Abstract
Background and aims
Certain plant species release root carboxylates in response to phosphorus (P) limitation; however, the prevalence of root exudate release in species in P-limited forest ecosystems remains unexplored due to challenges in field assessment.
Methods
Manganese (Mn) accumulation in mature leaves can indicate the presence of root carboxylate exudates in rhizosphere soil. To account for environmental factors such as soil pH, a negative reference species that does not release carboxylates is used for comparison. In this study, we assessed multiple forest stands across soil types and different levels of P availability in northern (Gansu) and southern (Guangxi) China. Leaf and soil samples were collected from 188 plant families representing various life forms, and leaf Mn concentration ([Mn]) was analyzed as a proxy for root carboxylate exudation patterns, using Dryopteridaceae as a negative reference.
Results
The results supported our hypotheses that leaf [Mn] was higher in P-limited forests of southern China compared to P-richer forests of northern China, even though the soil [Mn] was higher in the forests of northern China. Additionally, we observed a higher prevalence of species with high leaf [Mn] across various plant families in Guangxi (82%) than in Gansu (42%).
Conclusion
Our findings suggest a potential common strategy among plants in Guangxi forests, where root exudates are released in response to P limitation, possibly due to ineffective mycorrhizal symbiosis for nutrient acquisition. The diverse forest systems in China exhibit varying soil P availability, leading to the evolution of plant species with distinct P-acquisition strategies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Forestry ecosystems play a vital role in global carbon cycling, biodiversity conservation, and sustainable resource management (Miura et al. 2015; Watson et al. 2018; Migliavacca et al. 2021; Taye et al. 2021). Understanding nutrient dynamics within these ecosystems is crucial for effective management and conservation (Silver et al. 1996; Watson et al. 2018; Meier et al. 2019). Among essential nutrients, phosphorus (P) often limits plant growth, particularly in highly weathered and leached soils (Du et al. 2020; Hou et al. 2020), which are prevalent in southern China (Zhang et al. 2021; Yan et al. 2023). In response to P limitation, plants have evolved various adaptive strategies, such as the release of root exudates, especially carboxylates and phosphatases, to enhance P acquisition from P-impoverished soils (Lambers et al. 2015b; Güsewell and Schroth 2017; Lambers 2022). Investigating and quantifying root-exudation patterns in P-limited forestry ecosystems is therefore essential for understanding nutrient cycling processes and optimizing forest management practices. However, due to the challenge of directly accessing and measuring plant-soil nutrient dynamics in forests (Oburger and Jones 2018; Escolà Casas and Matamoros 2021), root exudation patterns across different forest ecosystems are largely unknown.
Leaf manganese (Mn) concentration ([Mn]) has been proposed as a proxy to estimate the extent of root exudation for P acquisition in a number of plant species (Lambers et al. 2015a, 2021; Pang et al. 2018; Zhou et al. 2022; Yu et al. 2023b). Some plants experiencing P limitation increase their root carboxylate exudation and, in turn, enhance Mn uptake from the soil (Shane and Lambers 2005; Lambers et al. 2015a). Plants absorb and transfer soil Mn through a combination of passive and active processes, lacking dedicated transporters for Mn (shared with iron and other elements), and the poor control of Mn uptake makes it possible to indicate its availability in rhizosphere soil (Page and Feller 2005; Millaleo et al. 2010). Manganese is transported to leaf cells through various transporters present in the plasma membrane, and subsequently accumulates in mature leaves. Manganese uptake rates may exceed the plant’s requirement by over 100-fold in some species, resulting in hyperaccumulation (Millaleo et al. 2010; Losfeld et al. 2015). Excessive Mn can be toxic, promoting plants to develop various mechanisms to accumulate Mn without adverse effects. These mechanisms include redistributing and compartmentalizing Mn within different tissues to maintain optimal [Mn] in essential tissues while sequestering excess Mn in less sensitive compartments such as cell walls (oxidized Mn and phenolic compounds) (Wissemeier and Horst 1992). In addition, plants can chelate excess Mn within cells in vacuoles by binding it to organic compounds such as citrate, oxalate, and malate (Memon and Yatazawa 1984; Millaleo et al. 2010). Due to the involvement of Mn in redox reactions and generation of reactive oxygen species (ROS), plants possess antioxidant defense systems to scavenge and neutralize ROS (Sytar et al. 2021; Kosakivska et al. 2021; Zhao et al. 2022). These findings suggest that leaf [Mn] has the potential to serve as a reliable indicator of rhizosphere carboxylate concentrations and P-acquisition strategies across various ecosystems (Lambers et al. 2015a; Huang et al. 2017).
Low soil P availability promotes leaf Mn accumulation in plants, and is often observed in P-deficient soils where plants adjust their nutrient-uptake and -allocation strategies to cope with the limited P availability (Millaleo et al. 2010; Lambers et al. 2015a, 2021). One of the mechanisms involves the exudation of carboxylates and phosphatases from their roots, enhancing the solubilization and mobilization of soil P. However, these carboxylates also influence the availability of other nutrients, including Mn (Shane and Lambers 2005). Under P-deficient conditions, increased secretion of carboxylates enhances the desorption of Mn from soil particles, making it more accessible to plant roots (Kochian et al. 2004). Subsequently, root uptake and shoot transport result in elevated [Mn] within mature leaves (Page and Feller 2005; Page et al. 2006; Losfeld et al. 2015).
There is a lack of comprehensive studies examining the correlation between root exudation, leaf [Mn], and P limitation in forestry ecosystems of southern China. Very few studies have explored the relationship between root exudates and P availability among different plant species and ecosystems in south China. For example, research conducted in subtropical rainforests has shown that root exudation is a major root functional trait (Sun et al. 2021), and root exudation is largely affected by soil P availability and mycorrhizal type (Jiang et al. 2022). However, how prevalent the release of root exudates is among different species in P-limited forest ecosystems remains unexplored (Wang and Lambers 2020). Are species in any plant family consistently exhibiting high levels of root exudation in both P-rich and P-limited environments? Does root carboxylate exudation demonstrate specific trends among plant life forms? Responses to these questions are essential to bridge the knowledge gap and provide valuable insights into the nutrient dynamics of significant forest ecosystems.
In the context of forestry ecosystems in southern China, characterized by acidic soils with low P availability, investigating the correlation between root exudation, leaf [Mn], and P limitation holds particular promise. This region also encompasses vast areas of plantation forests dominated by fast-growing tree species widely utilized for timber production and ecological restoration (Zhou et al. 2017; Sun et al. 2021; Yan et al. 2023). Understanding the mechanisms underlying nutrient acquisition and utilization in these ecosystems is crucial for sustainable forest management and ecosystem functioning. Additionally, using leaf [Mn] as a proxy can allow us to assess whether plants have the capacity to be hyperaccumulators and remove excessive soil Mn for soil restoration (Li et al. 2007; Liu et al. 2020; Wildová et al. 2021).
This study aimed to investigate the potential of using leaf [Mn] to proxy root exudation in different P-limited forestry ecosystems in southern China as well as in P-rich systems in northern China. Based on previous measurements (Lai et al. 2024), we assumed that the forest ecosystems in southern China likely experience P limitation due to their acidic soils and significant erosion. We hypothesized: 1) The leaf [Mn] would be higher in the forest ecosystems of southern China (low-P soils) than those in northern China (relatively P-rich soils). Therefore, we expected plants in the southern forest ecosystems to exhibit faster root carboxylate-exudation rates, leading to increased Mn uptake and consequently higher leaf [Mn] than those in the northern forest ecosystems. According to previous studies showing that root exudation is widespread across different plant families and plant forms, we further hypothesized: 2) high leaf [Mn] in P-limited southern China would be more common compared to that in the relatively P-rich ecosystems in northern China.
To test the above hypotheses, we assessed multiple forest stands across different soil types with different levels of P availability. Leaf and soil samples were collected from selected stands encompassing a range of plant families and life forms, and leaf [Mn] was analyzed to proxy root carboxylate-exudation patterns. Furthermore, we quantified soil P availability and characterized soil properties to assess their impact on the dynamics of root exudation.
Methods and materials
Study sites and species selection
The study was conducted in 2020, assessing multiple forest stands across different soil types with different P availability in two distinct natural forest ecosystems (Fig. 1; this map was made using R package “ggmap”; the source is “stadia” with a type “stamen_terrain”). One ecosystem is located in the Maijishan Region (34°20′N, 106°01′E) of Gansu Province, northern China. Known for its rich biodiversity, the Maijishan ecosystem has a total of 2371 species of vascular plants, representing 218 families and 962 genera (Lu 2006). Located within the Qinling Mountains, it spans altitudes ranging from 1200 to 1600 m above sea level. It experiences a continental climate characterized by cool, humid, and semi-humid zones, with an average annual rainfall of 700 mm, primarily occurring between July and September. The mean temperature is 9 °C with a relative air humidity of approximately 73% (Yang et al. 2023). The dominant soils in this region are mountain brown loams (Tudi et al. 2022).
Another ecosystem studied is situated in the Damingshan Region (23°24′N, 108°31′E), within the south-central Guangxi Zhuang Autonomous Region of southern China. Due to its unique geographic location, Damingshan exhibits rich biodiversity with a total of 2374 vascular plant species belonging to 918 genera across 234 families (Wen et al. 1998). This ecosystem lies between the northern tropics and southern subtropics at an average altitude of 1200 m, experiencing a southern subtropical monsoon climate. It receives an average precipitation of 2630 mm and an average temperature of 15.1℃ (Zhu et al. 2011).
According to the literature (Wen et al. 1998; Suo et al. 2008; Zhu et al. 2011; Yang et al. 2023) and recommendations from local botanists, we established a 200 m × 200 m study site in each ecosystem in 2020. Each site was subdivided into eight to 10 subsites based on the landscape for the purpose of investigating and selecting plant species. Taking into consideration factors such as abundance, life form, and endemism, a total of 188 species were collected from the two ecosystems: 38 plant families, 81 species from relatively P-rich soils of Gansu; 56 plant families, 107 species from low-P soils of Guangxi (Supplementary Table 1).
Reference species selection
The leaf [Mn] can serve as an indicator of root carboxylate release, implying that higher concentrations suggest plants release greater amount of carboxylates that mobilize Mn and enhance its uptake from the soil. To assess the relationship between different leaf [Mn] levels and the extent of root carboxylate release, a “negative reference” is necessary (Lambers et al. 2021; Lambers 2023). Therefore, we selected the family of Dryopteridaceae (ferns) species as the negative reference for several reasons: 1) Dryopteridaceae are adapted to efficiently utilize limited Mn in their habitats (Grosjean et al. 2019; Schmitt et al. 2017) which may not provide sufficient amounts of Mn for these ferns to accumulate high concentrations in their leaves (Cornara et al. 2007); these adaptations often involve conservative strategies for Mn uptake and allocation within the plant, resulting in lower leaf [Mn] (Bai et al. 2020; Zhou et al. 2022); 2) Dryopteridaceae typically exhibit slow growth rates compared with many vascular plant species (Pinson et al. 2017; Rünk and Zobel 2007), leading to reduced demand for Mn and consequently lower concentrations in their leaves (Reimann et al. 2007); 3) Dryopteridaceae occurred in both studied ecosystems. It is worth noting that leaf [Mn] in different fern families may vary due to their specific growth microhabitats, suggesting a discernible difference in leaf [Mn] among different fern families. In this study, when referring to “high” leaf [Mn], it indicates a comparison with the negative reference group of Dryopteridaceae.
Leaf and soil sampling
All samples were collected between August and October 2020. Five plants per species (except for a few species, which included three to four plants) were selected, and mature leaves were utilized for chemical analyses. The leaves were placed in envelopes for subsequent determination of dry weight (at 60℃ for seven days). Bulk soil samples were obtained by combining soil from three points within each plot of every subsite. All soil samples were collected in plastic bags, air-dried for one week, sieved to a particle size of 2 mm, and then ground.
Leaf and soil analyses
Aliquots of 80 mg sample were digested using concentrated HNO3 and HClO4 (v/v 3:1) and diluted to 10 ml with Milli-Q water, and leaf [Mn] was analyzed by inductively coupled plasma optical emission spectroscopy (ICP-OES) (Perkin Elmer, PE Avio 500, Shelton, CT, USA). Leaf P concentration ([P]) was analyzed using the molybdenum blue-based method (Ames 1966) with a microplate reader (Thermo Fisher Scientific, Varioskan Flash, Finland).
Aliquots of 0.5 g soil sample were weighed in a digestion tube and digested using concentrated H2SO4 and HClO4 at 360℃ for one hour. The total nitrogen concentration ([N]) and total [P] were then determined using an automatic chemical analyzer (AMS, Smartchem 450, Rome, Italy) and the molybdenum blue-based method (Olsen and Sommers 1982), respectively. Soil nitrate and ammonium were extracted with 2 M KCl and measured using a continuous segmented flow autoanalyzer (AA3, SEAL Analytical, Norderstedt, Germany). Anion exchange membranes (AEMs, VWR Chemical, Leuven, Belgium) were used to measure soil resin P (‘plant-available P’) (Bentley et al. 1999). Soil pH (water based) was measured using a pH meter.
Statistical analyses
All statistical analyses were performed using the R software platform (version 4.0.2, R Core Team 2020). Shapiro-Wilk normality was tested using ‘rstatix’ (version 0.7.2, Kassambara 2023), and homogeneity was tested using ‘stats’ (R Core Team 2020). Considering the varying sample sizes, such as three to five replicates for each species and one to 10 species from different families, the utilization of Welch t-test is more suitable for this study (Stewart-Oaten et al 1992; Ruxton 2006). The Welch t-test was used to compare the difference of mean concentrations between the “negative reference” and target families, and the difference between two locations using ‘stats’ (R Core Team 2020). All plant species from two locations were compared for significance (Figs. 2, 3 and 5), excluding the top 50% of species with the highest leaf [Mn], as depicted in Fig. 4. Appropriate generalised least square models (GLS) were selected based on the lowest Akaike's Information Criterion corrected (AICc) values and the Tukey's HSD post hoc test (Pinheiro & Bates 2000), to compare the significance among life forms at the same location (p < 0.05) in Fig. 5.
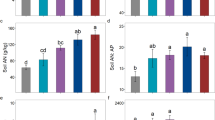
Leaf phosphorus (P) (a) and manganese (Mn) (b) concentration in different locations of relatively P-rich soils in Gansu and low-P soils in Guangxi. Each point indicates an individual plant, with a total of 327 and 395 individuals in Gansu and Guangxi, respectively. Asterisks **** indicate significant difference between values in Gansu and Guangxi using the Welch t-test, p < 0.0001
Leaf manganese (Mn) concentration of different plant life forms in relatively P-rich soils Gansu (a) and low-P soils of Guangxi (b). Different letters indicate significant difference among life forms within the same location using appropriate generalized least square models based Tukey's HSD post hoc test, p < 0.05
Results
Soil characteristic in different ecosystems
The soil in Guangxi exhibited significantly lower pH than that in Gansu (Table 1), accompanied by lower concentrations of total P and resin P (less than 20% of the levels observed in Gansu). Conversely, the soil total N concentration was higher in Guangxi, although not statistically significant; the concentration of NH4+-N in Gansu was 70% higher than that in Guangxi (p < 0.05), whereas the soil in Guangxi exhibited a higher level of NO3−-N (p < 0.05). Although we did not measure soil [Mn], literature data indicates a 1.9-fold higher level of soil [Mn] in Gansu compared with that in Guangxi (Wang et al. 2012, 2016).
Leaf P and Mn concentration in different ecosystems, families and species
In the ecosystem of low-P Guangxi, plants exhibited 79% lower leaf [P] (0.9 mg g−1, p < 0.0001, Fig. 2a), but 51% higher leaf [Mn] (230 mg kg−1; p < 0.0001, Fig. 2b) compared with those in the ecosystem of relatively P-rich soils of Gansu (1.6 mg g−1 leaf [P], and 113 mg kg−1 leaf [Mn]; Fig. 2).
We examined 38 and 56 families in relatively P-rich soils of Maijishan (Gansu) and low-P soils of Damingshan (Guangxi), respectively (Fig. 3). Approximately half of the families from relatively P-rich soils (16 out of 38) exhibited significantly higher leaf [Mn] than the reference group, while a substantial majority of the families from low-P soils (46 out of 56) showed significantly elevated leaf [Mn] relative to the negative reference. Leaf [Mn] was generally higher in low-P soils than in relatively P-rich soils (Fig. 3). Notably, in low-P soils, the families Altingiaceae, Verbenaceae, Clethraceae, Chloranthaceae, Araliaceae, Melastomataceae, and Selaginellaceae all displayed average leaf [Mn] values exceeding 500 mg kg−1, while in relatively P-rich soils, only the Fagaceae family exhibited an average leaf [Mn] above this threshold. When compared with the negative reference using a Welch t-test, the p-values were marginal for the Sapindaceae and Lauraceae families (0.052 and 0.051, respectively) in relatively P-rich soils, and for the Anacardiaceae, Juglandaceae, and Lindsaeeacea families (0.051, 0.056, and 0.05, respectively) in low-P soils.
Plant species with the highest leaf [Mn] in two ecosystems were presented in Fig. 4. In Maijishan, which had a higher soil P concentration, the top 10 species with the highest leaf [Mn] were Quercus spinosa (881 mg kg−1, Fagaceae), Taxus wallichiana var. chinensis (702 mg kg−1, Taxaceae), Quercus mongolica (516 mg kg−1, Fagaceae), Elaeagnus umbellate (442 mg kg−1, Elaeagnaceae), Symplocos tanakana (344 mg kg−1, Symplocaceae), Quercus aliena var. acutiserrata (310 mg kg−1, Fagaceae), Schisandra chinensis (303 mg kg−1, Schisandraceae), Deutzia grandiflora (247 mg kg−1, Hydrangeaceae), Litsea pungens (236 mg kg−1, Lauraceae) and Elaeagnus pungens (178 mg kg−1, Elaeagnaceae). In Damingshan where soil P concentration was lower than that in Majishan, the top 10 species with the highest leaf [Mn] were Clerodendrum kwangtungense (1506 mg kg−1, Verbenaceae), Altingia chinensis (1108 mg kg−1, Altingiaceae), Dendropanax dentiger (897 mg kg−1, Araliaceae), Selaginella doederleinii (852 mg kg−1, Selaginellaceae), Clethra delavayi (812 mg kg−1, Clethraceae), Blastus pauciflorus (756 mg kg−1, Melastomataceae), Betula austrosinensis (629 mg kg−1, Betulaceae), Elaeocarpus decipiens (628 mg kg−1, Elaeocarpaceae), Sarcandra glabra (625 mg kg−1, Chloranthaceae) and Lophatherum gracile (582 mg kg−1, Poaceae).
Compared with the top 10 species with the highest leaf [Mn] in relatively P-rich soils of Gansu (with an average of 416 mg Mn kg−1) and low-P soils of Guangxi (840 mg Mn kg−1), both the maximum and minimum leaf [Mn] in low-P soils were significantly higher than those observed on relatively P-rich soils, respectively. Amongst those top 10 species with highest leaf [Mn], Fagaceae (three species) and Elaeagnaceae (two species) were families most represented in relatively P-rich soils; in contrast, the top 10 highest leaf [Mn] species belonged to 10 different families in low-P soils.
In low-P soils of Guangxi, within the Verbenaceae family, two species demonstrated significant differences in leaf [Mn]: Callicarpa bodinieri exhibited 170 mg Mn kg−1, while Clerodendrum kwangtungense showed a value of 1500 mg Mn kg−1. Similarly, among Araliaceae, three species displayed high distinct levels of leaf [Mn], namely Dendropanax dentiger (894 mg Mn kg −1), Aralia armata (410 mg Mn kg−1), and Heptapleurum delavayi (454 mg Mn kg−1). In the Altingiaceae family, only one species was included: Altingia chinensis, and all five replicates exhibited high leaf [Mn], although there was considerable variation, ranging from 826 to 1813 mg Mn kg−1. Among the seven Poaceae there was considerable variation; Lophatherum gracile and Chimonobambusa damingshanensis had a leaf [Mn] of 582 mg Mn kg−1and 360 mg Mn kg−1, respectively. In contrast, Miscanthus floridulus and Setaria palmifolia exhibited low leaf [Mn], 12 mg Mn kg−1 and 24 mg Mn kg−1, respectively. However, all five Poaceae in relatively P-rich soils of Gansu exhibited relatively low leaf [Mn], with an average concentration of 38 ± 7 (standard error of the average) mg Mn kg−1.
Leaf Mn concentration as dependent on life form
When plant species were classified into different life forms (Fig. 5), in relatively P-rich soils of Gansu, trees exhibited the highest leaf [Mn], while herbs and ferns presented the lowest leaf [Mn]; in contrast, in low-P soils of Guangxi, shrubs demonstrated the highest leaf [Mn], whereas lianas had the lowest leaf [Mn]. Notably, relatively P-rich soils of Gansu showed more pronounced variation among plant life forms compared with relatively smaller differences in low-P soils of Guangxi.
Discussion
The present findings provide robust support for our hypotheses that leaf [Mn] in P-limited forests of southern China is higher than that in the forests of northern China with higher soil P, despite Gansu having a higher soil [Mn]. Additionally, we observed a more widespread distribution of high leaf [Mn] compared with the negative reference across various plant families in low-P soils than in relatively P-rich soils. This suggests a root carboxylate-release strategy commonly exhibited by plants in response to P limitation in Guangxi forests.
Soil P limitation promotes leaf Mn accumulation
Although soil [Mn] was higher in relatively P-rich soils of Gansu than in low-P soils of Guangxi (Table 1), Mn availability can vary. Soils that are more acidic generally exhibit higher Mn availability, while alkaline or highly weathered soils may have a very low Mn availability (Sims 1986). Additionally, a high availability of soil P can reduce the uptake and accumulation of Mn in leaves (Pedas et al. 2011). This is attributed to high levels of soil P that can induce alterations in soil pH and affect the availability of elements, including Mn. Furthermore, it is well-documented that P and Mn often exhibit antagonistic interactions within the soil environment, leading to mutual inhibition of their respective uptake (Barben et al. 2010). To mitigate the confounding effect of soil pH in different locations, the negative control is recommended to be used to standardize leaf [Mn] in response to soil P (Lambers et al. 2021). Leaf [Mn] was significantly higher (Fig. 2) across various plant families in P-limited Guangxi (Fig. 3), indicating enhanced mobilization and absorption of soil Mn by plants. The soil total P and resin P concentration both indicated a more severe P-deficiency in Guangxi than in Gansu (Table 1) which is consistent with previous studies (Zhang et al. 2005; Li et al. 2015; Liu et al. 2022). The combination of a lower soil pH, karst land, and higher precipitation in Guangxi exacerbates the severity of P deficiency when compared with that in relatively P-rich soils of Gansu (Zhang et al. 2021; Li et al. 2023).
Mycorrhizal fungi play a pivotal role in enhancing nutrient acquisition in plants through symbiotic interactions. However, the soil microbial diversity in low-P soils of Guangxi forests is comparatively lower than that at other locations (Zhao et al. 2020). Furthermore, the richness of arbuscular mycorrhizal fungi (AMF) exhibits a significant positive correlation with plant-available P and soil pH (Xiao et al. 2019). This symbiotic association and nutrient-uptake ability can be suppressed under severe P limitation (Abbott et al. 1984; Bolan et al. 1984; Treseder and Allen 2002; Albornoz et al. 2017), despite mycorrhizal symbiosis still playing a vital role in pathogen defense (Guillemin et al. 1994; Branzanti et al. 1999; Gille et al. 2024). In such scenarios, plants may rely on alternative strategies, such as root exudates, for P acquisition. This would partially explain the higher leaf [Mn] widely observed across various plant families in low-P soils of Guangxi.
A high leaf Mn concentration in mature leaves is a proxy for significant root exudation
Manganese accumulation in plants requires more study, as some species, such as Banksia (Proteaceae) and some Eucalyptus species (Myrtaceae) show stronger Mn enrichment characteristics than others in P-deficient conditions (Shane and Lambers 2005; Lambers et al. 2021; Zhou et al. 2022). Several families, including Fagaceae, Symplocaceae, Smilacaceae, Araliaceae, Pinaceae, Cupressaceae exhibited higher leaf [Mn] than the negative references in both relatively P-rich soils and low-P soils. Moreover, many of these species are known to release carboxylates from their roots (Table 2). For instance, Quercus species (Fagaceae) in temperate and tropical forests release significant amounts of oxalate from their fine roots (Sun et al. 2017; Wang et al. 2021; Nottingham et al. 2022); while Panax species (Araliaceae) release glycolate, propionate, glycerate and glutarate (Luo et al. 2022). Similarly, Picea species (Pinaceae) exude root carboxylates like oxalate and lactate into the rhizosphere (Sandnes et al. 2005). Monocarboxylates like lactate and glycolate do not chelate Mn, but they may affect Mn availability in soil when associated with a decrease in rhizosphere pH (Lambers et al. 2021).
Some plant families occurred at both locations but only showed significantly higher leaf [Mn] than the negative reference at the low-P location, such as Lauraceae, Asteraceae, Sapindaceae, Polypodiaceae, Fabaceae, Thelypteridaceae, Poaceae and Dennstaedtiaceae. Though previous studies found fern families in general have low leaf [Mn] (Grosjean et al. 2019; Schmitt et al. 2017), the present study observed that certain fern families (i.e. Thelypteridaceae and Dennstaedtiaceae) also responded to the soil P levels with the values at low-P soils of Guangxi being significantly higher than in relatively P-rich soils of Gansu (Fig. 5). This may be attributed to specific growth conditions, although such occurrences are not widespread (Reimann et al. 2007), which requires further investigation. The observation of high leaf [Mn] in some species aligns with the previous reports on the exudation of carboxylates from roots. For example, species belonging to Fabaceae and Poaceae are found to release root carboxylates such as citrate, oxalate and malate (Table 2). Additionally, species from Lauraceae release citrate (Aoki et al. 2012) and other root exudates (Sun et al. 2021); Asteraceae species release oxalate (Olivares et al. 2002). Interestingly, Cyperaceae showed higher leaf [Mn] than the negative reference in relatively P-rich soils of Gansu, but not in P-limited Guangxi (p = 0.07); many species of Cyperaceae produce dauciform roots and release root carboxylates and phosphatases (Playsted et al. 2006; Shane et al. 2006). The variation among Cyperaceae in P-limited Guangxi might be accounted for by cations other than protons accompanying the release of carboxylates (Roelofs et al. 2001; Zhu et al. 2005). Exudation of cations like K+ and Na+, rather than H+, contributes to maintaining the charge balance during the release of carboxylate anions. However, this affects the rhizosphere pH, and therefore the Mn availability, and subsequent leaf Mn accumulation (Fig. 3b) (Lambers et al. 2021). However, further investigation is required to fully comprehend these effects.
We observed significant variation in leaf [Mn] among certain families, such as Fagaceae, Taxaceae and Elaeagnaceae in relatively P-rich soils of Gansu. Moreover, a greater number of plant families exhibited significant variation in P-limited Guangxi, including Altingiaceae, Verbenaceae, Araliaceae, Melastomataceae, Selaginellaceae, Betulaceae, Zingiberaceae, Elaeocarpaceae, Polypodiaceae and Poaceae. These findings suggest that the capacity to accumulate leaf [Mn] varies within plant families. Additionally, some plants do not respond to low soil P by releasing root exudates and consequently maintain low leaf [Mn] (Lambers et al 2022).
Enhancing the perspectives on leaf manganese and root exudate research demands meticulous attention and focus
Investigating leaf [Mn] and root exudation of carboxylates is crucial for comprehending plant nutrient acquisition, rhizosphere dynamics, and plant-microbe interactions. Given the importance of further leaf Mn studies, it is important to note a number of factors affecting soil Mn availability and plant absorption and accumulation. It requires careful selection of the study sites. Climate conditions, soil moisture, and drainage can influence the availability and uptake of Mn by plants. Waterlogged or poorly-drained soils can enhance both Fe and Mn availability; however, plants down-regulate Fe uptake due to the tightly controlled Fe-acquisition mechanisms (Lambers et al. 2021), which consequently reduces Mn acquisition as Mn and Fe share the same transporter (Baxter et al. 2008). Conversely, dry soils may restrict root uptake and result in reduced leaf Mn accumulation. Soil characteristics such as pH, organic matter content, redox conditions, and [Mn] impact the availability of Mn for plant uptake.
Different plant species exhibit varying capacities for leaf [Mn]. Some plant species possess inherent adaptations for higher levels of [Mn], such as Schima superba (Theaceae), a Mn hyperaccumulator that can reach 10,000 mg Mn kg−1 in leaf in a pot experiment (Yang et al. 2008), Larix decidua (Pinaceae), Betula pendula (Betulaceae) and Vaccinium myrtillus (Ericaceae) accumulate 7,000 to 10,000 mg Mn kg−1 in leaf when growing in air-polluted mountain areas (Wildová et al. 2021). Certain plants form specialized roots such as cluster root in Proteaceae and many actinorhizal species and dauciform root in some Cyperaceae which enable them to release root carboxylates in an exudative burst thus efficiently mobilizing soil P to cope with P-impoverished environments in Western Australia, South Africa and South America (Neumann and Martinoia 2002; Shane et al. 2006; Lambers et al. 2015b). Additionally, an increasing number of species without specialized root systems have been reported to release root exudates for soil P acquisition (Table 2); however, it remains largely unknown how widespread this strategy is, globally.
Ericaceae exhibit a symbiosis with ericoid mycorrhizal fungi which is expected to enhance their P-acquisition (Smith et al. 2015). Additionally, the mycorrhizal hyphae can intercept mobilized Mn and prevent its accumulation in leaves (Hashem 1995). Interestingly, we collected 10 Ericaceae species in P-limited Guangxi, eight of them exhibiting relatively high leaf [Mn] ranging from 200 to 360 mg Mn kg−1 compared with the negative references. Similarly, other Ericaceae species have also been observed to accumulate high leaf Mn, such as Vaccinium myrtillus (Wildová et al. 2021), and Leucopogon verticillatus (Zhou et al. 2022). Notably, previous research has shown that Vaccinium species possess the ability to release root carboxylates like oxalate and citrate (Millaleo et al. 2020). Therefore, our findings suggest that symbiotic fungi did not intercept Mn and that they did not occupy a dominant position in the low-P soils, indicating that the symbiosis was possibly ineffective in facilitating plant P uptake. Instead, we propose that these Ericaceae species in Guangxi, which are limited by soil P availability, instead, rely on root carboxylate exudation for their P acquisition, similar to how some Eucalyptus plants that associate with AM and ECM fungi release root carboxylates to acquire P in severely P-depleted habitats in southwestern Australia (Zhou et al. 2022). These findings suggest that further research on leaf Mn dynamics along with investigations on mycorrhizal symbioses and root exudation is warranted, globally.
By integrating field observations (Lambers et al. 2021; Zhou et al. 2022), controlled-environment experiments (Pang et al. 2018; Yu et al. 2023b), and molecular analyses, we can gain deeper insights into the impact of environmental factors on leaf [Mn] and its correlation with root exudates. Long-term field studies offer valuable perspectives on the temporal and spatial patterns associated with these processes (Wildová et al. 2021). Molecular techniques such as transcriptomics, proteomics, and metabolomics can unveil the genetic and molecular mechanisms underlying leaf [Mn] and root carboxylate exudation (Sharma and Jha 2023; Yu et al. 2023a). Implementing these strategies will advance our understanding of leaf [Mn] and root carboxylate exudation to acquire deeper insights into plant nutrient-acquisition strategies and their ecological significance.
Conclusion
This study investigated leaf [Mn] in two forest ecosystems characterized by contrasting soil P availability. We observed that plants growing in low-P soils of Guangxi exhibited significantly higher average leaf [Mn] compared with those in relatively P-rich soils of Gansu, despite the fact that Gansu had higher soil [Mn]. By utilizing the same plant family, i.e. Dryopteridaceae, as a negative reference, the results indicated a greater number of species with high leaf [Mn] in low-P soils than in relatively P-rich soils. This trend was further confirmed across different plant families and life forms, suggesting that high leaf [Mn] is more prevalent in low-P soils. Furthermore, we synthesized literature data to demonstrate that a substantial proportion of plants with high leaf [Mn] also release significant amounts of root carboxylates, thereby establishing mature leaf [Mn] as a reliable proxy for root carboxylate release under field conditions. The utilization of root carboxylates for soil P acquisition represents a common strategy exhibited by plants inhabiting P-limited environments.
Data availability
The data that support the findings of this study are available now in [Figshare]: https://figshare.com/s/3cfbdbc16874ab7e9af8.
References
Abbott LK, Robson AD, De Boer G (1984) The effect of phosphorus on the formation of hyphae in soil by thevesicular-arbuscular mycorrhizal fungus, Glomus Fasciculatum. New Phytol 97:437–446. https://doi.org/10.1111/j.1469-8137.1984.tb03609.x
Albornoz FE, Burgess TI, Lambers H et al (2017) Native soilborne pathogens equalize differences in competitive ability between plants of contrasting nutrient-acquisition strategies. J Ecol 105:549–557. https://doi.org/10.1111/1365-2745.12638
Ames BN (1966) Assay of inorganic phosphate, total phosphate and phosphatases. In: Neufeld E, Ginsburg V (eds) Methods in enzymology, vol 8. Academic Press, New York, pp 115–118
Aoki M, Fujii K, Kitayama K (2012) Environmental control of root exudation of low-molecular weight organic acids in tropical Rainforests. Ecosystems 15:1194–1203. https://doi.org/10.1007/s10021-012-9575-6
Bai K, Wei Y, Zhang D et al (2020) Contrasting effects of light, soil chemistry and phylogeny on leaf nutrient concentrations in cave-dwelling plants. Plant Soil 448:105–120. https://doi.org/10.1007/s11104-020-04422-6
Barben SA, Hopkins BG, Jolley VD et al (2010) Phosphorus and manganese interactions and their relationships with zinc in chelator-buffered solution grown russet burbank potato. J Plant Nutr 33:752–769. https://doi.org/10.1080/01904160903575964
Baxter IR, Vitek O, Lahner B, et al (2008). The leaf ionome as a multivariable system to detect a plant's physiological status. Proc Natl Acad Sci U S A 105:12081–12086. https://doi.org/10.1073/pnas.0804175105
Bentley D, Grierson PF, Bennett LT, Adams MA (1999) Evaluation of anion exchange membranes to estimate bioavailable phosphorus in native grasslands of semi-arid Northwestern Australia. Commun Soil Sci Plant Anal 30:2231–2244. https://doi.org/10.1080/00103629909370368
Bolan NS, Robson AD, Barrow NJ (1984) Increasing phosphorus supply can increase the infection of plant roots by vesicular-arbuscular mycorrhizal fungi. Soil Biol Biochem 16:419–420. https://doi.org/10.1016/0038-0717(84)90043-9
Branzanti MB, Rocca E, Pisi A (1999) Effect of ectomycorrhizal fungi on chestnut ink disease. Mycorrhiza 9:103–109. https://doi.org/10.1007/s005720050007
Chao L, Liu Y, Zhang W et al (2023) Root functional traits determine the magnitude of the rhizosphere priming effect among eight tree species. Oikos 2023:1–14. https://doi.org/10.1111/oik.09638
Cornara L, Roccotiello E, Minganti V et al (2007) Level of trace elements in Pteridophytes growing on serpentine and metalliferous soils. J Plant Nutr Soil Sci 170:781–787. https://doi.org/10.1002/jpln.200720099
Deng L, Luo L, Li Y et al (2023) Autotoxic ginsenoside stress induces changes in root exudates to recruit the beneficial Burkholderia Strain B36 as revealed by transcriptomic and metabolomic approaches. J Agric Food Chem 71:4536–4549. https://doi.org/10.1021/acs.jafc.3c00311
Du E, Terrer C, Pellegrini AFA et al (2020) Global patterns of terrestrial nitrogen and phosphorus limitation. Nat Geosci 13:221–226. https://doi.org/10.1038/s41561-019-0530-4
Escolà Casas M, Matamoros V (2021) Analytical challenges and solutions for performing metabolomic analysis of root exudates. Trends Environ Anal Chem 31:e00130. https://doi.org/10.1016/j.teac.2021.e00130
Gille CE, Finnegan PM, Hayes PE et al (2024) Facilitative and competitive interactions between mycorrhizal and nonmycorrhizal plants in an extremely phosphorus-impoverished environment: role of ectomycorrhizal fungi and native oomycete pathogens in shaping species coexistence. New Phytol. https://doi.org/10.1111/nph.19489
Grosjean N, Blaudez D, Chalot M et al (2019) Identification of new hardy ferns that preferentially accumulate light rare earth elements: a conserved trait within fern species. Environ Chem 17:191–200. https://doi.org/10.1071/EN19182
Guillemin J-P, Gianinazzi S, Gianinazzi-Pearson V, Marchal J (1994) Contribution of arbuscular mycorrhizas to biological protection of micropropagated pineapple (Ananas comosus (L.) Merr) against Phytophthora cinnamomi Rands. Agric Food Sci 3:241–251. https://doi.org/10.23986/afsci.72702
Güsewell S, Schroth MH (2017) How functional is a trait? Phosphorus mobilization through root exudates differs little between Carex species with and without specialized dauciform roots. New Phytol 215:1438–1450. https://doi.org/10.1111/nph.14674
Hashem AR (1995) The role of mycorrhizal infection in the resistance of Vaccinium macrocarpon to manganese. Mycorrhiza 5:289–291. https://doi.org/10.1007/BF00204964
Hou E, Luo Y, Kuang Y et al (2020) Global meta-analysis shows pervasive phosphorus limitation of aboveground plant production in natural terrestrial ecosystems. Nat Commun 11:1–9. https://doi.org/10.1038/s41467-020-14492-w
Huang G, Hayes PE, Ryan MH et al (2017) Peppermint trees shift their phosphorus-acquisition strategy along a strong gradient of plant-available phosphorus by increasing their transpiration at very low phosphorus availability. Oecologia 185:387–400. https://doi.org/10.1007/s00442-017-3961-x
Jiang Z, Thakur MP, Liu R et al (2022) Soil P availability and mycorrhizal type determine root exudation in sub-tropical forests. Soil Biol Biochem 171:108722. https://doi.org/10.1016/j.soilbio.2022.108722
Kassambara A (2023) rstatix: pipe-friendly framework for basic statistical tests. R Package version 0.7.2. https://CRAN.R-project.org/package=rstatix
Kochian LV, Hoekenga OA, Piñeros MA (2004) How do crop plants tolerate acid soils? Mechanisms of aluminum tolerance and phosphorous efficiency. Annu Rev Plant Biol 55:459–493. https://doi.org/10.1146/annurev.arplant.55.031903.141655
Kosakivska IV, Babenko LM, Romanenko KO et al (2021) Molecular mechanisms of plant adaptive responses to heavy metals stress. Cell Biol Int 45:258–272. https://doi.org/10.1002/cbin.11503
Lai Y, Tang S, Lambers H et al (2024) Global change progressively increases foliar nitrogen–phosphorus ratios in China’s subtropical forests. Glob Change Biol 30:1–14. https://doi.org/10.1111/gcb.17201
Lambers H (2022) Phosphorus acquisition and utilization in plants. Annu Rev Plant Biol 73:17–42. https://doi.org/10.1146/annurev-arplant-102720-125738
Lambers H, Clements JC, Nelson MN (2013) How aphosphorus-acquisition strategy based on carboxylate exudation powers the success and agronomic potential of lupines (Lupinus, Fabaceae). Am J Bot 100:263–288. https://doi.org/10.3732/ajb.1200474
Lambers H, Hayes PE, Laliberté E et al (2015a) Leaf manganese accumulation and phosphorus-acquisition efficiency. Trends Plant Sci 20:83–90. https://doi.org/10.1016/j.tplants.2014.10.007
Lambers H, Martinoia E, Renton M (2015b) Plant adaptations to severely phosphorus-impoverished soils. Curr Opin Plant Biol 25:23–31. https://doi.org/10.1016/j.pbi.2015.04.002
Lambers H, Wright IJ, Guilherme Pereira C et al (2021) Leaf manganese concentrations as a tool to assess belowground plant functioning in phosphorus-impoverished environments. Plant Soil 461:43–61. https://doi.org/10.1007/s11104-020-04690-2
Lambers H, de Britto CP, Cawthray GR et al (2022) Strategies to acquire and use phosphorus in phosphorus-impoverished and fire-prone environments. Plant Soil 476:133–160. https://doi.org/10.1007/s11104-022-05464-8
Lambers H (2023) Chapter 17 - nutrient-use efficiency. In: Rengel Z, Cakmak I, White P (eds) Marschner’s Mineral Nutrition of Plants, 4th edn. Academic Press, San Diego, pp 651–66
Li MS, Luo YP, Su ZY (2007) Heavy metal concentrations in soils and plant accumulation in a restored manganese mineland in Guangxi, South China. Environ Pollut 147:168–175. https://doi.org/10.1016/j.envpol.2006.08.006
Li H, Liu J, Li G et al (2015) Past, present, and future use of phosphorus in Chinese agriculture and its influence on phosphorus losses. Ambio 44:274–285. https://doi.org/10.1007/s13280-015-0633-0
Li Y, Sun L, Zhu B (2022) Trade-offs among fine-root phosphorus-acquisition strategies of 15 tropical woody species. Forest Ecosystems 9:100055. https://doi.org/10.1016/j.fecs.2022.100055
Li J, Wu B, Zhang D, Cheng X (2023) Elevational variation in soil phosphorus pools and controlling factors in alpine areas of Southwest China. Geoderma 431:116361. https://doi.org/10.1016/j.geoderma.2023.116361
Liu K, Zhang H, Liu Y et al (2020) Investigation of plant species and their heavy metal accumulation in manganese mine tailings in Pingle Mn mine, China. Environ Sci Pollut Res 27:19933–19945. https://doi.org/10.1007/s11356-020-08514-9
Liu F, Wu H, Zhao Y et al (2022) Mapping high resolution national soil information grids of China. Science Bulletin 67:328–340. https://doi.org/10.1016/j.scib.2021.10.013
Losfeld G, L’Huillier L, Fogliani B et al (2015) Leaf-age and soil-plant relationships: key factors for reporting trace-elements hyperaccumulation by plants and design applications. Environ Sci Pollut Res 22:5620–5632. https://doi.org/10.1007/s11356-014-3445-z
Lu WZ (2006) Research on plant diversity in maiji mountain scenic and historic spot. Master thesis. Northwest Agriculture and Forestry University
Luo L, Zhang J, Ye C et al (2022) Foliar pathogen infection manipulates soil health through root exudate-modified rhizosphere microbiome. Microbiology Spectrum 10:e02418-e2422. https://doi.org/10.1128/spectrum.02418-22v
Ma X, Li X, Zou J et al (2021) Effects of Crucibulum laeve inoculation on metabolome in root exudate from Salix viminalis L. Forest Res 34:46–55. https://doi.org/10.13275/j.cnki.lykxyj.2021.03.005
Meier IC, Brunner I, Godbold DL et al (2019) Roots and rhizospheres in forest ecosystems: recent advances and future challenges. For Ecol Manage 431:1–5. https://doi.org/10.1016/j.foreco.2018.08.005
Memon AR, Yatazawa M (1984) Nature of manganese complexes in manganese accumulator plant - acanthopanax sciadophylloides. J Plant Nutr 7:961–974. https://doi.org/10.1080/01904168409363257
Migliavacca M, Musavi T, Mahecha MD et al (2021) The three major axes of terrestrial ecosystem function. Nature 598:468–472. https://doi.org/10.1038/s41586-021-03939-9
Millaleo R, Reyes-Díaz M, Ivanov AG et al (2010) Manganese as essential and toxic element for plants: transport, accumulation and resistance mechanisms. J Soil Sci Plant Nutr 10:476–494. https://doi.org/10.4067/s0718-95162010000200008
Millaleo R, Alvear M, Aguilera P et al (2020) Mn toxicity differentially affects physiological and biochemical features in highbush Blueberry (Vaccinium corymbosum L.) cultivars. J Soil Sci Plant Nutr 20:795–805. https://doi.org/10.1007/s42729-019-00166-0
Miura S, Amacher M, Hofer T et al (2015) Protective functions and ecosystem services of global forests in the past quarter-century. Forest Ecolgy and Management 352:35–46. https://doi.org/10.1016/j.foreco.2015.03.039
Nakib D, Slatni T, Di Foggia M et al (2021) Changes in organic compounds secreted by roots in two Poaceae species (Hordeum vulgare and Polypogon monspenliensis) subjected to iron deficiency. J Plant Res 134:151–163. https://doi.org/10.1007/s10265-020-01237-5
Neumann G, Martinoia E (2002) Cluster roots – an underground adaptation for survival in extreme environments. Trends Plant Sci 7:162–167. https://doi.org/10.1016/S1360-1385(02)02241-0
Neumann G, Römheld V (1999) Root excretion of carboxylic acids and protons in phosphorus-deficient plants. Plant Soil 211:121–130. https://doi.org/10.1023/A:1004380832118
Nottingham AT, Cheesman AW, Riutta T et al (2022) Large contribution of recent photosynthate to soil respiration in tropical dipterocarp forest revealed by girdling. J Ecol 110:387–403. https://doi.org/10.1111/1365-2745.13806
Oburger E, Jones DL (2018) Sampling root exudates – mission impossible? Rhizosphere 6:116–133. https://doi.org/10.1016/j.rhisph.2018.06.004
Olivares E, Peña E, Aguiar G (2002) Metals and oxalate in Tithonia diversifolia (Asteraceae): concentrations in plants growing in contrasting soils, and Al induction of oxalate exudation by roots. J Plant Physiol 159:743–749. https://doi.org/10.1078/0176-1617-0751
Olsen S, Sommers L (1982) Phosphorus. In: Miller R, Keeney D (eds) Methods of soil analysis, 2nd edn. Soil Science Society of America, Madison, pp 403–430
Osaro-Matthew RC, Ire FS, Frank-Peterside N (2020) Screening of actinomycetes from turmeric (Curcuma longa L.) and ginger (Zingiber officinale) rhizosphere for antifungal activity. J Adv Microbiol 18–28. https://doi.org/10.9734/jamb/2020/v20i230214
Page V, Feller U (2005) Selective transport of zinc, manganese, nickel, cobalt and cadmium in the root system and transfer to the leaves in young wheat plants. Ann Bot 96:425–434. https://doi.org/10.1093/aob/mci189
Page V, Weisskopf L, Feller U (2006) Heavy metals in white lupin: uptake, root-to-shoot transfer and redistribution within the plant. New Phytol 171:329–341. https://doi.org/10.1111/j.1469-8137.2006.01756.x
Pang J, Bansal R, Zhao H et al (2018) The carboxylate-releasing phosphorus-mobilizing strategy can be proxied by foliar manganese concentration in a large set of chickpea germplasm under low phosphorus supply. New Phytol 219:518–529. https://doi.org/10.1111/nph.15200
Pedas P, Husted S, Skytte K, Schjoerring JK (2011) Elevated phosphorus impedes manganese acquisition by barley plants. Front Plant Sci 2:1–12. https://doi.org/10.3389/fpls.2011.00037
Pinheiro JC, Bates DM (2000) Mixed-effects models in S and S-PLUS. Springer, New York
Pinson JB, Chambers SM, Nitta JH et al (2017) The separation of generations: biology and biogeography of long-lived sporophyteless fern gametophytes. Int J Plant Sci 178:1–8. https://doi.org/10.1086/688773
Playsted CWS, Johnston ME, Ramage CM et al (2006) Functional significance of dauciform roots: exudation of carboxylates and acid phosphatase under phosphorus deficiency in Caustis blakei (Cyperaceae). New Phytol 170:491–500. https://doi.org/10.1111/j.1469-8137.2006.01697.x
R Core Team (2020) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Reimann C, Arnoldussen A, Boyd R et al (2007) Element contents in leaves of four plant species (birch, mountain ash, fern and spruce) along anthropogenic and geogenic concentration gradients. Sci Total Environ 377:416–433. https://doi.org/10.1016/j.scitotenv.2007.02.011
Roelofs RF, Rengel Z, Cawthray GR et al (2001) Exudation of carboxylates in Australian Proteaceae: chemical composition. Plant Cell Environ 24:891–904. https://doi.org/10.1046/j.1365-3040.2001.00741.x
Rünk K, Zobel K (2007) Phenotypic plasticity and biomass allocation pattern in three Dryopteris (Dryopteridaceae) species on an experimental light-availability gradient. Plant Ecol 193:85–99. https://doi.org/10.1007/s11258-006-9250-0
Ruxton GD (2006) The unequal variance t-test is an underused alternative to student’s t-test and the Mann-Whitney U test. Behav Ecol 17:688–690. https://doi.org/10.1093/beheco/ark016
Ryan MH, Tibbett M, Edmonds-Tibbett T et al (2012) Carbon trading for phosphorus gain: the balance between rhizosphere carboxylates and arbuscular mycorrhizal symbiosis in plant phosphorus acquisition. Plant Cell Environ 35:2170–2180. https://doi.org/10.1111/j.1365-3040.2012.02547.x
Sandnes A, Eldhuset TD, Wollebæk G (2005) Organic acids in root exudates and soil solution of Norway spruce and silver birch. Soil Biol Biochem 37:259–269. https://doi.org/10.1016/j.soilbio.2004.07.036
Schmitt M, Mehltreter K, Sundue M et al (2017) The evolution of aluminum accumulation in ferns and lycophytes. Am J Bot 104:573–583. https://doi.org/10.3732/ajb.1600381
Shane MW, Lambers H (2005) Manganese accumulation in leaves of Hakea prostrata (Proteaceae) and the significance of cluster roots for micronutrient uptake as dependent on phosphorus supply. Physiol Plant 124:441–450. https://doi.org/10.1111/j.1399-3054.2005.00527.x
Shane MW, Cawthray GR, Cramer MD et al (2006) Specialized “dauciform” roots of Cyperaceae are structurally distinct, but functionally analogous with “cluster” roots. Plant, Cell Environ 29:1989–1999. https://doi.org/10.1111/j.1365-3040.2006.01574.x
Sharma D, Jha SR (2023) Current understanding of genomics, transcriptomics, proteomics, and metabolomics of plants upon heavy metal stress. In: Husn A, Ahmad A (eds) Genomics, transcriptomics, proteomics and metabolomics of crop plants. Academic Press, Chapter 15, pp 327–338. https://doi.org/10.1016/C2021-0-02329-4
Silver WL, Brown S, Lugo AE (1996) Effects of changes in biodiversity on ecosystem function in tropical forests. Conserv Biol 10:17–24. https://doi.org/10.1046/j.1523-1739.1996.10010017.x
Sims JT (1986) Soil pH effects on the distribution and plant availability of manganese, copper, and zinc. Soil Sci Soc Am J 50:367–373. https://doi.org/10.2136/sssaj1986.03615995005000020023x
Smith ES, Anderson CI, Smith FA (2015) Mycorrhizal associations and phosphorus acquisition: from cells to ecosystems. In: Plaxton WC, Lambers H (eds) Annual plant reviews volume 48: phosphorus metabolism in plants. John Wiley & Sons Inc, Hoboken, NJ, pp 409–439
Stewart-Oaten A, Bence JR, Osenberg CW (1992) Assessing effects of unreplicated perturbations: no simple solutions. Ecology 73:1396–1404. https://doi.org/10.2307/1940685
Sun L, Ataka M, Kominami Y, Yoshimura K (2017) Relationship between fine-root exudation and respiration of two Quercus species in a Japanese temperate forest. Tree Physiol 37:1011–1020. https://doi.org/10.1093/treephys/tpx026
Sun L, Ataka M, Han M et al (2021) Root exudation as a major competitive fine-root functional trait of 18 coexisting species in a subtropical forest. New Phytol 229:259–271. https://doi.org/10.1111/nph.16865
Suo AN, Ju TZ, Ge JP (2008) Relationship between species richness and biomass on environmental gradient in natural forest communities on Mt. Xiaolongshan, northwest China. Forestry Study in China 10:212–219. https://doi.org/10.1007/s11632-008-0041-7
Sytar O, Ghosh S, Malinska H et al (2021) Physiological and molecular mechanisms of metal accumulation in hyperaccumulator plants. Physiol Plant 173:148–166. https://doi.org/10.1111/ppl.13285
Taye FA, Folkersen MV, Fleming CM et al (2021) The economic values of global forest ecosystem services: A meta-analysis. Ecol Econ 189:107145. https://doi.org/10.1016/j.ecolecon.2021.107145
Treseder KK, Allen MF (2002) Direct nitrogen and phosphorus limitation of arbuscular mycorrhizal fungi: a model and field test. New Phytol 155:507–515. https://doi.org/10.1046/j.1469-8137.2002.00470.x
Tudi M, Li H, Li H et al (2022) Evaluation of soil nutrient characteristics in Tianshan Mountains. North-Western China Ecological Indicators 143:109431. https://doi.org/10.1016/j.ecolind.2022.109431
Valipour M, Khoshgoftarmanesh AH, Baninasab B (2018) Physiological responses of hawthorn (Crataegus persica Pojark.) and quince (Cydonia oblonga Mill.) rootstocks to bicarbonate-induced iron deficiency in nutrient solution. J Plant Nutr Soil Sci 181:905–913. https://doi.org/10.1002/jpln.201700576
Wang Y, Lambers H (2020) Root-released organic anions in response to low phosphorus availability: recent progress, challenges and future perspectives. Plant Soil 447:135–156. https://doi.org/10.1007/s11104-019-03972-8
Wang H, Sun X, Chen D et al (2012) Changes of soil physical and chemical properties at different developmental stages of Larix kaempferi plantations in Xiaolongshan, Gansu Province. For Res 25:294–301
Wang Z, Deng Q, Su Y (2016) Habitat soil properties of the natural population of Pseudotaxus chienii. Ecol Sci 35:208–213
Wang Y, Wang R, Lu B et al (2021) Mycorrhization of Quercus mongolica seedlings by Tuber melanosporum alters root carbon exudation and rhizosphere bacterial communities. Plant Soil 467:391–403. https://doi.org/10.1007/s11104-021-05112-7
Watanabe T, Osaki M (2002) Role of organic acids in aluminum accumulation and plant growth in Melastoma malabathricum. Tree Physiol 22:785–792. https://doi.org/10.1093/treephys/22.11.785
Watson JEM, Evans T, Venter O et al (2018) The exceptional value of intact forest ecosystems. Nat Ecol Evol 2:599–610. https://doi.org/10.1038/s41559-018-0490-x
Wen Y, Yuan C, Li X et al (1998) Development of species diversity in vegetation restoration process in mid-mountain region of Damingshan, Guangxi. Acta Phytoecol Sin 22:33–40
Wildová E, Elznicová J, Kula E (2021) Seasonal dynamics of manganese accumulation in European larch (Larix decidua Mill.), silver birch (Betula pendula Roth), and bilberry (Vaccinium myrtillus L.) over 10 years of monitoring. Environ Monit Assess 193:612. https://doi.org/10.1007/s10661-021-09415-1
Wissemeier AH, Horst WJ (1992) Effect of light intensity on manganese toxicity symptoms and callose formation in cowpea (Vigna unguiculata (L.) Walp.). Plant Soil 143:299–309. https://doi.org/10.1007/BF00007886
Xiao D, Che R, Liu X et al (2019) Arbuscular mycorrhizal fungi abundance was sensitive to nitrogen addition but diversity was sensitive to phosphorus addition in karst ecosystems. Biol Fertil Soils 55:457–469. https://doi.org/10.1007/s00374-019-01362-x
Yan L, Wen Y, Zhou X et al (2023) Adding Castanopsis hystrix to a Pinus massoniana plantation changed leaf phosphorus and nitrogen investment and soil nitrogen concentrations. Plant Soil. https://doi.org/10.1007/s11104-023-06097-1
Yang J, Xie B, Wang T, Erastus M-M (2023) Identification and optimization strategy of ecological security pattern in Maiji District of Gansu, China. Ecol Indic 157:111309. https://doi.org/10.1016/j.ecolind.2023.111309
Yang SX, Deng H, Li MS (2008) Manganese uptake and accumulation in a woody hyperaccumulator, Schima superba. Plant Soil Environ 54:441–446. https://doi.org/10.17221/401-pse
Yu G, Ullah H, Wang X et al (2023a) Integrated transcriptome and metabolome analysis reveals the mechanism of tolerance to manganese and cadmium toxicity in the Mn/Cd hyperaccumulator Celosia argentea Linn. J Hazard Mater 443:130206. https://doi.org/10.1016/j.jhazmat.2022.130206
Yu RP, Su Y, Lambers H et al (2023b) A novel proxy to examine interspecific phosphorus facilitation between plant species. New Phytol 239:1637–1650. https://doi.org/10.1111/nph.19082
Zhang C, Tian H, Liu J et al (2005) Pools and distributions of soil phosphorus in China. Glob Biogeochem Cycles 19:1–8. https://doi.org/10.1029/2004GB002296
Zhang Y-W, Guo Y, Tang Z et al (2021) Patterns of nitrogen and phosphorus pools in terrestrial ecosystems in China. Earth Syst Sci Data 13:5337–5351. https://doi.org/10.5194/essd-13-5337-2021
Zhao K, Wu Y (2014) Rhizosphere calcareous soil P-extraction at the expense of organic carbon from root-exuded organic acids induced by phosphorus deficiency in several plant species. Soil Sci Plant Nutr 60:640–650. https://doi.org/10.1080/00380768.2014.934191
Zhao M, Cong J, Cheng J et al (2020) Soil microbial community assembly and interactions are constrained by nitrogen and phosphorus in broadleaf forests of Southern China. Forests 11:1–12. https://doi.org/10.3390/f11030285
Zhao FJ, Tang Z, Song JJ et al (2022) Toxic metals and metalloids: Uptake, transport, detoxification, phytoremediation, and crop improvement for safer food. Mol Plant 15:27–44. https://doi.org/10.1016/j.molp.2021.09.016
Zhou X, Wen Y, Goodale UM et al (2017) Optimal rotation length for carbon sequestration in Eucalyptus plantations in subtropical China. New Forest 48:609–627. https://doi.org/10.1007/s11056-017-9588-2
Zhou XM, Ranathunge K, Cambridge ML et al (2022) A cool spot in a biodiversity hotspot: why do tall Eucalyptus forests in Southwest Australia exhibit low diversity? Plant Soil 476:669–688. https://doi.org/10.1007/s11104-022-05559-2
Zhu Y, Yan F, Zörb C, Schubert S (2005) A link between citrate and proton release by proteoid roots of white lupin (Lupinus albus L.) grown under phosphorus-deficient conditions? Plant Cell Physiol 46:892–901. https://doi.org/10.1093/pcp/pci094
Zhu H, Li YQ, Wen YG et al (2011) The dynamics of the structure and plant species diversity of evergreen broadleaved forests in Damingshan National Nature Reserve after a severe ice storm damage in 2008. Acta Ecol Sin 31:5571–5577
Acknowledgements
We thank Gansu Forestry Technological College, Guangxi Damingshan National Nature Reserve Administration Bureau, Yanlin Zhang, Juyuan Wang, Keming Pan, Xiongwen Yu, Wei Wang and Jinqi Zhang for the assistance in plant identification and sample collection.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. This study was financially supported by funds from Natural Science Foundation of Gansu Province, China (22JR5RA530) and the Fundamental Research Funds for the Central Universities (lzujbky-2021-pd07), Li Yan was supported by the International Postdoctoral Exchange Fellowship Program (PC2022030) by the Office of China Postdoctoral Council.
Author information
Authors and Affiliations
Contributions
Hans Lambers and Li Yan conceived the study. Li Yan and Dan Tang carried out the lab analyses. Li Yan finished statistical analyses and wrote the first draft of the manuscript, Hans Lambers, Jiayin Pang and Dan Tang contributed to revisions.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Additional information
Responsible Editor: Tim S. George.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yan, L., Tang, D., Pang, J. et al. Root carboxylate release is common in phosphorus-limited forest ecosystems in China: using leaf manganese concentration as a proxy. Plant Soil (2024). https://doi.org/10.1007/s11104-024-06791-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11104-024-06791-8