Abstract
The Philippines produce some 2.1–3.2 million t phosphogypsum (PG) per year. PG can contain elevated concentrations of rare earth elements (REEs). In this work, the leaching efficiency of the REEs from Philippine PG with H2SO4 was for the first time studied. A total of 18 experimental setups (repeated 3 times each) were conducted to optimize the acid concentration (1–10%), leaching temperature (40–80 °C), leaching time (5–120 min), and solid-to-liquid ratio (1:10–1:2) with the overall goal of maximizing the REE leaching efficiency. Applying different optimizations (Taguchi method, regression analysis and artificial neural network (ANN) analysis), a total REEs leaching efficiency of 71% (La 75%, Ce 72%, Nd 71% and Y 63%) was realized. Our results show the importance of the explanatory variables in the order of acid concentration > temperature > time > solid-to-liquid ratio. Based on the regression models, the REE leaching efficiencies are directly related to the linear combination of acid concentration, temperature, and time. Meanwhile, the ANN recognized the relevance of the solid-to-liquid ratio in the leaching process with an overall R of 0.97379. The proposed ANN model can be used to predict REE leaching efficiencies from PG with reasonable accuracy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Philippines is one of the largest phosphate fertilizer producers in the Southeast Asia, processing phosphate ore imported from different locations to wet phosphoric acid, an intermediate product in fertilizer production, and phosphogypsum (PG). PG is a powdery byproduct of which roughly 40% are presently used in the cement industry and as soil conditioners in the Philippines. The Mines and Geosciences Bureau of the Philippines is leaving no stone unturned in its quest to locate rare earth elements (REEs) that could support the country’s production sector while reducing metal imports from China [1, 2]. Ramirez et al. [3] recently pointed to the approximately 10.1 million t PG that are dry-stacked and accessible in the Philippines as a potential secondary resource of REEs.
Phosphate ore, the main raw material in phosphate fertilizer production is known for its elevated content of valuable trace elements, most notably REEs [4,5,6,7,8,9,10,11] and uranium [12,13,14,15,16]. During wet phosphoric acid production with sulfuric acid as it is done at most phosphate fertilizer plants worldwide, the majority of REEs (> 80%) transfers to the solid PG while most of the uranium (> 80%) transfers to the liquid phosphoric acid [17,18,19,20]. There is increased research activity to fully utilize PG stacks worldwide instead of just managing them [21,22,23,24,25,26,27,28,29,30]. Not processing PG but stacking the material could result in potential present and future environmental risks [31,32,33,34,35,36,37]. Recovering REEs that occur in relevant concentrations in PG [38,39,40,41,42,43] and ideally also removing actinides from the PG before using the remaining gypsum matrix as an inexpensive raw material in construction seems to be an attractive option [23, 44,45,46,47]. Although there have been considerable efforts to determine the concentrations and quantities of REEs in Philippine PG [3, 48] no work ever attempted to recover these resources. The present study aims at filling this gap by providing a simple as well as potentially economic process and optimization for REE leaching from Philippine PG that can be upscaled and applied in a next step.
Materials and methods
PG samples and elemental composition
PG samples were collected from 2-m-deep trenches in the tailing ponds of the main fertilizer plant in the Philippines as described in a previous work [3]. Although, the Philippines is one of the largest producers of fertilizer in Southeast Asia it has no domestic source of phosphate rock (PR). The PG in the Philippines is produced from a combination of sedimentary PR imported from China, Egypt, Israel, Jordan, Peru, Tunisia, the USA and Vietnam and igneous PR from Russia and South Africa [49, 50]. There are around 10.1 million t PG in the tailings ponds in the Philippines that have been accumulated since 1984 [48].
Five (5) of the samples with the highest total REE (TREE) concentrations from a previous study of the tailings ponds [3] were pulverized using mortar and pestle, and subsequently mixed to form a composite. To ensure homogeneity, the composite, weighing approximately 10 kg, was mixed in a Thermo Scientific bottle/tube roller for 24 h at 80 rpm. Samples were then sent to a third-party testing laboratory (Intertek Testing Services Philippines, Muntinlupa City, Philippines) for analysis. The laboratory is accredited by the Philippine Accreditation Bureau and also ISO/IEC 17025:2017-certified. Approximately 1 g of the composite was digested using a combination of analytical grade 37% HCl, 70% HNO3, 50% HF and 69–72% HClO4 and then analyzed for REEs using a combination of Inductively Coupled Plasma Mass Spectrometry (ICP-MS Agilent 7700x) and inductively coupled plasma optical emission spectrometry (ICP-OES Agilent 5100). Blank solutions and certified reference materials (i.e., OREAS 501c, 600, 623 90, and 44P) were used to ensure the accuracy of the results. The detection limits ranged from 0.05 to 0.1 mg kg−1. The REE composition of the PG composite is presented in Table 1.
REE leaching procedure
The leaching experiments were conducted following the patterns proposed by Al-Thyabat and Zhang [51, 52], Cánovas et al. [41], Lütke et al. [53], Rychkov et al. [54], and Walawalkar et al. [55] for other than Philippine PG. It is well known that PG from different locations shows different trace-element concentrations, so that leaching experiments successfully conducted at one PG location may lead to different results when a different PG stack is considered. The differences can be attributed to the different phosphate ore processed, the different processing conditions, as well as different qualities of the sulfuric acid used for wet-phosphoric acid processing [56]. The REE leaching optimization followed four succeeding steps: (1) optimizing acid concentration (C), (2) optimizing temperature (T), (3) optimizing time (t), and (4) optimizing solid-to-liquid ratio (S/L ratio) as summarized in Table 2.
There are a number of acids that are technically promising for leaching of REEs from PG [57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72]. In this work, H2SO4 (< 10% vol/vol) was chosen for its comparable leaching efficiency with HCl and HNO3, low solubility of PG in H2SO4 (i.e., resulting PG residue from leaching will enable secondary applications), and most importantly for onsite availability and economic reasons which could be beneficial for large scale extraction of REEs from PG in the Philippines [54].
Step 1: determination of optimum acid concentration
10 g PG was added to 50 mL (1:5 S/L ratio) of acid of varying concentrations in a 250 mL beaker. The mixture was leached at 380 rpm for 2 h at ambient temperature using 1% to 10% H2SO4. This concentration range was used to avoid common ion effect and formation of less soluble bisulfates that could inhibit the leaching of REEs from PG [73].
Step 2: determination of optimum temperature
10 g PG was added to 50 mL (1:5 S/L ratio) of the optimum acid concentration obtained in Step 1 in a 250 mL beaker. The mixture was leached at 380 rpm for 2 h at temperatures 40 to 80 °C.
Step 3: determination of optimum time
10 g PG was added to 50 mL (1:5 S/L ratio) of optimum acid concentration in a 250 mL beaker. The mixture was leached at 380 rpm at optimum temperatures obtained in Step 2 and at varying leaching times from 5 to 60 min.
Step 4: determination of optimum S/L ratio
10 g PG was added to varying volumes of optimum acid concentrations in 250 mL beaker to form 1:2, 1:3, 1:4, 1:5, and 1:10 S/L ratios. The mixture was leached for 30 min at 380 rpm at optimum temperatures.
The leaching experiment was performed in a hot bath (Fig. 1a). The temperature of the acid was stabilized prior to the addition of the PG. The acid-PG mixture was covered with a watch glass to prevent acid evaporation. After each leaching experiment, the PG and acid mixture was filtered using 125 mm Whatman ™ filter papers (Cat No 1440 125) and washed with 100 mL of distilled water (Fig. 1b). The collected residue was then dried in an oven at 105 °C for 24 h. Each experimental setup was repeated three (3) times which resulted in a total of 54 experiments. Due to the complexity of analyzing metals dissolved in sulfuric acid matrix [74], the dried residues were instead analyzed for REEs by the four-acid digestion method using ICP-MS and ICP-OES.
REE leaching efficiency
The efficiency of the leaching procedure was determined using the following formula:
where Ci and Cf are the REE concentrations in PG composite and PG residue, respectively.
Taguchi method
Taguchi method is an engineering technique used for process optimization which involves system design, parameter design, and tolerance design procedures [75, 76]. The signal/noise (S/N) ratio is used to examine the response in each experiment and the corresponding variance in the Taguchi method. The S/N ratio is a measure of deviation of quality characteristics from the ideal values [77]. There are usually three types of S/N ratios:
where m is the desired nominal value, n is the number of experiments, and y is the experimental result [78].
Multiple linear regression
A simple linear regression evaluates the relationship between the explanatory variable x and the response variable y. If there are multiple explanatory variables, Multiple linear regression (MLR) is utilized [79, 80]. Regression is normally used to make predictions. MLR assumes that the explanatory and response variables have a linear relationship, that the data has a normal distribution, that there are no extreme values, and that there are no multiple ties between the explanatory variables. MLR also synchronically accounts for the variance of the explanatory variable in the response variables [81].
Stepwise regression
Stepwise regression is also a multivariate modelling technique in which an explanatory variable is added or removed from the linear model at each step. In each step, the variable that increases the R2 coefficient the most is added to the model [82, 83]. In contrast to MLR, stepwise regression does not incorporate all the explanatory variables into the model but instead evaluates their statistical significance one at a time. It is typically used when investigating numerous explanatory variables. In this work, the regression models were performed in IBM SPSS Statistics version 25.
Artificial neural network (ANN)
Artificial neural network (ANN) is a machine learning technique that is now extensively used in mineral processing to identify complex relationships between input and output data using a series of nonlinear functions [84,85,86]. Unlike regression, ANN can be trained to learn and recognize patterns between the inputs and outputs [87]. One of the many benefits of using ANN is that it tolerates data noise [88]. ANN has been employed for optimization of leaching and extraction processes of precious metals (i.e., Cu, REEs, etc.) in several studies [75, 86, 88, 89].
ANN is essentially a computer model that simulates the brains learning mechanism. ANN consists of nodes or neurons which are processors that operate in a parallel way. The neurons are arranged in layers including an input layer, one or more hidden layers, and an output layer. The neurons are interconnected to one another through connection links carrying specific weights.
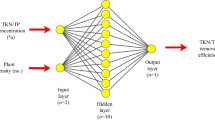
In this work, the feed-forward ANN using back-propagation algorithm was used to model the relationship between the explanatory variables and the REE leaching efficiency using MATLAB R2021b. Back-propagation algorithm is a method of reducing the error between output and input data by altering the weighted connections between neurons [90]. The architecture of the 4-9-5 neural network used in this work is shown in Fig. 2.
Results and discussion
Experimental leaching output
Acid concentration, temperature, time, and S/L ratio were optimized to maximize the efficiency of REE leaching from PG. The variables used in this work are based on previous experimental works [41, 51,52,53, 55]. Strong inorganic acids (i.e., HCl, HNO3, and H2SO4) were extensively used for the investigation of REE leaching from other than Philippine PG [91, 92]. H2SO4 was also used for leaching experiments from Florida PG by Gaetjens et al. [93] and Liang et al. [94], Russian PG by Lokshin et al. [95, 96], and Brazilian PG by Lütke et al. [53].
We performed a total of 18 leaching setups, repeated 3 times each to guarantee high accuracy of the results. The total experimental design matrix and the obtained REE leaching efficiencies are presented in Table 3. The leaching efficiencies for La, Ce, Nd, Y, and TREE ranged from 17 to 75%, 12 to 72%, 13 to 71%, 14 to 68%, and 14 to 71%. The setup with the highest TREE leaching efficiency was test number 6 which used 10% H2SO4, at 50 °C, a leaching time of 120 min, and a 1:5 S/L ratio.
For all the REEs, the leaching efficiencies followed the same trend in each of the leaching steps as shown in Figs. 3A–D. The efficiency of REE leaching increased with higher acid concentrations (Fig. 3A). This can be attributed to bisulfate formation that causes an increase in Ca2+ concentration after the reaction between H+ with SO42− as a result of increased gypsum solubility that controls the REE leaching efficiency since the gypsum hosts the REEs [32]. The temperature has a catalytic effect so that the leaching efficiency increased as the temperature increased from 40 °C to 50 °C (p < 0.05) as shown in Fig. 3B. The results for 50 °C to 80 °C are not significantly different (p > 0.05), although 50 °C leached the most REEs in the experiment. Generally, leaching efficiencies decrease at higher temperatures due to dissolution of fluoride precipitates which then reacts with the REEs and forms an insoluble precipitate [92]. The majority of the REEs leached from the PG after 15 min (Fig. 3C) although the setup with leaching time of 120 min leached the most REEs. Considering the economics in an industrial scale, we used 30 min for the final step of the optimization procedure. Leaching kinetic studies also show that the maximum REEs were leached from the PG after 20 min [53, 55]. Lastly, the most diluted mixture (1:10 S/L ratio) leached most of the REEs although the results of 1:3, 1:4, and 1:5 were not very different (Fig. 3D). The slight decrease in leaching efficiency observed for 1:5 is not significantly different (p > 0.05) with the results of 1:3 and 1:4. In some cases studied, a decrease in leaching efficiency could be explained with reaching the gypsum solubility limit [55]. In general, it is not desirable to have increased Ca2+ concentration in the solution because it can compete with REEs for available binding sites on the leaching agents. This means that if there is an excess of Ca2+ ions in the solution, they may bind to the leaching agents instead, which reduces the efficiency of the REE leaching process.
The Pearson correlation coefficients r between the independent variables (C H2SO4, T, t, and S/L ratio) and the dependent variables (La, Ce, Nd, Y, and TREE) is shown in Table 4. Among the explanatory variables, C H2SO4 has the highest r 0.918 to 0.956 (p < 0.01), followed by T with r 0.681–0.739 (p < 0.01). It is noteworthy that t has a negative r while the S/L ratio has a small positive r. Both were not significant (p > 0.05). Although the variables that should be used in regression models are for r > 0.3, we still used t and the S/L ratio in the regression models.
Determining the optimum leaching conditions using Taguchi method
Although the design of the experiment is not based on the orthogonal array suggested by Taguchi, we still used the Taguchi method to determine the optimum combination of variables. The result of the REE leaching efficiency was converted to signal-to-noise (S/N) ratios. The S/N ratio is a measure of deviation of quality characteristics from the ideal values [77]. There are three different types of S/N ratios (i.e., nominal value is better, smaller is better, and larger is better) depending on the data characteristics [78]. In this work, the larger is better type was used building on the work of Brest Kasongo and Mwanat [75]. Thus, the levels of the explanatory variables with the highest average S/N ratios are considered optimal. The result of this method can therefore determine the optimum levels and combination of the variables to maximize REEs leaching from PG. The square of responses, inverse of the square of responses, and S/N for the REE yield for each of the experiments is shown in Table 5.
The average S/N ratios of the explanatory variables at different levels for specific REE leaching is shown in Table 6. For all the REEs, the highest average S/N ratios corresponded to level 4 (10% H2SO4) for the acid concentration, level 6 (80 °C) for the temperature, level 3 (30 min—Y and TREE) to level 4 (45 min—La, Ce, and Nd) for the time, and level 1 (1:10) for the S/L ratio. Therefore, the optimum combination of the variables for the maximum leaching of REEs in PG is 10% H2SO4, 80 °C, 30–45 min, and 1:10 for the acid concentration, temperature, time, and S/L ratio, respectively.
Aside from finding the optimum combination of the variables, the Taguchi method also ranks the variables according to their overall importance to REE leaching efficiency. Also shown in Table 6 are the deltas and ranks of the explanatory variables which compare the relative magnitude of their effects. The delta is the difference between the highest and lowest average S/N ratio of each variable. And for this work, the higher the delta, the greater is the influence of the variable which assigns their rankings. Based on the delta, the ranking of the variables according to their overall importance to REE leaching in PG is as follows: acid concentration > temperature > time > S/L ratio.
Modelling of REE leaching efficiency using MLR
Multiple linear regression (MLR) models the linear association between the independent/explanatory variables (i.e., C, T, t, S/L ratio) and the dependent/response (i.e., Laeff, Ceeff, Ndeff, Yeff, and TREEeff) variables. We used the first order MLR to model the leaching efficiencies of REEs in PG using the explanatory variables. The MLR model used in this work follows the form proposed by Uyanık and Güler [81]:
where \({\beta }_{0}\) is the coefficient of the intercept or the constant and \({\beta }_{i}\) is the slope or the coefficient of the explanatory variable \({X}_{i}\) [89]. The significance of the explanatory variable for inclusion in the linear model was validated using p values (p ≤ 0.05) or Sig. For all the REEs, the regression statistics show an R2 of 0.938–0.961, adjusted R2 of 0.919–0.949, standard error of 3.534–4.025, and an overall p value of 0.000. The coefficients, p values, and the 95% confidence intervals of the explanatory variables are shown in Table 7.
Based on these values, the forms of the MLR models for the leaching efficiencies of the specific REEs are:
The regression models confirmed the results of the Pearson correlation and Taguchi method that the S/L ratio is not a particularly important variable in determining the leaching efficiency of REEs. T is significant for Laeff, Ceeff, and Ndeff whereas t is significant for all except for Laeff. For all the REEs, C H2SO4 is the most significant variable.
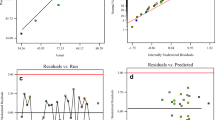
We validated the models using the experimental parameters in Table 3. Using the models, we found very good correspondence between the experimental and predicted values (Fig. 4A) with rExpt-Predicted (p < 0.01) of 0.983, 0.989, 0.990, 0.977, and 0.966 for Laeff, Ceeff, Ndeff, Yeff, and TREEeff models, respectively. We also computed the deviation of the predicted values from the experimental values using the % error. The performance of each model based on the average % error computed from the 18 experimental tests (Fig. 4B) is in the order of 7.08 ± 3.59% (Laeff) < 7.38 ± 9.66% (Ndeff) < 8.17 ± 8.90% (Ceeff) < 11.02 ± 8.48% (Yeff) < 13.53 ± 6.72 (TREEeff).
Modelling of REE leaching efficiency using stepwise regression
Leaching efficiency of TREEs can also be affected by an interaction among the different parameters. To determine such effects, we developed a first order multiple linear regression model with interaction effects between the explanatory variables using stepwise regression. The model follows the general form of:
where k is the number of explanatory variables [89]. A total of 18 interactions were analyzed including C H2SO4, T, t, S/L, C H2SO4·C H2SO4, C H2SO4·T, C H2SO4·t, C H2SO4·S/L, T·T, T·t, T·S/L, t∙t, t·S/L, S/L·S/LI, C H2SO4·T·t, C H2SO4·T·S/L, C H2SO4·t·S/L, and T·t∙S/L. The stepping method criteria used for inclusion in the model is the probability of F (entry ≤ 0.05 and removal ≥ 0.10). The coefficients, p values, 95% confidence interval, and other relevant statistics of the stepwise regression models of the possible interactions between the explanatory variables are presented in Table 8. For each of the REEs, two regression models were produced but the second model was selected for its non-multicollinearity such that Tolerance > 0.1 and VIF < 10 [89].
Among these possible interactions, we found that only C H2SO4 is significant for modelling the leaching efficiencies of all the REEs. The stepwise regression verifies the previous result of the MLR that the S/L ratio and its possible interaction with the other explanatory variables is not significant for the REE leaching efficiency from PG. And like the previous MLR models, there were very high accuracies in the regression models with R2 = 0.936–0.960, adjusted R2 = 0.928–0.955, a standard error of 3.3121–3.7875, and an overall p value of 0.000–0.011.
The analytical forms of the leaching models based on the interaction between the explanatory variables are therefore:
Validation of the model showed a very high correlation between the experimental and predicted values (Fig. 5A) with rExpt-Predicted (p < 0.01) = 0.914, 0.915, 0.918, 0.970, and 0.946 for La, Ce, Nd, Y, and TREE, respectively. Meanwhile, the average % error of the models is in the following order: Y (6.54 ± 9.24%) < L (7.58 ± 5.61%) < Ce (8.81 ± 6.63%) < TREE (8.89 ± 7.23%) < Nd (10.75 ± 8.03) as shown in Fig. 5B.
The regression models consistently eliminate the significance of the S/L ratio. This makes sense since the leaching efficiency only increased by 3–5% as the mixture became more diluted in Step 4 of the experimental procedure (Fig. 2D). Unlike the previous regression model, the stepwise regression of possible interaction between the parameters eliminated the significance of T and t in the leaching of REEs. To validate the role of the S/L ratio that may probably not observe linear patterns, the ANN was used to find hidden patterns that the regression models were not able to identify.
Modelling of REE leaching efficiency using ANN
The modelling was carried out using a 4-9-5 architecture based on the recommendation by the improved version of the Kolmogorov theorem called the Kolmogorov Mapping Neural Network Existence Theorem. This theorem recommends a three-layer neural network composing of n inputs, 2n + 1 hidden layers, and m outputs [97]. Thus, the 4-9-5 ANN architecture corresponds to 4 neurons in the input layer, 9 neurons in one hidden layer, and 5 neurons in the output layer. The nntool was used to perform the computation in MATLAB R2021b. By default, this tool uses 70% of the data for training, 15% for validation, and 15% for testing.
The Levenberg–Marquardt backpropagation algorithm that updates the values of weights according to the Levenberg–Marquardt optimization was used to train a feed-forward ANN. In a backpropagation algorithm, the network continues until convergence or a maximum number of iterations is reached [98]. The pureline function was used as the activation function in the ANN structure. The number of epochs was set at an initial of 100 but the training process stopped after 3 iterations. The training stops when the model with the lowest root mean squared error on single test points is found. The correlation coefficient R for the training of the final model was 0.98073. The R values for the testing and the overall model were 0.98039 and 0.97379, respectively. The results are shown in Figs. 6A–C. The leaching efficiencies of REEs from PG can be predicted at very high accuracy using ANN. In contrast to the results of the regression models, the ANN was able to accurately predict REE leaching efficiency with very high R values even after considering the S/L ratio.
Conclusions
This work investigated for the first time, the leaching efficiency of the REEs from Philippine PG with H2SO4 and optimized the relevant parameters: acid concentration > temperature > time > solid-to-liquid ratio using Taguchi method, regression, and ANN analysis. A TREEs leaching efficiency of 71% (La 75%, Ce 72%, Nd 71% and Y 63%) was realized and it could be shown that the modelling approaches are powerful tools to predict and optimize the leaching efficiencies of REEs from Philippine PG. The experiments described here, though very successful, were all conducted at laboratory scale, and it is recommended to conduct larger pilot plant scale experiments next, to better understand the potential of REE recovery from Philippine PG on larger scale.
References
Ilankoon I, Dushyantha NP, Mancheri N et al (2022) Constraints to rare earth elements supply diversification: evidence from an industry survey. J Clean Prod 331:129932. https://doi.org/10.1016/j.jclepro.2021.129932
Mancheri NA, Sprecher B, Bailey G et al (2019) Effect of Chinese policies on rare earth supply chain resilience. Resour Conserv Recycl 142:101–112. https://doi.org/10.1016/j.resconrec.2018.11.017
Ramirez JD, Diwa RR, Palattao BL et al (2022) Rare earths in Philippine phosphogypsum: use them or lose them. Extr Ind Soc 10:101082. https://doi.org/10.1016/j.exis.2022.101082
Chen M, Graedel TE (2015) The potential for mining trace elements from phosphate rock. J Clean Prod 91:337–346. https://doi.org/10.1016/j.jclepro.2014.12.042
Emsbo P, McLaughlin PI, Breit GN et al (2015) Rare earth elements in sedimentary phosphate deposits: Solution to the global REE crisis? Gondwana Res 27:776–785. https://doi.org/10.1016/j.gr.2014.10.008
Hakkar M, Arhouni FE, Mahrou A et al (2021) Enhancing rare earth element transfer from phosphate rock to phosphoric acid using an inexpensive fly ash additive. Miner Eng 172:107166. https://doi.org/10.1016/j.mineng.2021.107166
Roshdy OE, Haggag EA, Masoud AM et al (2023) Leaching of rare earths from Abu Tartur (Egypt) phosphate rock with phosphoric acid. J Mater Cycles Waste Manag 25:501–517. https://doi.org/10.1007/s10163-022-01558-8
Innocenzi V, De MI, Kopacek B, Vegliò F (2014) Yttrium recovery from primary and secondary sources: a review of main hydrometallurgical processes. Waste Manag 34:1237–1250. https://doi.org/10.1016/j.wasman.2014.02.010
Balaram V (2023) Potential future alternative resources for rare earth elements: opportunities and challenges. Minerals 13:425. https://doi.org/10.3390/min13030425
Al Khaledi N, Taha M, Hussein, A, Hussein E, El Yahyaoui A, Haneklaus N (2019) Direct leaching of rare earth elements and uranium from phosphate rocks. In: IOP conference series: materials science and engineering. vol 479, p 012065. https://doi.org/10.1088/1757-899X/479/1/012065
Pavon S, Haneklaus N, Meerbach K, Bertau M (2022) Iron(III) removal and rare earth element recovery from a synthetic wet phosphoric acid solution using solvent extraction. Miner Eng 182:182107569. https://doi.org/10.1016/j.mineng.2022.107569
Haneklaus N (2021) Unconventional uranium from phosphates. Encycl Nucl Energy. https://doi.org/10.1016/B978-0-12-819725-7.00152-5
Mwalongo DA, Haneklaus NH, Lisuma JB et al (2022) Uranium in phosphate rocks and mineral fertilizers applied to agricultural soils in East Africa. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-022-24574-5
Shang D, Geissler B, Mew M et al (2021) Unconventional uranium in China’s phosphate rock: review and outlook. Renew Sustain Energy Rev. https://doi.org/10.1016/j.rser.2021.110740
Steiner G, Geissler B, Haneklaus N (2020) Making uranium recovery from phosphates great again? Environ Sci Technol 54:1287–1289. https://doi.org/10.1021/acs.est.9b07859
Mwalongo DA, Haneklaus NH, Carvalho FP et al (2023) Influence of phosphate fertilizers on the radioactivity of agricultural soils and tobacco plants in Kenya, Tanzania, and Uganda. Environ Sci Pollut Res 30:83004–83023. https://doi.org/10.1007/s11356-023-27543-8
Haneklaus N, Sun Y, Bol R et al (2017) To extract, or not to extract uranium from phosphate rock, that is the question. Environ Sci Technol 51:753–754. https://doi.org/10.1021/acs.est.6b05506
Haneklaus N, Bayok A, Fedchenko V (2017) Phosphate rocks and nuclear proliferation. Sci Glob Secur 25:143–158. https://doi.org/10.1080/08929882.2017.1394061
Arhouni FE, Hakkar M, Mahrou A et al (2022) Better filterability and reduced radioactivity of phosphogypsum during phosphoric acid production in Morocco using a fly ash waste and pure silica additive. J Radioanal Nucl Chem 331:1609–1617. https://doi.org/10.1007/s10967-022-08235-y
Christophe NN, McCrindle R, Maree J, Ngole-Jeme V (2023) The behaviour of selected rare-earth elements during the conversion of phosphogypsum to calcium sulphide and residue. J Mater Cycles Waste Manag. https://doi.org/10.1007/s10163-023-01640-9
Chernysh Y, Yakhnenko O, Chubur V, Roubík H (2021) Phosphogypsum recycling: a review of environmental issues, current trends, and prospects. Appl Sci 11:1–22. https://doi.org/10.3390/app11041575
Cao Y, Cui Y, Yu X et al (2021) Bibliometric analysis of phosphogypsum research from 1990 to 2020 based on literatures and patents. Environ Sci Pollut Res 28:66845–66857. https://doi.org/10.1007/s11356-021-15237-y
Haneklaus N, Barbossa S, Basallote MD et al (2022) Closing the upcoming EU gypsum gap with phosphogypsum. Resour Conserv Recycl. https://doi.org/10.1016/j.resconrec.2022.106328
Cánovas CR, Macías F, Pérez-López R et al (2018) Valorization of wastes from the fertilizer industry: current status and future trends. J Clean Prod 174:678–690. https://doi.org/10.1016/j.jclepro.2017.10.293
El Zrelli R, Rabaoui L, Daghbouj N et al (2018) Characterization of phosphate rock and phosphogypsum from Gabes phosphate fertilizer factories ( SE Tunisia ): high mining potential and implications for environmental protection. Environ Sci Res 25:14690–14702
Wang C, Wang Z, Huang D et al (2023) Recovery and recycling core of phosphogypsum : characteristic hazardous elements risk assessment and analysis. Process Saf Environ Prot 170:738–756. https://doi.org/10.1016/j.psep.2022.12.062
Cui Y, Chang I, Yang S et al (2022) A novel dynamic business model to quantify the effects of policy intervention on solid waste recycling industry : a case study on phosphogypsum recycling in Yichang China. J Clean Prod 355:131779. https://doi.org/10.1016/j.jclepro.2022.131779
Qi M, Peng W, Wang W et al (2023) Simple and efficient method for purification and recovery of gypsum from phosphogypsum : reverse-direct flotation and mechanism. J Mol Liq 371:121111. https://doi.org/10.1016/j.molliq.2022.121111
Qin X, Cao Y, Guan H et al (2023) Resource utilization and development of phosphogypsum-based materials in civil engineering. J Clean Prod. https://doi.org/10.1016/j.jclepro.2023.135858
Qi J, Zhu H, Zhou P et al (2023) Application of phosphogypsum in soilization: a review. Int J Environ Sci Technol. https://doi.org/10.1007/s13762-023-04783-2
Millán-Becerro R, Pérez-López R, Cánovas CR et al (2023) Phosphogypsum weathering and implications for pollutant discharge into an estuary. J Hydrol 617:128943. https://doi.org/10.1016/j.jhydrol.2022.128943
Bisone S, Gautier M, Chatain V, Blanc D (2017) Spatial distribution and leaching behavior of pollutants from phosphogypsum stocked in a gypstack : geochemical characterization and modeling. J Environ Manage. https://doi.org/10.1016/j.jenvman.2017.02.055
Akfas F, Elghali A, Louis J et al (2023) Geochemical and mineralogical characterization of phosphogypsum and leaching tests for the prediction of the mobility of trace elements. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-023-25357-2
Li B, Danish K-D, Haneklaus N (2022) Ecological footprint analysis of the phosphorus industry in China. Environ Sci Pollut Res 29:73461–73479. https://doi.org/10.1007/s11356-022-20878-8
Neil W, Patrick T (2022) Radioactivity in future phosphogypsum : new predictions based on estimates of ‘Peak P’ and rock phosphate resources. J Environ Radioact 244–245:106828. https://doi.org/10.1016/j.jenvrad.2022.106828
Silva LFO, Oliveira MLS, Crissien TJ et al (2022) Chemosphere A review on the environmental impact of phosphogypsum and potential health impacts through the release of nanoparticles. Chemosphere 286:131513. https://doi.org/10.1016/j.chemosphere.2021.131513
Cánovas CR, Macías F, López RP, Nieto JM (2018) Science of the total environment mobility of rare earth elements, yttrium and scandium from a phosphogypsum stack : environmental and economic implications. Sci Total Environ 618:847–857. https://doi.org/10.1016/j.scitotenv.2017.08.220
Binnemans K, Jones PT, Blanpain B et al (2015) Towards zero-waste valorisation of rare-earth-containing industrial process residues: a critical review. J Clean Prod 99:17–38. https://doi.org/10.1016/j.jclepro.2015.02.089
Brahim JA, Hak SA, Achiou B et al (2022) Kinetics and mechanisms of leaching of rare earth elements from secondary resources. Miner Eng 177:107351. https://doi.org/10.1016/j.mineng.2021.107351
Gaustad G, Williams E, Leader A (2021) Rare earth metals from secondary sources: review of potential supply from waste and byproducts. Resour Conserv Recycl 167:105213. https://doi.org/10.1016/j.resconrec.2020.105213
Cánovas CR, Chapron S, Arrachart G, Pellet-Rostaing S (2019) Leaching of rare earth elements (REEs) and impurities from phosphogypsum: a preliminary insight for further recovery of critical raw materials. J Clean Prod 219:225–235. https://doi.org/10.1016/j.jclepro.2019.02.104
Jyothi RK, Thenepalli T, Ahn JW et al (2020) Review of rare earth elements recovery from secondary resources for clean energy technologies: grand opportunities to create wealth from waste. J Clean Prod 267:122048. https://doi.org/10.1016/j.jclepro.2020.122048
Shahbaz A (2022) A systematic review on leaching of rare earth metals from primary and secondary sources. Miner Eng 184:107632. https://doi.org/10.1016/j.mineng.2022.107632
Rosales J, Gázquez M, Cabrera M et al (2021) 6: application of phosphogypsum for the improvement of eco-efficient cements. In: Brito J, Thomas C, Medina C, Agrela FBT-W (eds) Woodhead publishing series in civil and structural engineering. Woodhead Publishing, pp 153–189
Fang K, Xu L, Yang M, Chen Q (2023) One-step wet-process phosphoric acid by-product CaSO 4 and its purification. Sep Purif Technol. https://doi.org/10.1016/j.seppur.2022.123048
Rashad AM (2017) Phosphogypsum as a construction material. J Clean Prod 166:732–743. https://doi.org/10.1016/j.jclepro.2017.08.049
Labrincha J, Puertas F, Schroeyers W et al (2017) 7: from NORM by-products to building materials. In: Schroeyers WBT-NORM (ed) Integration radiation protection in reuse. Woodhead Publishing, pp 183–252
Diwa RR, Tabora EU, Palattao BL et al (2021) Evaluating radiation risks and resource opportunities associated with phosphogypsum in the Philippines. J Radioanal Nucl Chem. https://doi.org/10.1007/s10967-021-08142-8
Haneklaus N, Reyes R, Lim WG et al (2015) Energy neutral phosphate fertilizer production using high temperature reactors: a Philippine case study. Philipp J Sci 44:69–79
Diwa RR, Ramirez JD, Haneklaus NH (2023) Uranium supply potential from imported phosphate rocks for the Philippine nuclear power program. The Extractive Industries and Society 15:101303. https://doi.org/10.1016/j.exis.2023.101303
Al-Thyabat S, Zhang P (2015) In-line extraction of REE from dihydrate (DH) and HEMIDIHYDRATE (HDH) wet processes. Hydrometallurgy 153:30–37. https://doi.org/10.1016/j.hydromet.2015.01.010
Al-Thyabat S, Zhang P (2015) REE extraction from phosphoric acid, phosphoric acid sludge, and phosphogypsum. Miner Process Extr Metall 124:143–150. https://doi.org/10.1179/1743285515Y.0000000002
Lütke SF, Oliveira MLS, Waechter SR et al (2022) Leaching of rare earth elements from phosphogypsum. Chemosphere 301:134661. https://doi.org/10.1016/j.chemosphere.2022.134661
Rychkov VN, Kirillov EV, Kirillov SV et al (2018) Recovery of rare earth elements from phosphogypsum. J Clean Prod 196:674–681. https://doi.org/10.1016/j.jclepro.2018.06.114
Walawalkar M, Nichol CK, Azimi G (2016) Process investigation of the acid leaching of rare earth elements from phosphogypsum using HCl, HNO3, and H2SO4. Hydrometallurgy 166:195–204. https://doi.org/10.1016/j.hydromet.2016.06.008
Bilal E, Bellefqih H, Bourgier V et al (2023) Phoshpogypsum circular economy considerations: a critical review from more than 65 storage sites worldwide. J Clean Prod. https://doi.org/10.1016/j.jclepro.2023.137561
Lambert A, Anawati J, Walawalkar M et al (2018) Innovative application of microwave treatment for recovering of rare earth elements from phosphogypsum. ACS Sustain Chem Eng 6:16471–16481. https://doi.org/10.1021/acssuschemeng.8b03588
Li S, Malik M, Azimi G (2022) Extraction of rare earth elements from phosphogypsum using mineral acids: process development and mechanistic investigation. Ind Eng Chem Res 61:102–114. https://doi.org/10.1021/acs.iecr.1c03576
Masmoudi-Soussi A, Hammas-Nasri I, Horchani-Naifer K, Férid M (2020) Hydrometallurgy rare earths recovery by fractional precipitation from a sulfuric leach liquor obtained after phosphogypsum processing. Hydrometallurgy 191:105253. https://doi.org/10.1016/j.hydromet.2020.105253
Mashifana TP (2019) Chemical treatment of phosphogypsum and its potential application for building and construction. Proced Manuf 35:641–648. https://doi.org/10.1016/j.promfg.2019.06.007
Golahdooz MR, Lashgari VA, Yoozbashizadeh H (2023) Separation of rare earth elements from phosphogypsum obtained from the processing of apatite concentrate from the chadormalu mine by acid leaching. Trans Indian Inst Met. https://doi.org/10.1007/s12666-023-02899-0
Bouargane B, Laaboubi K, Ghali M et al (2023) Effective and innovative procedures to use phosphogypsum waste in different application domains: review of the environmental, economic challenges and life cycle assessment. J Mater Cycles Waste Manag. https://doi.org/10.1007/s10163-023-01617-8
Lu S, Liu J (2023) Chemical engineering and processing: process intensification recovery of rare earth elements from phosphogypsum using subcritical water extraction. Chem Eng Process Process Intensif 190:1433. https://doi.org/10.1016/j.cep.2023.109433
Mukaba J-L, Eze CP, Pereao O, Petrik LF (2021) Rare earths’ recovery from phosphogypsum: an overview on direct and indirect leaching techniques. Minerals. https://doi.org/10.3390/min11101051
Hammas-Nasri I, Horchani-Naifer K, Férid M, Barca D (2016) Rare earths concentration from phosphogypsum waste by two-step leaching method. Int J Miner Process 149:78–83. https://doi.org/10.1016/j.minpro.2016.02.011
Valkov AV, Andreev VA, Anufrieva AV et al (2014) Phosphogypsum technology with the extraction of valuable components. Proced Chem 11:176–181. https://doi.org/10.1016/j.proche.2014.11.031
Gasser MS, Ismail ZH, Elgoud EMA et al (2022) Alkali treatment–acid leaching of rare earth elements from phosphogypsum fertilizer : insight for additional resource of valuable components. BMC Chem. https://doi.org/10.1186/s13065-022-00845-7
Brahim JA, Merroune A, Boulif R et al (2022) Efficient leaching process of rare earth, alkali and alkaline earth metals from phosphogypsum based on methanesulfonic acid (MSA) as green & eco-friendly lixiviant. RSC Adv 12:30639–30649. https://doi.org/10.1039/d2ra04124c
Tayar SP, Palmieri MC, Bevilaqua D (2022) Sulfuric acid bioproduction and its application in rare earth extraction from phosphogypsum. Miner Eng 185:107662. https://doi.org/10.1016/j.mineng.2022.107662
Kurkinen S, Virolainen S, Sainio T (2021) Hydrometallurgy recovery of rare earth elements from phosphogypsum waste in resin-in-leach process by eluting with biodegradable complexing agents. Hydrometallurgy 201:105569. https://doi.org/10.1016/j.hydromet.2021.105569
Brückner L, Elwert T, Schirmer T (2020) Extraction of rare earth elements from phospho-gypsum: concentrate digestion, leaching, and purification. Minerals. https://doi.org/10.3390/met10010131
Brahim JA, Merroune A, Mazouz H, Beniazza R (2023) Recovery of rare earth elements and sulfuric acid solution from phosphate byproducts via hydrofluoric acid conversion. J Ind Eng Chem. https://doi.org/10.1016/j.jiec.2023.07.028
Antonick PJ, Hu Z, Fujita Y et al (2019) Bio- and mineral acid leaching of rare earth elements from synthetic phosphogypsum. J Chem Thermodyn 132:491–496. https://doi.org/10.1016/j.jct.2018.12.034
Husáková L, Urbanová I, Šídová T, Mikysek T (2015) Multi-elemental analysis of sulfuric acid by oaTOF-ICP-MS after matrix modification with barium bromide. Anal Methods 7:5019–5027. https://doi.org/10.1039/C5AY00582E
Brest Kasongo K, Mwanat H-M (2021) Application of Taguchi method and artificial neural network model for the prediction of reductive leaching of cobalt (III) from oxidised low-grade ores. S Afr J Sci 117:1–8
Karna SK, Sahai R (2012) An overview on Taguchi method. Int J Eng Math Sci 1:1–7
Khare P, Kumar A (2012) Removal of phenol from aqueous solution using carbonized Terminalia chebula-activated carbon: process parametric optimization using conventional method and Taguchi’s experimental design, adsorption kinetic, equilibrium and thermodynamic study. Appl Water Sci 2:317–326. https://doi.org/10.1007/s13201-012-0047-0
Googerdchian F, Moheb A, Emadi R, Asgari M (2018) Optimization of Pb(II) ions adsorption on nanohydroxyapatite adsorbents by applying Taguchi method. J Hazard Mater 349:186–194. https://doi.org/10.1016/j.jhazmat.2018.01.056
Olive DJ (2017) Multiple linear regression. In: Olive DJ (ed) Linear regression. Springer International Publishing, Cham, pp 17–83
Tranmer M, Elliot M (2008) Multiple linear regression. Cathie Marsh Cent Census Surv Res 5:1–5
Uyanık GK, Güler N (2013) A study on multiple linear regression analysis. Proced Soc Behav Sci 106:234–240. https://doi.org/10.1016/j.sbspro.2013.12.027
Liao X, Li Q, Yang X et al (2008) Multiobjective optimization for crash safety design of vehicles using stepwise regression model. Struct Multidiscip Optim 35:561–569. https://doi.org/10.1007/s00158-007-0163-x
Wang M, Wright J, Brownlee A, Buswell R (2016) A comparison of approaches to stepwise regression on variables sensitivities in building simulation and analysis. Energy Build 127:313–326. https://doi.org/10.1016/j.enbuild.2016.05.065
Amnieh HB, Siamaki A, Soltani S (2012) Design of blasting pattern in proportion to the peak particle velocity (PPV): artificial neural networks approach. Saf Sci 50:1913–1916. https://doi.org/10.1016/j.ssci.2012.05.008
Chelgani SC, Shahbazi B, Rezai B (2010) Estimation of froth flotation recovery and collision probability based on operational parameters using an artificial neural network. Int J Miner Metall Mater 17:526–534. https://doi.org/10.1007/s12613-010-0353-1
Hoseinian FS, Abdollahzade A, Mohamadi SS, Hashemzadeh M (2017) Recovery prediction of copper oxide ore column leaching by hybrid neural genetic algorithm. Trans Nonferrous Met Soc China 27:686–693. https://doi.org/10.1016/S1003-6326(17)60076-1
Yang H, Ring Z, Briker Y et al (2002) Neural network prediction of cetane number and density of diesel fuel from its chemical composition determined by LC and GC–MS. Fuel 81:65–74. https://doi.org/10.1016/S0016-2361(01)00121-1
Jorjani E, Bagherieh AH, Mesroghli S, Chelgani SC (2008) Prediction of yttrium, lanthanum, cerium, and neodymium leaching recovery from apatite concentrate using artificial neural networks. J Univ Sci Technol Beijing Miner Metall Mater 15:367–374. https://doi.org/10.1016/S1005-8850(08)60070-5
Ma Y, Stopic S, Gronen L et al (2018) Neural network modeling for the extraction of rare earth elements from eudialyte concentrate by dry digestion and leaching. Metals (Basel). https://doi.org/10.3390/met8040267
Al-Thyabat S (2008) On the optimization of froth flotation by the use of an artificial neural network. J China Univ Min Technol 18:418–426. https://doi.org/10.1016/S1006-1266(08)60087-5
Yang X, Salvador D, Makkonen HT, Pakkanen L (2019) Phosphogypsum processing for rare earths recovery—a review. Nat Resour 10:325–336. https://doi.org/10.4236/nr.2019.109021
Ismail Z, Abu Elgoud EM, Gasser M et al (2015) Leaching of some lanthanides from phosphogypsum fertilizers by mineral acids. Arab J Nucl Sci Appl 48:37–50
Gaetjens T, Liang H, Zhang P, Moser R, Thomasson H, Dylewski H, Counce R, Watson J (2019) Economic optimization of rare earth element leaching kinetics from phosphogypsum with sulfuric acid. Int J Chem Reactor Eng 17(10). https://doi.org/10.1515/ijcre-2019-0061
Liang H, Zhang P, Jin Z, De Paoli D (2017) Rare earths recovery and gypsum upgrade from Florida phosphogypsum. Miner Metall Process 34:201–206. https://doi.org/10.19150/mmp.7615
Lokshin EP, Tareeva OA, Elizarov IR (2016) Agitation leaching of rare earth elements from phosphogypsum by weak sulfuric solutions. Theor Found Chem Eng 50:857–862. https://doi.org/10.1134/S0040579516050134
Lokshin EP, Tareeva OA, Elizarova IR (2015) Sorption of rare-earth elements from phosphogypsum sulfuric acid leaching solutions. Theor Found Chem Eng 49:773–778. https://doi.org/10.1134/S0040579515050127
Hecht-Nielsen R (1987) Kolmogorov’s mapping neural network existence theorem. Proceedings of the international conference on Neural networks. IEEE Press, New York, pp 11–14
Majdi A, Beiki M (2010) Evolving neural network using a genetic algorithm for predicting the deformation modulus of rock masses. Int J Rock Mech Min Sci 47:246–253. https://doi.org/10.1016/j.ijrmms.2009.09.011
Acknowledgements
This work is funded by the Department of Science and Technology—Philippine Council for Industry, Energy and Emerging Technology Research and Development (DOST-PCIEERD) and Austria's Agency for Education and Internationalization (OeAD) [Grant Numbers: Africa UNINET P006 and P058; HR 09/2022; KOEF 01/2019; TW 01/2021]. German Federal Ministry of Education and Research (Project Number: 033RU020A) support for this project is offered under the coordination of the ERA-MIN3 action, which has received funding from the European Union under the Horizon 2020 Program [European Commission Grant Agreement No. 101003575]. This work was further supported by the German Federal Ministry of Education and Research under Bridge2ERA2021 [Grant No. 100579052]. We are thankful to Mr. Dennis Mate and Mr. Antonino Varela, Jr. and his staff for their invaluable contribution to the success of this research project.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Diwa, R.R., Tabora, E.U., Haneklaus, N.H. et al. Rare earths leaching from Philippine phosphogypsum using Taguchi method, regression, and artificial neural network analysis. J Mater Cycles Waste Manag 25, 3316–3330 (2023). https://doi.org/10.1007/s10163-023-01753-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10163-023-01753-1