Abstract
Mangroves are highly productive and changing forests located in the intertidal zone of tropical regions. Leaf litter decomposition represents a substantial part of their carbon sink abilities. Little is known about the potential effect of climate change on this key process of ecosystem functioning. This study compared leaf litter microbial decay between fringe and riverine Avicennia germinans stands. A direct and reciprocal transplant experiment using litterbags was setup in French Guiana to test 3 hypotheses: (i) the activities and abundance of microbial decomposers are lowest in the fringe mangroves due to exposure to saline water and tidal immersion; (ii) for these reasons, litter decomposes faster in riverine stands; and (iii) according to the home-field advantage hypothesis, litter decomposes more rapidly in the environment from which it originates. Remaining litter masses, abundance of litter microbial community (phospholipid fatty acid signatures (PLFA)), and their functional capability (enzyme activities and Biolog) were assessed. Litter directly transplanted in riverine stands showed higher enzymatic activity (+ 77%), catabolic diversity (+ 10%), and microbial biomass (+ 60%) than litter transplanted directly in fringe stands. In contrast, both riverine and fringe derived litter showed faster decay at the fringe (14% mass loss) than riverine site (4% mass loss) between 30 and 45 days. Here, environmental conditions associated with different distances from the sea such as salinity and inundation regimes, rather than microbial features are suggested as main factors affecting decomposition process. Expected sea level rise in the coastal Guianas may therefore modify the mangroves productivity in the coming decades.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mangrove forests are located in the intertidal zone along coasts and river mouths in tropical and subtropical regions (Alongi 2002). These wetlands are characterized by seasonal variations and spatial gradients in environmental conditions (i.e., salinity gradients, tidal cycle, anoxic sediments, soil instability) (Feller et al. 2009). Despite low plant diversity and limited surface area (1% of tropical forest area and 0.4% of the world’s forests), mangroves provide many ecosystem goods and services, such as the stabilization of shorelines, the attenuation of extreme climatic events, and important carbon (C) sinks (Donato et al. 2011; Rovai et al. 2018; Kauffman et al. 2020). With an estimated mean C storage of 937 tC ha−1, they are among the most C-rich forests in the tropics. Worldwide, they represent only approximately 1% (13.5 Gt year−1) of forest C sequestration, but within coastal habitats they account for 14% of ocean C sequestration (Alongi 2014). Part of the biomass production, mainly leaf litter (40–85% of total litterfall), is consumed by fauna and decomposed by heterotrophic microbial communities that colonize intertidal sediments (Holguin et al. 2001; Luis et al. 2019). Forest litter, especially in mangroves, has thus an important place in the ecosystem as a source of energy and nutrients for many decomposing organisms, resulting in a substantial organic matter and nutrient storage or export to both marine and terrestrial environments through tidal water exchange and freshwater inputs (Feller et al. 2009).
Litter decomposition is controlled by climatic conditions (Meentemeyer 1978; Imgraben and Dittmann 2008; Kristensen et al. 2008; Arnaud et al. 2020), soil properties, organic matter quality (Aerts 1997; Chomel et al. 2016), bioturbation of benthic biodiversity (Lee 1989; Aschenbroich et al. 2017), and the characteristics of microbial communities (Coûteaux et al. 1995; Hättenschwiler et al. 2005; Gießelmann et al. 2010). In mangroves, litter decomposition can vary with duration of tidal inundation as shown by Dick and Osunkoya (2000) who observed a greater rate of Avicennia marina leaf litter decomposition in areas directly inundated by the tide than in areas further inland. However, if we focus on the effect of prolonged inundation, decomposition rates of mangrove soil organic matter (SOM) (which results mainly from decomposition of organic matter and the production of roots) do not decrease with simulated sea level rise as shown by Arnaud et al. (2020). Indeed, salinity of submerging waters also plays a role in this phenomenon. Chambers et al. (2013) showed that SOM decay is negatively affected by an increase of inundation time only with low salinity waters (15 ppt) while no effect was observed with high salinity waters (35 ppt). In C-rich soils such as mangroves, submersion by higher salinity waters which are rich in sulfates can induce a shift in C mineralization by triggering sulfate reduction under anaerobic conditions. This shift was suggested to compensate the slower SOM decomposition found normally under anaerobic conditions (Weston et al. 2006; Arnaud et al. 2020). Microbial communities exhibit a quick turnover rate and high sensitivity to salinity that was highlighted by the variations in C soil mineralization rates observed under different seawater concentrations (varying ionic strengths and sulfate concentrations) (Chambers et al. 2011). High ionic strength found in seawater can induce osmotic stress, disrupting microbes’ cellular integrity and functions, and inhibiting enzyme activity in the soil (Chambers et al. 2013). The salinity and anoxia gradients found in the intertidal zone therefore structure these communities and their functions, eventually affecting C cycling (Capdeville et al. 2019). Plant litter contains water-soluble compounds easily assimilated by microorganisms (e.g., carbohydrates, soluble phenolic acids, organic acids, amino acids), insoluble and refractory compounds (e.g., phenolic compounds, cellulose, hemicellulose, and lignin), and compounds that inhibit decomposition (e.g., alkaloids, terpenes) in various proportions (Marchand et al. 2005; Chomel et al. 2014).
The interaction between litter quality, environmental conditions, and decomposers can be explained by the home-field advantage (HFA) hypothesis; litter can decompose faster in its own habitat (at “home”) than “away” from it according to the sports analogy that states that team players in sports competition win more than 50% of the games played at home (Gholz et al. 2000). HFA suggests that a specialization of soil microbial decomposers, referred to as “team players,” for a given plant litter (i.e., its chemical quality), referred to as “coaches of decomposition” since it dictates how fast nutrients can be accessed by microbes, may occur through physiological adaptations of the soil biota in the short term (Perez et al. 2013). Ultimately, this specialization of decomposers for litter that they most often encounter, or that they are used to consuming under specific local environmental conditions, may allow a specific litter to decompose faster in the habitat from which it derives, referred to as a higher scoring necessary to win the “game” of decomposition (Chomel et al. 2015; Palozzi and Lindo 2018). This HFA could concern litter from different plant species (Ayres et al. 2009) but also litter from the same plant species growing in different locations as intraspecific variation in litter quality and decomposition rate can be similar to that reported for interspecific variation (Lecerf and Chauvet 2008). HFA hypothesis is still scarcely studied (Ayres et al. 2009; Veen et al. 2015), particularly in tropical ecosystems (Wang et al. 2013; Wu et al. 2019; Elias et al. 2020). To our knowledge, only one previous work tested the HFA hypothesis in mangroves, where seedlings growth exposed to leaf litter of different species were assessed (Chapman and Feller 2011).
In French Guiana, mangroves cover 600 km2 and are part of the world longest muddy shoreline (1500 km) of the Guianas coast presenting high river discharge (Orseau et al. 2017; Diop et al. 2019). Through a spatial dynamic alternating the colonization by mangroves of Amazonian mudflats and intense erosion of mature mangroves during high tides (Walcker et al. 2015), the Guyanese mangrove can be considered as a mobile forest moving along the coast. In addition to this dynamic, which favors the close coexistence of young and old stands (Fromard et al. 2004), tides cause a significant variation in the submergence time and salinity and structure the mangrove stands (Orseau et al. 2017). Hence, a zonation appears between the main species Rhizophora mangle L. (Rhizophoraceae), Laguncularia racemosa (L.) C.F.Gaertn. (Combretaceae), and Avicennia germinans L. (Acanthaceae) along an axis of distance from the sea sometimes modulated by the freshwater supply from rivers (Fromard et al. 1998). In the context of climatic change in the coming decades, rising sea levels, changes in hydrology due to impoundments or higher precipitation, increasing frequency and intensity of storms, increasing temperatures, and atmospheric CO2 concentrations could induce changes in the regimes and levels of salinization of mangroves and thus potentially lead to spatial and functional reorganization of these ecosystems (Lovelock et al. 2015), particularly their productivity (Alongi 2018). Robertson (1988) suggests that the fate of leaf litter may vary with site and forest type, with flooding frequency being one of the most important parameters. Lee (1989) demonstrated that leaf decomposition of Kandelia candel could vary between the landward and seaward edges of the forest. Expected sea level rise in the Amazon Delta and coastal Guianas will increase the exposure of mangroves to prolonged flooding. Sea level rise will also affect freshwater supply by exacerbating saltwater intrusion (Anthony et al. 2021).
This study aims to assess the potential effects of climate change (e.g., salinity and tidal exposure) on litter decomposition and to test the HFA hypothesis in mangroves. For that purpose, we set up a in situ crossed experiment in three main rivers of French Guiana, by comparing the decomposition of A. germinans litter and the biomass, composition, and activity of microbial decomposers between fringe mangroves (i.e., mangroves that experience more frequent marine salinity) and riverine mangroves (i.e., subjected to more abundant freshwater inputs). Our hypotheses were that (i) environmental factors associated with fringe mangroves induce a reduction of the catabolic fitness and abundance of leaf litter associated microbial communities compared to riverine stands; (ii) therefore, leaf litter decomposition is slowest in fringe stands; (iii) finally, litter decomposes more rapidly in the environment from which it originates (home-field advantage hypothesis).
Material and methods
Study sites
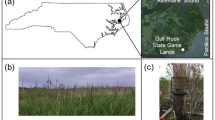
The experiment was conducted on 3 rivers in French Guiana selected among the 8 rivers flowing into the Atlantic Ocean: the Mahury, the Kourou, and the Sinnamary (Fig. 1). Each river benefits at its mouth from a humid equatorial climate whose intra-annual variability is determined by the phenomenon of intertropical convergence and makes it possible to distinguish 2 wet seasons and 2 dry seasons. The average annual temperature is 26.5 °C with a thermal amplitude of 3 °C at the most between the hottest and the coldest month and the average annual rainfall ranges from 2500 to 3000 mm y−1 (Marchand et al. 2004). For each of the 3 rivers, two sites for sampling A. germinans green leaves were selected (Fig. 1) according to the definition of two types of mangroves: fringe mangroves or tide-dominated mangroves (they receive most of the tides so seawater) and riverine mangroves or river-dominated mangroves (inundated by river water as well as tides, so salinity is more variable) according to Lugo and Snedaker (1974), Cintrón et al. (1985), and Ewel et al. (1998). The two harvesting sites were chosen in these two environments (riverine and fringe mangrove) in the limit of colonization by A. germinans at the pioneer Avicennia stage as defined in Fromard et al. (1998). The main physicochemical characteristics of water were measured and recorded by a multi-parameter (temperature and salinity) water quality probe—Aquaprobe® AP-7000, England (Aquameter)—equipped with a GPS receiver and a barometric sensor (Online Resource 1). For Mahury and Kourou rivers, two types of stations were chosen according to their delimitation of polyhaline (18 to 30 g L−1 salinity) and mesohaline (5 to 20 g L−1) zones (source: DEAL Guyane-EDF). In the case of Sinnamary river, their study conducted in both dry and wet seasons and during 6 tidal cycles did not allow to clearly set a delimitation between levels of salinity. Still, salinity measurements conducted in surface waters at the beginning of our study showed contrasted salinity between the two selected fringe and riverine sites at low tide (Online Resource 1). French Guiana coast is mesotidal with semi-diurnal tides (Ray et al. 2020). Litterbags were therefore submerged twice a day during our assay, where tide elevation ranged from 1.11 to 3.04 m above hydrographic reference for Sinnamary and Kourou rivers and from 0.92 m and 3.06 m for Mahury. These data were collected from the Service Hydrographique et Oceanique de la Marine for the Iles du Salut harbor which was closest to the corresponding Sinnamary and Kourou study sites and the Degrad de Cannes harbor for Mahury sites (SHOM: www.shom.fr). Experimental sites map figure was obtained using QGIS Geographic Information System software (QGIS Association, QGIS.org, 2021).
Geographical location of experimental sites (white dot: fringe mangrove, black dot: riverine mangrove) for each studied river in French Guiana (a Sinnamary, b Kourou, c Mahury). Black lines and dashed areas indicate hydrographical network and mangrove distribution respectively. Abbreviation: km, kilometers (cartographic backgrounds source: ©2018 GADM; mangrove distribution source: ©2020 CARNAMA(@PRZHT))
Experimental setup and litter bag processing
At each of the 6 sites, approximately 300 green leaves (500gWM) were collected from five trees of A. germinans in October 2018 and air-dried during 2 days before the experiment. Litter decomposition was assessed by using the litter bag method (Swift et al. 1979). Two-millimeter mesh litter bags (20 × 20 cm polypropylene mesh) containing 30 g of fresh leaves were used to perform the experiment. To test the HFA hypothesis, litterbags were transplanted directly and reciprocally between each site for the five trees under consideration and for each river separately, i.e., one bag containing fresh leaves from each tree was placed at each site. Thus, 10 litterbags were deposited at the tidal sway zone of each site, which corresponded to five replicates of 2 transplants at the site of origin or “home” (from fringe mangrove to fringe mangrove: FF, from riverine mangrove to riverine mangrove: RR) and 2 transplants at the foreign leaf site or “away” (from fringe mangrove to riverine mangrove: FR, from riverine mangrove to fringe mangrove: RF). Thus, the experiment consisted in 2 modalities corresponding to the 3 rivers (Kourou, Mahury and Sinnamary) × 2 sites (fringe and riverine). In total, 120 litter bags (3 rivers × 2 mangrove types (i.e., riverine vs fringe) × 2 transplants (home vs away) × 2 collection times × 5 replicates) were analyzed. Litter bags were sealed and secured in the river with stainless metallic rings slipped over steel rods, which were anchored in the sediment.
The litter mass loss, catabolic enzyme activity, microbial catabolic profiles, microbial community composition, and biomasses were followed after 30 and 45 days of decomposition (t1 and t2). At each sampling date, 60 litter bags were retrieved from each experimental site. Litter bags were placed in plastic bags to prevent the loss of biological material. In the laboratory, leaves contained in litter bags were individually rinsed with distilled water to remove the greater part of extraneous sediments and adhering invertebrates. A subsample of the leaf material was kept at 4 °C for the determination of microbial parameters (enzymatic activities and catabolic functions profile). The remaining leaves were stored at – 18 °C and then freeze-dried (Lyovac GT2), weighted to the nearest 0.1 g, and ground to powder (MM400, Retsch GmbH.D, Hann Germany). Subsamples of about 250 mg were ashed for 3 h in a muffle furnace at 550 °C to determine ash-free dry mass (AFDM). C and nitrogen (N) contents were determined by thermal combustion using a CHN elemental analyzer (Flash EA 1112 series, ThermoScientific®, Waltham, USA).
At the beginning of the experiment (t0), 30 samples (3 rivers × 2 sites × 5 replicates) were used to determine initial dry weight (g), C and N contents, leaf area, physiological leaf status, and microbial catabolic profiles.
Leaf area
Thirty green leaves collected on each 5 trees per site (t0) were used to assess leaf area (LA). Leaves were scanned and resulting images were processed by using Winfolia software (Regent Instruments Canada Inc.) to obtain surfaces expressed in cm2.
Leaf physiological indices by epidermal fluorescence
To assess the plant physiological status, we measured several leaf physiological indices. Leaf epidermal fluorescence of 50 leaves collected on each tree (t0) was assessed under ambient light conditions by using a hand-held Multiplex® multiparametric fluorescence sensor (Multiplex®, Force-A, Orsay, France). Based on fluorescence intensities, several signals associated with leaf epidermal constituents were calculated according to Agati et al. (2011) and are detailed in Online Resource 2. Anthocyanins and flavonols are plant secondary compounds that can be affected by stress and thus reveal a physiological dysfunction. Without N limitation, a plant promotes its primary metabolism and synthesizes proteins (nitrogenous molecules) containing chlorophyll, and few flavonols. The N balance index which corresponds to the chlorophyll:flavonols ratio is a useful indicator of N deficiency.
Microbial enzymatic activities
Two extracellular enzyme activities (EEA) involved in organic C cycle were assessed to determine the catabolic potential of microbial communities extracted from litter: tyrosinase and fluorescein diacetate hydrolase (FDAse) activities at t1 and t2. Tyrosinase is involved in the degradation of aromatic compounds such as tannins, phenolic acids, or lignin (Zimmer 2005). FDAse is a pool of enzymes comprising phosphatase, cellulase, and lipase involved in degradation of cellulose and carbohydrates (Guénon et al. 2017). It corresponds to the potential hydrolysis activity by different hydrolases. They therefore reflect the degradation of the most abundant constituents of leaf litter that are related to our hypothesis. For each activity, two repeated assays were performed per litter sample. Tyrosinase activity was assessed according to the modified method of Saiya-Cork et al. (2002) and detailed in Online Resource 2. FDAse activity was determined according to the method of Schnürer and Rosswall (1982) and detailed in Online Resource 2. FDAse is a pool of enzymes produced by microorganisms as well as plants. Their non-specificity to microorganisms is one of the limitations of this assay since we chose to relate it specifically to litter microorganisms. Moreover, this assay measures an activity potential rather than the exact activity taking place in environmental conditions, where abiotic conditions are variable compared to controlled conditions in our assay. For example, exposure to metals that might modify enzyme activity are not considered in our controlled bioassay and should be kept in mind for the interpretation of our results.
Microbial catabolic functions profiles and diversity
Catabolic profiles of the cultivable microbial litter communities were analyzed by the microplate Biolog® EcoPlates (Biolog Inc., Hayward, California, USA) method adapted from Garland and Mills (1991) and detailed in Online Resource 2. Each plate contains 3 repeated blocks of a substrate-free blank and 31 of the most used C sources for soil community analysis. Oxidation of carbonaceous substrates induces a reduction in violet tetrazolium, the intensity of which depends on the intensity of catabolism. Catabolic richness (S) represents the total number of substrates used. Catabolic diversity was calculated using Shannon’s index \({\mathrm{H}}^{\mathrm{^{\prime}}} = - \sum {P}_{i}(\mathrm{ln}{P}_{i})\) where Pi is the ratio of the OD from one well to the sum of the positive ODs from all wells in the sample. Catabolic functional evenness was calculated using Simpson’s evenness index \(E = D/S\) where D is Simpson’s dominance index expressed as \(D = 1/\sum {{P}_{i}}^{2}\) (Morris et al. 2014). The main limitation of this bioassay that should be considered for the interpretation of our results is that only cultivable microorganisms with fast growing ability (conducted during 7 days) are assessed.
Microbial community composition and biomass
Phospholipid fatty acids (PLFA) were extracted from t1 and t2 samples using the Buyer and Sasser (2012) and Aupic-Samain et al. (2021) method and detailed in Online Resource 2. Total PLFA amount (μg g−1 dry mass of litter) was used as a measure of total microbial biomass, while PLFA biomarkers specific to bacterial and fungal biomass (μg g−1 dry mass of litter) were summed (Frostegård and Bååth 1996) to estimate the biomass of fungi and bacteria. We analyzed 11 specific biomarkers out of 29 identified PLFAs. Gram-positive bacteria (G+) were represented by the PLFAs: i15:0, a15:0, i16:0, i17:0, a17:0 and Gram-negative bacteria (G−) by the PLFAs: 16:1 ω7c and 18:1 ω7c. Other saturated straight-chain PLFAs (15:0, 16:0, 17:0) were identified as general bacterial biomarkers (GBM). The PLFA 18:2 ω6,9c were used as the indicators of fungi. G+, G−, and GBM markers were added to obtain total bacterial PLFA (Frostegård et al. 1993).
Home-field advantage index
HFA indices (HFAi) were calculated according to Ayres et al. (2009) adapted for each studied river (Mahury, Kourou and Sinnamary (n = 5); Online Resource 1), on AFDM and on all microbial properties (EEAs, catabolic diversity H’ and EvInd indices and microbial, fungal, and bacterial biomass) measured after 30 days (t1) and 45 days (t2) of decomposition. Since the HFAi reflects all possible interactions between the transplanted litter and the environmental conditions of the different sites, we referred to the percentages of AFDM losses and microbial properties studied, between the site of origin and the incubation site, in the form of site-binding interactions. These interactions may be positive or negative depending on whether the percentage of relative loss is accelerated or slowed down at the site of origin (“home”) or at the foreign site (“away”), according to the following equation: \({A}_{RMLa}= \frac{{A}_{a}}{{A}_{a}+{B}_{a}} \times 100\) where ARMLa represents the relative loss of mass or of each microbial property of a litter type A at the site of origin, noted a, or the foreign site, noted b. Aa and Ab represent the percentage of considered measure at site a and site b respectively. Measurements of relative losses were used to calculate the HFA. \(HFAi=\left[\frac{{A}_{RMLa}+{B}_{RMLb}}{2}/\frac{{A}_{RMLb}+{B}_{RMLa}}{2}\right]\times 100-100\) where HFAi represents the fastest percentage loss in mass and highest value of microbial properties when decomposing “home” compared to “away.” It thus represents a net value for both of considered litter origins (i.e., fringe or riverine stands) involved in the reciprocal transplantation.
Statistical analyses
To analyze the effects of distance from the sea (i.e., fringe (F) or riverine (R) mangrove) on initial t0 leaves C and N contents, LA, physiological indices and catabolic indices of epiphytic microbes, linear-mixed-effect models (LMMs) (R package “lme4”) were applied including the type of mangroves as fixed factor and the river as random factor. When necessary, data were transformed using the “bestNormalize” package (Peterson and Cavanaugh 2020) to meet the assumptions of normality and homoscedasticity of variances. Differences were tested using generalized linear-mixed-effect models (GLMMs) with binomial distribution (lme4 package) for C:N ratio since transformation did not allow normality and homoscedasticity. The same approach was used to test the effects of litter transplants (i.e., RR, RF, FF, and FR) and decomposition time (i.e., 30 and 45 days) on t1 and t2 leaf litter C and N contents, EEAs, microbial catabolic diversity indices, and PLFA biomarker abundances while AFDM remaining, total PLFA abundance, and total bacterial PLFA abundance differences where tested using GLMMs since transformation did not allow normality and homoscedasticity. All implemented LMMs and GLMMs are shown in Online Resource 3 for each variable. In the case of a significant litter transplant effect, a Tukey HSD post hoc test was used to test which types of transplants are different from others. The used R scripts are shown in Online Resource 4 with EEAs (Online Resource 5) and AFDM remaining (Online Resource 6) datasets as an example. To test HFA on AFDM remaining and microbial properties of litters collected after 30 and 45 days of decomposition, Student’s t tests were performed to determine if calculated HFA is were significantly different from 0. All statistical analyses were performed with the R software (version 3.6.0).
Results
Initial leaf traits and microbial catabolic diversity
Leaves originating from riverine sites showed higher C and N contents than fringe sites (Online Resource 7) while no significant difference in C:N ratio and LA was observed (Online Resource 7). The chlorophyll index was significantly higher in riverine sites than in fringe sites (Online Resource 7). Anthocyanin, flavonols, and N balance indices were not significantly different between the two stand types. No difference in epiphytic microbial catabolic diversity indices was observed between A. germinans leaves originating from fringe or riverine stands.
Carbon and nitrogen contents during decomposition
C and N contents of decomposed leaf litter were significantly affected by the litter transplant (χ2 = 70.68, p < 0.05 for C; χ2 = 13.39, p < 0.05 for N) while C:N ratios did not show any difference (χ2 = 2.17, p > 0.05). Moreover, C and N litter contents were not significantly different between the two dates of decomposition (χ2 = 2.10, p > 0.05 for C, χ2 = 0.68, p > 0.05 for N and χ2 = 1.01, p > 0.05 for C:N ratio). C and N contents from FF litters were lower than in RR litters, for both dates of decomposition (Fig. 2). C content in FR and RF litters was similar but significantly lower than in RR litters and significantly higher than in FF litters. C:N ratios were similar for all the modalities.
Total carbon (C) and nitrogen (N) concentrations expressed as a percentage of dry mass and C:N ratios of Avicennia germinans leaf litter originated from fringe stands (a, c, e) transplanted home (FF, white) or away (FR, light gray) and riverine stands (b, d, f) transplanted home (RR, black) or away (RF, dark gray) after 30 and 45 days of decomposition. Solid and dashed lines indicate when litterbags are transplanted at “home” or “away” respectively. Means and SE. Different letters indicate statistical differences among groups with a < b < c and A < B < C after 30 and 45 days respectively (post hoc pairwise comparison p < 0.05)
Microbial activities during decomposition process
Microbial catabolic activity increased significantly between 30 and 45 days of decomposition for the FF, FR, and RF litters for tyrosinase and for FF litter for FDAse (Fig. 3a,b). We observed a significant interaction of time of decomposition and litter transplant on tyrosinase (χ2 = 8.49, p < 0.05) and FDAse activities (χ2 = 8.33, p < 0.05). After 30 days of decomposition, tyrosinase and FDAse of FF litter were both significantly lower than in RR litter (Fig. 3a,b) while FR and RF litters did not show any difference.
Microbial catabolic activities (a tyrosinase; b FDAse) and functional diversity (c Shannon index H’) and evenness (d Evenness index EvInd) of Avicennia germinans leaf litter originated from fringe stands transplanted home (FF, white) or away (FR, light gray) and from riverine stands transplanted home (RR, black) or away (RF, dark gray) after 30 and 45 days of decomposition. The activities are expressed as μmoles of reaction product formed per minute (U) per gram of dry matter (U g.−1 DM). Different letters indicate statistical differences among groups with a < b and A < B after 30 and 45 days respectively; bars indicate statistical differences between 30 and 45 days within one type of transplant (post hoc pairwise comparison p < 0.05)
A significant effect of interaction between the time of decomposition and litter transplant was observed on the catabolic diversity indices (H’) (χ2 = 7.84, p < 0.05) while for the catabolic evenness (EvInd), main effects of litter transplant (χ2 = 9.38, p < 0.05) and time (χ2 = 5.40, p < 0.05) were shown but without significant interaction (χ2 = 3.05, p > 0.05). After 30 days of decomposition, H’ of FF and FR litters were lower than RR litter and EvInd of FF litter was found to be lower than RR litter (Fig. 3c,d). After 45 days of decomposition, H’ index of FF litter was lower than FR litter and no difference among transplants was observed for EvInd. Moreover, increases in H’ of FF and FR litters and for EvInd of FF litters were observed between 30 and 45 days of decomposition.
Litter microbial biomass
No significant change during time was observed for total microbial (χ2 = 0.29, p > 0.05), fungal (χ2 = 1.88, p > 0.05), and bacterial biomass (χ2 = 0.05, p > 0.05) in litters (Fig. 4). A significant effect of transplants was observed for total microbial biomass (χ2 = 8.44, p < 0.05), fungal biomass (χ2 = 11.27, p < 0.05), and bacterial biomass (χ2 = 8.42, p < 0.05) such as G+ bacteria (χ2 = 21.97, p < 0.05), G− bacteria (χ2 = 14.51, p < 0.05) and GBM (χ2 = 17.47, p < 0.05). Litter from FF transplant showed significantly lower microbial (1.4- and 1.6-fold), fungal (1.8- and 2-fold), and bacterial biomass (1.5- and 1.5-fold) than litter from RR after 30 and 45 days of decomposition. Total microbial and bacterial biomass of FR and RF litters were equivalent and did not show significant differences with transplants “home” after 30 days of decomposition. After 45 days, FR litters showed a similar microbial and bacterial biomass to RR litters that were significantly higher than FF litters (1.6- and 1.5-fold respectively). Fungal biomass of RF litters was significantly more abundant than in FF litters and equivalent to RR litters after 30 days of decomposition. No significant differences of litter fungal biomass were observed between “away” (FR and RF) and “home” transplants (FF and RR) with similar values for FR and RF litters after 45 days of decomposition.
Microbial biomass (a), fungal biomass (b), and bacterial biomass (c) of Avicennia germinans leaf litter originated from riverine stands transplanted home (RR, black) or away (RF, dark gray) and from fringe stands transplanted home (FF, white) or away (FR, light gray) after 30 and 45 days of decomposition. Grids, dots, and plain bars indicate general bacterial markers (GBM), G− bacteria biomarkers and G+ bacteria biomarkers biomasses respectively. Biomasses are expressed as phospholipid fatty acid (PLFA) biomarkers abundance (μg g litter.−1 dry matter). Means and SE. Different letters indicate statistical differences among groups with a < b after 30 and 45 days respectively (post hoc pairwise comparison p < 0.05)
Litter mass loss
No significant effect of transplants was shown on remaining litter mass (χ2 = 1.68, p > 0.05). For all tested litter transplants, more than 50% of initial litter mass was lost after 30 days of decomposition (Fig. 5). We observed a significant decrease in AFDM remaining between 30 and 45 days (χ2 = 6.14, p < 0.05) for FF and RF litters with a 14.78% and 14.39% loss, respectively.
Litter ash-free dry mass (AFDM) remaining expressed as a relative fraction of initial mass of Avicennia germinans leaf litter originated from fringe stands (a) transplanted home (FF, white) or away (FR, light gray) and riverine stands (b) transplanted home (RR, black) or away (RF, dark gray) after 30 and 45 days of decomposition. Solid and dashed lines indicate when litterbags are transplanted at “home” or “away” respectively. Means and SE. Bars indicate statistical differences between 30 and 45 days within one type of transfer (post hoc pairwise comparison p < 0.05)
Home-field advantage
HFA indices of FDAse activity were significantly different than 0 with a positive value after 45 days of decomposition (Table 1). No HFA effect was observed for all assessed parameters.
Discussion
Among the factors impacting litter decomposition and ultimately nutrient cycling in mangroves, we compared decomposition process in fringe and riverine mangroves in a similar climate and community composition (i.e., A. germinans). This process was also assessed on the abundance, catabolic activity, and functional diversity of microbial decomposers associated with the litter. Finally, the HFA hypothesis was tested by considering fringe and riverine stand litters as coaches of the decomposition “game” and their associated microbes as team players.
Leaf litter traits
When considering the initial characteristics of litters from fringe and riverine stands prior to their decay in the litter bag experiment, few of the parameters measured on green leaves showed a significant variation. For C and N contents and chlorophyll index, however, the observed higher values in riverine stands were slight but significant. These findings indicate that the distance from the sea of A. germinans communities has low effect on their foliar nutrient and epidermal specialized metabolites contents, as well as their epiphytic microbial catabolic diversity. Still, these results go along with the known nutrient-rich conditions found in riverine systems (Feller et al. 2009) that are associated with higher leaf nutrient content.
Through the litter decomposition process, this nutrient content difference remained for litters transplanted at “home”. Riverine originating litters (RR) were found richer in C and N than fringe originating litters (FF) after 30 and 45 days of decomposition with an observed average of 1.5-fold and 1.1-fold, respectively. Even if subtle, the higher proportions of N particularly may also indicate a more labile substrate for microbial decomposers, as suggested by Vinh et al. (2020). Nevertheless, C:N ratios, which are also related to litter decomposition dynamics (Mfilinge et al. 2002), were found equivalent for all litter transplants and time of measurement.
Microbial catabolic activities
For both assessed extracellular enzymes, a difference occurred between the litters that were transplanted home after 30 days of decomposition only: activities of fringe litters transplanted home (FF) were lower than riverine litters transplanted home (RR). This dissimilarity disappeared after 45 days of decomposition, highlighting an impact of transplants on microbial catabolic activities in the early phase of decomposition. Similarly, Lewis et al. (2014) showed that C mineralization decreased when A. germinans leaf litter was exposed to longer inundation linked to a decrease of microbial activity. Furthermore, FDAse enzymes activity, which involves a pool of many hydrolases reflecting total microbial activity (Guénon et al. 2017), increased between 30 and 45 days of decomposition only for fringe litters transplanted home (FF). In the case of tyrosinase activity, an increase was shown for FF litters and for both the away transplants (FR and RF), highlighting an increase of phenol oxidation activity involved in lignin and polyphenol degradation for these conditions (Zimmer 2005). In a similar pattern, FF litter catabolic diversity and evenness were lower than RR litters after 30 days, indicating a better capacity of microbial communities to degrade a higher number of different organic substrates and a more equal repartition of their consumption in riverine mangroves. Similarly, between 30 and 45 days, these indices increased to reach equivalent values to RR litters.
Functional shift of decomposers during decay
That increase of FF litter decomposer activity and catabolic diversity, however, was not observed for their biomass. Indeed, FF litters total microbial, bacterial, and fungal biomass were lower than RR after 30 days, but these differences remained after 45 days, as opposed to their activity and functional breadth. A shift in catabolic functions and fitness may have taken place for FF litters, suggesting a substantial plasticity of these communities towards the environmental constraints associated with mangrove fringe zones and particularly higher exposure to salinity. The osmotic stress exerted by more frequent exposure to high saline concentrations may have contributed to a selection of more functionally diverse and effective microbes while reducing their biomass as shown by Zhang et al. (2019). Moreover, the potential presence in higher amounts of more recalcitrant compounds or specialized metabolites inducing higher microbial catabolic activity (Musilova et al. 2016) in the fringe originating litters may also be responsible for the observed functional shift, as previously shown by Uhlik et al. (2013). This hypothesis is further supported by the possible accumulation of sulfide ions in soils (which can have deleterious effects on soil bacteria) as byproducts of sulfate reduction that may have taken place through prolonged exposure to saline waters (Chambers et al. 2013). Tyrosinase activity is mostly associated with fungi (Zimmer 2005). Our current results of fungal biomass found lower in EE than RR litter may indicate a higher enzyme fitness that could explain the observed increase in activity since no increase of fungal biomass was observed between 30 and 45 days. We formulate the same hypothesis for observed FDAse activities which is a more ubiquitous extracellular enzyme (i.e., associated with fungal, bacterial, and plant communities) (Schnürer and Rosswall 1982).
Implications for litter mass loss
Throughout litter decomposition, no differences in remaining masses were observed between all tested litter transplants. Yet, our results reveal a significant mass loss between 30 and 45 days only for litters transplanted in fringe stands (FF and RF). Broadly, the higher microbial features found in litters originating from river stands than fringe stands, when transferred home, confirms our first hypothesis of more advantageous environmental conditions taking place in riverine stand for microbial decomposer development (and fitness, in the early phases of decomposition only). Still, contrary to our second hypothesis, these higher microbial features were not associated with a faster mass loss in riverine stands. This highlights that microbial abundance and nutrient content are not the main factors controlling leaf litter decomposition dynamics in the present study. Both litters transplanted in fringe stands (i.e., originating from fringe or riverine stands) showed the same faster mass loss dynamic. Between 30 and 45 days of decomposition, the observed increase of catabolic enzyme activities and functional breadth indices of FF litters may partly explain the more dynamic mass loss found in fringe stands. Nevertheless, these improved catabolic abilities were less pronounced for RF litters since tyrosinase activity only was enhanced. These heightened features allowed both FF and RF litters to reach equivalent catabolic fitness to RR and FR litters (i.e., transplanted in riverine stands) after 45 days, suggesting that another factor may be responsible for the faster mass loss taking place in fringe stands. In fact, these results underline that the site of decomposition may have more impact on mass loss than litter microbial features. The faster decomposition in fringe stands may thus be explained by higher physical leaching dynamics due to stronger tide force, as suggested by Contreras et al. (2017). This hypothesis is further supported by the French Guiana coasts widely described as particularly dynamic, with an alternation between the formation of mudbanks and rapid sediment erosion (Marchand et al. 2003; Orseau et al. 2017) and a high wave energy season described by Gratiot et al. (2007) from October to May which corresponds to the period of our assay.
Influence of tidal inundation on the speed of decay
Furthermore, increased submergence times of litter occurring in the seaward fringe zones of mangroves were previously described as a factor accelerating their speed of decay, as reported by Imgraben and Dittmann (2008) and Dick and Osunkoya (2000) for A. marina leaf litter. Ashton et al. (1999) suggested that the tide’s physical impact on the leaves may cause fragmentation and thus increased weight loss. Moreover, exposure to saline water inundation in assessed fringe zones may have triggered litter decay through sulfate reduction, compensating the generally reduced decomposition occurring under anaerobic conditions, as previously suggested by Arnaud et al. (2020) in mangrove soils and Chambers et al. (2013) in outdoor mangrove mesocosms. In the second phase of decomposition described by Valiela et al. (1985) in salt marsh ecosystems (approximately a year), 40 to 70% of litter initial mass is lost through microbial degradation, suggesting that the effect of lower microbial abundance in fringe stands may actually affect decomposition dynamics on a broader timescale (i.e., above 45 days in the case of our study) as shown by Elias et al. (2020). After 45 days, catabolic enzyme activities reached the same levels for all tested transplants while after 30 days, FF transfers were lower than RR. These results may indicate the beginning of decomposition’s second phase, where microbial degradation becomes the main factor for mass loss. Overall, when considering litters that were transplanted home (i.e., direct transplantations FF and RR), our study allowed to show marked differences in nutrient content and microbial decomposer community fitness shifts taking place in fringe and riverine mangrove zones in the short term. Yet, for litters transplanted away (i.e., reciprocal transplantations RF and FR), these differences were scarcer, indicating a potential buffer effect.
Home-field advantage hypothesis
Finally, we observed a HFA effect only for FDAse activity after 45 days of decomposition. This generalist catabolic activity was higher at home only after 45 days of decomposition, showing that microbial communities shifted towards more efficient local communities at the end of decomposition. Apart from FDAase activity, no HFA effect was observed. These results are in accordance with Gießelmann et al. (2011) findings of a lack of HFA in the Atlantic rainforest of Brazil suggesting that a stronger difference in litter chemical quality may be necessary to underline such an effect. The slight differences of nutrient content, and the equivalent C:N ratios observed in the litters during our assay support this assumption.
Overall, the differences observed in the present study suggest a different productivity for each stand type, where fringe leaf litter is more exposed to leaching with higher exposure to tidal inundation and thus potentially exporting more organic C to the seawater and riverine litter decomposing slower, which is often associated to a higher C accumulation in the soil (Vinh et al. 2020).
Conclusions
By comparing fringe and riverine mangrove stands in A. germinans dominated mangroves of French Guiana, our study highlighted a difference of nutrient content, microbial decomposer abundance and fitness, and leaf litter decomposition dynamics. Litters decomposed under fringe stands, characterized by higher salinity regimes, higher tidal influence, and higher hydrological dynamics showed lower microbial abundances than those decomposed in riverine stands, supporting our first hypothesis of facilitating conditions taking place in riverine stands for microbial decomposers. Still, our study highlighted a strong metabolic/physiological plasticity of these communities towards fringe environmental constraints.
Moreover, our results showed more marked differences in microbial decomposer community structure between fringe and riverine litter when transplanted home, suggesting a potential buffer effect when they are transplanted reciprocally. The site of decomposition, particularly exposure to tidal water movement, is suggested as the main factor for decomposition velocity in the first phases of decomposition. Finally, no home-field advantage was observed on litter mass losses between A. germinans leaf litter from fringe and riverine stands.
This study contributes to the understanding of specialized adaptations that may occur in different zones from the same mangrove forest that could influence ecosystem processes and ultimately the services they provide, particularly their C sink abilities. Expected sea level rise in the coastal Guianas may therefore modify these services in the coming decades.
References
Aerts R (1997) Climate, leaf litter chemistry and leaf litter decomposition in terrestrial ecosystems: a triangular relationship. Oikos 79:439–449. https://doi.org/10.2307/3546886
Agati G, Cerovic ZG, Pinelli P, Tattini M (2011) Light-induced accumulation of ortho-dihydroxylated flavonoids as non-destructively monitored by chlorophyll fluorescence excitation techniques. Environ Exp Bot 73:3–9. https://doi.org/10.1016/j.envexpbot.2010.10.002
Alongi DM (2002) Present state and future of the world’s mangrove forests. Environ Conserv 29:331–349. https://doi.org/10.1017/S0376892902000231
Alongi DM (2014) Carbon cycling and storage in mangrove forests. Annu Rev Mar Sci 6:195–219. https://doi.org/10.1146/annurev-marine-010213-135020
Alongi DM (2018) Impact of global change on nutrient dynamics in mangrove forests. Forests 9:596. https://doi.org/10.3390/f9100596
Anthony EJ, Brondizio ES, dos Santos VF, Gardel A, Besset M (2021) Sustainable management, conservation, and restoration of the Amazon River Delta and Amazon-influenced Guianas coast: A Review. Water 13:1371. https://doi.org/10.3390/w13101371
Arnaud M, Baird AJ, Morris PJ, Dang TH, Nguyen TT (2020) Sensitivity of mangrove soil organic matter decay to warming and sea level change. Glob Change Biol 26:1899–1907. https://doi.org/10.1111/gcb.14931
Aschenbroich A, Michaud E, Gilbert F, Fromard F, Alt A et al (2017) Bioturbation functional roles associated with mangrove development in French Guiana, South America. Hydrobiologia 794:179–202. https://doi.org/10.1007/s10750-017-3093-7
Ashton E, Hogarth P, Ormond R (1999) Breakdown of mangrove leaf litter in a managed mangrove forest in Peninsular Malaysia. Hydrobiologia 413:77–88. https://doi.org/10.1023/A:1003842910811
Aupic-Samain A, Santonja M, Chomel M, Pereira S, Quer E et al (2021) Soil biota response to experimental rainfall reduction depends on the dominant tree species in mature northern Mediterranean forests. Soil Biol Biochem 154:108122. https://doi.org/10.1016/j.soilbio.2020.108122
Ayres E, Steltzer H, Simmons BL, Simpson RT, Steinweg JM et al (2009) Home-field advantage accelerates leaf litter decomposition in forests. Soil Biol Biochem 41:606–610. https://doi.org/10.1016/j.soilbio.2008.12.022
Buyer JS, Sasser M (2012) High throughput phospholipid fatty acid analysis of soils. Appl Soil Ecol 61:127–130. https://doi.org/10.1016/j.apsoil.2012.06.005
Capdeville C, Pommier T, Gervaix J, Fromard F, Rols JL et al (2019) Mangrove facies drives resistance and resilience of sediment microbes exposed to anthropic disturbance. Front Microbiol 9:3337. https://doi.org/10.3389/fmicb.2018.03337
Chambers LG, Reddy KR, Osborne TZ (2011) Short-term response of carbon cycling to salinity pulses in a freshwater wetland. Soil Sci Soc Am J 75:2000–2007. https://doi.org/10.2136/sssaj2011.0026
Chambers LG, Osborne TZ, Reddy KR (2013) Effect of salinity-altering pulsing events on soil organic carbon loss along an intertidal wetland gradient: a laboratory experiment. Biogeochemistry 115:363–383. https://doi.org/10.1007/s10533-013-9841-5
Chapman SK, Feller IC (2011) Away-field advantage: mangrove seedlings grow best in litter from other mangrove species. Oikos 120:1880–1888. https://doi.org/10.1111/j.1600-0706.2011.19381.x
Chomel M, Fernandez C, Bousquet-Mélou A, Gers C, Monnier Y et al (2014) Secondary metabolites of Pinus halepensis alter decomposer organisms and litter decomposition during afforestation of abandoned agricultural zones. J Ecol 102:411–424. https://doi.org/10.1111/1365-2745.12205
Chomel M, Guittonny-Larchevêque M, DesRochers A, Baldy V (2015) Home field advantage of litter decomposition in pure and mixed plantations under boreal climate. Ecosystems 18:1014–1028. https://doi.org/10.1007/s10021-015-9880-y
Chomel M, Guittonny-Larchevêque M, Fernandez C, Gallet C, DesRochers A et al (2016) Plant secondary metabolites: a key driver of litter decomposition and soil nutrient cycling. J Ecol 104:1527–1541. https://doi.org/10.1111/1365-2745.12644
Cintrón G, Lugo A, Martinez R (1985) Structural and functional properties of mangrove forests. In: D’Arcy WG, Correa MD (eds) The Botany and Natural History of Panama, vol 10. Monographs in Systemic Botany, pp 53–66
Contreras LM, Fierro-Cabo A, Cintra-Buenrostro CE (2017) Early drivers of Black Mangrove (Avicennia germinans) leaf litter decomposition in the water column. Hydrobiologia 803:147–157. https://doi.org/10.1007/s10750-017-3167-6
Coûteaux MM, Bottner P, Berg B (1995) Litter decomposition, climate and litter quality. Trends Ecol Evol 10:63–66. https://doi.org/10.1016/S0169-5347(00)88978-8
Dick T, Osunkoya O (2000) Influence of tidal restriction floodgates on decomposition of mangrove litter. Aquat Bot 68:273–280. https://doi.org/10.1016/S0304-3770(00)00119-4
Diop B, Sanz N, Blanchard F, Walcker R, Gardel A (2019) The role of mangrove in the French Guiana shrimp fishery. J Environ Econ Policy 8:147–158. https://doi.org/10.1080/21606544.2018.1522601
Donato DC, Kauffman JB, Murdiyarso D, Kurnianto S, Stidham M et al (2011) Mangroves among the most carbon-rich forests in the tropics. Nat Geosci 4:293–297. https://doi.org/10.1038/ngeo1123
Elias DMO, Robinson S, Both S, Goodall T, Majalap-Lee N et al (2020) Soil microbial community and litter quality controls on decomposition across a tropical forest disturbance gradient. Front For Glob Change 3:81. https://doi.org/10.3389/ffgc.2020.00081
Ewel KC, Twilley RR, Ong JE (1998) Different kinds of mangrove forests provide different goods and services. Glob Ecol Biogeogr Lett 7:83–94. https://doi.org/10.2307/2997700
Feller IC, Lovelock CE, Berger U, McKee KL, Joye SB et al (2009) Biocomplexity in mangrove ecosystems. Annu Rev Mar Sci 2:395–417. https://doi.org/10.1146/annurev.marine.010908.163809
Fromard F, Puig H, Mougin E, Marty G, Betoulle JL et al (1998) Structure, above-ground biomass and dynamics of mangrove ecosystems: new data from French Guiana. Oecologia 115:39–53. https://doi.org/10.1007/s004420050489
Fromard F, Vega C, Proisy C (2004) Half a century of dynamic coastal change affecting mangrove shorelines of French Guiana. A case study based on remote sensing data analyses and field surveys. Mar Geol 208:265–280. https://doi.org/10.1016/j.margeo.2004.04.018
Frostegård A, Bååth E (1996) The use of phospholipid fatty acid analysis to estimate bacterial and fungal biomass in soil. Biol Fertil Soils 22:59–65. https://doi.org/10.1007/BF00384433
Frostegård Å, Tunlid A, Bååth E (1993) Phospholipid fatty acid composition, biomass, and activity of microbial communities from two soil types experimentally exposed to different heavy metals. Appl Environ Microbiol 59:3605–3617. https://doi.org/10.1128/aem.59.11.3605-3617.1993
Garland JL, Mills AL (1991) Classification and characterization of heterotrophic microbial communities on the basis of patterns of community-level sole-carbon-source utilization. Appl Environ Microbiol 57:2351–2359. https://doi.org/10.1128/AEM.57.8.2351-2359.1991
Gholz HL, Wedin DA, Smitherman SM, Harmon ME, Parton WJ (2000) Long-term dynamics of pine and hardwood litter in contrasting environments: toward a global model of decomposition. Glob Change Biol 6:751–765. https://doi.org/10.1046/j.1365-2486.2000.00349.x
Gießelmann UC, Martins KG, Brändle M, Schädler M, Marques R et al (2010) Diversity and ecosystem functioning: Litter decomposition dynamics in the Atlantic Rainforest. Appl Soil Ecol 46:283–290. https://doi.org/10.1016/j.apsoil.2010.07.006
Gießelmann UC, Martins KG, Brändle M, Schädler M, Marques R et al (2011) Lack of home-field advantage in the decomposition of leaf litter in the Atlantic Rainforest of Brazil. Appl Soil Ecol 49:5–10. https://doi.org/10.1016/j.apsoil.2011.07.010
Gratiot N, Gardel A, Anthony EJ (2007) Trade-wind waves and mud dynamics on the French Guiana coast, South America: Input from ERA-40 wave data and field investigations. Mar Geol 236:15–26. https://doi.org/10.1016/j.margeo.2006.09.013
Guénon R, Day TA, Velazco-Ayuso S, Gros R (2017) Mixing of Aleppo pine and Holm oak litter increases biochemical diversity and alleviates N limitations of microbial activity. Soil Biol Biochem 105:216–226. https://doi.org/10.1016/j.soilbio.2016.11.023
Hättenschwiler S, Tiunov AV, Scheu S (2005) Biodiversity and litter decomposition in terrestrial ecosystems. Annu Rev Ecol Evol Syst 36:191–218. https://doi.org/10.1146/annurev.ecolsys.36.112904.151932
Holguin G, Vazquez P, Bashan Y (2001) The role of sediment microorganisms in the productivity, conservation, and rehabilitation of mangrove ecosystems: an overview. Biol Fertil Soils 33:265–278. https://doi.org/10.1007/s003740000319
Imgraben S, Dittmann S (2008) Leaf litter dynamics and litter consumption in two temperate South Australian mangrove forests. J Sea Res 59:83–93. https://doi.org/10.1016/j.seares.2007.06.004
Kauffman JB, Adame MF, Arifanti VB, Schile-Beers LM, Bernardino AF et al (2020) Total ecosystem carbon stocks of mangroves across broad global environmental and physical gradients. Ecol Monogr 90:e01405. https://doi.org/10.1002/ecm.1405
Kristensen E, Bouillon S, Dittmar T, Marchand C (2008) Organic carbon dynamics in mangrove ecosystems: A review. Aquat Bot 89:201–219. https://doi.org/10.1016/j.aquabot.2007.12.005
Lecerf A, Chauvet E (2008) Intraspecific variability in leaf traits strongly affects alder leaf decomposition in a stream. Basic Appl Ecol 9:598–605. https://doi.org/10.1016/j.baae.2007.11.003
Lee SY (1989) The importance of sesarminae crabs Chiromanthes spp. and inundation frequency on mangrove (Kandelia candel (L.) Druce) leaf litter turnover in a Hong Kong tidal shrimp pond. J Exp Mar Biol Ecol 131:23–43. https://doi.org/10.1016/0022-0981(89)90009-9
Lewis DB, Brown JA, Jimenez KL (2014) Effects of flooding and warming on soil organic matter mineralization in Avicennia germinans mangrove forests and Juncus roemerianus salt marshes. Estuar Coast Shelf Sci 139:11–19. https://doi.org/10.1016/j.ecss.2013.12.032
Lovelock CE, Cahoon DR, Friess DA, Guntenspergen GR, Krauss KW et al (2015) The vulnerability of Indo-Pacific mangrove forests to sea-level rise. Nature 526:559–563. https://doi.org/10.1038/nature15538
Lugo AE, Snedaker SC (1974) The ecology of mangroves. Annu Rev Ecol Syst 5:39–64. https://doi.org/10.1146/annurev.es.05.110174.000351
Luis P, Saint-Genis G, Vallon L, Bourgeois C, Bruto M et al (2019) Contrasted ecological niches shape fungal and prokaryotic community structure in mangroves sediments. Environ Microbiol 21:1407–1424. https://doi.org/10.1111/1462-2920.14571
Marchand C, Lallier-Vergès E, Baltzer F (2003) The composition of sedimentary organic matter in relation to the dynamic features of a mangrove-fringed coast in French Guiana. Estuar Coast Shelf Sci 56:119–130. https://doi.org/10.1016/S0272-7714(02)00134-8
Marchand C, Baltzer F, Lallier-Vergès E, Albéric P (2004) Pore-water chemistry in mangrove sediments: relationship with species composition and developmental stages (French Guiana). Mar Geol 208:361–381. https://doi.org/10.1016/j.margeo.2004.04.015
Marchand C, Disnar JR, Lallier-Vergès E, Lottier N (2005) Early diagenesis of carbohydrates and lignin in mangrove sediments subject to variable redox conditions (French Guiana). Geochim Cosmochim Acta 69:131–142. https://doi.org/10.1016/j.gca.2004.06.016
Meentemeyer V (1978) Macroclimate and lignin control of litter decomposition rates. Ecology 59:465–472. https://doi.org/10.2307/1936576
Mfilinge P, Atta N, Tsuchiya M (2002) Nutrient dynamics and leaf litter decomposition in a subtropical mangrove forest at Oura Bay, Okinawa, Japan. Trees 16:172–180. https://doi.org/10.1007/s00468-001-0156-0
Morris EK, Caruso T, Buscot F, Fischer M, Hancock C et al (2014) Choosing and using diversity indices: insights for ecological applications from the German Biodiversity Exploratories. Ecol Evol 4:3514–3524. https://doi.org/10.1002/ece3.1155
Musilova L, Ridl J, Polivkova M, Macek T, Uhlik O (2016) Effects of secondary plant metabolites on microbial populations: changes in community structure and metabolic activity in contaminated environments. Int J Mol Sci 17:1205. https://doi.org/10.3390/ijms17081205
Orseau S, Lesourd S, Huybrechts N, Gardel A (2017) Hydro-sedimentary processes of a shallow tropical estuary under Amazon influence. The Mahury Estuary. French Guiana. Estuar Coast Shelf Sci 189:252–266. https://doi.org/10.1016/j.ecss.2017.01.011
Palozzi JE, Lindo Z (2018) Are leaf litter and microbes team players? Interpreting home-field advantage decomposition dynamics. Soil Biol Biochem 124:189–198. https://doi.org/10.1016/j.soilbio.2018.06.018
Perez G, Aubert M, Decaëns T, Trap J, Chauvat M (2013) Home-field advantage: a matter of interaction between litter biochemistry and decomposer biota. Soil Biol Biochem 67:245–254. https://doi.org/10.1016/j.soilbio.2013.09.004
Peterson RA, Cavanaugh JE (2020) Ordered quantile normalization: a semiparametric transformation built for the cross-validation era. J Appl Stat 47:2312–2327. https://doi.org/10.1080/02664763.2019.1630372
Pradisty NA, Amir AA, Zimmer M (2021) Plant species- and stage-specific differences in microbial decay of mangrove leaf litter: the older the better? Oecologia 195:843–858. https://doi.org/10.1007/s00442-021-04865-3
Ray R, Thouzeau G, Walcker R, Vantrepotte V, Gleixner G et al (2020) Mangrove-derived organic and inorganic carbon exchanges between the Sinnamary estuarine system (French Guiana, South America) and Atlantic Ocean. J Geophys Res Biogeosciences 125:e2020JG005739. https://doi.org/10.1029/2020JG005739
Robertson AI (1988) Decomposition of mangrove leaf litter in tropical Australia. J Exp Mar Biol Ecol 116:235–247. https://doi.org/10.1016/0022-0981(88)90029-9
Rovai AS, Twilley RR, Castañeda-Moya E, Riul P, Cifuentes-Jara M et al (2018) Global controls on carbon storage in mangrove soils. Nat Clim Change 8:534–538. https://doi.org/10.1038/s41558-018-0162-5
Saiya-Cork KR, Sinsabaugh RL, Zak DR (2002) The effects of long term nitrogen deposition on extracellular enzyme activity in an Acer saccharum forest soil. Soil Biol Biochem 34:1309–1315. https://doi.org/10.1016/S0038-0717(02)00074-3
Schnürer J, Rosswall T (1982) Fluorescein diacetate hydrolysis as a measure of total microbial activity in soil and litter. Appl Environ Microbiol 43:1256–1261. https://doi.org/10.1128/aem.43.6.1256-1261.1982
Swift MJ, Heal OW, Anderson JM, Anderson JM (1979) Decomposition in terrestrial ecosystems. University of California Press
Talbot JM, Treseder KK (2012) Interactions among lignin, cellulose, and nitrogen drive litter chemistry–decay relationships. Ecology 93:345–354. https://doi.org/10.1890/11-0843.1
Uhlik O, Musilova L, Ridl J, Hroudova M, Vlcek C et al (2013) Plant secondary metabolite-induced shifts in bacterial community structure and degradative ability in contaminated soil. Appl Microbiol Biotechnol 97:9245–9256. https://doi.org/10.1007/s00253-012-4627-6
Valiela I, Teal JM, Allen SD, Van Etten R, Goehringer D et al (1985) Decomposition in salt marsh ecosystems: The phases and major factors affecting disappearance of above-ground organic matter. J Exp Mar Biol Ecol 89:29–54. https://doi.org/10.1016/0022-0981(85)90080-2
Veen GF(C), Freschet GT, Ordonez A, Wardle DA (2015) Litter quality and environmental controls of home-field advantage effects on litter decomposition. Oikos 124:187–195. https://doi.org/10.1111/oik.01374
Vinh TV, Allenbach M, Linh KTV, Marchand C (2020) Changes in leaf litter quality during its decomposition in a tropical planted mangrove forest (Can Gio, Vietnam). Front Environ Sci 8:10. https://doi.org/10.3389/fenvs.2020.00010
Walcker R, Anthony EJ, Cassou C, Aller RC, Gardel A et al (2015) Fluctuations in the extent of mangroves driven by multi-decadal changes in North Atlantic waves. J Biogeogr 42:2209–2219. https://doi.org/10.1111/jbi.12580
Wang Q, Zhong M, He T (2013) Home-field advantage of litter decomposition and nitrogen release in forest ecosystems. Biol Fertil Soils 49:427–434. https://doi.org/10.1007/s00374-012-0741-y
Weston NB, Dixon R, Joye S (2006) Ramifications of increased salinity in tidal freshwater sediments: Geochemistry and microbial pathways of organic matter mineralization. J Geophys Res-Biogeosci 111:G01009. https://doi.org/10.1029/2005JG000071
Wu C, Zhang Z, Wang H, Huang G, Shu C et al (2019) Home-field advantage of CWD decomposition in subtropical forests varied by field sites. For Ecol Manag 444:127–137. https://doi.org/10.1016/j.foreco.2019.04.051
Zhang W, Wang C, Xue R, Wang L (2019) Effects of salinity on the soil microbial community and soil fertility. J Integr Agric 18:1360–1368. https://doi.org/10.1016/S2095-3119(18)62077-5
Zimmer M (2005) Phenol Oxidation. In: Graça MAS, Bärlocher F, Gessner MO (eds) Methods to study litter decomposition: A practical guide. Springer, Netherlands, Dordrecht, pp 279–282. https://doi.org/10.1007/1-4020-3466-0_38
Acknowledgements
We are grateful to the Ecologie, Evolution, et Interactions des Systèmes amazoniens laboratory (USR LEEISA, Cayenne) and to the Ecologie des Forêts de Guyane laboratory (UMR EcoFoG, Cayenne), in particular to Antoine Gardel, Yann Rousseau and Christophe Duplais for making their facilities available during our missions in French Guiana. We are also grateful to the Common Services of Biological and Chemical Analysis and of Chemical Ecology and Metabolomics (Mediterranean Institute of marine and terrestrial Biodiversity, IMBE, Marseille) technical facilities for producing a part of the data used in this study. We thank all members of the DFME team from IMBE, Marseille.
Funding
This study was supported by the French National Center for Scientific Research (CNRS) through the Pépinière Interdisciplinaire de Guyane (PIG) funding program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by Robert Aller and accepted by Topical Collection Chief Editor Christopher Reyer.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on The highly dynamic French Guiana littoral under the Amazon influence: the last decade of multidisciplinary research.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dhaou, D., Gros, R., Baldy, V. et al. Comparison of leaf litter decomposition and microbial decomposer communities in fringe and riverine mangroves in French Guiana. Reg Environ Change 22, 102 (2022). https://doi.org/10.1007/s10113-022-01956-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10113-022-01956-6