Gerngross TU (2004) Advances in the production of human therapeutic proteins in yeasts and filamentous fungi. Nat Biotechnol 22(11):1409–1414. doi:10.1038/Nbt1028
Gienow U, Kunze G, Schauer F, Bode R, Hofemeister J (1990) The yeast genus Trichosporon spec. LS3-molecular characterization of genomic complexity. Zbl Mikrobiol 145(1):3–12
Good NE, Winget GD, Winter W, Connolly TN, Izawa S, Singh RMM (1966) Hydrogen ion buffers for biological research. Biochemistry 5(2):467–477. doi:10.1021/Bi00866a011
Greasham RL (1993) Bioprocessing. In: Media for microbial fermentation. Wiley-VCH, Weinheim
Guez JS, Müller CH, Danze PM, Büchs J, Jacques P (2008) Respiration activity monitoring system (RAMOS), an efficient tool to study the influence of the oxygen transfer rate on the synthesis of lipopeptide by Bacillus subtilis ATCC6633. J Biotechnol 134(1–2):121–126. doi:10.1016/j.jbiotec.2008.01.003
Hellwig S, Stöckmann C, Gellissen G, Büchs J (2005) Production of recombinant proteins-novel microbial and eukaryotic expression systems. In: Comparative fermentation. Wiley-VCH, Weinheim
Jones RP, Greenfield PF (1984) A review of yeast ionic nutrition. I. Growth and fermentation requirements. Process Biochem 19(2):48–60
Kaiser C, Uhlig S, Gerlach T, Korner M, Simon K, Kunath K, Florschutz K, Baronian K, Kunze G (2010) Evaluation and validation of a novel Arxula adeninivorans estrogen screen (nAES) assay and its application in analysis of wastewater, seawater, brackish water and urine. Sci Total Environ 408(23):6017–6026. doi:10.1016/j.scitotenv.2010.08.050
Kaur P, Lingner A, Singh B, Boer E, Polajeva J, Steinborn G, Bode R, Gellissen G, Satyanarayana T, Kunze G (2007) APHO1 from the yeast Arxula adeninivorans encodes an acid phosphatase of broad substrate specificity. Anton Leeuw Int J G 91(1):45–55. doi:10.1007/s10482-006-9094-6
Kleiner D (1985) Bacterial ammonium transport. FEMS Microbiol Rev 32(2):87–100. doi:10.1111/j.1574-6968.1985.tb01185.x
Knabben I, Regestein L, Grumbach C, Steinbusch S, Kunze G, Büchs J (2010) Online determination of viable biomass up to very high cell densities in Arxula adeninivorans fermentations using an impedance signal. J Biotechnol 149(1–2):60–66. doi:10.1016/j.jbiotec.2010.06.007
Knoll A, Bartsch S, Husemann B, Engel P, Schroer K, Ribeiro B, Stöckmann C, Seletzky J, Büchs J (2007) High cell density cultivation of recombinant yeasts and bacteria under non-pressurized and pressurized conditions in stirred tank bioreactors. J Biotechnol 132(2):167–179. doi:10.1016/j.jbiotec.2007.06.010
Kottmeier K, Müller C, Huber R, Büchs J (2010) Increased product formation induced by a directed secondary substrate limitation in a batch Hansenula polymorpha culture. Appl Microbiol Biotechnol 86(1):93–101. doi:10.1007/s00253-009-2285-0
Losen M, Frolich B, Pohl M, Büchs J (2004) Effect of oxygen limitation and medium composition on Escherichia coli fermentation in shake-flask cultures. Biotechnol Progr 20(4):1062–1068. doi:10.1021/Bp034821
MacCabe AP, Ramon D (2001) Expression of the Aspergillus nidulans xlnC gene encoding the X-34 endo-xylanase is subject to carbon catabolite repression and pH control. World J Microbiol Biotechnol 17(1):57–60. doi:10.1023/A:1016615817331
Maier U, Losen M, Büchs J (2004) Advances in understanding and modeling the gas-liquid mass transfer in shake flasks. Biochem Eng J 17(3):155–167. doi:10.1016/S1369-703x(03)00174-8
Martin JF (1989) Regulation of secondary metabolism in actinomycetes. Molecular mechanisms for the control by phosphate of the biosynthesis of antibiotics and other secondary metabolites. CRC Press, Boca Raton
Middelhoven WJ, Dejong IM, Dewinter M (1991) Arxula-Adeninivorans, a yeast assimilating many nitrogenous and aromatic compounds. Anton Leeuw Int J G 59(2):129–137. doi:10.1007/Bf00445657
Minocha N, Kaur P, Satyanarayana T, Kunze G (2007) Acid phosphatase production by recombinant Arxula adeninivorans. Appl Microbiol Biotechnol 76(2):387–393. doi:10.1007/s00253-007-1021-x
Peter CP, Lotter S, Maier U, Büchs J (2004) Impact of out-of-phase conditions on screening results in shaking flask experiments. Biochem Eng J 17(3):205–215. doi:10.1016/S1369-703x(03)00179-7
Pham HTM, Kunath K, Gehrmann L, Giersberg M, Tuerk J, Uhlig S, Hanke G, Simon K, Baronian K, Kunze G (2013) Application of modified Arxula adeninivorans yeast cells in an online biosensor for the detection of estrogenic compounds in wastewater samples. Sensor Actuator B Chem 185:628–637. doi:10.1016/j.snb.2013.05.065
Qin YJ, Cabral JMS (1994) Kinetic-studies of the urease-catalyzed hydrolysis of urea in a buffer-free system. Appl Biochem Biotechnol 49(3):217–240. doi:10.1007/Bf02783059
Roos W, Luckner M (1984) Relationships between proton extrusion and fluxes of ammonium-ions and organic acids in Penicillium cyclopium. J Gen Microbiol 130:1007–1014
Rosel H, Kunze G (1995) Cloning and characterization of a tef gene for elongation-factor 1-alpha from the yeast Arxula adeninivorans. Curr Genet 28(4):360–366. doi:10.1007/Bf00326434
Saito I, Honda H, Kawabe T, Mukumoto F, Shimizu M, Kobayashi T (2000) Comparison of biotin production by recombinant Sphingomonas sp under various agitation conditions. Biochem Eng J 5(2):129–136. doi:10.1016/S1369-703x(00)00050-4
Sedzielewska KA, Boer E, Bellebna C, Wartmann T, Bode R, Melzer M, Baronian K, Kunze G (2012) Role of the AFRD1-encoded fumarate reductase in hypoxia and osmotolerance in Arxula adeninivorans. FEMS Yeast Res 12(8):924–937. doi:10.1111/j.1567-1364.2012.00842.x
Seletzky JM, Noack U, Hahn S, Knoll A, Amoabediny G, Büchs J (2007) An experimental comparison of respiration measuring techniques in fermenters and shake flasks: exhaust gas analyzer vs. RAMOS device vs. respirometer. J Ind Microbiol Biotechnol 34(2):123–130. doi:10.1007/s10295-006-0176-2
Steinborn G, Gellissen G, Kunze G (2007) A novel vector element providing multicopy vector integration in Arxula adeninivorans. FEMS Yeast Res 7(7):1197–1205. doi:10.1111/j.1567-1364.2007.00280.x
Stöckmann C, Losen M, Dahlems U, Knocke C, Gellissen G, Büchs J (2003) Effect of oxygen supply on passaging, stabilising and screening of recombinant Hansenula polymorpha production strains in test tube cultures. FEMS Yeast Res 4(2):195–205. doi:10.1016/S1567-1356(03)000147-8
Stöckmann C, Maier U, Anderlei T, Knocke C, Gellissen G, Büchs J (2003) The oxygen transfer rate as key parameter for the characterization of Hansenula polymorpha screening cultures. J Ind Microbiol Biotechnol 30(10):613–622. doi:10.1007/s10295-003-0090-9
Stöckmann C, Scheidle M, Dittrich B, Merckelbach A, Hehmann G, Melmer G, Klee D, Büchs J, Kang HA, Gellissen G (2009) Process development in Hansenula polymorpha and Arxula adeninivorans, a re-assessment. Microb Cell Fact 8. doi:10.1186/1475-2859-8-22
Walker GM (1998) Yeast physiology and biotechnology. Wiley-VCH, Weinheim
Wartmann T, Kruger A, Adler K, Duc BM, Kunze I, Kunze G (1995) Temperature-dependent dimorphism of the yeast Arxula adeninivorans LS3. Anton Leeuw Int J G 68(3):215–223. doi:10.1007/Bf00871818
Wartmann T, Stoltenburg R, Boer E, Sieber H, Bartelsen O, Gellissen G, Kunze G (2003) The ALEU2 gene-a new component for an Arxula adeninivorans-based expression platform. FEMS Yeast Res 3(2):223–232. doi:10.1016/S1567-1356(02)00190-3
Yang XX, Wartmann T, Stoltenburg R, Kunze G (2000) Halotolerance of the yeast Arxula adeninivorans LS3. Anton Leeuw Int J G 77(4):303–311. doi:10.1023/A:1002636606282
Acknowledgments
Part of the work was carried out in a project funded by the Ministry of Economy NRW, Germany (TPW-9910v08).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stöckmann, C., Palmen, T.G., Schroer, K. et al. Definition of culture conditions for Arxula adeninivorans, a rational basis for studying heterologous gene expression in this dimorphic yeast. J Ind Microbiol Biotechnol 41, 965–976 (2014). https://doi.org/10.1007/s10295-014-1433-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-014-1433-4