Abstract
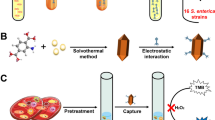
A label-free biosensor based on cupric oxide (CuO) nanoparticles was constructed for the selective detection of Gram-negative bacteria. CuO possesses oxidase-like activity and can catalyze the oxidation of o-phenylenediamine (OPD) to produce oxidized OPD, which has a fluorescence emission at 573 nm under excitation at 423 nm. The mechanism study suggests that the oxygen vacancies of CuO can activate the dissolved oxygen to form superoxide anions, which in turn oxidize OPD. Gram-negative bacteria can reduce part of Cu(II) in CuO to Cu(I) based on their copper homeostasis system, thus inhibiting the oxidation of OPD and decreasing the fluorescence intensity of the catalytic system. This principle was utilized to construct a biosensor to realize the selective detection of Gram-negative bacteria successfully. The biosensor exhibited a good linear correlation toward the logarithm concentration of three Gram-negative bacteria with R2 ≥ 0.985. It was applied to detect three Gram-negative bacteria in eggshell, Chinese cabbage, and the Pearl River water samples, with recoveries ranging from 92.4 to 107%. Moreover, a smartphone-based portable device was designed and fabricated to realize the on-site detection of bacteria. The results of the portable device were comparable to those of fluorescence spectrophotometry, suggesting that the portable device has tremendous potential in the on-site detection of bacteria.
Graphical Abstract








Similar content being viewed by others
References
Furst AL, Francis MB (2019) Impedance-based detection of bacteria. Chem Rev 119:700–726. https://doi.org/10.1021/acs.chemrev.8b00381
Sundström K (2018) Cost of illness for five major foodborne illnesses and sequelae in Sweden. Appl Health Econ Health Policy 16:243–257. https://doi.org/10.1007/s40258-017-0369-z
Jasson V, Jacxsens L, Luning P et al (2010) Alternative microbial methods: an overview and selection criteria. Food Microbiol 27:710–730. https://doi.org/10.1016/j.fm.2010.04.008
Roda A, Mirasoli M, Roda B et al (2012) Recent developments in rapid multiplexed bioanalytical methods for foodborne pathogenic bacteria detection. Microchim Acta 178:7–28. https://doi.org/10.1007/s00604-012-0824-3
Rajkovic A, Jovanovic J, Monteiro S et al (2020) Detection of toxins involved in foodborne diseases caused by Gram-positive bacteria. Compr Rev Food Sci Food Saf 19:1605–1657. https://doi.org/10.1111/1541-4337.12571
Rubab M, Shahbaz HM, Olaimat AN, Oh DH (2018) Biosensors for rapid and sensitive detection of Staphylococcus aureus in food. Biosens Bioelectron 105:49–57. https://doi.org/10.1016/j.bios.2018.01.023
Jin B, Wang S, Lin M et al (2017) Upconversion nanoparticles based FRET aptasensor for rapid and ultrasenstive bacteria detection. Biosens Bioelectron 90:525–533. https://doi.org/10.1016/j.bios.2016.10.029
Saad SM, Abdullah J, Rashid SA et al (2019) A fluorescence quenching based gene assay for Escherichia coli O157:H7 using graphene quantum dots and gold nanoparticles. Microchim Acta 186:804. https://doi.org/10.1007/s00604-019-3913-8
Bahari D, Babamiri B, Salimi A, Salimizand H (2021) Ratiometric fluorescence resonance energy transfer aptasensor for highly sensitive and selective detection of Acinetobacter baumannii bacteria in urine sample using carbon dots as optical nanoprobes. Talanta 221:121619. https://doi.org/10.1016/j.talanta.2020.121619
Li J, Fang Y, Lin X et al (2021) Universal nanoplatform for ultrasensitive ratiometric fluorescence detection and highly efficient photothermal inactivation of pathogenic bacteria. ACS Appl Bio Mater 4:6361–6370. https://doi.org/10.1021/acsabm.1c00583
Meng J, Shen H, Luo L et al (2022) Dual-wavelength ratiometric immunosensor for Bacillus cereus: oxidase-like MnO2-Au trigged “OFF-ON” detection strategy. Sensors Actuators B Chem 365:131925. https://doi.org/10.1016/j.snb.2022.131925
Chen W, Chen J, Liu AL et al (2011) Peroxidase-like activity of cupric oxide nanoparticle. ChemCatChem 3:1151–1154. https://doi.org/10.1002/cctc.201100064
Chen W, Hong L, Liu AL et al (2012) Enhanced chemiluminescence of the luminol-hydrogen peroxide system by colloidal cupric oxide nanoparticles as peroxidase mimic. Talanta 99:643–648. https://doi.org/10.1016/j.talanta.2012.06.061
Bin HS, Hu AL, Zhuang QQ et al (2020) Ascorbate oxidase mimetic activity of copper(II) oxide nanoparticles. ChemBioChem 21:978–984. https://doi.org/10.1002/cbic.201900595
Hu AL, Deng HH, Zheng XQ et al (2017) Self-cascade reaction catalyzed by CuO nanoparticle-based dual-functional enzyme mimics. Biosens Bioelectron 97:21–25. https://doi.org/10.1016/j.bios.2017.05.037
Yang X, Wang E (2011) A nanoparticle autocatalytic sensor for Ag+ and Cu2+ ions in aqueous solution with high sensitivity and selectivity and its application in test paper. Anal Chem 83:5005–5011. https://doi.org/10.1021/ac2008465
Hofmann L, Hirsch M, Ruthstein S (2021) Advances in understanding of the copper homeostasis in Pseudomonas aeruginosa. Int J Mol Sci 22:2050. https://doi.org/10.3390/ijms22042050
Espariz M, Checa SK, Audero MEP et al (2007) Dissecting the Salmonella response to copper. Microbiology 153:2989–2997. https://doi.org/10.1099/mic.0.2007/006536-0
Rensing C, Grass G (2003) Escherichia coli mechanisms of copper homeostasis in a changing environment. FEMS Microbiol Rev 27:197–213. https://doi.org/10.1016/S0168-6445(03)00049-4
Bondarczuk K, Piotrowska-Seget Z (2013) Molecular basis of active copper resistance mechanisms in Gram-negative bacteria. Cell Biol Toxicol 29:397–405. https://doi.org/10.1007/s10565-013-9262-1
Solioz M, Abicht HK, Mermod M, Mancini S (2010) Response of Gram-positive bacteria to copper stress. J Biol Inorg Chem 15:3–14. https://doi.org/10.1007/s00775-009-0588-3
Cheng Z, Chu X, Xu J et al (2016) Synthesis of various CuO nanostructures via a Na3PO4-assisted hydrothermal route in a CuSO4-NaOH aqueous system and their catalytic performances. Ceram Int 42:3876–3881. https://doi.org/10.1016/j.ceramint.2015.11.053
Zhao B, Liu P, Zhuang H et al (2013) Hierarchical self-assembly of microscale leaf-like CuO on graphene sheets for high-performance electrochemical capacitors. J Mater Chem A 1:367–373. https://doi.org/10.1039/c2ta00084a
Bandara J, Kiwi J (1999) Fast kinetic spectroscopy, decoloration and production of H2O2 induced by visible light in oxygenated solutions of the azo dye Orange II. New J Chem 23:717–724. https://doi.org/10.1039/a902425e
Shi W, Guo F, Wang H et al (2017) Carbon dots decorated the exposing high-reactive (111) facets CoO octahedrons with enhanced photocatalytic activity and stability for tetracycline degradation under visible light irradiation. Appl Catal B Environ 219:36–44. https://doi.org/10.1016/j.apcatb.2017.07.019
Mousavi M, Habibi-Yangjeh A, Seifzadeh D (2018) Novel ternary g-C3N4/Fe3O4/MnWO4 nanocomposites: synthesis, characterization, and visible-light photocatalytic performance for environmental purposes. J Mater Sci Technol 34:1638–1651. https://doi.org/10.1016/j.jmst.2018.05.004
Shang H, Li M, Li H et al (2019) Oxygen vacancies promoted the selective photocatalytic removal of NO with blue TiO2 via simultaneous molecular oxygen activation and photogenerated hole annihilation. Environ Sci Technol 53:6444–6453. https://doi.org/10.1021/acs.est.8b07322
Wang Y, Feng C, Zhang M et al (2010) Enhanced visible light photocatalytic activity of N-doped TiO2 in relation to single-electron-trapped oxygen vacancy and doped-nitrogen. Appl Catal B Environ 100:84–90. https://doi.org/10.1016/j.apcatb.2010.07.015
Zhu J, Zhu Y, Zhou W (2022) Cu-doped Ni-LDH with abundant oxygen vacancies for enhanced methyl 4-hydroxybenzoate degradation via peroxymonosulfate activation: key role of superoxide radicals. J Colloid Interface Sci 610:504–517. https://doi.org/10.1016/j.jcis.2021.11.097
Vallar S, Houivet D, El Fallah J et al (1999) Oxide slurries stability and powders dispersion: optimization with zeta potential and rheological measurements. J Eur Ceram Soc 19:1017–1021. https://doi.org/10.1016/S0955-2219(98)00365-3
Abu-Serie MM, Eltarahony M (2021) Novel nanoformulated diethyldithiocarbamate complexes with biosynthesized or green chemosynthesized copper oxide nanoparticles: an in vitro comparative anticancer study. Int J Pharm 609:121149. https://doi.org/10.1016/j.ijpharm.2021.121149
Robert T, Bartel M, Offergeld G (1972) Characterization of oxygen species adsorbed on copper and nickel oxides by X-ray photoelectron spectroscopy. Surf Sci 33:123–130. https://doi.org/10.1016/0039-6028(72)90103-3
National Institute of Standards and Technology, NIST X-ray photoelectron spectroscopy database version 4.1. https://srdata.nist.gov/xps/Default.aspx. Accessed 22 June 2022
Sahai A, Goswami N, Kaushik SD, Tripathi S (2016) Cu/Cu2O/CuO nanoparticles: novel synthesis by exploding wire technique and extensive characterization. Appl Surf Sci 390:974–983. https://doi.org/10.1016/j.apsusc.2016.09.005
Shahbazi P, Kiani A (2016) Fabricated Cu2O porous foam using electrodeposition and thermal oxidation as a photocatalyst under visible light toward hydrogen evolution from water. Int J Hydrogen Energy 41:17247–17256. https://doi.org/10.1016/j.ijhydene.2016.07.080
Applerot G, Lellouche J, Lipovsky A et al (2012) Understanding the antibacterial mechanism of CuO nanoparticles: revealing the route of induced oxidative stress. Small 8:3326–3337. https://doi.org/10.1002/smll.201200772
García Campaña AM, Rodríguez LC, Muñoz JA, Barrero FA (1997) Chemometric protocol to validate an analytical method in the presence of corrigible constant and proportional systematic errors. J AOAC Int 80:657–664. https://doi.org/10.1093/jaoac/80.3.657
Funding
This work was supported by the National Natural Science Foundation of China (21675056).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file2 (MP4 40933 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Deng, S., Tu, Y., Fu, L. et al. A label-free biosensor for selective detection of Gram-negative bacteria based on the oxidase-like activity of cupric oxide nanoparticles. Microchim Acta 189, 471 (2022). https://doi.org/10.1007/s00604-022-05571-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-022-05571-4