Abstract
Reactive silica additives, such as clays, can increase the filterability of phosphogypsum (PG) during wet phosphoric acid production from phosphate rock (PR). In this study, the effect of adding inexpensive fly ash waste (34 kg per t PR) together with lower quantities of pure silica (8.5 kg per t PR) on the radioactivity of PG was investigated. The addition of fly ash waste/pure silica reduced the radiological activity of the PG by roughly 30%. The reduction was attributed to decreased activities from 238U (60% reduction) and 226Ra (30% reduction) in PG. Besides, P2O5 losses were slightly decreased.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Morocco is one of the largest phosphate rock (PR) producing countries in the world [1]. Mined PR is further processed to mineral fertilizer using the wet phosphoric acid (WPA) process. During the WPA process some 5 t phosphogypsum (PG) (dry weight) are produced per 1 t WPA. PR can contain elevated concentrations of naturally occurring radionuclides that transfer in parts to the WPA and the PG [2]. Although the activity of PG is relatively low (0.2–3.0 Bq/g for 226Ra which is the most relevant radionuclide) it permits utilization of PG as a building material under most national regulations [3, 4] so that some 100–280 million t PG per year are stacked in the vicinity of fertilizer plants worldwide today.
The purification of PG is difficult since the radium (Ra) is chemically very similar to calcium (Ca), so that separation from the PG matrix is complex, usually inefficient, and as a result costly [5, 6]. Economic PG treatment and potential utilization is an active field of research with increased pressure to process PG due to the environmental risks associated with storage and management of this bulk commodity [7,8,9,10].
In PR processing the main objective of the WPA industry is to obtain the highest concentration of WPA with maximum yield [11, 12]. The size and shape of the PG crystals is a major factor that effects filterability. If the size of the PG crystals is for instance too small, the resulting low filterability creates undesirable P2O5 losses [11]. There are options to modify size and shape of the PG particles. Flocculation agents for instance, produce larger aggregates which can be separated easier from WPA. Increasing PG crystal size is an effective strategy to increase WPA efficiency [13]. Different additives can further increase the rate of PG precipitation. These additives include aluminum sulfate, clay, and calcined clay [14], aluminum hydroxide, perlite, active charcoal, and manganese dioxide [15, 16], surfactants [17, 18], phosphonates [19, 20], and active silica [21].
WPA production units in Morocco currently use (relatively) expensive clay as a reactive silica additive. Hakkar et al. [22] recently suggested the use of fly ash instead of clay as a low-cost reactive silica source for WPA production from Khouribga PR in Morocco. This practice would further support valuable rare earths recovery from WPA since it increases the share of rare earths transferring from the PR to the liquid WPA instead of the solid PG [23, 24]. Such a practice may be particularly suitable at the Jorf Lasfar industrial zone in El Jadida, Morocco, where PR is processed to WPA. Furthermore, nearly 50% of the country’s electricity is produced at the Jorf Lasfar coal power plant, which produces approximately 500,000 t fly ash and an additional 140,000 t bottom ash per year of which only 30% fly ash is currently utilized in construction [25,26,27,28].
While Hakkar et al. [22] focused on the transfer of rare earth from PR to WPA and PG, this work investigates the possibility of using inexpensive fly ash as a mineral crystal modifier to (1) enhance the filtration rate of PG from WPA and (2) reduce the overall radioactivity of the produced PG so that the PG may find increased use in construction or agriculture in Morocco.
Experimental
Experimental procedure
Experiments were carried out using a laboratory scale unit at conditions simulating the industrial dihydrate process of WPA production in Morocco. The reaction was carried out in a 2 L reactor fitted with a stirrer and placed in a water bath. The phosphate pulp was added continuously using a spatula, while the sulfuric acid (65% H2SO4) and the recycled acid (18% P2O5) were added drop by drop, using two burettes. All the above feeding was done on continuous bases and the reaction temperature was maintained at 80 °C with constant agitation of 250 rpm. The slurry was agitated for an additional hour to assure sufficient time for PG crystal growth before the PG was filtered. The vacuum filtration was carried out using a Buchner type filter. In the end, the filter cake was washed. These tests were carried out repeatedly until the operating parameters representing industrial conditions that result in WPA with 29% P2O5 could be well simulated. All experiments used the same amount of sulfuric acid (250 g per 200 g PR) independent of the use of additives.
A second series of tests were performed in the presence of fly ash, pure silica and a mixture of both materials. The masses of the additives were chosen to maintain a SiO2/F ratio ≥ 0.53. This ratio was evaluated based on the stoichiometry of the complex forming reaction between F and the equivalent SiO2 to obtain a total conversion of HF to H2SiF6, following (1):
In total, 32 tests (8 baseline experiments without additives, 8 experiments with pure silica, 8 experiments with fly ash and 8 experiments with the fly ash/silica mixture) were carried out to simulate industrial WPA production with and without fly ash/silica additive in Morocco. Mean values of these experiments are presented here.
Chemicals and instrumentation.
Atomic absorption spectroscopy (AAS) was performed using a Perkin Elmer Pinnacle 900 T (Perkin Elmer Inc., Waltham, MA, USA). Elemental analysis was conducted using inductively coupled plasma optical emission spectrometry (ICP-OES) on an iCAP PRO XPS (Thermo Fischer Scientific, Thermo Jarrell Ash IRIS, Waltham, MA, USA) and on an Avio 560 Max (Perkin Elmer, Waltham, MA, USA). X-ray diffraction (XRD) was performed on a Bruker D8 (Bruker Inc., Billerica, MA, USA). The chemicals were sourced from Sigma Aldrich (St. Louis, LA, USA). Analytical grade purity was used if not specified otherwise.
Mineralogical characterization
Silica and active silica were analyzed using AAS. Other major compounds such as Al2O3, F, MgO, CaO, and P2O5 were determined using ICP-OES. Crystalline phases in PR/PG samples were analyzed using XRD with CuKα radiation (acceleration voltage of 40 kV and a current of 40 mA). The patterns of diffraction were obtained in a 2\(\theta\) scanning range from 5 to 70°, with a step of 0.02° and counting time of 11 min. Rietveld method with HighScore software and the current ICSD and PDF4 + databases was used to determine the shares of the mineral phases. The chemical composition of the PR, the fly ash, and the pure silica are provided in Table 1.
The particle distribution of the washed and floated Khouribga PR was further determined using different sieves. More than 85% of the PR showed particle sizes > 40 µm and only 7% of the PR showed particle sizes > 250 µm (Table 2).
Filtration rate
In phosphate industry, the filtration rate is usually expressed in t of P2O5 produced per m2 per day and can be calculated as shown in (2).
with FR being the filtration rate (t P2O5/m2/day), W the suspension weight (g), SC the solids content (%), F the filtration factor = 0.122, T the total filtration, washing and drying time (s). The filtration factor F is related to filter area, conversion of time, weight, and area units, as well as P2O5 recovery.
Reaction efficiency, P2O5 recovery and washing efficiency
The reaction efficiency is defined as the percentage of P2O5 passing from the phosphate concentrate to the WPA. It was calculated as following [29]:
with: P2O5PG being the concentration of P2O5 in the PG (%). P2O5water−soluble: percentage (%) of water-soluble P2O5 in the gypsum cake; CaOPR: % CaO in the PR used to make the acid; P2O5PR: percentage (%) P2O5 in the PR used to make the acid; CaOPG: percentage (%) CaO in the PG.
P2O5 recovery is defined as the percentage of P2O5 extracted from the PR into solution, because part of the P2O5 is lost if the PG is not fully washed and filtered.
The washing efficiency is defined as the percentage of water-soluble P2O5 that passes from the suspension into the WPA. It is determined as following:
Spectroscopic analysis
The analyses of PR and PG samples were carried out by gamma ray spectroscopy at the National Center for Energy Sciences and Nuclear Techniques (CNESTEN) in Morocco, using a broad energy germanium (BEGe) detector. The PR and PG samples were homogenized, and then packaged in cylindrical bottles with a volume of 175 mL and 75 mL. The energy measurement was 1332.5 keV with a resolution of 1.8 keV. For the energy and efficiency calibration of the BEGe detector, a certified multi energy standard was analyzed under the same conditions and geometry as the samples. The data acquisition, display, and analysis of the amplitude spectrum were carried out using the GENIE 2000 analysis software [30]. These analyses allowed the determination of the specific activity in Bq/kg for each radionuclide present in the samples using the following equation:
\(N_{{{\text{net}}}} \left( {E_{\gamma } } \right)\) represents the net number of strokes of the energy peak \(E_{\gamma }\); m denotes the mass of the sample in kg; \(\varepsilon_{{E_{\gamma } }}\) stands for the counting efficiency for the energy \(E_{\gamma }\); t is the time in s; \(I_{\gamma }\) is the branching ratio to the energy \(E_{\gamma }\); \(f_{{E_{\gamma } }}\) is the correction factor that is dependent on the energy \(E_{\gamma }\).
Radiological effects and dose estimation
To evaluate the radiological risks of gamma radiation emitted by radionuclides present in the PR and the PG samples, the radium equivalent activity (Raeq) was determined. The Raeq, due to the non-uniform distribution of natural radionuclides in the samples, is generally represented as the sum of the specific activities of 226Ra, 232Th and 40K, based on the understanding that 10 Bq/kg of 226Ra, 7 Bq/kg of 232Th and 130 Bq/kg of 40K would produce an identical gamma dose rate. It is the most widely used index to assess radiological risks. Raeq was calculated using the following equation [31]
with \(A_{226Ra}\), \(A_{232Th}\) and \(A_{40K}\) being the specific activities in (Bq/kg) of 226Ra, 232Th and 40K in the samples that were analyzed.
Internal and external hazard indices
The hazard indices are defined by a model considering the maximum activity of Raeq (370 Bq/kg). The external hazard index (Hex) is defined in (9).
In addition to the Hex, the respiratory organs are threatened because of the decay of 226Ra into 222Rn and its daughter products. To account for this, the maximum permissible activity for 226Ra is reduced by half to 185 Bq/kg. This internal hazard index (Hin) is defined in (10).
Hex and Hin should be < 1 or the unity, which is equivalent to an external dose rate of 1.5 mGy y−1 absorbed dose rate and annual effective dose.
Absorbed gamma dose rate
The absorbed gamma dose rate Ḋ (nGy/h) in air at 1 m height is assessed from natural radionuclides originating from 226Ra, 232Th and 40K. The outdoor external dose and indoor external dose rate were determined as depicted in (11) and (12) based on guidelines provided by UNSCEAR [31]:
The annual outdoor effective dose rate (\(\dot{E}_{{{\text{out}}}}\)) as well as the annual indoor effective dose rate \(\left( {\dot{E}_{{{\text{in}}}} } \right)\) were calculated as depicted in (13) and (14):
These doses consider a conversion coefficient from the absorbed dose in air as well as an indoor and outdoor occupancy factor to determine the effective dose received by adults. UNSCEAR [31] reports a value of 0.7 Sv/Gy for the conversion coefficient and 0.2 and 0.8 for the outdoor and indoor occupancy factors.
Results and discussion
Effect of the additives on the filtration rate and process efficiency
To avoid losses of P2O5 in the PG and to assure a high rate of P2O5 extraction, pure silica, fly ash and a mixture thereof was added to PR deficient in silica and alumina so that the crystal size is modified, and filterability is improved. For each experiment, the filtration rate, the P2O5 yields, and losses were determined. Table 3 compares the filtration and reaction data obtained when no additive was applied (Baseline) to those observed in the presence additives.
The best filtration rate was obtained with the addition of fly ash and the mixture, for which the improvement was increased by 0.13 and 0.42%, respectively, compared with the baseline. Improving the efficiency of PG filtration is critical to economic utilization of PR. Both reaction efficiency and P2O5 recovery are higher with the addition of the additives. The highest efficiency was recorded when the fly ash and the pure silica were mixed. This was attributed to the decrease of crystallized P2O5 and unreacted P2O5 losses as shown in Fig. 1.
The increase in reaction efficiencies were 1.05, 3.55 and 3.9%; and the increase in P2O5 recoveries were 0.5, 2.45 and 2.7% with the addition of pure silica, fly ash, and the mixture, respectively. The obtained washing efficiencies were within the industrial limits of 97.7 to 98.8%.
Effect of the additives on the PG morphology
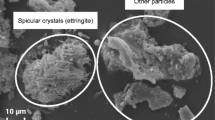
PG crystals of uniform size are most desirable for filtration and washing. Figure 2 shows the morphology of the PG samples without additives (Fig. 2a), with a pure silica additive (Fig. 2b), with a fly ash additive (Fig. 2c) and with the fly ash/silica additive (Fig. 2d).
The microscopic analysis reveals the formation of needle-shaped crystals and small fine diamonds that are characteristic for PG [32, 33]. The increased presence of small crystals decreases filtration by blocking the tissue. The different PG crystal shapes indicate that the additives had a positive effect on the crystal growth. Specifically, the crystals growth was promoted in one direction leading to fewer small crystals and increased needle-shaped crystals that have a larger length-to-width ratio and can be filtered more easily.
Effect of the additives on the produced WPA quality
WPA prepared from PR has a typical dark green color whose intensity depends on the rock’s organic matter content. This coloration is a nuisance, particularly in the preparation of food-grade acid and liquid fertilizers. A quantitative analysis method has also been developed in the laboratory to determine the amount of organic matter contained in the acid. It was based on an analysis by UV/vis spectrophotometer. For a characteristic wavelength of \(\lambda =408 \mathrm{nm}\), the absorbance of the strong acid 29% was measured. The results showed a drop of the absorbance from 0.477 to an average of 0.220 in the presence of additives. Doping with additives, thus allowed a clarification of the WPA by decreasing the content of organic matter and fluorine, which opens great possibilities of exploitation such as liquid fertilizers, the production of calcium and ammonium phosphates for animal feed, detergent industry, and surface treatment products. Figure 3b shows the produced WPA in its typical dark green and light green color produced with and without fly ash/pure silica additives.
Effect of the additives on the radioactivity of the PG
The specific activities of the three radioactive families (238U, 232Th and 235U) as well as 40K present in the PR and PG samples are shown in Fig. 3a. Most of the radioactivity measured from the PR can be attributed to the presence of radionuclides of the 238U family, particularly 226Ra. The specific activity of 238U was 1,191 Bq/kg and the specific activity for 226Ra was 1,032 Bq/kg. As expected, as PR is a material of natural origin derived from natural geological and precipitation processes, 232Th and 235U families contributed little to the activity of the PR, and the activity of 40K was below the detection limit.
During the production of WPA, the radioactive equilibrium between the three radioactive families and their daughters in the raw PR was broken and each radionuclide was distributed differently depending on its solubility. The results indicate an elevation in the concentration of 226Ra and 232Th in the analyzed PG samples with a percentage of about 85 and 81%, respectively. At the same time, the activities of 238U and 235U in PG is systematically decreased by 81 and 85%. This demonstrates the strong association of the 226Ra, originally existing in the PR, to the PG fraction. The results are consistent with the findings of other studies that had already found that uranium is quite soluble and tends to associate with PA while radium tends to be present in PG [34,35,36,37].
The presence of a fraction of 238U detected in the PG was associated with the presence of the remaining P2O5 [38, 39]. Whereas radium has been proposed to co-precipitate with CaSO4·nH2O [40]. Radium can be adsorbed by organic material or be mainly associated to BaSO4 or SrSO4 solid solutions. The affinity of the radium ions with the barite and strontium crystal lattice can be explained through their similar ionic radius [41]. When the fly ash was added, the fraction of P2O5 in the PG decreased and, consequently the activity of associated uranium decreased by 40% from 224 to 89 Bq/kg. The specific activity of 226Ra decreased by 30% from 879 to 617 Bq/kg as shown in Fig. 3a.
The Raeq value of the PG produced without additives was 897 Bq/kg (Fig. 3b). This value is 2.4 times greater than the current admissible limit for PG use in construction, which is ~ 370 Bq/kg [3, 31]. With the addition of fly ash, the Raeq value decreases to 641 Bq/kg. It must be concluded that the approach used in this work does not allow for the direct use of the PG as raw material in construction or soil amendment although the radioactivity can be notably reduced.
Radiation hazard indices
Based on the measured specific activity values of 226Ra, 232Th, and 40K in the different samples, the levels of radiological health hazard parameters were calculated, and the results are listed in Table 4.
The calculated values of the Hex and Hin for all samples varied from 1.73 to 2.85 and from 3.40 to 5.64, respectively. These values were significantly > 1, which is the maximum value recommended by UNSCEAR [31]. With the use of the fly ash/pure silica additive, the Hin and Hex values could be reduced by 40% due to the reduction of the concentration of 226Ra. Despite the considerable reduction in activity the PG still showed activity levels above the recommended limits so that it cannot directly be used as a raw material in construction.
The measured outdoor (\(\dot{D}_{{{\text{out}}}}\)) and indoor (\(\dot{D}_{{{\text{in}}}}\)) absorbed gamma dose rate ranged from 294 for PG (with additives) to 484 nGy h−1 for PR and from 586 for PG (with additives) to 967 n Gyh−1 for PR. These measurements result in outdoor and the indoor annual effective dose of 0.36 to 0.59 mSv/y for \(\dot{E}_{{{\text{out}}}}\) and 2.88 to 4.74 mSv/y for \(\dot{E}_{{{\text{in}}}}\). Most importantly the values of the outdoor annual dose (\(\dot{E}_{{{\text{out}}}}\)) do not exceed the annual effective dose limit of 1 mSv/y. Not surprisingly, the additives did again reduce the activity of the PG.
By assessing the radiological health hazards associated with PG in the presence of the additives, it can be concluded that even with additives the PG cannot directly be used as a raw material in construction given current regulations. The additives can, however, significantly reduce the activity of the PG so that more of the material could be used in a diluted mixture (if regulations would allow this practice) with natural material while fully complying with the relevant safety standards.
In this context it is noteworthy that sedimentary PR from Morocco and the resulting PG show much higher levels of radiological activity than PR and PG from other regions, particularly igneous PR and resulting PG have considerably lower radiological activity []. The findings of this work may thus be most relevant for regions that could reduce the radiological activity of the produced PG by adding inexpensive fly ash/pure silica additives to a point that the PG can be utilized in construction or elsewhere while fully complying to the relevant national regulations. In this study the measured Hex value of PG could for instance be reduced by nearly 30% (28.81%) from 2.43 to 1.73. Qamouche et al. [42] report an average Hex value of 1.48 for Moroccan PG. Assuming a similarly high reduction potential if the fly ash/pure silica additive is used, a Hex value of 1.05 that is very close to the level (< 1) recommended by UNSCEAR [31] could be realized. The same is true for PG and corresponding Hex values reported for Egypt (1.86) [37], the Philippines (1.80) [43] and Serbia (1.64) [44].
Conclusions
The effect of (1) pure silica, (2) fly ash, and (3) a fly ash/pure silica mixture as additives during PR processing to WPA has been investigated. Specifically, the form of the PG crystals, the rate of filtration and the P2O5 yields, as well as the quality of the produced acid and filtered PG was investigated. The results show an increase of the PG filtration rate of 0.13% for fly ash and 0.42% for the fly ash/pure silica mixture. These enhancements of the filtration rate are a result of the modified morphology of the PG crystals that allow slightly better filterability. The reaction efficiency could be increased by 1.05% (pure silica), 2.45% (fly ash) and 3.90% (fly ash/pure silica mixture), and the P2O5 recovery was increased by 0.50% (pure silica), 3.55% (fly ash), and 2.70% (fly ash/pure silica mixture). Besides, the use of additives resulted in a cleaner WPA with fewer organic material and reduced fluorine content. The specific activity of 238U and 226Ra in PG could also be reduced by 60% and 30%, respectively. Negative effects such as an increased need for sulfuric acid as a result of the additives was not observed.
Although the activity of the PG was significantly reduced using the fly ash/pure silica additives, the PG measured in this study still shows activity levels that do not permit direct utilization under current regulations in Morocco. The results are, however, a promising first step in the right direction and we strongly recommend additional studies that aim at further reducing the activity of PG in WPA production. We recommend that these studies will be conducted with larger material flows to better determine the average radiological activity of the produced PG (that can vary considerably within single stacks) and to better understand if the promising lab-scale results presented here can be reproduced on pilot plant- and subsequently industrial scale.
References
USGS (2022) United States Geological Survey - Phosphate rock. https://pubs.usgs.gov/periodicals/mcs2022/mcs2022-phosphate.pdf
Rutherford PM, Dudas MJ, Arocena JM (1996) Heterogeneous distribution of radionuclides, barium and strontium in phosphogypsum by-product. Sci Total Environ 180:201–209. https://doi.org/10.1016/0048-9697(95)04939-8
IAEA (2013) Safety reports series No. 78 radiation protection and management of NORM residues in the phosphate industry. ISBN 978-92-0-135810-3.
Haneklaus N, Sun Y, Bol R et al (2017) To extract, or not to extract uranium from phosphate rock, that is the question. Environ Sci Technol 51:753–754. https://doi.org/10.1021/acs.est.6b05506
Haneklaus N (2021) Unconventional uranium resources from phosphates. Encycl Nucl Energy. https://doi.org/10.1016/B978-0-12-819725-7.00152-5
Beltrami D, Cote G, Mokhtari H et al (2014) Recovery of uranium from wet process phosphoric acid by solvent extraction. Chem Rev 114:12002–12023. https://doi.org/10.1021/cr5001546
Chernysh Y, Yakhnenko O, Chubur V, Roubík H (2021) Phosphogypsum recycling: a review of environmental issues, current trends, and prospects. Appl Sci 11:1–22. https://doi.org/10.3390/app11041575
Hermann L, Kraus F, Hermann R (2018) Phosphorus processing-potentials for higher efficiency. Sustain. https://doi.org/10.3390/su10051482
Silva LFO, Oliveira MLS, Crissien TJ et al (2022) A review on the environmental impact of phosphogypsum and potential health impacts through the release of nanoparticles. Chemosphere 286:131513. https://doi.org/10.1016/j.chemosphere.2021.131513
Saadaoui E, Ghazel N, Ben Romdhane C, Massoudi N (2017) Phosphogypsum: potential uses and problems–a review. Int J Environ Stud 74:558–567. https://doi.org/10.1080/00207233.2017.1330582
Becker P (1983) Phosphates and phosphoric acid—raw materials, Technology, and economics of the wet process
Valdez Salas B, Schorr Wiener M, Salinas Martinez JR (2017) Phosphoric acid industry: problems and solutions. INTECH. https://doi.org/10.5772/intechopen.70031
Li B, Peng H, Guo J (2019) Effect of surfactant on water content of phosphogypsum. Appl Sci. https://doi.org/10.3390/app9081684
Abdel-Aal (1989) Industrial simulation for wet process phosphoric acid production. PhD Thesis: Inorganic Chemistry, Faculty of Science, Cairo University.
Boumnijel I, Ben Amor H, Chtara C (2013) Effect of calcinated and activated perlite on improving efficiency of dihydrate process for phosphoric acid. Int J Miner Process 125:112–117. https://doi.org/10.1016/j.minpro.2013.10.005
Derhy M, Taha Y, Hakkou R, Benzaazoua M (2020) Review of the main factors affecting the flotation of phosphate ores. Minerals 10:1–22. https://doi.org/10.3390/min10121109
El-Shall H, Abdel-Aal EA, Moudgil BM (2000) Effect of surfactants on phosphogypsum crystallization and filtration during wet-process phosphoric acid production. Sep Sci Technol 35:395–410. https://doi.org/10.1081/SS-100100164
Mahmoud MHH, Rashad MM, Ibrahim IA, Abdel-Aal EA (2004) Crystal modification of calcium sulfate dihydrate in the presence of some surface-active agents. J Colloid Interface Sci 270:99–105. https://doi.org/10.1016/j.jcis.2003.09.023
El-Shall H, Rashad MM, Abdel-Aal EA (2002) Effect of phosphonate additive on crystallization of gypsum in phosphoric and sulfuric acid medium. Cryst Res Technol 37:1264–1273. https://doi.org/10.1002/crat.200290001
Tadros ME, Mayes I (1979) Linear growth rates of calcium sulfate dihydrate crystals in the presence of additives. J Colloid Interface Sci 72:245–254. https://doi.org/10.1016/0021-9797(79)90106-1
Manar S (2016) Increasing the filtration rate of phosphor-gypsum by using mineral additives. Procedia Eng 138:151–163. https://doi.org/10.1016/j.proeng.2016.02.073
Hakkar M, Arhouni FE, Mahrou A et al (2021) Enhancing rare earth element transfer from phosphate rock to phosphoric acid using an inexpensive fly ash additive. Miner Eng 172:107166. https://doi.org/10.1016/j.mineng.2021.107166
Mahrou A, Hakkar M, Jouraiphy R et al (2021) Rare earth elements distribution during phosphoric acid production. Mining Metall Explor. https://doi.org/10.1007/s42461-021-00513-9
Qamouche K, Chetaine A, El Yahyaoui A et al (2021) Uranium and other heavy metal sorption from moroccan phosphoric acid with argan nutshell sawdust. Miner Eng 171:2019–2022. https://doi.org/10.1016/j.mineng.2021.107085
Boukhair A, Belahbib L, Azkour K et al (2016) Assessment of the radiological impact on the environment near a storage site of coal ashes in a thermal power plant. World J Nucl Sci Technol 06:206–216. https://doi.org/10.4236/wjnst.2016.64022
Taoufiq L, Laamyem A, Boukhair A et al (2018) Radiological assessment of wastewater treatment processes based on the use of coal ashes as a filters. J Radiat Res Appl Sci 11:217–224. https://doi.org/10.1016/j.jrras.2018.01.006
Kassi B, Boukhair A, Azkour K et al (2018) Assessment of exposure due to technologically enhanced natural radioactivity in various samples of moroccan building materials. World J Nucl Sci Technol 08:176–189. https://doi.org/10.4236/wjnst.2018.84015
Boukhair A, Belahbib L, Azkour K et al (2016) Measurement of natural radioactivity and radon exhalation rate in coal ash samples from a thermal power plant. World J Nucl Sci Technol 06:153–160. https://doi.org/10.4236/wjnst.2016.63017
Moudgil B (1995) Enhanced Filtration of Phosphogypsum. Gainesville, Florida
Canberra (2005) Genie 2000, version 3.2. Canberra: canberra industries, Canberra France HQ & Europe Coordination Bois Mouton
UNSCEAR (2000) United Nations Scientific Committee on the effect of atomic radiation. Sources and effects of ionizing radiation. New York
Grabas K, Pawełczyk A, Stręk W et al (2019) Study on the properties of waste apatite phosphogypsum as a raw material of prospective applications. Waste and Biomass Valorization 10:3143–3155. https://doi.org/10.1007/s12649-018-0316-8
Li X, Zhang Q (2020) Effect of molecular structure of organic acids on the crystal habit of a-CaSO4 0.5H2O from Phosphogypsum. Crystals. https://doi.org/10.3390/cryst10010024
Rutherford PM, Dudas MJ, Samek RA (1994) Environmental impacts of phosphogypsum. Sci Total Environ 149:1–38. https://doi.org/10.1016/0048-9697(94)90002-7
Haneklaus N, Bayok A, Fedchenko V (2017) Phosphate rocks and nuclear proliferation. Sci Glob Secur 25:143–158. https://doi.org/10.1080/08929882.2017.1394061
Saueia CHR, Mazzilli BP (2006) Distribution of natural radionuclides in the production and use of phosphate fertilizers in Brazil. J Environ Radioact 89:229–239. https://doi.org/10.1016/j.jenvrad.2006.05.009
Korany KA, Masoud AM, Rushdy OE et al (2021) Phosphate, phosphoric acid and phosphogypsum natural radioactivity and radiological hazards parameters. J Radioanal Nucl Chem 329:391–399. https://doi.org/10.1007/s10967-021-07796-8
Bolivar JP, Perez-Moreno JP, Martín JE, et al Occupational exposures and distribution of natural radionuclides in phosphoric acid production by the wet process (Spain). In: 9th EAN ALARA Workshop. Augsburg, pp 1–13
Hull CD, Burnett WC (1996) Radiochemistry of Florida phosphogypsum. J Environ Radioact 32:213–238. https://doi.org/10.1016/0265-931X(95)00061-E
IAEA (2014) The Environmental Behaviour of Radium: Revised Edition. ISBN 978-92-1-143310-7.
Rutherford PM, Dudas MJ, Arocena JM (1995) Radioactivity and elemental composition of phosphogypsum produced from three phosphate rock sources. Waste Manag Res 13:407–423. https://doi.org/10.1016/S0734-242X(05)80021-7
Qamouche K, Chetaine A, Elyahyaoui A et al (2020) Radiological characterization of phosphate rocks, phosphogypsum, phosphoric acid and phosphate fertilizers in Morocco: an assessment of the radiological hazard impact on the environment. Mater Today Proc 27:3234–3242. https://doi.org/10.1016/j.matpr.2020.04.703
Diwa RR, Tabora EU, Palattao BL et al (2021) Evaluating radiation risks and resource opportunities associated with phosphogypsum in the Philippines. J Radioanal Nucl Chem. https://doi.org/10.1007/s10967-021-08142-8
Kuzmanović P, Todorović N, Forkapić S et al (2020) Radiological characterization of phosphogypsum produced in Serbia. Radiat Phys Chem 166:108463. https://doi.org/10.1016/j.radphyschem.2019.108463
Acknowledgements
The authors are grateful for samples provided by the OCP Group, the continuous discussions with the experts of the Td-Lab Sustainable Mineral Resources at the University for Continuous Education Krems. This work was supported by Austria's Agency for Education and Internationalization [Grant Numbers: Africa UNINET P006 and P058; HR 09/2022] and the Max-Buchner-Forschungsstiftung from the DECHEMA Gesellschaft für Chemische Technik und Biotechnologie e.V. (Society for Chemical Engineering and Biotechnology) [Grant number 3824]. German Federal Ministry of Education and Research (Project Number: 033RU020A) support for this project is offered under the coordination of the ERA-MIN3 action, which has received funding from the European Union under the Horizon 2020 Program (European Commission Grant Agreement No. 101003575).
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Arhouni, F.E., Hakkar, M., Mahrou, A. et al. Better filterability and reduced radioactivity of phosphogypsum during phosphoric acid production in Morocco using a fly ash waste and pure silica additive. J Radioanal Nucl Chem 331, 1609–1617 (2022). https://doi.org/10.1007/s10967-022-08235-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-022-08235-y