Abstract
Although cyanobacteria have specialized for a photolithoautotrophic mode of life during evolution many cyanobacterial strains have been identified as being capable of photoheterotrophy or even chemoheterotrophy. The mutant strain of Synechocystis sp. PCC 6803, which lacks the gtr gene coding for the strain’s glucose/fructose permease, has been believed to be a strict photolithoautotroph in the past as it has lost the wild type’s facility to use external glucose for both photoheterotrophy and light-induced chemoheterotrophy. However, recent experiments revealed the strain’s capacity to use fructose for mixotrophic and photoheterotrophic growth, a sugar which is toxic for the wild type. Both the growth rate and the amount of fructose incorporated into the cells increased along with the fructose concentrations in the surrounding medium. Furthermore an increase of the total carbon mass of the cells within a liquid culture over a period of photoheterotrophic growth could be demonstrated. Contrary to the wild type, glucose could not be used for photoheterotrophic growth, and chemoheterotrophic growth failed with fructose as well as with glucose.
Graphic abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The major interest in cyanobacteria rests on their capacity for oxygenic photosynthesis. This process has been acquired later in evolution by some eukaryotes caused by endosymbiosis with cyanobacteria-like prokaryotes [1]. Characteristic for cyanobacteria is the photolithoautotrophic growth mode using light as their energy source, water as their electron source, and carbon dioxide as their carbon source.
Although many cyanobacteria are strict photolithoautotrophs unable to use other carbon compounds than CO2 as their sole carbon source, some have the capacity for photoheterotrophy and some can even grow chemoheterotrophically [2,3,4]. In some cases the inability to grow heterotrophically may be due to the incapacity for taking up organic substrates (for a review see [5]). For instance Synechocystis sp. PCC 6803 loses its capacity for both photoheterotrophic [2, 3] and light-activated chemoheterotrophic growth [6] on glucose when the gtr gene encoding the glucose transporter [7] is knocked out [8, 9]. When this gene was introduced into Synechococcus sp. PCC 7942 on a self-replicating plasmid, the new transgenic strain acquired the capacity for photoheterotrophic growth on glucose; however, this plasmid could not be maintained stably [10]. Integration of the gtr gene into the chromosome of PCC 7942 led to a strain for which glucose was highly toxic [10]. Similarly glucose was toxic for Anabaena sp. PCC 7120 if the Synechocystis gtr gene was introduced on a stably replicating plasmid [11].
Wolk and Shaffer [12] demonstrated that Anabaena sp. ATCC 29413 can grow in permanent darkness if fructose is added to the growth medium. Ungerer et al. [13] introduced the gene locus frtRABC encoding the transport system for fructose from Anabaena sp. ATCC 29413 into the closely related Anabaena sp. strain PCC 7120, which had been characterized earlier as strictly photolithoautotrophic [3]. The resulting derivative of Anabaena sp. PCC 7120 acquired the capacity for chemoheterotrophic growth on fructose [13]. Later the strain was discovered to grow without any genetic modification both photoheterotrophically and chemoheterotrophically on fructose, if very high concentrations (50–200 mM) were added to the growth medium [11]. These results led us to study the behaviour of a mutant strain of Synechocystis sp. strain PCC 6803, whose gtr gene encoding the glucose/fructose permease had been deleted [7]. Strain PCC 6803 gtr− exhibited both mixotrophic and photoheterotrophic growth dependent on high concentrations of fructose.
Results and discussion
Synechocystis sp. PCC 6803 gtr − can grow mixotrophically and photoheterotrophically on high concentrations of fructose
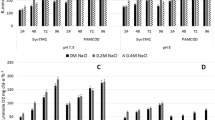
We tested Synechocystis sp. strain PCC 6803 gtr− [7], which had been considered to be an obligate photolithoautotroph prior to the results described in this paper. Strain PCC 6803 gtr− was cultivated at various concentrations of fructose (50 mM, 100 mM, and 200 mM) in the presence (photoheterotrophic conditions) as well as in the absence of 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU) (mixotrophic conditions). DCMU blocks the electron transfer from photosystem II to the quinone pool thereby preventing oxygenic photosynthesis [14,15,16]. In addition to OD730 values (Figs. 1a, 2a) we also measured the chlorophyll concentration/cm3 culture (Figs. 1b, 2b) as a further control to exclude the possibility that the long durations of the experiments as well as the high sugar concentrations may enhance the danger that the cultures have turned xenic.
Photoheterotrophic growth of Synechocystis sp. PCC 6803 gtr− (a) OD730 values, (b) chlorophyll concentration: blue diamond: 0 mM, red square: 50 mM, green triangle: 100 mM, violet cross mark: 200 mM fructose, the bars indicate the deviation within the triplicate of measurements (color figure online)
Both mixotrophic (Fig. 1) and photoheterotrophic growth (Fig. 2, Tables 1S and 2S) were found with high concentrations of fructose, which indicates the strain’s capacity to grow in the absence of oxygenic photosynthesis. DCMU-resistant mutants in diverse cyanobacterial strains have been known for a long time [15, 17]; however, in the photoheterotrophic experiments, no growth occurred in the negative control, where DCMU but not fructose had been added (Fig. 2, Tables 1S and 2S).
In contrast to fructose 200 mM glucose did not support mixotrophic or photoheterotrophic growth. Since Anabaena sp. PCC 7120 can grow in permanent darkness dependent on high concentrations of fructose [11] we tested whether Synechocystis sp. PCC 6803 gtr− is able to grow in the dark on 200 mM glucose or fructose. No growth at all was observed for both sugars either in complete darkness or at a daily illumination of 5 min. The latter mode has been discovered to be essential for chemoheterotrophic growth of Synechocystis sp. strain PCC 6803 wild type dependent on glucose [6]. Nieves-Morion and Flores [18] have identified five genes encoding ABC sugar transporter components in PCC 7120 and single mutations in each of them resulted in a reduction of mixotrophic growth dependent on fructose. No homologous genes are present in Synechocystis sp. PCC 6803.
Since low concentrations (10 mM) of fructose are toxic for Synechocystis sp. PCC 6803 wild type [8], the concentrations that enabled photoheterotrophic growth of strain PCC 6803 gtr− (Fig. 2) were also tested on Synechocystis sp. PCC 6803 wild type both in the presence and absence of DCMU. Under none of the tested conditions the cells could survive.
Fructose is taken up by Synechocystis sp. PCC 6803 gtr −
The uptake of radioactively labelled fructose by Synechocystis sp. PCC 6803 gtr− was tested at different external fructose concentrations (1 mM, 10 mM, and 100 mM). The strain took up fructose from the surrounding medium and incorporated it inside the cells (Fig. 3a). A higher fructose concentration in the medium also increased the amount of fructose taken up by strain PCC 6803 gtr−, although the increase of uptake was not proportional to the surrounding concentrations (Fig. 3a). Although glucose did not stimulate photoheterotrophic growth in PCC 6803 gtr−, it was taken up roughly 8 times faster compared to fructose (Fig. 3b, c). Therefore, the growth supporting effect of fructose but not of glucose is rather caused by metabolic instead of uptake mechanisms.
Uptake of a fructose, b glucose and c fructose compared with glucose under different external sugar concentrations by Synechocystis sp. PCC 6803 gtr−; light blue diamond: 1 mM fructose, blue square: 10 mM fructose, dark blue triangle: 100 mM fructose, light red diamond: 1 mM glucose, red square: 10 mM glucose, dark red triangle: 100 mM glucose, the bars indicate the deviation within the triplicate of measurements (color figure online)
According to these data an unidentified carrier protein must allow the import through the cytoplasmic membrane, since large polar molecules like fructose cannot pass the cytoplasmic membrane without such a transporter. The possibility that simple diffusion is the way of entry into the cells can also be excluded, because for simple diffusion the uptake rate of fructose should increase proportionally to the concentration in the surrounding medium, which is not the case (Fig. 3a). This fact rather indicates an import of this sugar by a specific transporter protein. The total sequence of PCC 6803 does not contain a sugar transporter beyond the gtr gene so that the transporter(s) responsible for the entry of fructose and/or glucose into Synechocystis sp. PCC 6803 gtr− may have another primary function. Niederholtmeyer et al. [19] demonstrated that the glucose importer Glf of Zymomonas mobilis [20] acted as an exporter within Synechococcus sp. PCC 7942 if the intracellular glucose concentration was artificially increased. Therefore fructose may be imported as well by an actual exporter for the carbohydrate portion of LPS (lipopolysaccharides of the outer membrane), if there is an excess of a similar substrate like fructose in the surrounding medium.
It is currently unknown, why the entering fructose molecule cannot be used for dark growth of Synechocystis sp. PCC 6803 gtr− as it is the case for Anabaena sp. PCC 7120. A number of instances is known from the literature [10, 19, 21], where the entry of a sugar into a cyanobacterium using different transporters leads to drastically different effects (growth or death). For example Anabaena sp. PCC 7120 wild type can utilize 200 mM fructose and to a much lesser extent 200 mM glucose for mixotrophic growth, while 200 mM fructose and even 5 mM glucose are toxic for PCC 7120 mutant strain containing the gtr gene from Synechocystis sp. PCC 6803 on a stably self-replicating plasmid [11].
The glucose carrier Gtr from Synechocystis sp. PCC 6803 has been identified as interacting with glucose and to a lesser extent with fructose [8]. Inactivation of the gtr gene in PCC 6803 abolished both the wild type’s capacity for photoheterotrophic growth and light-activated chemoheterotrophic growth on glucose and its sensitivity towards fructose [8, 9, 22]. In contrast to the PCC 6803 wild type, which was even a little sensitive against its only known heterotrophic substrate glucose under mixotrophic conditions [8], no such characteristics could be observed for fructose on strain PCC 6803 gtr−. Concentrations of fructose, which supported photoheterotrophy (Fig. 2), also allowed mixotrophy in PCC 6803 gtr− (Fig. 1).
Photoheterotrophic growth is due to fructose as carbon source
To rule out the possibility that any contaminant of commercially available fructose is the source for the growth of Synechocystis sp. PCC 6803 gtr− the fructose used was analysed by HPLC/ESI–MS. The data revealed that the fructose used contained a contamination of 0.16% glucose. Beside glucose no further organic contaminants in significant amounts were found.
To exclude the possibility that photoheterotrophic growth is supported by the detected glucose contamination the increase of the total carbon mass in the cells during photoheterotrophic growth was measured applying elemental analysis. Several cultures of Synechocystis sp. PCC 6803 gtr− were inoculated with the same density in pure BG11T medium. After they had reached an OD730 between 0.8 and 0.9 200 mM fructose and DCMU were added. For half of these cultures the absolute carbon amount present in the cells was determined immediately after fructose/DCMU had been added, while the other cultures grew further under photoheterotrophic conditions. The carbon amount present in their cells was measured 10 days later when they had reached an OD730 between 4.1 and 4.2 (for details see “Experimental”). The dry masses of each culture, the % and the absolute mass of carbon are listed in Table 1.
The amount of carbon incorporated into the cells during 10 days of photoheterotrophic growth can be calculated as the difference between the carbon present in the cells immediately after the addition of fructose and DCMU (4.19 mg) and the carbon present in the cells after 10 days (15.77 mg), which means that 11.58 mg carbon must have been incorporated from the surrounding medium. The 200 mM fructose present in 50 cm3 medium contains 60 mmoles or 720 mg carbon, which are much more than the incorporated 11.58 mg. The glucose added as contaminant in each culture contains 0.096 mmoles or 1.152 mg carbon, while the BG11 medium [3] also contains a total of 0.0538 mM citric acid + citrate, which corresponds to 0.016 mmoles or 0.194 mg carbon in the photoheterotrophic cultures. Together, the glucose contamination, the citric acid and the citrate contain 1.346 mg carbon in the surrounding medium and therefore cannot supply the 11.58 mg carbon incorporated into the cells. As a consequence the photoheterotrophic growth must be due to fructose.
Conclusion
Despite having been classified as a strict photolithoautotroph for decades, Synechocystis sp. PCC 6803 gtr− can use fructose for mixotrophic as well as for photoheterotrophic growth and fructose is able to enter the cells of this mutant strain. Combining these results with previous research about Anabaena sp. PCC 7120 we believe that more cyanobacteria than hitherto believed are facultative (photo)heterotrophs and among these strains fructose may be a widespread substrate.
Experimental
Cyanobacterial strains used
-
Synechocystis sp. PCC 6803 gtr− [7]
-
Synechocystis sp. PCC 6803 wild type
Cultivation of the strains
PCC 6803 gtr− was cultivated either in 50 cm3 liquid BG11 medium [3] supplemented with 10 mM 2-[[1,3-dihydroxy-2-(hydroxymethyl)propan-2-yl]amino]ethanesulfonic acid (TES) in 100 cm3 Erlenmeyer flasks or on solid plates of 20–30 cm3 BG11 supplemented with 10 mM TES, 3 g/dm3 Na2S2O3·5 H2O and 1.5% Difco bacto-agar in petri dishes. The liquid cultures were shaken at 150 rpm, 32 °C and an average illumination of 50 µE m−2 s−1 in a New Brunswick shaker 2300. The solid cultures were incubated in a Heraeus Vötsch climat chamber at 32 °C. When necessary, 20 µg/cm3 of kanamycin was added to liquid or solid cultures. Photoheterotrophic growth was tested in the presence of 10 µM DCMU and varying concentrations of glucose or fructose. The capacity for mixotrophic growth was tested with glucose or fructose in the absence of DCMU. For experiments concerning chemoheterotrophic growth the Erlenmeyer flasks containing the cultures were wrapped with aluminum foils to prevent exposure to light.
Measurements of growth
Growth was determined by measuring the OD730 as well as the chlorophyll concentration/cm3 culture. For the latter 1 cm3 culture was centrifuged for 10 min at 14,000 rpm. The supernatant was removed and the pellet was resuspended in 1 cm3 methanol. After centrifugation for 10 min at 14,000 rpm the OD665 of the supernatant was determined. To obtain µg chlorophyll/cm3 culture the OD665 values were divided by 0.0745 [23]. Every day samples were taken. All experiments were performed in triplicate and the average values and the standard deviations were calculated.
Measurement of sugar uptake into the cells of Synechocystis sp. PCC 6803 gtr −
Cells were cultivated under photolithoautotrophic conditions until they had reached an OD730 ~ 1.0. They were centrifuged for 10 min at 4000 rpm. The supernatant was removed and the pellet was washed in BG11T medium and centrifuged again for 10 min at 4000 rpm. Then the cells were resuspended in BG11T medium to an OD730 = 1.10 cm3 of this suspension were transferred into a cell chamber, where they were rotated by a magnetic stirrer and permanently illuminated. After an incubation phase of 10 min the required amount of stable glucose or fructose and 50 nCi of uniformly labeled 14C-d-glucose or uniformly labeled 14C-d-fructose from Hartmann Analytic (Braunschweig, Germany) were added. In regular intervals 1 cm3 samples were taken and transferred onto a Whatman nitrocellulose filter (25 mm diameter, pore size 0.45 µm) connected to an oil vacuum pump apparatus. The supernatant was sucked off and the filter with the attached cells was washed immediately afterwards with 5 cm3 BG11T medium. Then the filter was transferred into a scintillation tube and dissolved in 10 cm3 scintillation cocktail (60 g naphthalene and 4 g 2,5-diphenyl-1,3,4-oxadiazol dissolved in 1 dm3 dioxan). As a negative control 10 cm3 scintillation cocktail without a sample were measured. For determination of the total radioactivity 1 cm3 unfiltrated suspension was mixed with 10 cm3 scintillation cocktail in a tube. The samples were evaluated in a Perkin-Elmer Tri-Carb 2800 TR liquid scintillation counter. All experiments were performed in triplicate and the average values and the standard deviations were calculated.
HPLC instrumentation and chromatographic conditions for HPLC/ESI–MS analysis of the fructose reagent
HPLC/ESI–MS analysis was performed on a Dionex HPCL Ultimate 3000 system equipped with LPG3400A low-pressure gradient pump, WPS-3000SL autosampler, TCC3000 thermostatted column compartment and VWD-3400 variable wavelength detector. Separation was performed on a Phenomenex (Torrance, California, United States) column, Rezex RCM-Monosaccharide Ca2+, 300 × 7.80 mm, particle size 8 µm.
The mobile phase was 100% water (VWR Chemicals, manufactured by: VWR international S.A.S. Fontenay-sous-Bois Cedex, France) at a flow rate of 0.6 cm3/min with a split-flow volume of 0.12 cm3/min into the mass spectrometer. Run time was 30 min under isocratic conditions at 80 °C.
Fructose was dissolved in double-distilled water in a concentration of 100 µg/cm3 and 20 mm3 were injected. The separated sugars were detected by mass spectrometer instrument (maXis classic QqTOF, Bruker Daltonik, Bremen, Germany). The scan range was 50–350 Da at a scan rate of 1 Hz. Capillary voltage was 4500 V, nebular gas flow was 1.2 bar, dry gas flow was 6.0 dm3/min N2, and the transfer capillary temperature was at 180 °C.
Measurement of total carbon mass of a culture
Six 50 cm3 cultures of PCC 6803 gtr− were inoculated at an OD730 of ~ 0.1 and grown to an OD730 between 0.8 and 0.9. Then 10 µM DCMU and 200 mM fructose were added. Three of the cultures were immediately harvested, while the other cultures were further grown. The latter were harvested after they had reached an OD730 ~ 4.2. When harvested the cultures were centrifuged for 10 min at room temperature. The pellets were resuspended in 50 cm3 pure H2O and centrifuged once more for 10 min at room temperature. The pellets were resuspended in 1 cm3 pure H2O and centrifuged once more for 10 min at room temperature. All supernatant was removed and the pellets were dried in the vacuum desiccator for 2 h. The amount of carbon in the dried pellet was determined by elemental analysis (see “Determination of the amount of carbon present in the cell”). The total carbon amount of each culture was calculated by mass of the pellet x % of carbon/100. Since all experiments have been performed in triplicate both the average and standard deviation were calculated.
Determination of the amount of carbon present in the cells
The amount of carbon was determined at the laboratory for microanalysis services at the faculty of chemistry of the University of Vienna. In short: the amount of carbon in the sample was determined using C, H, N, S analysis in a Eurovector EA 3000 CHNS-O Elemental Analyser (built 2009) with sulphanilamide as a standard substance. The samples were exposed to O2 at 1000 °C. The resulting gases were transported through a gas chromatograph with high-purity helium as the carrier gas. The amount of CO2 was determined using a thermal conducting detector (TCD).
References
Mereschkowski C (1905) Biol Zentralbl 25:593
Rippka R (1972) Arch Mikrobiol 87:93
Rippka R, Deruelles J, Waterbury JB, Herdman M, Stanier RY (1979) J Gen Microbiol 111:1
Smith AJ (1983) Modes of cyanobacterial carbon metabolism. In: Carr NG, Whitton BA (eds) The biology of the cyanobacteria. University of California Press, Berkeley, p 47
Stebegg R, Schmetterer G, Rompel A (2019) Phytochemistry 157:206
Anderson SL, McIntosh L (1991) J Bacteriol 173:2761
Schmetterer G (1990) Plant Mol Biol 14:697
Flores E, Schmetterer G (1986) J Bacteriol 166:693
Zhang CC, Durand MC, Jeanjean R, Joset F (1989) Mol Microbiol 3:1221
Zhang CC, Jeanjean R, Joset F (1998) FEMS Microbiol Lett 161:285
Stebegg R, Wurzinger B, Mikulic M, Schmetterer G (2012) J Bacteriol 194:4601
Wolk CP, Shaffer PW (1976) Arch Microbiol 110:145
Ungerer JL, Pratte BS, Thiel T (2008) J Bacteriol 190:8115
Hirschberg J, McIntosh L (1983) Science 222:346
Golden SS, Haselkorn R (1985) Science 229:1104
Metz JG, Pakrasi HB, Seibert M, Arntzer CJ (1986) FEBS Lett 205:269
Astier C, Meyer I, Vernotte C, Etienne AL (1986) FEBS Lett 207:234
Nieves-Morion M, Flores E (2018) Environ Microbiol Rep 10:40
Niederholtmeyer H, Wolfstädter BT, Savage DF, Silver PA, Way JC (2010) Appl Environ Microbiol 76:3462
Barnell WO, Yi KC, Conway T (1990) J Bacteriol 172:7227
McEwen JT, Machado IMP, Connor MR, Atsumi S (2012) Appl Environ Microbiol 79:1668
Joset F, Buchou T, Zhang CC, Jeanjean R (1988) Arch Microbiol 149:417
Mackinney G (1941) J Biol Chem 140:315
Acknowledgements
Open access funding provided by University of Vienna. This work was supported by the project “Verbesserung des Verfahrens zur Herstellung sowie der Methoden der Qualitätskontrolle und Reinheitsprüfung von hochgereinigtem Chlorophyll a” of the Universität Wien [Grant no. ET524001]. We thank Mag. Johannes Theiner, Jürgen Bach and their team for performing the elemental analysis to determine the carbon content of the cyanobacterial cells. We also thank Peter Unteregger for performing HPLC/ESI–MS to determine the amount of impurities in the fructose used.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Stebegg, R., Schmetterer, G. & Rompel, A. Photoheterotrophic growth of unicellular cyanobacterium Synechocystis sp. PCC 6803 gtr− dependent on fructose. Monatsh Chem 150, 1863–1868 (2019). https://doi.org/10.1007/s00706-019-02484-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-019-02484-6