Abstract
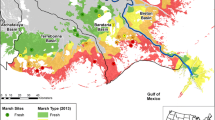
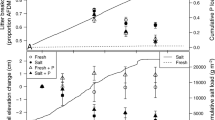
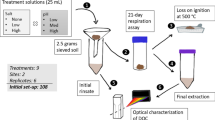
Salinity changes resulting from storm surge, tides, precipitation, and stormwater run-off are common in coastal wetlands. Soil microbial communities respond quickly to salinity changes, altering the rate of soil organic carbon (SOC) loss and associated biogeochemical processes. This study quantified the impact of salinity-altering pulses on SOC loss, defined as microbial respiration (CO2 flux) at high and low tide, CH4 flux, and dissolved OC (DOC) release, in 3 intertidal wetlands (Jacksonville, FL, USA). Intact soil cores from a freshwater tidal, brackish, and salt marsh were exposed to simulated tides and 3 salinity pulsing events during a 53-day laboratory experiment. Soil and water physio-chemical properties, nutrient release, and microbial indicators were measured. Microbial respiration was the dominate pathway of SOC loss (>97 %). Soil hydraulic conductivity was greater in brackish and salt marshes and was critical to overall soil respiration. High tide CO2 flux was greatest in the freshwater marsh (58 % of SOC loss) and positively correlated with DOC concentration; low tide CO2 flux was greatest in brackish and salt marshes (62 and 70 % of SOC loss, respectively) and correlated with NH4 + and microbial biomass. The freshwater marsh was sensitive to brackish pulses, causing a 112 % increase in respiration, presumably from accelerated sulfate reduction and N-cycling. SOC loss increased in the salt marsh pulsed with freshwater, suggesting freshwater run-off may reduce a salt marsh’s ability to keep-pace with sea level rise. Increased inundation from storm surges could accelerate SOC loss in freshwater marshes, while decreasing SOC loss in brackish and salt marshes.






Similar content being viewed by others
References
Alef K (1995) Dehydrogenase activity. In: Alef K, Nannipieri P (eds) Methods in applied soil microbiology and biochemistry. Academic Press, London, pp 228–230
Armentano TV, Menges ES (1986) Patterns of change in the carbon balance of organic soil- wetlands of the temperate zone. J Ecol 74(3):755–774
Azam F, Ifzal M (2006) Microbial populations immobilizing NH4 +–N and NO3 −–N differ in their sensitivity to sodium chloride salinity in soil. Soil Biol Biochem 38(8):2491–2494
Baldwin DS, Rees GN, Mitchell AM, Watson G, Williams J (2006) The short-term effects of salinization on anaerobic nutrient cycling and microbial community structure in sediment from a freshwater wetland. Wetlands 26:455–464
Blodau C, Moore TR (2003) Micro-scale CO2 and CH4 dynamics in a peat soil during a water fluctuation and sulfate pulse. Soil Biol Biochem 35(4):535–547
Blum LK, Roberts MS, Garland JL, Mills AL (2004) Distribution of microbial communities associated with the dominant high marsh plants and sediments of the United States east coast. Microb Ecol 48(3):375–388
Boelter DH (1965) Hydraulic conductivity of peats. Soil Sci 100:227–231
Brady NC, Weil RR (2004) Elements of the nature and properties of soils, 2nd edn. Pearson Prentice Hall, New Jersey
Bridgham SD, Megonigal JP, Keller JK, Bliss NB, Trettin C (2006) The carbon balance of North American wetlands. Wetlands 26(4):889–916
Bruland GL, DeMent G (2009) Phosphorus sorption dynamics of Hawaii’s coastal wetlands. Estuaries Coasts 32(5):844–854
Capone DG, Kiene RP (1988) Comparison of microbial dynamics in marine and fresh-water sediments: contrasts in anaerobic carbon catabolism. Limnol Oceanogr 33(4):725–749
Chambers LG, Reddy KR, Osborne TZ (2011) Short-term response of carbon cycling to salinity pulses in a freshwater wetland. Soil Sci Soc Am J 75:2000–2007
Clymo RS (1983) Peat. Mines: swamp, bog, fen and moor. Gen Stud A:159–224
Cordova-Kreylos AL, Cao YP, Green PG, Hwang HM, Kuivila KM, LaMontagne MG, Van De Werfhorst LC, Holden PA, Scow KM (2006) Diversity, composition, and geographical distribution of microbial communities in California salt marsh Sediments. Appl Environ Microbiol 72:3357–3366
Coultas CL (1996) Soils of the intertidal marshes of Florida’s Gulf Coast. In: Coultas CL, Hesieh Y-P (eds) Ecology and management of tidal marshes. CRC Press, Boca Raton, pp 61–69
Craft C (2007) Freshwater input structures soil properties, vertical accretion, and nutrient accumulation of Georgia and U.S. tidal marshes. Limnol Oceanogr 52:1220–1230
Craft C, Clough J, Ehman J, Joye S, Park R, Pennings S, Guo HY, Machmuller M (2009) Forecasting the effects of accelerated sea-level rise on tidal marsh ecosystem services. Front Ecol Environ 7(2):73–78
D’Angelo EM, Reddy KR (1999) Regulators of heterotrophic microbial potentials in wetland soils. Soil Biol Biochem 31(6):815–830
Davis CS (2002) Statistical methods for the analysis of repeated measurements. Springer, New York
DeLaune RD, White JR (2011) Will coastal wetlands continue to sequester carbon in response to an increase in global sea level?: a case study of the rapidly subsiding Mississippi river deltaic plain. Clim Change 110(1–2):297–314
DeLaune RD, Smith CJ, Patrick WH (1983) Methane release from Gulf-coast wetlands. Tellus B 35:8–15
DeLaune RD, Devai I, Crozier CR, Kelle P (2002) Sulfate reduction in Louisiana marsh soils of varying salinities. Commun Soil Sci Plant Anal 33:79–94
Dinesh R, Ramanathan G, Singh H (1995) Influence of chloride and sulfate-ions on soil enzymes. J Agron Crop Sci-Zeitschrift Fur Acker Und Pflanzenbau 175(2):129–133
Edmonds JW, Weston NB, Joye SB, Mou XZ, Moran MA (2009) Microbial community response to seawater amendment in low-salinity tidal sediments. Microb Ecol 58:558–568
Fagherazzi S, Kirwan ML, Mudd SM, Guntenspergen GR, Temmerman S, D’Alpaos A, van de Koppel J, Rybczyk JM, Reyes E, Craft C, Clough J (2012) Numerical models of salt marsh evolution: ecological, geomorphic, and climate factors. Rev Geophys 50:28
Frankenberger WT, Bingham FT (1982) Influence of salinity on soil enzyme-activities. Soil Sci Soc Am J 46:1173–1177
Freeman C, Lock MA, Reynolds B (1993) Fluxes of CO2, CH4 and N2O from a Welsh peatland following simulation of water table draw down- potential feedback to climatic-change. Biogeochemistry 19:51–60
Freeman C, Liska G, Ostle NJ, Lock MA, Hughes S, Reynolds B, Hudson J (1997) Enzymes and biogeochemical cycling in wetlands during a simulated drought. Biogeochemistry 39(2):177–187
Gardner LR (2005) Role of geomorphic and hydraulic parameters in governing pore water seepage from salt marsh sediments. Water Resour Res 41:11
Gennari M, Abbate C, La Porta V, Baglieri A, Cignetti A (2007) Microbial response to Na2SO4 additions in a volcanic soil. Arid Land Res Manag 21:211–227
Gregory J (1989) Fundamentals of flocculation. Crit Rev Environ Control 19(3):185–230
Gribsholt B, Kristensen E (2003) Benthic metabolism and sulfur cycling along an inundation gradient in a tidal Spartina anglica salt marsh. Limnol Oceanogr 48(6):2151–2162
Gribsholt B, Boschker HTS, Struyf E, Andersson M, Tramper A, De Brabandere L, van Damme S, Brion N, Meire P, Dehairs F, Middelburg JJ, Heip CHR (2005) Nitrogen processing in a tidal freshwater marsh: a whole-ecosystem (15)N labeling study. Limnol Oceanogr 50(6):1945–1959
Hale RL, Groffman PM (2006) Chloride effects on nitrogen dynamics in forested and suburban stream debris dams. J Environ Qual 35(6):2425–2432
Henman J, Poulter B (2008) Inundation of freshwater peatlands by sea level rise: Uncertainty and potential carbon cycle feedbacks. J Geophys Res Biogeosci 113(G1):11
Ikenaga M, Guevara R, Dean AL, Pisani C, Boyer JN (2010) Changes in community structure of sediment bacteria along the Florida coastal everglades marsh-mangrove-seagrass salinity gradient. Microb Ecol 59(2):284–295
IPCC (2007) Climate change 2007: a synthesis report. Valencia, Spain
Jackson CR, Vallaire SC (2009) Effects of salinity and nutrients on microbial assemblages in Louisiana wetland sediments. Wetlands 29(1):277–287
Jolly ID, McEwan KL, Holland KL (2008) A review of groundwater-surface water interactions in arid/semi-arid wetlands and the consequences of salinity for wetland ecology. Ecohydrology 1(1):43–58
Joye SB, Hollibaugh JT (1995) Influence of sulfide inhibition of nitrification on nitrogen regeneration in sediments. Science 270(5236):623–627
Kathilankal JC, Mozdzer TJ, Fuentes JD, D’Odorico P, McGlathery KJ, Zieman JC (2008) Tidal influences on carbon assimilation by a salt marsh. Environ Res Lett 3:6
Kester DR, Duedall IW, Connors DN, Pytkowic RM (1967) Preparation of artificial seawater. Limnol Oceanogr 12:176–178
King GM, Wiebe WJ (1980) Regulation of sulfate concentrations and methanogenesis in salt-marsh soils. Estuar Coast Mar Sci 10:215–223
King GM, Klug MJ, Wiegert RG, Chalmers AG (1982) Relation of soil-water movement and sulfide concentration to Spartina alterniflora production in a Georgia salt marsh. Science 218:61–63
Kirwan ML, Mudd SM (2012) Response of salt-marsh carbon accumulation to climate change. Nature 489(7417):550–554
Krauss KW, Whitbeck JL (2012) Soil greenhouse gas fluxes during wetland forest retreat along the lower Savannah River, Georgia (USA). Wetlands 32(1):73–81
Li CY, Weeks E, Rego JL (2009) In situ measurements of saltwater flux through tidal passes of Lake Pontchartrain estuary by Hurricanes Gustav and Ike in September 2008. Geophys Res Lett 36:5
Makoi J, Ndakidemi PA (2008) Selected soil enzymes: Examples of their potential roles in the ecosystem. Afr J Biotechnol 7(3):181–191
Marx MC, Wood M, Jarvis SC (2001) A microplate fluorimetric assay for the study of enzyme diversity in soils. Soil Biol Biochem 33:1633–1640
McKee KL, Mendelssohn IA (1989) Response of a fresh-water marsh plant community to increased salinity and increased water level. Aquat Bot 34(4):301–316
McLeod E, Chmura GL, Bouillon S, Salm R, Bjork M, Duarte CM, Lovelock CE, Schlesinger WH, Silliman BR (2011) A blueprint for blue carbon: toward an improved understanding of the role of vegetated coastal habitats in sequestering CO(2). Front Ecol Environ 9(10):552–560
Mendelssohn IA, Sorrell BK, Brix H, Schierup HH, Lorenzen B, Maltby E (1999) Controls on soil cellulose decomposition along a salinity gradient in a Phragmites australis wetland in Denmark. Aquat Bot 64(3–4):381–398
Michener WK, Blood ER, Bildstein KL, Brinson MM, Gardner LR (1997) Climate change, hurricanes and tropical storms, and rising sea level in coastal wetlands. Ecol Appl 7:770–801
Mousavi ME, Irish JL, Frey AE, Olivera F, Edge BL (2011) Global warming and hurricanes: the potential impact of hurricane intensification and sea level rise on coastal flooding. Clim Change 104:575–597
Muhammad S, Muller T, Joergensen RG (2006) Decomposition of pea and maize straw in Pakistani soils along a gradient in salinity. Biol Fertil Soils 43:93–101
Mulholland PJ, Best GR, Coutant CC, Hornberger GM, Meyer JL, Robinson PJ, Stenberg JR, Turner RE, VeraHerrera F, Wetzel RG (1997) Effects of climate change on freshwater ecosystems of the south-eastern United States and the Gulf Coast of Mexico. Hydrol Process 11:949–970
Neubauer SC (2011) Ecosystem responses of a tidal freshwater marsh experiencing saltwater intrusion and altered hydrology. Estuar Coasts DIO. doi:10.1007/s12237-011-9455-x
Neubauer SC, Miller WD, Anderson IC (2000) Carbon cycling in a tidal freshwater marsh ecosystem: a carbon gas flux study. Mar Ecol Prog Ser 199:13–30
Neubauer SC, Givler K, Valentine SK, Megonigal JP (2005) Seasonal patterns and plant-mediated controls of subsurface wetland biogeochemistry. Ecology 86(12):3334–3344
Nicholls RJ, Hoozemans FMJ, Marchand M (1999) Increasing flood risk and wetland losses due to global sea-level rise: regional and global analyses. Glob Environ Change-Hum Policy Dimens 9:S69–S87
NOAA (2011) National weather service Jacksonville. FL Weather Forecast Office. http://www.srh.noaa.gov/jax/, Accessed 2 February 2012
Nyman JA, Delaune RD, Patrick WH (1990) Wetland soil formation in the rapidly subsiding Mississippi River deltaic plain- mineral and organic-matter relationships. Estuar Coast Shelf Sci 31:57–69
Odum WE (1988) Comparative ecology of tidal fresh-water and salt marshes. Annu Rev Ecol Syst 19:147–176
Poffenbarger HJ, Needelman BA, Megonigal JP (2011) Salinity influence on methane emissions from tidal marshes. Wetlands 31(5):831–842
Portnoy JW, Giblin AE (1997) Biogeochemical effects of seawater restoration to diked salt marshes. Ecol Appl 7:1054–1063
Quintino V, Sangiorgio F, Ricardo F, Mamede R, Pires A, Freitas R, Rodrigues AM, Basset A (2009) In situ experimental study of reed leaf decomposition along a full salinity gradient. Estuar Coast Shelf Sci 85(3):497–506
Rasul G, Appuhn A, Muller T, Joergensen RG (2006) Salinity-induced changes in the microbial use of sugarcane filter cake added to soil. Appl Soil Ecol 31(1–2):1–10
Roberts J, Rowland A (1998) Cellulose fractionation in decomposition studies using detergent fiber pretreatment methods. Commun Soil Sci Plant Anal 25(11–14):269–277
Roseberg RJ, Christensen NW, Jackson TL (1986) Chloride, soil solution osmotic potential, and soil-pH effects on nitrification. Soil Sci Soc Am J 50(4):941–945
Saviozzi A, Cardelli R, Di Puccio R (2011) Impact of salinity on soil biological activities: a laboratory experiment. Commun Soil Sci Plant Anal 42(3):358–367
Seo DC, Yu K, Delaune RD (2008) Influence of salinity level on sediment denitrification in a Louisiana estuary receiving diverted Mississippi River water. Arch Agron Soil Sci 54:249–257
Smith SM (2009) Multi-decadal changes in salt marshes of Cape Cod, MA: Photographic analyses of vegetation loss, species shifts, and geomorphic change. Northeast Nat 16(2):183–208
Sparling GP, Feltham CW, Reynolds J, West AW, Singleton P (1990) Estimation of soil microbial C by fumigation extraction method- use on soils of high organic matter content and a reassessment of the KEC-factor. Soil Biol Biochem 22(3):301–307
Standard Methods (1997) Standard methods for the examination of water and wastewater, 20th edn. 4110 Determination of Anions by Ion Chromatography with chemical suppression of eluent conductivity
Thalmann A (1968) Zur Methodik der Bestimmung der Dehydrogenaseaktivität im Boden mittels Triphenyltetrazoliumchlorid (TTC). Landwirtsch Forsch 21:249–259
Torzilli AP, Sikaroodi M, Chalkley D, Gillevet PM (2006) A comparison of fungal communities from four salt marsh plants using automated ribosomal intergenic spacer analysis (ARISA). Mycologia 98(5):690–698
Tripathi S, Kumari S, Chakraborty A, Gupta A, Chakrabarti K, Bandyapadhyay BK (2006) Microbial biomass and its activities in salt-affected coastal soils. Biol Fertil Soils 42(3):273–277
Tripathi S, Chakraborty A, Chakrabarti K, Bandyopadhyay BK (2007) Enzyme activities and microbial biomass in coastal soils of India. Soil Biol Biochem 39(11):2840–2848
Tuxen K, Schile L, Stralberg D, Siegel S, Parker T, Vasey M, Callaway J, Kelly M (2011) Mapping changes in tidal wetland vegetation composition and pattern across a salinity gradient using high spatial resolution imagery. Wetlands Ecol Manage 19(2):141–157
USDA (1978) Soil survey of city of Jacksonville. Duval County, Florida
USEPA (1993) Methods for the determination of inorganic substances in environmental samples. USEPA 600/R-93/100: method 365.1
Van Ryckegem G, Verbeken A (2005) Fungal diversity and community structure on Phragmites australis (Poaceae) along a salinity gradient in the Scheldt estuary (Belgium). Nova Hedwigia 80:173–197
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass-C. Soil Biol Biochem 19:703–707
Weston NB, Dixon RE, Joye SB (2006) Ramifications of increased salinity in tidal freshwater sediments: Geochemistry and microbial pathways of organic matter mineralization. J Geophys Res Biogeosci 111:14
Weston NB, Vile MA, Neubauer SC, Velinsky DJ (2011) Accelerated microbial organic matter mineralization following salt-water intrusion into tidal freshwater marsh soils. Biogeochemistry 102(1–3):135–151
White JR, Reddy KR (2001) Influence of selected inorganic electron acceptors on organic nitrogen mineralization in everglades soils. Soil Sci Soc Am J 65(3):941–948
White JR, Reddy KR (2003) Nitrification and denitrification rates of everglades wetland soils along a phosphorus-impacted gradient. J Environ Qual 32(6):2436–2443
Wieski K, Guo HY, Craft CB, Pennings SC (2010) Ecosystem functions of tidal fresh, brackish, and salt marshes on the Georgia coast. Estuaries Coasts 33:161–169
Williams K, Ewel KC, Stumpf RP, Putz FE, Workman TW (1999) Sea-level rise and coastal forest retreat on the west coast of Florida, USA. Ecology 80(6):2045–2063
Wong VNL, Dalal RC, Greene RSB (2008) Salinity and sodicity effects on respiration and microbial biomass of soil. Biol Fertil Soils 44(7):943–953
Wright AL, Reddy KR (2001) Heterotrophic microbial activity in northern Everglades wetland soils. Soil Sci Soc Am J 65(6):1856–1864
Wu Y, Tam NFY, Wong MH (2008) Effects of salinity on treatment of municipal wastewater by constructed mangrove wetland microcosms. Mar Pollut Bull 57:727–734
Acknowledgments
This research was supported in part by the Florida Sea Grant Nutrient Dynamics Fellowship, with funds from Scotts Miracle-Gro, Inc. The views expressed are those of the authors and do not necessarily reflect the view of these organizations. The authors would like to thank Matt Norton and Gavin Wilson for field and laboratory assistance during this project and Dr. Kanika Inglett for assistance with the soil enzyme analysis. The helpful comments of two anonymous reviewers improved the quality of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chambers, L.G., Osborne, T.Z. & Reddy, K.R. Effect of salinity-altering pulsing events on soil organic carbon loss along an intertidal wetland gradient: a laboratory experiment. Biogeochemistry 115, 363–383 (2013). https://doi.org/10.1007/s10533-013-9841-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-013-9841-5