Abstract
Purpose
Four joint arthroplasty registries (JARs) levels exist based on the recorded data type. Level I JARs are national registries that record primary data. Hospital or institutional JARs (Level II–IV) document further data (patient-reported outcomes, demographic, radiographic). A worldwide list of Level II–IV JARs must be created to effectively assess and categorize these data.
Methods
Our study is a systematic scoping review that followed the PRISMA guidelines and included 648 studies. Based on their publications, the study aimed to map the existing Level II–IV JARs worldwide. The secondary aim was to record their lifetime, publications’ number and frequency and recognise differences with national JARs.
Results
One hundred five Level II–IV JARs were identified. Forty-eight hospital-based, 45 institutional, and 12 regional JARs. Fifty JARs were found in America, 39 in Europe, nine in Asia, six in Oceania and one in Africa. They have published 485 cohorts, 91 case-series, 49 case–control, nine cross-sectional studies, eight registry protocols and six randomized trials. Most cohort studies were retrospective. Twenty-three per cent of papers studied patient-reported outcomes, 21.45% surgical complications, 13.73% postoperative clinical and 5.25% radiographic outcomes, and 11.88% were survival analyses. Forty-four JARs have published only one paper. Level I JARs primarily publish implant revision risk annual reports, while Level IV JARs collect comprehensive data to conduct retrospective cohort studies.
Conclusions
This is the first study mapping all Level II–IV JARs worldwide. Most JARs are found in Europe and America, reporting on retrospective cohorts, but only a few report on studies systematically.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Joint arthroplasty registries (JARs) are databases that report the outcomes of joint arthroplasties (JAs). Their primary goal is the JAs quality improvement [1], and they are considered the gold standard source for informed medical decision-making. There are four levels of JARs based on the type of data recorded. Level I JARs record basic data, Level II demographic and comorbidity data, Level III patient-reported outcome data and Level IV imaging and radiographic data. Most well-known national JARs collect type I data because further data collection is expensive. Fewer regional, institutional, or hospital-based JARs collect more detailed patients’ data (type II–IV) [1, 2].
National arthroplasty registries (Level I) report primary data on patients and procedures using revision arthroplasty as the endpoint. They collect large data volume nationally, reporting annual survival outcomes and revision risk of specific implants [3, 4]. However, national JARs reports are extensive and interpreted with difficulty by clinicians having little statistical training. The reports’ interpretation may also be misleading due to the absence of more comprehensive registry data (type II–IV) [5, 6].
On the other hand, Level II–IV regional or hospital-based registries collect smaller volumes but more inclusive data correlating efficiently radiologic or patient history data with arthroplasty outcomes. These JARs may complement national JARs, allowing further scrutiny and deeper causative correlation of JAs failure, improving outcomes [5]. Currently, an attempt to record the hip and knee JARs in Europe is being made [6]. However, a worldwide list of hospital-based JARs does not exist, and their contribution to assessing arthroplasty results remains unclear.
Level I JARs have been thoroughly researched, but there is a lack of information on the importance of Level II–IV JARs in the literature. To accurately evaluate and classify the more specific and patient-centric data they provide, we require a comprehensive inventory of both institutional and hospital-based JARs. Knowing the quantity and location of these JARs and the number and variety of publications they produce can enhance our comprehension of their value and necessity.
The present study is a systematically performed scoping review. The primary aim of this study was to map the existing institutional and hospital-based (Level II–IV) JARs worldwide and their lifetime. The secondary aim was to record their lifetime, publications’ number and frequency and recognize differences with national JARs. Countries with national and hospital-based JARs were also recorded.
Materials and methods
Our study is a systematic scoping review that followed the PRISMA 2020 statement [7].
Search strategy
A systematic review of published articles from several databases such as MEDLINE (PubMed), Cochrane Database of Systematic Reviews and Clinical Trials by the U.S. National Library of Medicine was conducted from conception to July 2022. The following keywords and Mesh terms were utilized with “AND” or “OR”: “arthroplasty, replacement, knee,” “arthroplasty, replacement, hip,” “arthroplasty, replacement, ankle,” “arthroplasty, replacement, shoulder,” “registries,” “arthroplasty registry,” “joint registry,” “regional registry,” “hospital registry”,” registry level,” “national registry.” The authors created the keywords, drawing on their own experience, and employed different names to refer to the term "registry". They did not involve any input from a librarian.
Inclusion and exclusion criteria
Specific inclusion criteria were the following: i) randomized (RCTs) and non-randomized control trials, prospective and retrospective cohorts, case series and comparative studies, (ii) studies involving adult patients (> 18 years) that underwent elective total joint arthroplasty (TJA), (iii) studies evaluating joint arthroplasty outcomes based on Level II–IV JARs data (regional, institutional or hospital-based arthroplasty registries), (iv) studies providing extractable data (studies that have organized and fully structured data that can be extracted from the manuscript).
On the other hand, studies were excluded if they i) reported national type I JARs data, (ii) used non-arthroplasty registries data (hospital discharge or other ailments registries), (iii) were narrative reviews, letters to the editor, editorial comments, meta-analysis or systematic reviews related to the topic, (iv) were conducted in animals or cadavers, (v) were written in a non-English language, (vi) had no full-text available.
Data extraction
The searched papers with abstract information were managed in Mendeley to remove duplicated citations. The remaining studies were screened independently by two authors. Firstly, titles and abstracts were screened using the search strategy to fulfil the inclusion criteria. The data extraction process was done by the two authors independently. The final extracted data were cross-checked. A third senior author resolved any disagreement.
Data synthesis
Data synthesis was performed and analyzed by the same two authors that recorded the following information for JARs: i) the location (country, city, hospital name); (ii) the quality of reported studies (study type, methodology, population and other characteristics) (iii) their lifetime calculated from the time of the first and last found publication and (iv) if the countries of hospital-based registries had also a national registry. Differences in the published information between national and hospital-based registries were also evaluated.
Results
Search results
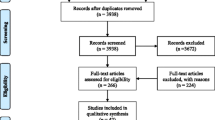
The initial electronic search yielded 4251 studies. After eliminating 48 duplicated studies, 4203 were reviewed on their title and abstract. According to our inclusion and exclusion criteria, 3269 records were excluded based on title and abstract, and 934 papers were deemed suitable and screened in the full article text. Finally, 648 studies were included in this systematic review. The flow diagram of the search strategy is shown in Fig. 1.
Demographics and patient characteristics, study type, design & primary aim
The included studies were published from 1997 to 2022 [8,9,10,11,12]. The sample size of the studied population ranged from 9 to 84,998 patients [13, 14]. Almost 60 per cent of the patients were women. The follow-up of patients varied from three months to twenty-five years [15, 16].
According to the study type, 485 (74.85%) were cohorts [2, 3, 8,9,10,11,12, 14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182,183,184,185,186,187,188,189,190,191,192,193,194,195,196,197,198,199,200,201,202,203,204,205,206,207,208,209,210,211,212,213,214,215,216,217,218,219,220,221,222,223,224,225,226,227,228,229,230,231,232,233,234,235,236,237,238,239,240,241,242,243,244,245,246,247,248,249,250,251,252,253,254,255,256,257,258,259,260,261,262,263,264,265,266,267,268,269,270,271,272,273,274,275,276,277,278,279,280,281,282,283,284,285,286,287,288,289,290,291,292,293,294,295,296,297,298,299,300,301,302,303,304,305,306,307,308,309,310,311,312,313,314,315,316], 91 (14.04%) were case-series [13, 317,318,319,320,321,322,323,324,325,326,327,328,329,330,331,332,333,334,335,336,337,338,339,340,341,342,343,344,345,346,347,348,349,350,351,352,353,354,355,356,357,358,359,360,361,362,363,364,365,366,367,368,369,370,371,372,373,374,375,376,377,378,379,380,381,382,383,384,385,386,387,388,389,390,391,392,393,394,395,396,397,398,399,400,401,402,403,404,405,406], 49 (7.56%) were case–control studies [407,408,409,410,411,412,413,414,415,416,417,418,419,420,421,422,423,424,425,426,427,428,429,430,431,432,433,434,435,436,437,438,439,440,441,442,443,444,445,446,447,448,449,450,451,452,453,454,455], nine (1.39%) cross-sectional studies [456,457,458,459,460,461,462,463,464], eight (1.23%) protocols for registry-based studies [465,466,467,468,469,470,471,472] and six (0.93%) randomised control trials (RCT) [473,474,475,476,477,478]. Three hundred twenty-seven cohort studies were retrospective [2, 8, 11, 14, 17, 18, 21,22,23,24,25,26, 29,30,31,32,33,34, 36, 38, 41, 42, 44,45,46, 54,55,56, 64,65,66,67,68,69,70,71, 83,84,85,86,87,88,89,90,91, 106,107,108,109,110,111,112,113,114,115,116,117,118, 123, 137,138,139,140,141,142,143,144,145,146, 155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182,183,184,185,186,187,188,189,190, 200,201,202,203,204,205,206,207,208,209,210,211,212,213,214,215,216,217, 233,234,235,236,237,238,239,240,241,242,243,244,245,246,247,248,249,250,251,252,253,254,255,256,257,258,259,260,261,262,263,264,265,266, 273, 281,282,283,284,285,286,287,288,289,290,291,292,293,294,295,296,297,298,299,300,301,302,303,304,305,306,307,308, 311,312,313,314,315, 479,480,481,482,483,484,485,486,487,488,489,490,491,492,493,494,495,496,497,498,499,500,501,502,503,504,505,506,507,508,509,510,511,512,513,514,515,516,517,518,519,520,521,522,523,524,525,526,527,528,529,530,531,532,533,534,535,536,537,538,539,540,541,542,543,544,545,546,547,548,549,550,551,552,553,554,555,556,557,558,559,560,561,562,563,564,565,566,567,568,569,570,571,572,573,574,575,576,577,578,579,580,581,582,583,584,585,586,587,588,589,590], and 158 were prospective [9, 10, 12, 15, 16, 19, 20, 27, 28, 35, 37, 39, 40, 43, 47,48,49,50,51,52,53, 58,59,60,61,62,63, 73,74,75,76,77,78,79,80,81,82, 92,93,94,95,96,97,98,99,100,101,102,103,104,105, 119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136, 147,148,149,150,151,152,153,154, 191,192,193,194,195,196,197,198,199, 210, 218,219,220,221,222,223,224,225,226,227,228,229,230,231,232, 267,268,269,270,271,272, 274,275,276,277,278,279,280, 309, 310, 316, 591,592,593,594,595,596,597,598,599,600,601,602,603,604,605,606,607,608,609,610,611,612,613,614,615,616,617,618,619,620,621,622,623,624,625,626,627,628,629]. In thirty-seven cohort studies, a comparison between two cohorts was made [57, 72, 147, 188,189,190,191, 200, 233, 265, 309,310,311,312,313,314,315,316, 479,480,481,482, 526,527,528, 560,561,562,563,564,565,566,567, 630,631,632,633].
Almost half of the included papers studied the patient-reported outcome measures (PROMs), the Quality-of-Life Years (QALYs) and the intra- and postoperative complications rate. In detail, the primary outcome in 149 (23%) studies were PROMs and QALYs [18, 19, 23, 34, 40, 55, 59, 61, 68, 81, 92, 99, 100, 108, 111, 117, 124, 127, 129, 135, 137, 139, 150, 153, 161, 168, 170, 173, 182, 187, 191, 193, 196,197,198,199,200, 206, 207, 210, 212, 215, 217, 222, 223, 225, 227, 236, 242, 244, 245, 263, 265, 267, 268, 273, 275,276,277,278, 281, 283, 300, 301, 307, 310, 314, 339, 352, 353, 357, 368, 378, 382, 395, 396, 400, 401, 403, 405, 408, 412, 417, 425, 426, 434, 439, 440, 445, 446, 449, 460,461,462,463, 481, 483,484,485, 487, 492, 494, 499, 501, 512, 513, 516, 523, 525, 528, 530, 533, 542, 546, 548, 549, 564, 565, 574,575,576, 579, 587, 589,590,591, 593,594,595, 601, 602, 605,606,607,608,609, 612,613,614, 619, 621, 622, 625, 628, 629, 634,635,636], while in 139 (21.45%) studies were surgical complications (i.e., infections, fractures, thrombosis etc.) [9, 13, 15,16,17, 20, 21, 25, 45, 58, 63, 66, 69, 73, 77,78,79,80, 84, 85, 88, 101,102,103,104,105, 107, 109, 110, 113, 119, 121, 125, 130, 133, 146, 147, 152, 157, 159, 160, 162, 163, 166, 172, 174, 175, 180, 183, 194, 195, 202, 204, 209, 213, 234, 237, 239, 240, 250, 252, 254, 257, 264, 266, 269,270,271, 279, 282, 287, 289, 290, 295, 298, 306, 311, 312, 316, 317, 319, 327, 334, 336, 341, 354, 356, 365, 366, 370, 377, 381, 388, 392, 397, 398, 409, 413, 416, 422, 427, 432, 437, 441, 442, 444, 451, 452, 474, 475, 478, 480, 488, 498, 500, 504, 509, 520, 522, 529, 534, 536, 545, 547, 550, 554,555,556, 558, 560, 561, 568, 596, 597, 600, 630, 637,638,639]. Eighty-nine studies (13.73%) evaluated postoperative patients’ clinical outcomes [11, 27, 47, 50, 62, 74, 83, 91, 95,96,97, 106, 112, 114, 120, 128, 134, 149, 151, 176, 178, 181, 185, 188, 190, 192, 203, 205, 208, 210, 216, 221, 224, 229, 230, 235, 238, 247, 261, 286, 291, 292, 296, 302, 303, 305, 348, 349, 369, 372, 373, 375, 379, 380, 386, 387, 391, 411, 415, 419, 420, 424, 429, 430, 436, 454, 493, 496, 497, 503, 521, 526, 531, 537, 553, 559, 566, 567, 570, 573, 584, 616, 617, 633, 640,641,642,643], 34 (5.25%) postoperative radiographic outcomes [35, 56, 116, 148, 167, 169, 171, 246, 248, 274, 284, 304, 309, 324, 335, 343, 363, 364, 376, 447, 450, 453, 459, 464, 477, 489, 490, 514, 517, 535, 539, 572, 618, 644] and 46 (7.10%) studies assessed the efficacy of a specific implant [22, 24, 41,42,43,44, 71, 72, 86, 87, 118, 126, 165, 184, 201, 219, 233, 241, 243, 256, 262, 297, 325, 332, 337, 347, 359, 394, 410, 423, 431, 443, 479, 506, 541, 544, 580, 586, 598, 615, 624, 627, 632, 645, 646]. Seventy-seven (11.88%) studies were survival analyses [2, 10, 26, 37, 38, 46, 64, 67, 70, 115, 122, 136, 154, 155, 164, 177, 179, 189, 211, 231, 232, 251, 260, 272, 280, 288, 293, 308, 321, 329, 331, 340, 342, 344,345,346, 351, 360, 361, 371, 385, 402, 414, 421, 433, 438, 482, 491, 495, 502, 507, 508, 511, 515, 518, 519, 524, 527, 532, 540, 551, 557, 563, 569, 581,582,583, 585, 588, 592, 599, 603, 611, 631, 647, 648] but 21 (3.24%) studied the long-term arthroplasty outcomes [29, 48, 49, 82, 93, 141, 218, 228, 285, 322, 383, 384, 407, 458, 505, 543, 562, 623, 626, 649]. Besides, 17 (2.62%) studies compared different surgical techniques [28, 53, 144, 145, 186, 253, 338, 358, 362, 374, 390, 428, 538, 552, 578, 650, 651] and seven (1.08%) studies evaluated various levels of surgeons’ experience [30, 33, 36, 214, 255, 294, 299], while in 18 (2.78%) studies, a prediction of pre- or postoperative risk factors was made [14, 39, 52, 54, 98, 131, 132, 143, 355, 367, 389, 404, 435, 448, 456, 457, 604, 620]. Finally, 15 (2.31%) were cost analysis studies [57, 65, 75, 90, 138, 140, 142, 156, 158, 226, 315, 326, 328, 399, 486], 13 papers (2%) studied the patients’ mortality rate [31, 60, 76, 258, 259, 313, 320, 323, 333, 418, 577, 652, 653], 12 (1.85%) studies offered general registry information [8, 51, 89, 123, 220, 249, 318, 330, 350, 393, 571, 610], nine (1.39%) were protocols [465,466,467,468,469,470,471,472,473] and two (0.31%) genetic studies [32, 476].
Global mapping of Level ΙΙ–IV registries
105 Level II–IV registries were identified. Forty-eight (45.71%) were hospital-based, forty-five (42.86%) were institutional, and twelve (11.43%) were regional JARs. Tables 1, 2, and 3 show the distribution of the included JARs per continent. Specifically, 50 (47.62%) Level II–IV JARs were found in America (USA:44, Canada:5, SouthAmerica:1), 39 (37.14%) in Europe (Switzerland:7, UK:7, France:5, Germany:4, Italy:3, Spain:2, Greece:2, Ireland:2, Sweden:2, Norway:1, Denmark:1, Austria:1, Scotland:1, Turkey:1), nine (8.57%) in Asia (China:4, Taiwan:1, Japan:1, Hong Kong:1, Korea:1, Singapore:1), six (5.71%) in Oceania (Australia:5, New Zealand:1) and one (0.95%) in Africa (Tunisia) (Tables 1, 2 and 3). Some countries have more than one institutional JARs in different cities, while others have only one hospital-based arthroplasty JAR. The global geographic distribution of the included type II–IV JARs is depicted in Fig. 2.
Table 4 shows the number of publications and the time of the first and last publication for those JARs with more than one published study. Forty-four JARs have published only one paper, and 74 JARs have a publication lifetime of fewer than five years. The “Mayo Clinic Total Joint Registry” has been reporting studies for twenty-five consecutive years, followed by the “Trent” JAR for twenty-one years and the “Register of Orthopaedic Prosthetic Implant (RIPO) of Emilia-Romagna region” for twenty years. The “Mayo Clinic Total Joint Registry” has published 149 papers from 1997 to 2022 [8, 650], including 120 cohort studies [8, 25, 31, 45, 58, 60, 62, 64, 67, 69, 73, 76,77,78,79,80,81,82, 84, 85, 88, 92,93,94,95,96,97, 99,100,101, 106, 110, 112, 114, 118, 120, 122, 123, 125,126,127, 129, 132, 134, 135, 137, 138, 140, 142, 143, 152, 154, 157, 159, 163, 166, 169, 174, 179, 180, 190, 194, 195, 201, 232, 234, 240, 244, 247, 249, 251, 252, 257,258,259,260, 263, 264, 269, 289, 290, 293, 305, 306, 308, 312, 480, 486, 495, 497, 500, 507, 509,510,511, 515, 518, 519, 524, 529, 532, 543, 548, 550, 551, 559, 568, 576, 578, 582, 583, 586, 593, 596, 598, 631, 639, 648, 650, 652, 653], 24 case series [13, 320,321,322,323, 325,326,327, 332, 333, 335, 338, 348, 354, 356, 363, 371, 374, 375, 380, 383, 385, 390] and five case–control studies [407, 409, 416, 449, 450]. Among the most frequent study types were 48 documents that focused on surgical complications [13, 25, 45, 58, 69, 73, 77,78,79,80, 84, 85, 88, 101, 110, 125, 152, 157, 159, 163, 166, 174, 180, 194, 195, 234, 240, 252, 257, 264, 269, 289, 290, 306, 312, 327, 354, 356, 409, 416, 480, 500, 509, 529, 550, 568, 596, 639], 27 on implant survival [64, 67, 122, 154, 179, 232, 251, 260, 293, 308, 321, 371, 385, 495, 507, 510, 511, 515, 518, 519, 524, 532, 551, 582, 583, 631, 648], 17 on postoperative clinical outcomes [62, 95,96,97, 106, 112, 114, 120, 134, 190, 247, 305, 348, 375, 380, 497, 559] and 15 on PROMs and QALYs [81, 92, 99, 100, 127, 129, 135, 137, 244, 263, 449, 548, 576, 593, 636]. Besides, ten studies evaluated the patients’ mortality rate [31, 60, 76, 258, 259, 320, 323, 333, 652, 653], seven different implant types [118, 126, 201, 325, 332, 586, 598] and seven the long-term postoperative outcomes [82, 93, 94, 322, 383, 407, 543]. The “Trent” JAR published ten studies from 1997 to 2018 [9, 276], including nine cohort studies [9, 20,21,22, 27, 53, 218, 256, 276] and one case series [329]. Postoperative complications, short and long-term clinical outcomes, PROMs and QALYs, the efficacy of specific implants, comparison of different surgical techniques and survival analyses were among the main outcome of the published studies. The “Register of Orthopaedic Prosthetic Implant (RIPO) of Emilia-Romagna region” published 30 papers from 2002 to 2022 [24, 645]. Among them, twenty-eight were cohort studies [24, 26, 39, 43, 57, 145, 164, 189, 231, 288, 297, 482, 491, 502, 506, 508, 527, 540, 562, 563, 569, 585, 592, 597, 599, 624, 630, 645], one was RCT [476] and one registry protocol [468]. Of these 30 studies, 16 were survival [26, 164, 189, 231, 288, 482, 491, 502, 508, 527, 540, 563, 569, 585, 592, 599], six analysed implant types [24, 43, 297, 506, 624, 645], and two studied postoperative complications [597, 630]. Long-term postoperative outcomes [562], cost [57] and risk factors analysis [39], genetic studies [476], a protocol for registry study [468] and comparison of different surgical techniques [145] were among the primary outcomes of other study types.
Concerning the publications’ frequency (number of publications/years of the JAR’s operation), the "THR Registry in Hospital for Special Surgery" is in the first place, with more than eight publications per year (89 papers from 2011 to 2022). "Mayo Clinic Total Joint Registry" is in second place with almost six publications per year (149 papers in twenty-five years), followed by the "Partners Arthroplasty Registry Massachusetts (PAR)" with four publications per year (4 articles in one year). More details are shown in Table 4.
Countries with Level I and Level IV arthroplasty registries
Nine countries have national (Level I) and institutional (Level II–IV) JARs. In these countries, institutional JARs belong to the national JARs but publish their results independently. There are two national JARs in Oceania (Australian Orthopaedic Association National Joint Replacement Registry, New Zealand national joint registry) and six institutional JARs (Repatriation General Hospital, St. Vincent’s Hospital SMART, The Alfred Hospital, The Hollywood Hospital, Barwon in St John of God Hospital, Tauranga Public Hospital) The last one is distinct from but complementary to the New Zealand National Joint Registry. In Europe, six countries (Denmark, Germany, Norway, Sweden, Switzerland and the United Kingdom) own both Levels I and IV JARs. There is a national registry in Germany (Endoprosthesenregister Deutschland EPRD), and two out of four hospital-based registries (Registries at the University of Heidelberg and Regensburg University) work independently. In the United States of America, there are national JARs and 44 hospital-based or institutional registries. More details are shown in Tables 1, 2 and 3.
Some countries have only institutional JARs. Seven Asian and African countries (China, Hong Kong, Japan, Korea, Republic of China-Taiwan, Singapore and Tunisia) have hospital-based JARs. Still, no national JAR can be found on these continents (Table 1). Besides, seven European countries (Austria, France, Greece, Italy, Scotland, Spain, and Turkey) do not have national but only institutional JARs.
Discussion
Our study mapped all Level II–IV JARs worldwide systematically based on their publications. Few hospital or institutional JARs have been found in Asia and Africa, with limited published studies. In Australia, Europe and the United States of America, all JARs levels can be found. The northern European countries (Scandinavia, United Kingdom) have well-known national JARs and institutional registries. In contrast, southern countries (Greece, Spain, Italy) lack a national but own hospital-based JARs publishing data. Due to financial, legal, and regulatory challenges in the United States of America [654], hospital-based JARs prevail, along with the existence of national registries: American Joint Replacement Registry (AJRR) and Kaiser Permanente. Most Level II–IV JARs are found in Europe and America. Some publish their data independently, while others also report through their relative national registries. All institutional or hospital-based JARs in the United Kingdom are part of the National Joint Registry. On the other hand, the "Endoprothesenregister in Regensburg University" and the "Endoprosthesis Register in Department of Orthopedic Surgery, University of Heidelberg" are institutional JARs that do not transfer data to the German national registry "Endoprothesenregister Deutschland (ERPD) ".
It is imperative to obtain a comprehensive inventory of institutional and hospital-based JARs, including Level II–IV, to thoroughly assess and categorize the patient-focused data they offer. The significance and necessity of these JARs can only be fully understood by identifying their quantity, location, and the variety and number of publications they generate. Therefore, we must prioritize acquiring this information to advance our understanding and improve patient care.
Hospital-based and institutional JARs worldwide reported all types of evidence-based pyramid studies. A few published studies are randomized clinical trials; most are cohorts, case-series and case–control studies. The published data are mainly retrospective, with the prospective studies being a minority. The study types differ among Level I and II–IV JARs. Level I national registries publish prospectively annual reports, including revision risk data for various implants. Level II–IV JARs collect more inclusive data to perform cohort and comparative studies; however, most are retrospective. Level I JARs data mainly control implant survival by monitoring the revision rate, the institutional performance and evaluating the quality offered pre-, intra- and postoperatively by all surgeons [8]. Level II–IV JARs data come mainly from senior high-volume surgeons and specialized centres worldwide and cannot be quickly adopted and generalized. However, Level II–IV JARs data are more inclusive. Demographics and baseline characteristics of patients, the type of implants, surgeons, surgical approaches and other procedural features and clinical and radiographical data are usually more detailed. Quality of patients’ life and medical complications other than implant failure as infections, deep vein thrombosis or pulmonary embolism, are also frequently recorded [63, 518, 655, 656]. The Harris Hip Score (HHS) and Hip disability and Osteoarthritis Outcome Score (HOOS) are used to report patients’ quality of life from most registries [2, 647]. There are also implant survival studies from various institutional JARs simultaneously reporting clinical outcomes, complications, PROMs and radiological implants’ data as secondary study outcomes [64, 321, 511, 524, 647]. Radiological data are beneficial to follow implants and understanding the reasons for failure, but they are only available by Level IV JARs [2, 116, 335, 450]. Several surgical approaches and comparative clinical studies of surgical outcomes between specialist orthopaedic surgeons and trainees have been evaluated [255, 428]. Level II–IV JARs often report studies that analyze risk factors (obesity/rheumatic diseases) of TJA outcomes, but also cost analysis studies of the length of hospital stay following TJA improving the cost-effectiveness of joint replacements [76, 158, 333, 425, 456, 486]. Genetic studies have also been performed [350, 476].
The lifetime and publishing frequency varies considerably between JARs. As previously mentioned, among the longest-running Level IV JARs are the "Mayo Clinic Total Joint Registry" from 1997 to 2022, followed by the "Trent" from 1997 to 2018 and the "Register of Orthopedic Prosthetic Implant (RIPO) of Emilia-Romagna Region" from 2002 to 2022. These JARs have published 147, 10 and 30 papers, respectively. The "Mayo Clinic Total Joint Registry" seems to be the oldest institutional registry and has published the most articles. However, the "Total Hip Registry in the Hospital for Special Surgery" is the JAR with the highest publishing frequency, with more than eight published papers per year, followed by the "Mayo Clinic Total Joint Registry" with almost six publications per year, and the "Partners Arthroplasty Registry Massachusetts (PAR)" with four publications per year. On the other hand, twenty-eight JARs publish less than one paper per year in their lifetime.
Besides, many institutional or hospital-based JARs have published only one article during their lifetime. This may be attributed to several factors. The patients and data enrollment of institutional registries is lower than the national ones, and a longer time is needed to complete and report studies. So, their lifetime may be longer than the actual measures from the first and last publication. However, the existence of some long-lasting low-frequency publishing JARs may be disputed in the future.
Our study has some limitations. The principal limit is that the institutional or hospital-based JARs data may not be fully accessible for several reasons. First, there are Level IV JARs, such as the “ German Orthopaedic Foot and Ankle Association's (D. A. F.)” registry [657], that only publish studies in their native language. Thus they are not included in this report. Secondly, only a few regional and hospital-based JARs manage a website to publish annual reports, such as national JARs, due to a lack of funding. Thirdly, most Level IV JARs do not have yearly reports available. If the reports are available online, they are not open to the public, contrary to national JARs [654]. Lastly, many Level IV JARs publish studies only once or twice in their lifetime [21, 24]. That way, a lot of helpful information may be lost.
Conclusion
To our knowledge, this is the first systematic review mapping all institutional or hospital-based JARs worldwide. Most of these registries are found in Europe and America, reporting all types of evidence-based pyramid studies. The reported studies may have data missing from national registry reports as radiographic data, but they are often retrospective. The frequency of data reporting varies considerably among Level II–IV JARs, but this is generally not systematic. Their contribution is undeniable, mainly due to the detailed and variable data they collect. Further studies are needed to evaluate the quality of the offered knowledge in the clinical setting, especially for Level IV registries that do not publish their data annually or in a non-English language.
References
Franklin PD, Lewallen D, Bozic K et al (2014) Implementation of patient-reported outcome measures in U.S. Total joint replacement registries: rationale, status, and plans. J Bone Joint Surg Am 96(Suppl 1):104–109. https://doi.org/10.2106/JBJS.N.00328
Kenanidis E, Kakoulidis P, Anagnostis P et al (2021) Constrained liners revisited: favourable mid-term results in patients with high-risk of dislocation: technical considerations for the optimal outcome. Hip Int. https://doi.org/10.1177/11207000211010712
Labek G, Janda W, Agreiter M et al (2011) Organisation, data evaluation, interpretation and effect of arthroplasty register data on the outcome in terms of revision rate in total hip arthroplasty. Int Orthop 35:157–163. https://doi.org/10.1007/s00264-010-1131-4
Labek G, Sekyra K, Pawelka W et al (2011) Outcome and reproducibility of data concerning the Oxford unicompartmental knee arthroplasty: a structured literature review including arthroplasty registry data. Acta Orthop 82:131–135. https://doi.org/10.3109/17453674.2011.566134
Malchau H, Garellick G, Berry D et al (2018) Arthroplasty implant registries over the past five decades: development, current, and future impact. J Orthop Res 36:2319–2330. https://doi.org/10.1002/jor.24014
Lübbeke A, Silman AJ, Barea C et al (2018) Mapping existing hip and knee replacement registries in Europe. Health Policy 122:548–557. https://doi.org/10.1016/j.healthpol.2018.03.010
Page MJ, McKenzie JE, Bossuyt PM et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. The BMJ. https://doi.org/10.1136/bmj.n71
Berry DJ, Kessler M, Morrey BF (1997) Maintaining a hip registry for 25 years. Mayo Clinic experience. Clin Orthop Relat Res. https://doi.org/10.1097/00003086-199711000-00007
Fender D, Harper WM, Thompson JR, Gregg PJ (1997) Mortality and fatal pulmonary embolism after primary total hip replacement. Results from a regional hip register. J Bone Joint Surg Br 79:896–899. https://doi.org/10.1302/0301-620x.79b6.7677
Girard J, Epinette JA, Martinot P, Dartus J (2022) French hip resurfacing registry: a study of 1650 cases. Orthop Traumatol Surg Res 108:103087. https://doi.org/10.1016/j.otsr.2021.103087
Bendich I, Tarity TD, Alpaugh K et al (2022) Minimal Clinically Important Difference (MCID) at one year postoperatively in aseptic revision total hip arthroplasty. J Arthroplasty 37:S954–S957. https://doi.org/10.1016/j.arth.2022.01.044
Kenanidis E, Paparoidamis G, Milonakis N et al (2022) Comparative outcomes between a new robotically assisted and a manual technique for total knee arthroplasty in patients with osteoarthritis: a prospective matched comparative cohort study. Eur J Orthop Surg Traumatol. https://doi.org/10.1007/s00590-022-03274-3
Turner NS 3rd, Pagnano MW, Sim FH (2001) Total knee arthroplasty after ipsilateral peripheral arterial bypass graft: acute arterial occlusion is a risk with or without tourniquet use. J Arthroplasty 16:317–321. https://doi.org/10.1054/arth.2001.21502
Layson JT, Markel DC, Hughes RE et al (2022) John N. Insall Award: MARCQI’s Pain-Control Optimization Pathway (POP): impact of registry data and education on opioid utilization. J Arthroplasty 37:S19–S26. https://doi.org/10.1016/j.arth.2022.02.109
Gandhi R, Razak F, Tso P et al (2009) Metabolic syndrome and the incidence of symptomatic deep vein thrombosis following total knee arthroplasty. J Rheumatol 36:2298–2301. https://doi.org/10.3899/jrheum.090282
Carothers JT, White RE, Tripuraneni KR et al (2013) Lessons learned from managing a prospective, private practice joint replacement registry: a 25-year experience. Clin Orthop Relat Res 471:537–543. https://doi.org/10.1007/s11999-012-2541-y
Inneh IA, Lewis CG, Schutzer SF (2014) Focused risk analysis: regression model based on 5,314 total hip and knee arthroplasty patients from a single institution. J Arthroplasty 29:2031–2035. https://doi.org/10.1016/j.arth.2014.05.007
Berliner JL, Brodke DJ, Chan V et al (2017) Can preoperative patient-reported outcome measures be used to predict meaningful improvement in function after TKA? Clin Orthop Relat Res 475:149–157. https://doi.org/10.1007/s11999-016-4770-y
Poultsides LA, Ghomrawi HMK, Lyman S et al (2012) Change in preoperative expectations in patients undergoing staged bilateral primary total knee or total hip arthroplasty. J Arthroplasty 27:1609-1615.e1. https://doi.org/10.1016/j.arth.2012.02.004
Fender D, Harper WM, Gregg PJ (1999) Outcome of Charnley total hip replacement across a single health region in England: the results at five years from a regional hip register. J Bone Joint Surg Br 81:577–581. https://doi.org/10.1302/0301-620x.81b4.9859
Fender D, Harper WM, Gregg PJ (2000) The trent regional arthroplasty study. Experiences with a hip register. J Bone Joint Surg Br 82:944–947. https://doi.org/10.1302/0301-620x.82b7.10762
Hassan T, Birtwistle S, Power RA, Harper WM (2000) Revision hip arthroplasty activity in a single UK health region: an audit of 1265 cases. Ann R Coll Surg Engl 82:283–286
Bourne RB, Sibbald WJ, Doig G et al (2001) The Southwestern Ontario Joint Replacement Pilot Project: electronic point-of-care data collection. Southwestern Ontario Study Group. Can J Surg 44:199–202
Stea S, Bordini B, Sudanese A, Toni A (2002) Registration of hip prostheses at the Rizzoli Institute. 11 years’ experience. Acta Orthop Scand Suppl 73:40–44. https://doi.org/10.1080/000164702760379549
Mantilla CB, Horlocker TT, Schroeder DR et al (2002) Frequency of myocardial infarction, pulmonary embolism, deep venous thrombosis, and death following primary hip or knee arthroplasty. Anesthesiology 96:1140–1146. https://doi.org/10.1097/00000542-200205000-00017
Toni A, Stea S, Bordini B, Traina F (2002) Lost to follow-up in a hip prosthesis register. Experience of R.I.P.O. Acta Orthop Scand Suppl 73:49–53. https://doi.org/10.1080/000164702760379567
Fender D, van der Meulen JHP, Gregg PJ (2003) Relationship between outcome and annual surgical experience for the charnley total hip replacement. Results from a regional hip register. J Bone Joint Surg Br 85:187–190. https://doi.org/10.1302/0301-620x.85b2.12759
Gioe TJ, Killeen KK, Hoeffel DP et al (2003) Analysis of unicompartmental knee arthroplasty in a community-based implant registry. Clin Orthop Relat Res. https://doi.org/10.1097/01.blo.0000093004.90435.d1
Kennedy LG, Newman JH, Ackroyd CE, Dieppe PA (2003) When should we do knee replacements? Knee 10:161–166. https://doi.org/10.1016/s0968-0160(02)00138-2
Malik MHA, Gambhir AK, Bale L et al (2004) Primary total hip replacement: a comparison of a nationally agreed guide to best practice and current surgical technique as determined by the North West Regional Arthroplasty Register. Ann R Coll Surg Engl 86:113–118. https://doi.org/10.1308/003588404322827509
Parvizi J, Ereth MH, Lewallen DG (2004) Thirty-day mortality following hip arthroplasty for acute fracture. J Bone Joint Surg Am 86:1983–1988. https://doi.org/10.2106/00004623-200409000-00017
Meenagh GK, McGibbon D, Nixon J et al (2005) Lack of support for the presence of an osteoarthritis susceptibility locus on chromosome 6p. Arthritis Rheum 52:2040–2043. https://doi.org/10.1002/art.21120
Malik MHA, Chougle A, Pradhan N et al (2005) Primary total knee replacement: a comparison of a nationally agreed guide to best practice and current surgical technique as determined by the North West Regional Arthroplasty Register. Ann R Coll Surg Engl 87:117–122. https://doi.org/10.1308/1478708051676
Harse JD, Holman CDJ (2005) Charlson’s Index was a poor predictor of quality of life outcomes in a study of patients following joint replacement surgery. J Clin Epidemiol 58:1142–1149. https://doi.org/10.1016/j.jclinepi.2005.02.017
Parvizi J, Kim K-I, Goldberg G et al (2006) Recurrent instability after total hip arthroplasty: beware of subtle component malpositioning. Clin Orthop Relat Res 447:60–65. https://doi.org/10.1097/01.blo.0000218749.37860.7c
White SP, Smith EJ (2007) Minimal access surgery for total hip arthroplasty—current beliefs and activity profile in the UK. Ann R Coll Surg Engl 89:36–40. https://doi.org/10.1308/003588407X160800
Gioe TJ, Sinner P, Mehle S et al (2007) Excellent survival of all-polyethylene tibial components in a community joint registry. Clin Orthop Relat Res 464:88–92. https://doi.org/10.1097/BLO.0b013e31812f7879
Le Duff MJ, Amstutz HC, Dorey FJ (2007) Metal-on-metal hip resurfacing for obese patients. J Bone Joint Surg Am 89:2705–2711. https://doi.org/10.2106/JBJS.F.01563
Stea S, Bordini B, Viceconti M et al (2008) Is laterality associated with a higher rate of hip arthroplasty on the dominant side? Artif Organs 32:73–77. https://doi.org/10.1111/j.1525-1594.2007.00457.x
Gandhi R, Davey JR, Mahomed NN (2008) Predicting patient dissatisfaction following joint replacement surgery. J Rheumatol 35:2415–2418. https://doi.org/10.3899/jrheum.080295
Middernacht B, De Wilde L, Molé D et al (2008) Glenosphere disengagement: a potentially serious default in reverse shoulder surgery. Clin Orthop Relat Res 466:892–898. https://doi.org/10.1007/s11999-007-0090-6
Dudley TE, Gioe TJ, Sinner P, Mehle S (2008) Registry outcomes of unicompartmental knee arthroplasty revisions. Clin Orthop Relat Res 466:1666–1670. https://doi.org/10.1007/s11999-008-0279-3
Stea S, Bordini B, De Clerico M et al (2009) First hip arthroplasty register in Italy: 55,000 cases and 7 year follow-up. Int Orthop 33:339–346. https://doi.org/10.1007/s00264-007-0465-z
Restrepo C, Ghanem E, Houssock C et al (2009) Isolated polyethylene exchange versus acetabular revision for polyethylene wear. Clin Orthop Relat Res 467:194–198. https://doi.org/10.1007/s11999-008-0533-8
Galat DD, McGovern SC, Larson DR et al (2009) Surgical treatment of early wound complications following primary total knee arthroplasty. J Bone Joint Surg Am 91:48–54. https://doi.org/10.2106/JBJS.G.01371
Springer BD, Fehring TK, Griffin WL et al (2009) Why revision total hip arthroplasty fails. Clin Orthop Relat Res 467:166–173. https://doi.org/10.1007/s11999-008-0566-z
Hyer CF, Portera WB, Haglund EM (2010) Lower extremity implant registries: Has the time come in the United States? Foot Ankle Spec 3:148–152. https://doi.org/10.1177/1938640010369254
Gandhi R, Razak F, Davey JR, Mahomed NN (2010) Metabolic syndrome and the functional outcomes of hip and knee arthroplasty. J Rheumatol 37:1917–1922. https://doi.org/10.3899/jrheum.091242
Gandhi R, Dhotar H, Razak F et al (2010) Predicting the longer term outcomes of total knee arthroplasty. Knee 17:15–18. https://doi.org/10.1016/j.knee.2009.06.003
Gandhi R, Dhotar H, Davey JR, Mahomed NN (2010) Predicting the longer-term outcomes of total hip replacement. J Rheumatol 37:2573–2577. https://doi.org/10.3899/jrheum.100149
Lübbeke A, Garavaglia G, Barea C, Hoffmeyer P (2010) Why do we need hospital-based registries? The Geneva Hip Arthroplasty Registry. Hug 22:66
Schäfer T, Krummenauer F, Mettelsiefen J et al (2010) Social, educational, and occupational predictors of total hip replacement outcome. Osteoarthr Cartil 18:1036–1042. https://doi.org/10.1016/j.joca.2010.05.003
Ibrahim T, Bloch B, Esler CN et al (2010) Temporal trends in primary total hip and knee arthroplasty surgery: results from a UK regional joint register, 1991–2004. Ann R Coll Surg Engl 92:231–235. https://doi.org/10.1308/003588410X12628812458572
Gandhi R, Dhotar H, Tsvetkov D, Mahomed NN (2010) The relation between body mass index and waist-hip ratio in knee osteoarthritis. Can J Surg 53:151–154
Bourne RB, Chesworth B, Davis A et al (2010) Comparing patient outcomes after THA and TKA: Is there a difference? Clin Orthop Relat Res 468:542–546. https://doi.org/10.1007/s11999-009-1046-9
Mall NA, Nunley RM, Smith KE et al (2010) The fate of grafting acetabular defects during revision total hip arthroplasty. Clin Orthop Relat Res 468:3286–3294. https://doi.org/10.1007/s11999-010-1427-0
Di Tanna GL, Ferro S, Cipriani F et al (2011) Modeling the cost-effectiveness for cement-less and hybrid prosthesis in total hip replacement in Emilia Romagna, Italy. J Surg Res 169:227–233. https://doi.org/10.1016/j.jss.2009.10.031
Singh JA, Lewallen DG (2011) Association of peptic ulcer disease and pulmonary disease with risk of periprosthetic fracture after primary total knee arthroplasty. Arthritis Care Res 63:1471–1476. https://doi.org/10.1002/acr.20548
Ghomrawi HMK, Mandl LA, Rutledge J et al (2011) Is there a role for expectation maximization imputation in addressing missing data in research using WOMAC questionnaire? Comparison to the standard mean approach and a tutorial. BMC Musculoskelet Disord 12:109. https://doi.org/10.1186/1471-2474-12-109
Singh JA, Sperling JW, Cofield RH (2011) Ninety day mortality and its predictors after primary shoulder arthroplasty: an analysis of 4,019 patients from 1976–2008. BMC Musculoskelet Disord 12:231. https://doi.org/10.1186/1471-2474-12-231
Ebrahimpour PB, Do HT, Bornstein LJ, Westrich GH (2011) Relationship between demographic variables and preoperative pain and disability in 5945 total joint arthroplasties at a single institution. J Arthroplasty 26:133-137.e1. https://doi.org/10.1016/j.arth.2011.04.011
Singh JA, Sperling JW, Cofield RH (2011) Revision surgery following total shoulder arthroplasty: analysis of 2588 shoulders over three decades (1976 to 2008). J Bone Joint Surg Br 93:1513–1517. https://doi.org/10.1302/0301-620X.93B11.26938
Dushey CH, Bornstein LJ, Alexiades MM, Westrich GH (2011) Short-term coagulation complications following total knee arthroplasty: a comparison of patient-reported and surgeon-verified complication rates. J Arthroplasty 26:1338–1342. https://doi.org/10.1016/j.arth.2010.11.007
Howard JL, Kremers HM, Loechler YA et al (2011) Comparative survival of uncemented acetabular components following primary total hip arthroplasty. J Bone Joint Surg Am 93:1597–1604. https://doi.org/10.2106/JBJS.J.00195
Dowsey MM, Liew D, Choong PFM (2011) Economic burden of obesity in primary total knee arthroplasty. Arthritis Care Res 63:1375–1381. https://doi.org/10.1002/acr.20563
Zmistowski B, Restrepo C, Kahl LK et al (2011) Incidence and reasons for nonrevision reoperation after total knee arthroplasty. Clin Orthop Relat Res 469:138–145. https://doi.org/10.1007/s11999-010-1558-3
Abdel MP, Morrey ME, Jensen MR, Morrey BF (2011) Increased long-term survival of posterior cruciate-retaining versus posterior cruciate-stabilizing total knee replacements. J Bone Joint Surg Am 93:2072–2078. https://doi.org/10.2106/JBJS.J.01143
Alzahrani K, Gandhi R, Debeer J et al (2011) Prevalence of clinically significant improvement following total knee replacement. J Rheumatol 38:753–759. https://doi.org/10.3899/jrheum.100233
Jacob AK, Mantilla CB, Sviggum HP et al (2011) Perioperative nerve injury after total knee arthroplasty: regional anesthesia risk during a 20-year cohort study. Anesthesiology 114:311–317. https://doi.org/10.1097/ALN.0b013e3182039f5d
Gioe TJ, Sharma A, Tatman P et al (2011) Do “premium” joint implants add value?: analysis of high cost joint implants in a community registry. Clin Orthop Relat Res 469:48–54. https://doi.org/10.1007/s11999-010-1436-z
Callanan MC, Jarrett B, Bragdon CR et al (2011) The John Charnley Award: risk factors for cup malpositioning: quality improvement through a joint registry at a tertiary hospital. Clin Orthop Relat Res 469:319–329. https://doi.org/10.1007/s11999-010-1487-1
Johnson TC, Tatman PJ, Mehle S, Gioe TJ (2012) Revision surgery for patellofemoral problems: should we always resurface? Clin Orthop Relat Res 470:211–219. https://doi.org/10.1007/s11999-011-2036-2
Singh JA, Sperling JW, Cofield RH (2012) Cardiopulmonary complications after primary shoulder arthroplasty: a cohort study. Semin Arthritis Rheum 41:689–697. https://doi.org/10.1016/j.semarthrit.2011.09.003
Choi JK, Geller JA, Yoon RS et al (2012) Comparison of total hip and knee arthroplasty cohorts and short-term outcomes from a single-center joint registry. J Arthroplasty 27:837–841. https://doi.org/10.1016/j.arth.2012.01.016
Wang W, Geller JA, Nyce JD et al (2012) Does ipsilateral knee pain improve after hip arthroplasty? Clin Orthop Relat Res 470:578–583. https://doi.org/10.1007/s11999-011-2116-3
Singh JA, Lewallen DG (2012) Ninety-day mortality in patients undergoing elective total hip or total knee arthroplasty. J Arthroplasty 27:1417-1422.e1. https://doi.org/10.1016/j.arth.2012.03.008
Singh JA, Jensen MR, Lewallen DG (2012) Patient factors predict periprosthetic fractures after revision total hip arthroplasty. J Arthroplasty 27:1507–1512. https://doi.org/10.1016/j.arth.2011.12.010
Singh JA, Lewallen DG (2012) Peptic ulcer disease and heart disease are associated with periprosthetic fractures after total hip replacement. Acta Orthop 83:353–359. https://doi.org/10.3109/17453674.2012.717844
Sviggum HP, Jacob AK, Mantilla CB et al (2012) Perioperative nerve injury after total shoulder arthroplasty: assessment of risk after regional anesthesia. Reg Anesth Pain Med 37:490–494. https://doi.org/10.1097/AAP.0b013e31825c258b
Singh JA, Sperling JW, Schleck C et al (2012) Periprosthetic infections after shoulder hemiarthroplasty. J Shoulder Elbow Surg 21:1304–1309. https://doi.org/10.1016/j.jse.2011.08.067
Singh JA, Lewallen DG (2012) Predictors of use of pain medications for persistent knee pain after primary Total Knee Arthroplasty: a cohort study using an institutional joint registry. Arthritis Res Ther 14:R248. https://doi.org/10.1186/ar4091
Singh JA, Sperling JW, Cofield RH (2012) Risk factors for revision surgery after humeral head replacement: 1,431 shoulders over 3 decades. J Shoulder Elbow Surg 21:1039–1044. https://doi.org/10.1016/j.jse.2011.06.015
Yu Y-H, Chen AC-Y, Hu C-C et al (2012) Acute delirium and poor compliance in total hip arthroplasty patients with substance abuse disorders. J Arthroplasty 27:1526–1529. https://doi.org/10.1016/j.arth.2011.12.003
Singh JA, Sperling J, Schleck C et al (2012) Periprosthetic fractures associated with primary total shoulder arthroplasty and primary humeral head replacement: a thirty-three-year study. J Bone Joint Surg Am 94:1777–1785. https://doi.org/10.2106/JBJS.J.01945
Kubista B, Hartzler RU, Wood CM et al (2012) Reinfection after two-stage revision for periprosthetic infection of total knee arthroplasty. Int Orthop 36:65–71. https://doi.org/10.1007/s00264-011-1267-x
Plate JF, Seyler TM, Stroh DA et al (2012) Risk of dislocation using large- vs. small-diameter femoral heads in total hip arthroplasty. BMC Res Notes 5:553. https://doi.org/10.1186/1756-0500-5-553
Labek G, Kovac S, Levasic V et al (2012) The outcome of the cementless tapered SL-Plus stem: an analysis of arthroplasty register data. Int Orthop 36:1149–1154. https://doi.org/10.1007/s00264-011-1421-5
Severson EP, Singh JA, Browne JA et al (2012) Total knee arthroplasty in morbidly obese patients treated with bariatric surgery: a comparative study. J Arthroplasty 27:1696–1700. https://doi.org/10.1016/j.arth.2012.03.005
Barr CJ, Barbalace RJ, Wessinger SJ et al (2012) Validation of a hospital-based joint registry: quantification of errors and maximizing utility. J Arthroplasty 27:1766–1771. https://doi.org/10.1016/j.arth.2012.04.028
Lawless BM, Greene M, Slover J et al (2012) Does age or bilateral disease influence the value of hip arthroplasty? Clin Orthop Relat Res 470:1073–1078. https://doi.org/10.1007/s11999-011-2118-1
Farshad M, Grögli M, Catanzaro S, Gerber C (2012) Revision of reversed total shoulder arthroplasty Indications and outcome. BMC Musculoskelet Disord 13:160. https://doi.org/10.1186/1471-2474-13-160
Singh JA, Lewallen DG (2013) Better functional and similar pain outcomes in osteoarthritis compared to rheumatoid arthritis after primary total knee arthroplasty: a cohort study. Arthritis Care Res 65:1936–1941. https://doi.org/10.1002/acr.22090
Riddle DL, Singh JA, Harmsen WS et al (2013) Clinically important body weight gain following knee arthroplasty: a five-year comparative cohort study. Arthritis Care Res 65:669–677. https://doi.org/10.1002/acr.21880
Riddle DL, Singh JA, Harmsen WS et al (2013) Clinically important body weight gain following total hip arthroplasty: a cohort study with 5-year follow-up. Osteoarthr Cartil 21:35–43. https://doi.org/10.1016/j.joca.2012.09.010
Singh JA, Lewallen DG (2013) Diabetes: a risk factor for poor functional outcome after total knee arthroplasty. PLoS ONE 8:e78991. https://doi.org/10.1371/journal.pone.0078991
Singh JA, Lewallen DG (2013) Medical and psychological comorbidity predicts poor pain outcomes after total knee arthroplasty. Rheumatology 52:916–923. https://doi.org/10.1093/rheumatology/kes402
Singh JA, Lewallen DG (2013) Medical comorbidity is associated with persistent index hip pain after total hip arthroplasty. Pain Med 14:1222–1229. https://doi.org/10.1111/pme.12153
Vulcano E, Lee Y-Y, Yamany T et al (2013) Obese patients undergoing total knee arthroplasty have distinct preoperative characteristics: an institutional study of 4718 patients. J Arthroplasty 28:1125–1129. https://doi.org/10.1016/j.arth.2012.10.028
Singh JA, Lewallen DG (2013) Operative diagnosis for revision total hip arthroplasty is associated with patient-reported outcomes (PROs). BMC Musculoskelet Disord 14:210. https://doi.org/10.1186/1471-2474-14-210
Singh JA, Lewallen DG (2013) Patient-level clinically meaningful improvements in activities of daily living and pain after total hip arthroplasty: data from a large US institutional registry. Rheumatology 52:1109–1118. https://doi.org/10.1093/rheumatology/kes416
Singh JA, Jensen M, Lewallen D (2013) Predictors of periprosthetic fracture after total knee replacement: an analysis of 21,723 cases. Acta Orthop 84:170–177. https://doi.org/10.3109/17453674.2013.788436
Girard J, Kern G, Migaud H et al (2013) Primary total hip arthroplasty revision due to dislocation: prospective French multicenter study. Orthop Traumatol Surg Res 99:549–553. https://doi.org/10.1016/j.otsr.2013.03.026
Lübbeke A, Garavaglia G, Rothman KJ et al (2013) Statins may reduce femoral osteolysis in patients with total Hip arthroplasty. J Orthop Res 31:814–820. https://doi.org/10.1002/jor.22262
Delaunay C, Hamadouche M, Girard J, Duhamel A (2013) What are the causes for failures of primary hip arthroplasties in France? Clin Orthop Relat Res 471:3863–3869. https://doi.org/10.1007/s11999-013-2935-5
Olivecrona C, Lapidus LJ, Benson L, Blomfeldt R (2013) Tourniquet time affects postoperative complications after knee arthroplasty. Int Orthop 37:827–832. https://doi.org/10.1007/s00264-013-1826-4
Duncan CM, Moeschler SM, Horlocker TT et al (2013) A self-paired comparison of perioperative outcomes before and after implementation of a clinical pathway in patients undergoing total knee arthroplasty. Reg Anesth Pain Med 38:533–538. https://doi.org/10.1097/AAP.0000000000000014
Wasserstein D, Farlinger C, Brull R et al (2013) Advanced age, obesity and continuous femoral nerve blockade are independent risk factors for inpatient falls after primary total knee arthroplasty. J Arthroplasty 28:1121–1124. https://doi.org/10.1016/j.arth.2012.08.018
Siow WM, Chin PL, Chia SL et al (2013) Comparative demographics, ROM, and function after TKA in Chinese, Malays, and Indians. Clin Orthop Relat Res 471:1451–1457. https://doi.org/10.1007/s11999-012-2776-7
Beyer-Westendorf J, Lützner J, Donath L et al (2013) Efficacy and safety of thromboprophylaxis with low-molecular-weight heparin or rivaroxaban in hip and knee replacement surgery: findings from the ORTHO-TEP registry. Thromb Haemost 109:154–163. https://doi.org/10.1160/TH12-07-0510
Singh JA, Lewallen DG (2013) Income and patient-reported outcomes (PROs) after primary total knee arthroplasty. BMC Med 11:62. https://doi.org/10.1186/1741-7015-11-62
Baker P, Muthumayandi K, Gerrand C et al (2013) Influence of body mass index (BMI) on functional improvements at 3 years following total knee replacement: a retrospective cohort study. PLoS ONE 8:e59079. https://doi.org/10.1371/journal.pone.0059079
Singh JA, Lewallen DG (2013) Ipsilateral lower extremity joint involvement increases the risk of poor pain and function outcomes after hip or knee arthroplasty. BMC Med 11:144. https://doi.org/10.1186/1741-7015-11-144
Johnson BK, Goodman SM, Alexiades MM et al (2013) Patterns and associated risk of perioperative use of anti-tumor necrosis factor in patients with rheumatoid arthritis undergoing total knee replacement. J Rheumatol 40:617–623. https://doi.org/10.3899/jrheum.121171
Sierra RJ, Kassel CA, Wetters NG et al (2013) Revision of unicompartmental arthroplasty to total knee arthroplasty: not always a slam dunk! J Arthroplasty 28:128–132. https://doi.org/10.1016/j.arth.2013.02.040
Argenson J-N, Boisgard S, Parratte S et al (2013) Survival analysis of total knee arthroplasty at a minimum 10 years’ follow-up: a multicenter French nationwide study including 846 cases. Orthop Traumatol Surg Res 99:385–390. https://doi.org/10.1016/j.otsr.2013.03.014
Suero EM, Citak M, Claps C et al (2013) Variations in ankle registration using two different anatomic landmarks: a radiographic study. Knee Surg Sports Traumatol Arthrosc 21:2759–2763. https://doi.org/10.1007/s00167-012-2165-5
Dakin H, Gray A, Murray D (2013) Mapping analyses to estimate EQ-5D utilities and responses based on Oxford Knee Score. Qual Life Res 22:683–694. https://doi.org/10.1007/s11136-012-0189-4
Kamath AF, Prieto H, Lewallen DG (2013) Alternative bearings in total hip arthroplasty in the young patient. Orthop Clin N Am 44:451–462. https://doi.org/10.1016/j.ocl.2013.06.001
Colwell CWJ, Froimson MI, Anseth SD et al (2014) A mobile compression device for thrombosis prevention in hip and knee arthroplasty. J Bone Joint Surg Am 96:177–183. https://doi.org/10.2106/JBJS.L.01031
Singh JA, Lewallen DG (2014) Cerebrovascular disease is associated with outcomes after total knee arthroplasty: a US total joint registry study. J Arthroplasty 29:40–43. https://doi.org/10.1016/j.arth.2013.04.003
Ast MP, Gorab AH, Banka TR et al (2014) Clinical outcomes of patients with non-fatal VTE after total knee arthroplasty. J Arthroplasty 29:37–39. https://doi.org/10.1016/j.arth.2013.04.013
Kremers HM, Sierra RJ, Schleck CD et al (2014) Comparative survivorship of different tibial designs in primary total knee arthroplasty. J Bone Joint Surg Am 96:e121. https://doi.org/10.2106/JBJS.M.00820
Hansen VJ, Greene ME, Bragdon MA et al (2014) Registries collecting level-I through IV Data: institutional and multicenter use: AAOS exhibit selection. J Bone Joint Surg Am 96:e160. https://doi.org/10.2106/JBJS.M.01458
Seah RB, Yeo SJ, Chin PL et al (2014) Evaluation of medial-lateral stability and functional outcome following total knee arthroplasty: results of a single hospital joint registry. J Arthroplasty 29:2276–2279. https://doi.org/10.1016/j.arth.2014.04.015
Singh JA, Lewallen DG (2014) Increasing obesity and comorbidity in patients undergoing primary total hip arthroplasty in the U.S.: a 13-year study of time trends. BMC Musculoskelet Disord 15:441. https://doi.org/10.1186/1471-2474-15-441
Owens CJ, Sperling JW, Cofield RH (2014) Long-stemmed humeral components in primary shoulder arthroplasty. J Shoulder Elbow Surg 23:1492–1498. https://doi.org/10.1016/j.jse.2014.01.008
Singh JA, Lewallen DG (2014) Patient-level improvements in pain and activities of daily living after total knee arthroplasty. Rheumatology 53:313–320. https://doi.org/10.1093/rheumatology/ket325
Goodman SM, Ramsden-Stein DN, Huang W-T et al (2014) Patients with rheumatoid arthritis are more likely to have pain and poor function after total hip replacements than patients with osteoarthritis. J Rheumatol 41:1774–1780. https://doi.org/10.3899/jrheum.140011
Singh JA, Lewallen DG (2014) Predictors of pain medication use for arthroplasty pain after revision total knee arthroplasty. Rheumatology 53:1752–1758. https://doi.org/10.1093/rheumatology/ket443
Ehlinger M, Delaunay C, Karoubi M et al (2014) Revision of primary total hip arthroplasty for peri-prosthetic fracture: a prospective epidemiological study of 249 consecutive cases in France. Orthop Traumatol Surg Res 100:657–662. https://doi.org/10.1016/j.otsr.2014.03.030
Lübbeke A, Rothman KJ, Garavaglia G et al (2014) Strong association between smoking and the risk of revision in a cohort study of patients with metal-on-metal total hip arthroplasty. J Orthop Res 32:762–768. https://doi.org/10.1002/jor.22603
Singh JA, Lewallen DG (2014) Time trends in the characteristics of patients undergoing primary total knee arthroplasty. Arthritis Care Res 66:897–906. https://doi.org/10.1002/acr.22233
Brophy RH, Gray BL, Nunley RM et al (2014) Total knee arthroplasty after previous knee surgery: expected interval and the effect on patient age. J Bone Joint Surg Am 96:801–805. https://doi.org/10.2106/JBJS.M.00105
Noiseux NO, Long WJ, Mabry TM et al (2014) Uncemented porous tantalum acetabular components: early follow-up and failures in 613 primary total hip arthroplasties. J Arthroplasty 29:617–620. https://doi.org/10.1016/j.arth.2013.07.037
Singh JA, Lewallen DG (2014) Underlying diagnosis predicts patient-reported outcomes after revision total knee arthroplasty. Rheumatology 53:361–366. https://doi.org/10.1093/rheumatology/ket357
Salassa T, Hoeffel D, Mehle S et al (2014) Efficacy of revision surgery for the dislocating total hip arthroplasty: report from a large community registry. Clin Orthop Relat Res 472:962–967. https://doi.org/10.1007/s11999-013-3344-5
Singh JA, Lewallen DG (2014) Are outcomes after total knee arthroplasty worsening over time? A time-trends study of activity limitation and pain outcomes. BMC Musculoskelet Disord 15:440. https://doi.org/10.1186/1471-2474-15-440
Ashraf A, Larson AN, Maradit-Kremers H et al (2014) Hospital costs of total hip arthroplasty for developmental dysplasia of the hip. Clin Orthop Relat Res 472:2237–2244. https://doi.org/10.1007/s11999-014-3587-9
Imam MA, Barke S, Stafford GH et al (2014) Loss to follow-up after total hip replacement: a source of bias in patient reported outcome measures and registry datasets? Hip Int 24:465–472. https://doi.org/10.5301/hipint.5000141
Maradit Kremers H, Visscher SL, Kremers WK et al (2014) Obesity increases length of stay and direct medical costs in total hip arthroplasty. Clin Orthop Relat Res 472:1232–1239. https://doi.org/10.1007/s11999-013-3316-9
Adelani MA, Mall NA, Nyazee H et al (2014) Revision total hip arthroplasty with retained acetabular component. J Bone Joint Surg Am 96:1015–1020. https://doi.org/10.2106/JBJS.L.01177
Kremers HM, Visscher SL, Kremers WK et al (2014) The effect of obesity on direct medical costs in total knee arthroplasty. J Bone Joint Surg Am 96:718–724. https://doi.org/10.2106/JBJS.M.00819
Ahmadi S, Lawrence TM, Sahota S et al (2014) The incidence and risk factors for blood transfusion in revision shoulder arthroplasty: our institution’s experience and review of the literature. J Shoulder Elbow Surg 23:43–48. https://doi.org/10.1016/j.jse.2013.03.010
Christensen CP, Karthikeyan T, Jacobs CA (2014) Greater prevalence of wound complications requiring reoperation with direct anterior approach total hip arthroplasty. J Arthroplasty 29:1839–1841. https://doi.org/10.1016/j.arth.2014.04.036
Bordini B, Stea S, Falcioni S et al (2014) Unicompartmental knee arthroplasty: 11-year experience from 3929 implants in RIPO register. Knee 21:1275–1279. https://doi.org/10.1016/j.knee.2014.02.012
Inacio MCS, Kritz-Silverstein D, Raman R et al (2014) The impact of pre-operative weight loss on incidence of surgical site infection and readmission rates after total joint arthroplasty. J Arthroplasty 29:458–64.e1. https://doi.org/10.1016/j.arth.2013.07.030
Issa K, Rifai A, Boylan MR et al (2015) Do various factors affect the frequency of manipulation under anesthesia after primary total knee arthroplasty? Clin Orthop Relat Res 473:143–147. https://doi.org/10.1007/s11999-014-3772-x
Esposito CI, Gladnick BP, Lee Y-Y et al (2015) Cup position alone does not predict risk of dislocation after hip arthroplasty. J Arthroplasty 30:109–113. https://doi.org/10.1016/j.arth.2014.07.009
Dowsey MM, Smith AJ, Choong PFM (2015) Latent Class Growth Analysis predicts long term pain and function trajectories in total knee arthroplasty: a study of 689 patients. Osteoarthr Carti 23:2141–2149. https://doi.org/10.1016/j.joca.2015.07.005
Patel J, Lee JH, Li Z et al (2015) Predictors of low patient-reported outcomes response rates in the california joint replacement registry. J Arthroplasty 30:2071–2075. https://doi.org/10.1016/j.arth.2015.06.029
Wiater BP, Koueiter DM, Maerz T et al (2015) Preoperative deltoid size and fatty infiltration of the deltoid and rotator cuff correlate to outcomes after reverse total shoulder arthroplasty. Clin Orthop Relat Res 473:663–673. https://doi.org/10.1007/s11999-014-4047-2
Singh JA, Inacio MCS, Namba RS, Paxton EW (2015) Rheumatoid arthritis is associated with higher ninety-day hospital readmission rates compared to osteoarthritis after hip or knee arthroplasty: a cohort study. Arthritis Care Res 67:718–724. https://doi.org/10.1002/acr.22497
Morris BJ, Haigler RE, Laughlin MS et al (2015) Workers’ compensation claims and outcomes after reverse shoulder arthroplasty. J Shoulder Elbow Surg 24:453–459. https://doi.org/10.1016/j.jse.2014.07.009
Wilke B, Wagner E, Trousdale R (2015) Long-term survival of a semi-constrained implant following revision for infection. J Arthroplasty 30:808–812. https://doi.org/10.1016/j.arth.2014.10.037
Lampropoulou-Adamidou K, Macheras GA, Hartofilakidis G (2015) Bilateral character of total hip replacement does not change the overall survival. Hip Int 25:138–141. https://doi.org/10.5301/hipint.5000202
Soukup DS, O’Malley MJ, Ellis SJ (2015) Costs versus benefits of routine histopathological examination in total ankle replacement. Foot Ankle Int 36:801–805. https://doi.org/10.1177/1071100715576371
Maradit Kremers H, Lewallen LW, Mabry TM et al (2015) Diabetes mellitus, hyperglycemia, hemoglobin A1C and the risk of prosthetic joint infections in total hip and knee arthroplasty. J Arthroplasty 30:439–443. https://doi.org/10.1016/j.arth.2014.10.009
Peel TN, Cheng AC, Liew D et al (2015) Direct hospital cost determinants following hip and knee arthroplasty. Arthritis Care Res 67:782–790. https://doi.org/10.1002/acr.22523
Maradit Kremers H, Lewallen LW, Lahr BD et al (2015) Do claims-based comorbidities adequately capture case mix for surgical site infections? Clin Orthop Relat Res 473:1777–1786. https://doi.org/10.1007/s11999-014-4083-y
Naranje S, Lendway L, Mehle S, Gioe TJ (2015) Does operative time affect infection rate in primary total knee arthroplasty? Clin Orthop Relat Res 473:64–69. https://doi.org/10.1007/s11999-014-3628-4
Foster SA, Hambright DS, Antoci V et al (2015) Effects of obesity on health related quality of life following total hip arthroplasty. J Arthroplasty 30:1551–1554. https://doi.org/10.1016/j.arth.2015.03.023
Helwani MA, Avidan MS, Ben Abdallah A et al (2015) Effects of regional versus general anesthesia on outcomes after total hip arthroplasty: a retrospective propensity-matched cohort study. J Bone Joint Surg Am 97:186–193. https://doi.org/10.2106/JBJS.N.00612
Wagner ER, Demark RV 3rd, Wilson GA et al (2015) Intraoperative periprosthetic fractures associated with metacarpophalangeal joint arthroplasty. J Hand Surg Am 40:945–950. https://doi.org/10.1016/j.jhsa.2014.12.038
Fitch DA, Ancarani C, Bordini B (2015) Long-term survivorship and complication rate comparison of a cementless modular stem and cementless fixed neck stems for primary total hip replacement. Int Orthop 39:1827–1832. https://doi.org/10.1007/s00264-015-2894-4
Fehring TK, Fehring K, Odum SM (2015) Metal artifact reduction sequence MRI abnormalities occur in metal-on-polyethylene hips. Clin Orthop Relat Res 473:574–580. https://doi.org/10.1007/s11999-014-3873-6
Houdek MT, Wagner ER, Watts CD et al (2015) Morbid obesity: a significant risk factor for failure of two-stage revision total hip arthroplasty for infection. J Bone Joint Surg Am 97:326–332. https://doi.org/10.2106/JBJS.N.00515
Gulotta LV, Chambers KL, Warren RF et al (2015) No differences in early results of a hybrid glenoid compared with a pegged implant. Clin Orthop Relat Res 473:3918–3924. https://doi.org/10.1007/s11999-015-4558-5
Thambiah MD, Nathan S, Seow BZX et al (2015) Patient satisfaction after total knee arthroplasty: an Asian perspective. Singapore Med J 56:259–263. https://doi.org/10.11622/smedj.2015074
Yuan BJ, Lewallen DG, Hanssen AD (2015) Porous metal acetabular components have a low rate of mechanical failure in THA after operatively treated acetabular fracture. Clin Orthop Relat Res 473:536–542. https://doi.org/10.1007/s11999-014-3852-y
Maratt JD, Lee Y, Lyman S, Westrich GH (2015) Predictors of satisfaction following total knee arthroplasty. J Arthroplasty 30:1142–1145. https://doi.org/10.1016/j.arth.2015.01.039
Hurst JM, Berend KR, Adams JB, Lombardi AVJ (2015) Radiographic comparison of mobile-bearing partial knee single-peg versus twin-peg design. J Arthroplasty 30:475–478. https://doi.org/10.1016/j.arth.2014.10.015
Stryker LS, Odum SM, Fehring TK, Springer BD (2015) Revisions of monoblock metal-on-metal THAs have high early complication rates. Clin Orthop Relat Res 473:469–474. https://doi.org/10.1007/s11999-014-3791-7
Ast MP, Abdel MP, Lee Y-Y et al (2015) Weight changes after total hip or knee arthroplasty: prevalence, predictors, and effects on outcomes. J Bone Joint Surg Am 97:911–919. https://doi.org/10.2106/JBJS.N.00232
Wagner ER, Houdek MT, Elhassan BT et al (2015) What are risk factors for intraoperative humerus fractures during revision reverse shoulder arthroplasty and do they influence outcomes? Clin Orthop Relat Res 473:3228–3234. https://doi.org/10.1007/s11999-015-4448-x
Blomfeldt R, Kasina P, Ottosson C et al (2015) Prosthetic joint infection following hip fracture and degenerative hip disorder: a cohort study of three thousand, eight hundred and seven consecutive hip arthroplasties with a minimum follow-up of five years. Int Orthop 39:2091–2096. https://doi.org/10.1007/s00264-015-2989-y
Winther SB, Foss OA, Wik TS et al (2015) 1-year follow-up of 920 hip and knee arthroplasty patients after implementing fast-track. Acta Orthop 86:78–85. https://doi.org/10.3109/17453674.2014.957089
Asaad A, Hart A, Khoo MMY et al (2015) Frequent femoral neck osteolysis with Birmingham mid-head resection resurfacing arthroplasty in young patients. Clin Orthop Relat Res 473:3770–3778. https://doi.org/10.1007/s11999-015-4348-0
Mackie A, Muthumayandi K, Shirley M et al (2015) Association between body mass index change and outcome in the first year after total knee arthroplasty. J Arthroplasty 30:206–209. https://doi.org/10.1016/j.arth.2014.09.003
Watts CD, Abdel MP, Lewallen DG et al (2015) Increased risk of periprosthetic femur fractures associated with a unique cementless stem design. Clin Orthop Relat Res 473:2045–2053. https://doi.org/10.1007/s11999-014-4077-9
Watts CD, Wagner ER, Houdek MT et al (2015) Morbid obesity: increased risk of failure after aseptic revision TKA. Clin Orthop Relat Res 473:2621–2627. https://doi.org/10.1007/s11999-015-4283-0
McLawhorn AS, Bjerke-Kroll BT, Blevins JL et al (2015) Patient-reported allergies are associated with poorer patient satisfaction and outcomes after lower extremity arthroplasty: a retrospective cohort study. J Arthroplasty 30:1132–1136. https://doi.org/10.1016/j.arth.2015.01.043
Morris BJ, Laughlin MS, Elkousy HA et al (2015) Preoperative opioid use and outcomes after reverse shoulder arthroplasty. J Shoulder Elbow Surg 24:11–16. https://doi.org/10.1016/j.jse.2014.05.002
Morris BJ, O’Connor DP, Torres D et al (2015) Risk factors for periprosthetic infection after reverse shoulder arthroplasty. J Shoulder Elbow Surg 24:161–166. https://doi.org/10.1016/j.jse.2014.05.020
Keeney JA, Martell JM, Pashos G et al (2015) Highly cross-linked polyethylene improves wear and mid-term failure rates for young total hip arthroplasty patients. Hip Int 25:435–441. https://doi.org/10.5301/hipint.5000242
Hightower CD, Hightower LS, Tatman PJ et al (2016) How often is the office visit needed? Predicting total knee arthroplasty revision risk using pain/function scores. BMC Health Serv Res 16:429. https://doi.org/10.1186/s12913-016-1669-y
Maratt JD, Gagnier JJ, Butler PD et al (2016) No difference in dislocation seen in anterior vs posterior approach total hip arthroplasty. J Arthroplasty 31:127–130. https://doi.org/10.1016/j.arth.2016.02.071
Goodman SM, Mandl LA, Parks ML et al (2016) Disparities in TKA outcomes: census tract data show interactions between race and poverty. Clin Orthop Relat Res 474:1986–1995. https://doi.org/10.1007/s11999-016-4919-8
Lum ZC, Lombardi AV, Hurst JM et al (2016) Early outcomes of twin-peg mobile-bearing unicompartmental knee arthroplasty compared with primary total knee arthroplasty. Bone Joint J 98-B:28–33. https://doi.org/10.1302/0301-620X.98B10.BJJ-2016-0414.R1
Bordini B, Ancarani C, Fitch DA (2016) Long-term survivorship of a medial-pivot total knee system compared with other cemented designs in an arthroplasty registry. J Orthop Surg Res 11:44. https://doi.org/10.1186/s13018-016-0388-8
Watts CD, Martin JR, Houdek MT et al (2016) Prior bariatric surgery may decrease the rate of re-operation and revision following total hip arthroplasty. Bone Joint J 98-B:1180–1184. https://doi.org/10.1302/0301-620X.98B9.37943
Hansen LE, Stone GL, Matson CA et al (2016) Total joint arthroplasty in patients taking methadone or buprenorphine/naloxone preoperatively for prior heroin addiction: a prospective matched cohort study. J Arthroplasty 31:1698–1701. https://doi.org/10.1016/j.arth.2016.01.032
Bin Abd Razak HR, Tan C-S, Chen YJD et al (2016) Age and preoperative knee society score are significant predictors of outcomes among asians following total knee arthroplasty. J Bone Joint Surg Am 98:735–741. https://doi.org/10.2106/JBJS.15.00280
Werner BC, Wong AC, Mahony GT et al (2016) Causes of poor postoperative improvement after reverse total shoulder arthroplasty. J Shoulder Elbow Surg 25:e217–e222. https://doi.org/10.1016/j.jse.2016.01.002
Wagner ER, Kamath AF, Fruth KM et al (2016) Effect of body mass index on complications and reoperations after total hip arthroplasty. J Bone Joint Surg Am 98:169–179. https://doi.org/10.2106/JBJS.O.00430
Wagner ER, Kamath AF, Fruth K et al (2016) Effect of body mass index on reoperation and complications after total knee arthroplasty. J Bone Joint Surg Am 98:2052–2060. https://doi.org/10.2106/JBJS.16.00093
Haase E, Kopkow C, Beyer F et al (2016) Patient-reported outcomes and outcome predictors after primary total hip arthroplasty: results from the Dresden Hip Surgery Registry. Hip Int 26:73–81. https://doi.org/10.5301/hipint.5000300
Schilling C, Dowsey MM, Clarke PM, Choong PF (2016) Using patient-reported outcomes for economic evaluation: getting the timing right. Value Health 19:945–950. https://doi.org/10.1016/j.jval.2016.05.014
Benditz A, Drescher J, Greimel F et al (2016) Implementing a benchmarking and feedback concept decreases postoperative pain after total knee arthroplasty: a prospective study including 256 patients. Sci Rep 6:38218. https://doi.org/10.1038/srep38218
Hussey DK, Madanat R, Donahue GS et al (2016) Worse health-related quality of life and hip function in female patients with elevated chromium levels. Acta Orthop 87:485–491. https://doi.org/10.1080/17453674.2016.1213596
D’Apuzzo MR, Villa JM, Alcerro JC et al (2016) Total joint arthroplasty: a granular analysis of outcomes in the economically disadvantaged patient. J Arthroplasty 31:41–44. https://doi.org/10.1016/j.arth.2016.02.066
Houdek MT, Wagner ER, Wyles CC et al (2016) All-polyethylene tibial components: an analysis of long-term outcomes and infection. J Arthroplasty 31:1476–1482. https://doi.org/10.1016/j.arth.2015.12.048
Markel DC, Allen MW, Zappa NM (2016) Can an arthroplasty registry help decrease transfusions in primary total joint replacement? A quality initiative. Clin Orthop Relat Res 474:126–131. https://doi.org/10.1007/s11999-015-4470-z
Guerrero-Ludueña RE, Comas M, Espallargues M et al (2016) Predicting the Burden of Revision Knee Arthroplasty: simulation of a 20-year Horizon. Value Health 19:680–687. https://doi.org/10.1016/j.jval.2016.02.018
Long G, Hao C, Li G et al (2016) Predictive value of B-type natriuretic peptide (BNP) for adverse cardiac events in patients undergoing primary total knee arthroplasty (TKA). J Orthop Sci 21:826–830. https://doi.org/10.1016/j.jos.2016.08.003
Hallstrom B, Singal B, Cowen ME et al (2016) The Michigan experience with safety and effectiveness of tranexamic acid use in hip and knee arthroplasty. J Bone Joint Surg Am 98:1646–1655. https://doi.org/10.2106/JBJS.15.01010
Lyman S, Lee Y-Y, Franklin PD et al (2016) Validation of the HOOS, JR: a short-form hip replacement survey. Clin Orthop Relat Res 474:1472–1482. https://doi.org/10.1007/s11999-016-4718-2
Lyman S, Lee Y-Y, Franklin PD et al (2016) Validation of the KOOS, JR: a short-form knee arthroplasty outcomes survey. Clin Orthop Relat Res 474:1461–1471. https://doi.org/10.1007/s11999-016-4719-1
Werner BC, Chang B, Nguyen JT et al (2016) What change in american shoulder and elbow surgeons score represents a clinically important change after shoulder arthroplasty? Clin Orthop Relat Res 474:2672–2681. https://doi.org/10.1007/s11999-016-4968-z
Tetreault MW, Della Valle CJ, Bohl DD et al (2016) What factors influence the success of medial gastrocnemius flaps in the treatment of infected TKAs? Clin Orthop Relat Res 474:752–763. https://doi.org/10.1007/s11999-015-4624-z
Goodman SM, Johnson B, Zhang M et al (2016) Patients with rheumatoid arthritis have similar excellent outcomes after total knee replacement compared with patients with osteoarthritis. J Rheumatol 43:46–53. https://doi.org/10.3899/jrheum.150525
Emerson RH, Alnachoukati O, Barrington J, Ennin K (2016) The results of Oxford unicompartmental knee arthroplasty in the United States: a mean ten-year survival analysis. Bone Joint J 98-B:34–40. https://doi.org/10.1302/0301-620X.98B10.BJJ-2016-0480.R1
Jacobs CA, Morris BJ, Sciascia AD, Edwards TB (2016) Comparison of satisfied and dissatisfied patients 2 to 5 years after anatomic total shoulder arthroplasty. J Shoulder Elbow Surg 25:1128–1132. https://doi.org/10.1016/j.jse.2015.12.001
Haynes JA, Stambough JB, Sassoon AA et al (2016) Contemporary surgical indications and referral trends in revision total hip arthroplasty: a 10-year review. J Arthroplasty 31:622–625. https://doi.org/10.1016/j.arth.2015.09.026
Wilson MD, Dowsey MM, Spelman T, Choong PFM (2016) Impact of surgical experience on outcomes in total joint arthroplasties. ANZ J Surg 86:967–972. https://doi.org/10.1111/ans.13513
Berliner JL, Brodke DJ, Chan V et al (2016) John Charnley Award: preoperative patient-reported outcome measures predict clinically meaningful improvement in function after THA. Clin Orthop Relat Res 474:321–329. https://doi.org/10.1007/s11999-015-4350-6
Morris BJ, Sciascia AD, Jacobs CA, Edwards TB (2016) Preoperative opioid use associated with worse outcomes after anatomic shoulder arthroplasty. J Shoulder Elbow Surg 25:619–623. https://doi.org/10.1016/j.jse.2015.09.017
Liu JN, Garcia GH, Mahony G et al (2016) Sports after shoulder arthroplasty: a comparative analysis of hemiarthroplasty and reverse total shoulder replacement. J Shoulder Elbow Surg 25:920–926. https://doi.org/10.1016/j.jse.2015.11.003
Shannak O, Palan J, Esler C (2017) A regional registry study of 216 patients investigating if patient satisfaction after total knee arthroplasty changes over a time period of five to 20years. Knee 24:824–828. https://doi.org/10.1016/j.knee.2017.03.005
Gonzalez AI, Bartolone P, Lubbeke A et al (2017) Comparison of dual-mobility cup and unipolar cup for prevention of dislocation after revision total hip arthroplasty. Acta Orthop 88:18–23. https://doi.org/10.1080/17453674.2016.1255482
Bautista MP, Bonilla GA, Mieth KW et al (2017) Data quality in institutional arthroplasty registries: description of a model of validation and report of preliminary results. J Arthroplasty 32:2065–2069. https://doi.org/10.1016/j.arth.2017.02.030
Charles RJ, Singal BM, Urquhart AG et al (2017) Data sharing between providers and quality initiatives eliminate unnecessary nursing home admissions. J Arthroplasty 32:1418–1425. https://doi.org/10.1016/j.arth.2016.11.041
Werner BC, Wong AC, Chang B et al (2017) Depression and patient-reported outcomes following total shoulder arthroplasty. J Bone Joint Surg Am 99:688–695. https://doi.org/10.2106/JBJS.16.00541
Ghomrawi HMK, Mancuso CA, Dunning A et al (2017) Do surgeon expectations predict clinically important improvements in WOMAC scores after THA and TKA? Clin Orthop Relat Res 475:2150–2158. https://doi.org/10.1007/s11999-017-5331-8
Singleton N, Poutawera V (2017) Does preoperative mental health affect length of hospital stay and functional outcomes following arthroplasty surgery? A registry-based cohort study. J Orthop Surg 25:2309499017718902. https://doi.org/10.1177/2309499017718902
Franklin PD, Miozzari H, Christofilopoulos P et al (2017) Important patient characteristics differ prior to total knee arthroplasty and total hip arthroplasty between Switzerland and the United States. BMC Musculoskelet Disord 18:14. https://doi.org/10.1186/s12891-016-1372-5
Sanz-Ruiz P, Matas-Diez JA, Sanchez-Somolinos M et al (2017) Is the commercial antibiotic-loaded bone cement useful in prophylaxis and cost saving after knee and hip joint arthroplasty? The transatlantic paradox. J Arthroplasty 32:1095–1099. https://doi.org/10.1016/j.arth.2016.11.012
Ang J-GE, Bin Abd Razak HR, Howe T-S et al (2017) Obesity does not affect outcomes in hybrid versus cemented total knee arthroplasty in Asians. J Arthroplasty 32:3643–3646. https://doi.org/10.1016/j.arth.2017.06.043
Schilling CG, Dowsey MM, Petrie DJ et al (2017) Predicting the long-term gains in health-related quality of life after total knee arthroplasty. J Arthroplasty 32:395-401.e2. https://doi.org/10.1016/j.arth.2016.07.036
bin Abd Razak HR, Acharyya S, Tan S-M et al (2017) Predictors of midterm outcomes after medial unicompartmental knee arthroplasty in Asians. Clin Orthop Surg 9:432–438. https://doi.org/10.4055/cios.2017.9.4.432
Rothermich MA, Nam D, Brophy RH et al (2017) The impact of prior surgery after total knee arthroplasty. J Knee Surg 30:57–62. https://doi.org/10.1055/s-0036-1579666
Castagnini F, Sudanese A, Bordini B et al (2017) Total knee replacement in young patients: survival and causes of revision in a registry population. J Arthroplasty 32:3368–3372. https://doi.org/10.1016/j.arth.2017.05.052
Wagner ER, Houdek MT, Schleck C et al (2017) Increasing body mass index is associated with worse outcomes after shoulder arthroplasty. J Bone Joint Surg Am 99:929–937. https://doi.org/10.2106/JBJS.15.00255
Barlow BT, Oi KK, Lee Y-Y et al (2017) Incidence, indications, outcomes, and survivorship of stems in primary total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc 25:3611–3619. https://doi.org/10.1007/s00167-016-4227-6
Kosola J, Kaipia A, Laitinen MK, Nieminen J (2017) Complications after surgical treatment of femoral neck fractures in men with alcohol dependence syndrome: retrospective register analysis of 154 cases. Arch Orthop Trauma Surg 137:967–973. https://doi.org/10.1007/s00402-017-2713-z
Ghomrawi HMK, Lee Y-Y, Herrero C et al (2017) A crosswalk between UCLA and lower extremity activity scales. Clin Orthop Relat Res 475:542–548. https://doi.org/10.1007/s11999-016-5130-7
McLawhorn AS, Steinhaus ME, Southren DL et al (2017) Body mass index class is independently associated with health-related quality of life after primary total hip arthroplasty: an institutional registry-based study. J Arthroplasty 32:143–149. https://doi.org/10.1016/j.arth.2016.06.043
Fehring TK, Fehring KA, Anderson LA et al (2017) Catastrophic varus collapse of the tibia in obese total knee arthroplasty. J Arthroplasty 32:1625–1629. https://doi.org/10.1016/j.arth.2016.12.001
Moussa ME, Lee Y-Y, Patel AR, Westrich GH (2017) Clinical outcomes following the use of constrained condylar knees in primary total knee arthroplasty. J Arthroplasty 32:1869–1873. https://doi.org/10.1016/j.arth.2017.01.001
Sendi P, Lötscher PO, Kessler B et al (2017) Debridement and implant retention in the management of hip periprosthetic joint infection: outcomes following guided and rapid treatment at a single centre. Bone Joint J 99-B:330–336. https://doi.org/10.1302/0301-620X.99B3.BJJ-2016-0609.R1
Maradit Kremers H, Schleck CD, Lewallen EA et al (2017) Diabetes mellitus and hyperglycemia and the risk of aseptic loosening in total joint arthroplasty. J Arthroplasty 32:S251–S253. https://doi.org/10.1016/j.arth.2017.02.056
Rowan FE, Salvatore AJ, Lange JK, Westrich GH (2017) Dual-mobility vs fixed-bearing total hip arthroplasty in patients under 55 years of age: a single-institution, matched-cohort analysis. J Arthroplasty 32:3076–3081. https://doi.org/10.1016/j.arth.2017.05.004
Swarup I, Henn CM, Nguyen JT et al (2017) Effect of pre-operative expectations on the outcomes following total shoulder arthroplasty. Bone Joint J 99-B:1190–1196. https://doi.org/10.1302/0301-620X.99B9.BJJ-2016-1263.R1
Christ AB, Baral E, Koch C et al (2017) Patellofemoral arthroplasty conversion to total knee arthroplasty: retrieval analysis and clinical correlation. Knee 24:1233–1239. https://doi.org/10.1016/j.knee.2017.06.015
Yao JJ, Maradit Kremers H, Schleck CD et al (2017) Patient-reported outcomes can be used to streamline post-total hip arthroplasty follow-up to high-risk patients. J Arthroplasty 32:3319–3321. https://doi.org/10.1016/j.arth.2017.05.033
Goh GS-H, Liow MHL, Bin Abd Razak HR et al (2017) Patient-reported outcomes, quality of life, and satisfaction rates in young patients aged 50 years or younger after total knee arthroplasty. J Arthroplasty 32:419–425. https://doi.org/10.1016/j.arth.2016.07.043
Li Z, Esposito CI, Koch CN et al (2017) Polyethylene damage increases with varus implant alignment in posterior-stabilized and constrained condylar knee arthroplasty. Clin Orthop Relat Res 475:2981–2991. https://doi.org/10.1007/s11999-017-5477-4
Viste A, Perry KI, Taunton MJ et al (2017) Proximal femoral replacement in contemporary revision total hip arthroplasty for severe femoral bone loss: a review of outcomes. Bone Joint J 99-B:325–329. https://doi.org/10.1302/0301-620X.99B3.BJJ-2016-0822.R1
Glanzmann CM, Kolling C, Schwyzer H-K et al (2017) Radiological and functional 24-month outcomes of resurfacing versus stemmed anatomic total shoulder arthroplasty. Int Orthop 41:375–384. https://doi.org/10.1007/s00264-016-3310-4
Maradit Kremers H, Salduz A, Schleck CD et al (2017) Referral bias in primary total knee arthroplasty: retrospective analysis of 22,614 surgeries in a Tertiary Referral Center. J Arthroplasty 32:390–394. https://doi.org/10.1016/j.arth.2016.08.014
Crawford DA, Berend KR, Morris MJ et al (2017) Results of a modular revision system in total knee arthroplasty. J Arthroplasty 32:2792–2798. https://doi.org/10.1016/j.arth.2017.03.076
Hernandez NM, Chalmers BP, Wagner ER et al (2017) Revision to reverse total shoulder arthroplasty restores stability for patients with unstable shoulder prostheses. Clin Orthop Relat Res 475:2716–2722. https://doi.org/10.1007/s11999-017-5429-z
Ledford CK, Statz JM, Chalmers BP et al (2017) Revision total hip and knee arthroplasties after solid organ transplant. J Arthroplasty 32:1560–1564. https://doi.org/10.1016/j.arth.2016.11.047
Lim JBT, Chong HC, Pang HN et al (2017) Revision total knee arthroplasty for failed high tibial osteotomy and unicompartmental knee arthroplasty have similar patient-reported outcome measures in a two-year follow-up study. Bone Joint J 99-B:1329–1334. https://doi.org/10.1302/0301-620X.99B10.BJJ-2017-0034.R1
Courtney PM, Huddleston JI, Iorio R, Markel DC (2017) Socioeconomic risk adjustment models for reimbursement are necessary in primary total joint arthroplasty. J Arthroplasty 32:1–5. https://doi.org/10.1016/j.arth.2016.06.050
Weber M, Benditz A, Woerner M et al (2017) Trainee surgeons affect operative time but not outcome in minimally invasive total hip arthroplasty. Sci Rep 7:6152. https://doi.org/10.1038/s41598-017-06530-3
Kumar A, Bloch BV, Esler C (2017) Trends in total hip arthroplasty in young patients—results from a regional register. Hip Int 27:443–448. https://doi.org/10.5301/hipint.5000485
Wagner ER, Srnec JJ, Mehrotra K, Rizzo M (2017) What are the risk factors and complications associated with intraoperative and postoperative fractures in total wrist arthroplasty? Clin Orthop Relat Res 475:2694–2700. https://doi.org/10.1007/s11999-017-5442-2
Chalmers BP, Ledford CK, Statz JM et al (2017) What risks are associated with primary THA in recipients of hematopoietic stem cell transplantation? Clin Orthop Relat Res 475:475–480. https://doi.org/10.1007/s11999-016-5029-3
Alentorn-Geli E, Clark NJ, Assenmacher AT et al (2017) What are the complications, survival, and outcomes after revision to reverse shoulder arthroplasty in patients older than 80 years? Clin Orthop Relat Res 475:2744–2751. https://doi.org/10.1007/s11999-017-5406-6
Ledford CK, Chalmers BP, Statz JM et al (2017) Primary total knee arthroplasty after solid organ transplant: survivorship and complications. J Arthroplasty 32:101–105. https://doi.org/10.1016/j.arth.2016.07.018
Hurwit DJ, Liu JN, Garcia GH et al (2017) A comparative analysis of work-related outcomes after humeral hemiarthroplasty and reverse total shoulder arthroplasty. J Shoulder Elbow Surg 26:954–959. https://doi.org/10.1016/j.jse.2016.10.004
Brüggemann A, Fredlund E, Mallmin H, Hailer NP (2017) Are porous tantalum cups superior to conventional reinforcement rings? Acta Orthop 88:35–40. https://doi.org/10.1080/17453674.2016.1248315
Maradit Kremers H, Kremers WK, Berry DJ, Lewallen DG (2017) Patient-reported outcomes can be used to identify patients at risk for total knee arthroplasty revision and potentially individualize postsurgery follow-up. J Arthroplasty 32:3304–3307. https://doi.org/10.1016/j.arth.2017.05.043
Njathi CW, Johnson RL, Laughlin RS et al (2017) Complications after continuous posterior lumbar plexus blockade for total hip arthroplasty: a retrospective cohort study. Reg Anesth Pain Med 42:446–450. https://doi.org/10.1097/AAP.0000000000000589
Lyman S, Lee Y-Y, McLawhorn AS et al (2018) What are the minimal and substantial improvements in the HOOS and KOOS and JR versions after total joint replacement? Clin Orthop Relat Res 476:2432–2441. https://doi.org/10.1097/CORR.0000000000000456
Lange JK, Spiro SK, Westrich GH (2018) Utilizing dual mobility components for first-time revision total hip arthroplasty for instability. J Arthroplasty 33:505–509. https://doi.org/10.1016/j.arth.2017.09.029
Garriga C, Sanchez-Santos MT, Judge A et al (2018) Development of a model predicting non-satisfaction 1 year after primary total knee replacement in the UK and transportation to Switzerland. Sci Rep 8:3380. https://doi.org/10.1038/s41598-018-21713-2
Goodman SM, Mehta B, Zhang M et al (2018) Disparities in total hip arthroplasty outcomes: census tract data show interactions between race and community deprivation. J Am Acad Orthop Surg 26:e457–e464. https://doi.org/10.5435/JAAOS-D-17-00393
Christensen TC, Wagner ER, Harmsen WS et al (2018) Effect of physical parameters on outcomes of total knee arthroplasty. J Bone Joint Surg Am 100:1829–1837. https://doi.org/10.2106/JBJS.18.00248
Pitta M, Esposito CI, Li Z et al (2018) Failure after modern total knee arthroplasty: a prospective study of 18,065 knees. J Arthroplasty 33:407–414. https://doi.org/10.1016/j.arth.2017.09.041
Gonzalez AI, Luime JJ, Uçkay I et al (2018) Is there an association between smoking status and prosthetic joint infection after primary total joint arthroplasty? J Arthroplasty 33:2218–2224. https://doi.org/10.1016/j.arth.2018.02.069
Gladnick BP, Fehring KA, Odum SM et al (2018) Midterm survivorship after revision total hip arthroplasty with a custom triflange acetabular component. J Arthroplasty 33:500–504. https://doi.org/10.1016/j.arth.2017.09.026
Goh GS-H, Liow MHL, Pang H-N et al (2018) Patients with poor baseline mental health undergoing unicompartmental knee arthroplasty have poorer outcomes. J Arthroplasty 33:2428–2434. https://doi.org/10.1016/j.arth.2018.02.074
Matuszak SJ, Galea VP, Connelly JW et al (2018) Periprosthetic acetabular radiolucency progression in mid-term follow-up of the articular surface replacement hip system. Arch Orthop Trauma Surg 138:1021–1028. https://doi.org/10.1007/s00402-018-2962-5
Halawi MJ, Cote MP, Singh H et al (2018) The effect of depression on patient-reported outcomes after total joint arthroplasty is modulated by baseline mental health: a registry study. J Bone Joint Surg Am 100:1735–1741. https://doi.org/10.2106/JBJS.17.01677
Thomas C, Patel V, Mallick E et al (2018) The outcome of secondary resurfacing of the patella following total knee arthroplasty: results from the Trent and Wales Arthroplasty Register. Knee 25:146–152. https://doi.org/10.1016/j.knee.2017.10.004
Goh GS-H, Bin Abd Razak HR, Tay DK-J et al (2018) Unicompartmental knee arthroplasty achieves greater flexion with no difference in functional outcome, quality of life, and satisfaction vs total knee arthroplasty in patients younger than 55 years. A propensity score-matched cohort analysis. J Arthroplasty 33:355–361. https://doi.org/10.1016/j.arth.2017.09.022
Ingelsrud LH, Roos EM, Terluin B et al (2018) Minimal important change values for the Oxford Knee Score and the Forgotten Joint Score at 1 year after total knee replacement. Acta Orthop 89:541–547. https://doi.org/10.1080/17453674.2018.1480739
Mahony GT, Werner BC, Chang B et al (2018) Risk factors for failing to achieve improvement after anatomic total shoulder arthroplasty for glenohumeral osteoarthritis. J Shoulder Elbow Surg 27:968–975. https://doi.org/10.1016/j.jse.2017.12.018
Franck F, Ouanezar H, Jacquel A et al (2018) The predictive factors of secondary patellar resurfacing in computer-assisted total knee arthroplasty. A prospective cohort study. Int Orthop 42:1051–1060. https://doi.org/10.1007/s00264-017-3630-z
Ponzio DY, Chiu Y-F, Salvatore A et al (2018) An analysis of the influence of physical activity level on total knee arthroplasty expectations, satisfaction, and outcomes: increased revision in active patients at five to ten years. J Bone Joint Surg Am 100:1539–1548. https://doi.org/10.2106/JBJS.17.00920
Siu KT, Ng FY, Chan PK et al (2018) Bacteriology and risk factors associated with periprosthetic joint infection after primary total knee arthroplasty: retrospective study of 2543 cases. Hong Kong Med J 24:152–157. https://doi.org/10.12809/hkmj176885
Padgett DE, Christ AB, Joseph AD et al (2018) Discharge to inpatient rehab does not result in improved functional outcomes following primary total knee arthroplasty. J Arthroplasty 33:1663–1667. https://doi.org/10.1016/j.arth.2017.12.033
Tan SM, Bin Abd Razak HR, Singh Rikhraj I (2018) Early functional, radiological outcomes and satisfaction rates of total ankle arthroplasty in an Asian population. Foot Ankle Spec 11:425–432. https://doi.org/10.1177/1938640017750252
Ford MC, Hellman MD, Kazarian GS et al (2018) Five to ten-year results of the birmingham hip resurfacing implant in the U.S.: a single institution’s experience. J Bone Joint Surg Am 100:1879–1887. https://doi.org/10.2106/JBJS.17.01525
Menendez ME, Lawler SM, Ring D, Jawa A (2018) High pain intensity after total shoulder arthroplasty. J Shoulder Elbow Surg 27:2113–2119. https://doi.org/10.1016/j.jse.2018.08.001
Garfinkel JH, Gladnick BP, Roland N, Romness DW (2018) Increased incidence of bleeding and wound complications with Factor-Xa Inhibitors after total joint arthroplasty. J Arthroplasty 33:533–536. https://doi.org/10.1016/j.arth.2017.08.039
Boyer B, Bordini B, Caputo D et al (2018) Is Cross-linked polyethylene an improvement over conventional ultra-high molecular weight polyethylene in total knee arthroplasty? J Arthroplasty 33:908–914. https://doi.org/10.1016/j.arth.2017.10.005
Hartzler MA, Abdel MP, Sculco PK et al (2018) Otto Aufranc Award: dual-mobility constructs in revision THA reduced dislocation, rerevision, and reoperation compared with large femoral heads. Clin Orthop Relat Res 476:293–301. https://doi.org/10.1007/s11999.0000000000000035
Statz JM, Wagner ER, Sperling JW, Cofield RH (2018) Outcomes of shoulder arthroplasty in diabetic patients as assessed by peri-operative A1C. Int Orthop 42:1923–1934. https://doi.org/10.1007/s00264-018-3874-2
Bautista M, Muskus M, Llinás A et al (2018) Peri-articular injection of an analgesic mixture in primary total hip arthroplasty: an effective strategy for pain control during the first post-operative day. Int Orthop 42:1803–1810. https://doi.org/10.1007/s00264-018-3788-z
Weber M, Craiovan B, Woerner ML et al (2018) Predictors of outcome after primary total joint replacement. J Arthroplasty 33:431–435. https://doi.org/10.1016/j.arth.2017.08.044
Chalmers BP, Tibbo ME, Trousdale RT et al (2018) Primary total hip arthroplasty for charcot arthropathy is associated with high complications but improved clinical outcomes. J Arthroplasty 33:2912–2918. https://doi.org/10.1016/j.arth.2018.04.002
Bao MH, Keeney BJ, Moschetti WE et al (2018) Resident participation is not associated with worse outcomes after TKA. Clin Orthop Relat Res 476:1375–1390. https://doi.org/10.1007/s11999.0000000000000002
Lombardi AVJ, Kolich MT, Berend KR et al (2018) Revision of unicompartmental knee arthroplasty to total knee arthroplasty: Is it as good as a primary result? J Arthroplasty 33:S105–S108. https://doi.org/10.1016/j.arth.2018.03.023
Weber M, Renkawitz T, Voellner F et al (2018) Revision surgery in total joint replacement is cost-intensive. Biomed Res Int 2018:8987104. https://doi.org/10.1155/2018/8987104
Giardina F, Castagnini F, Stea S et al (2018) Short stems versus conventional stems in cementless total hip arthroplasty: a long-term registry study. J Arthroplasty 33:1794–1799. https://doi.org/10.1016/j.arth.2018.01.005
Boddapati V, Fu MC, Tetreault MW et al (2018) Short-term complications after revision hip arthroplasty for prosthetic joint infection are increased relative to noninfectious revisions. J Arthroplasty 33:2997–3002. https://doi.org/10.1016/j.arth.2018.05.001
Weber M, Worlicek M, Voellner F et al (2018) Surgical training does not affect operative time and outcome in total knee arthroplasty. PLoS ONE 13:e0197850. https://doi.org/10.1371/journal.pone.0197850
Zhang L, Lix LM, Ayilara O et al (2018) The effect of multimorbidity on changes in health-related quality of life following hip and knee arthroplasty. Bone Joint J 100-B:1168–1174. https://doi.org/10.1302/0301-620X.100B9.BJJ-2017-1372.R1
Konopka JF, Buly RL, Kelly BT et al (2018) The effect of prior hip arthroscopy on patient-reported outcomes after total hip arthroplasty: an institutional registry-based, matched cohort study. J Arthroplasty 33:1806–1812. https://doi.org/10.1016/j.arth.2018.01.012
Sershon RA, Tecle N, Della Valle CJ et al (2018) The impact of an acute, traumatic wound dehiscence on clinical outcomes following primary knee arthroplasty. J Arthroplasty 33:2613–2615. https://doi.org/10.1016/j.arth.2018.02.090
Murphy BP d’S, Dowsey MM, Spelman T, Choong PFM (2018) The impact of older age on patient outcomes following primary total knee arthroplasty. Bone Joint J 100-B:1463–1470. https://doi.org/10.1302/0301-620X.100B11.BJJ-2017-0753.R6
Webb BT, Ulrich SD, MacKinlay KGW et al (2018) Use of shorter intramedullary guide for ipsilateral total knee arthroplasty following prior total hip arthroplasty. J Knee Surg 31:348–351. https://doi.org/10.1055/s-0037-1603796
Hernandez NM, Parry JA, Mabry TM, Taunton MJ (2018) Patients at risk: preoperative opioid use affects opioid prescribing, refills, and outcomes after total knee arthroplasty. J Arthroplasty 33:S142–S146. https://doi.org/10.1016/j.arth.2018.01.004
Yao JJ, Maradit Kremers H, Kremers WK et al (2018) Perioperative inpatient use of selective serotonin reuptake inhibitors is associated with a reduced risk of THA and TKA revision. Clin Orthop Relat Res 476:1191–1197. https://doi.org/10.1007/s11999.0000000000000098
Goodman SM, Mandl LA, Mehta B et al (2018) Does education level mitigate the effect of poverty on total knee arthroplasty outcomes? Arthritis Care Res 70:884–891. https://doi.org/10.1002/acr.23442
Parry JA, Hernandez NM, Berry DJ et al (2018) Risk factors for subsidence of modular fluted tapered stems used during revision total hip arthroplasty for periprosthetic hip fractures. J Arthroplasty 33:2967–2970. https://doi.org/10.1016/j.arth.2018.05.006
Galea VP, Botros MA, Madanat R et al (2019) Promising early outcomes of a novel anatomic knee system. Knee Surg Sports Traumatol Arthrosc 27:1067–1074. https://doi.org/10.1007/s00167-018-5248-0
Perneger T V, Hannouche D, Miozzari HH, Lübbeke A (2019) Symptoms of osteoarthritis influence mental and physical health differently before and after joint replacement surgery: A prospective study. PLoS ONE 14:e0217912. https://doi.org/10.1371/journal.pone.0217912
Ferguson RJ, Silman AJ, Combescure C et al (2019) ASA class is associated with early revision and reoperation after total hip arthroplasty: an analysis of the Geneva and Swedish Hip Arthroplasty Registries. Acta Orthop 90:324–330. https://doi.org/10.1080/17453674.2019.1605785
Hernandez NM, Fruth KM, Larson DR et al (2019) Conversion of hemiarthroplasty to THA carries an increased risk of reoperation compared with primary and revision THA. Clin Orthop Relat Res 477:1392–1399. https://doi.org/10.1097/CORR.0000000000000702
Arias-de la Torre J, Valderas JM, Evans JP et al (2019) Differences in risk of revision and mortality between total and unicompartmental knee arthroplasty. The influence of hospital volume. J Arthroplasty 34:865–871. https://doi.org/10.1016/j.arth.2019.01.046
Halawi MJ, Cote MP, Savoy L et al (2019) The effect of payer type on patient-reported outcomes in total joint arthroplasty is modulated by baseline patient characteristics. J Arthroplasty 34:1072–1075. https://doi.org/10.1016/j.arth.2019.01.069
Harris WH, Muratoglu OK (2019) The role of crosslinked polyethylene in reducing aggregated costs of total hip arthroplasty in the United States. J Arthroplasty 34:1089–1092. https://doi.org/10.1016/j.arth.2019.02.038
Halawi MJ, Caminiti N, Cote MP et al (2019) The most significant risk factors for urinary retention in fast-track total joint arthroplasty are iatrogenic. J Arthroplasty 34:136–139. https://doi.org/10.1016/j.arth.2018.08.042
Amanatullah DF, Lawson KA, Li Z et al (2020) Risk adjustment in the California Joint Replacement Registry: Is patient complexity accurately assessed in academic versus nonacademic hospitals? J Arthroplasty 35:3437–3444. https://doi.org/10.1016/j.arth.2020.06.075
Hauser DL, Wessinger SJ, Condon RT et al (2001) An electronic database for outcome studies that includes digital radiographs. J Arthroplasty 16:71–75. https://doi.org/10.1054/arth.2001.28366
Akisue T, Yamaguchi M, Bauer TW et al (2003) “Backside” polyethylene deformation in total knee arthroplasty. J Arthroplasty 18:784–791. https://doi.org/10.1016/s0883-5403(03)00255-9
Pagnano MW, McLamb LA, Trousdale RT (2003) Primary and revision total hip arthroplasty for patients 90 years of age and older. Mayo Clin Proc 78:285–288. https://doi.org/10.4065/78.3.285
Rand JA, Trousdale RT, Ilstrup DM, Harmsen WS (2003) Factors affecting the durability of primary total knee prostheses. J Bone Joint Surg Am 85:259–265. https://doi.org/10.2106/00004623-200302000-00012
Parvizi J, Sullivan T, Duffy G, Cabanela ME (2004) Fifteen-year clinical survivorship of Harris-Galante total hip arthroplasty. J Arthroplasty 19:672–677. https://doi.org/10.1016/j.arth.2004.01.005
Schneiderbauer MM, von Knoch M, Schleck CD et al (2004) Patient survival after hip arthroplasty for metastatic disease of the hip. J Bone Joint Surg Am 86:1684–1689. https://doi.org/10.2106/00004623-200408000-00011
Field RE, Singh PJ, Latif AMH et al (2006) Five-year prospective clinical and radiological results of a new cannulated cemented polished Tri-Taper femoral stem. J Bone Joint Surg Br 88:315–320. https://doi.org/10.1302/0301-620X.88B3.17314
Parvizi J, Wade FA, Rapuri V et al (2006) Revision hip arthroplasty for late instability secondary to polyethylene wear. Clin Orthop Relat Res 447:66–69. https://doi.org/10.1097/01.blo.0000218751.14989.a6
Sanchez-Sotelo J, Haidukewych GJ, Boberg CJ (2006) Hospital cost of dislocation after primary total hip arthroplasty. J Bone Joint Surg Am 88:290–294. https://doi.org/10.2106/JBJS.D.02799
Haidukewych GJ, Jacofsky DJ, Hanssen AD, Lewallen DG (2006) Intraoperative fractures of the acetabulum during primary total hip arthroplasty. J Bone Joint Surg Am 88:1952–1956. https://doi.org/10.2106/JBJS.E.00890
Burns AWR, Bourne RB, Chesworth BM et al (2006) Cost effectiveness of revision total knee arthroplasty. Clin Orthop Relat Res 446:29–33. https://doi.org/10.1097/01.blo.0000214420.14088.76
Allami MK, Fender D, Khaw FM et al (2006) Outcome of Charnley total hip replacement across a single health region in England. The results at ten years from a regional arthroplasty register. J Bone Joint Surg Br 88:1293–1298. https://doi.org/10.1302/0301-620X.88B10.17933
Cipriano LE, Chesworth BM, Anderson CK, Zaric GS (2007) Predicting joint replacement waiting times. Health Care Manag Sci 10:195–215. https://doi.org/10.1007/s10729-007-9013-z
Gioe TJ, Novak C, Sinner P et al (2007) Knee arthroplasty in the young patient: survival in a community registry. Clin Orthop Relat Res 464:83–87. https://doi.org/10.1097/BLO.0b013e31812f79a9
Cheung EV, Sperling JW, Cofield RH (2007) Polyethylene insert exchange for wear after total shoulder arthroplasty. J Shoulder Elbow Surg 16:574–578. https://doi.org/10.1016/j.jse.2006.12.009
Sierra RJ, Timperley JA, Gie GA (2009) Contemporary cementing technique and mortality during and after Exeter total hip arthroplasty. J Arthroplasty 24:325–332. https://doi.org/10.1016/j.arth.2008.01.301
Pinaroli A, Piedade SR, Servien E, Neyret P (2009) Intraoperative fractures and ligament tears during total knee arthroplasty. A 1795 posterostabilized TKA continuous series. Orthop Traumatol Surg Res 95:183–189. https://doi.org/10.1016/j.otsr.2008.04.002
Larson AN, McIntosh AL, Trousdale RT, Lewallen DG (2010) Avascular necrosis most common indication for hip arthroplasty in patients with slipped capital femoral epiphysis. J Pediatr Orthop 30:767–773. https://doi.org/10.1097/BPO.0b013e3181fbe912
Kempton LB, Ankerson E, Wiater JM (2011) A complication-based learning curve from 200 reverse shoulder arthroplasties. Clin Orthop Relat Res 469:2496–2504. https://doi.org/10.1007/s11999-011-1811-4
Noble PC, Durrani SK, Usrey MM et al (2012) Constrained cups appear incapable of meeting the demands of revision THA. Clin Orthop Relat Res 470:1907–1916. https://doi.org/10.1007/s11999-011-2212-4
Khan FA, Rose PS, Yanagisawa M et al (2012) Surgical technique: Porous tantalum reconstruction for destructive nonprimary periacetabular tumors. Clin Orthop Relat Res 470:594–601. https://doi.org/10.1007/s11999-011-2117-2
Greenbaum JN, Bornstein LJ, Lyman S et al (2012) The validity of self-report as a technique for measuring short-term complications after total hip arthroplasty in a joint replacement registry. J Arthroplasty 27:1310–1315. https://doi.org/10.1016/j.arth.2011.10.031
Epinette J-A, Brunschweiler B, Mertl P et al (2012) Unicompartmental knee arthroplasty modes of failure: wear is not the main reason for failure: a multicentre study of 418 failed knees. Orthop Traumatol Surg Res 98:S124–S130. https://doi.org/10.1016/j.otsr.2012.07.002
Summers JC, Bedi HS (2013) Reoperation and patient satisfaction after the Mobility total ankle arthroplasty. ANZ J Surg 83:371–375. https://doi.org/10.1111/ans.12002
Clauss M, Gersbach S, Butscher A, Ilchmann T (2013) Risk factors for aseptic loosening of Müller-type straight stems: a registry-based analysis of 828 consecutive cases with a minimum follow-up of 16 years. Acta Orthop 84:353–359. https://doi.org/10.3109/17453674.2013.810517
Goldvasser D, Marchie A, Bragdon LK et al (2013) Incidence of osteolysis in total knee arthroplasty: comparison between radiographic and retrieval analysis. J Arthroplasty 28:201–206. https://doi.org/10.1016/j.arth.2012.06.008
Bergeson AG, Berend KR, Lombardi AVJ et al (2013) Medial mobile bearing unicompartmental knee arthroplasty: early survivorship and analysis of failures in 1000 consecutive cases. J Arthroplasty 28:172–175. https://doi.org/10.1016/j.arth.2013.01.005
Wechter J, Comfort TK, Tatman P et al (2013) Improved survival of uncemented versus cemented femoral stems in patients aged < 70 years in a community total joint registry. Clin Orthop Relat Res 471:3588–3595. https://doi.org/10.1007/s11999-013-3182-5
Figgie MP, Wright TM, Drinkwater D (2014) What design and material factors impact the wear and corrosion performance in total elbow arthroplasties? Clin Orthop Relat Res 472:3770–3776. https://doi.org/10.1007/s11999-014-3781-9
Heekin RD, Fokin AA (2014) Incidence of bicompartmental osteoarthritis in patients undergoing total and unicompartmental knee arthroplasty: is the time ripe for a less radical treatment? J Knee Surg 27:77–81. https://doi.org/10.1055/s-0033-1349401
Streubel PN, Simone JP, Sperling JW, Cofield R (2014) Thirty and ninety-day reoperation rates after shoulder arthroplasty. J Bone Joint Surg Am 96:e17. https://doi.org/10.2106/JBJS.M.00127
Ramaskandhan JR, Kakwani R, Kometa S et al (2014) Two-year outcomes of MOBILITY Total Ankle Replacement. J Bone Joint Surg Am 96:e53. https://doi.org/10.2106/JBJS.L.00536
Allepuz A, Martínez O, Tebé C et al (2014) Joint registries as continuous surveillance systems: the experience of the Catalan Arthroplasty Register (RACat). J Arthroplasty 29:484–490. https://doi.org/10.1016/j.arth.2013.07.048
Bachmann M, Bolliger L, Ilchmann T, Clauss M (2014) Long-term survival and radiological results of the Duracon™ total knee arthroplasty. Int Orthop 38:747–752. https://doi.org/10.1007/s00264-013-2154-4
Jacobs CA, Christensen CP, Karthikeyan T (2015) Chronic non-orthopedic conditions more common in patients with less severe degenerative changes that have elected to undergo total knee arthroplasty. J Arthroplasty 30:1146–1149. https://doi.org/10.1016/j.arth.2015.01.051
SooHoo NF, Li Z, Chenok KE, Bozic KJ (2015) Responsiveness of patient reported outcome measures in total joint arthroplasty patients. J Arthroplasty 30:176–191. https://doi.org/10.1016/j.arth.2014.09.026
von Roth P, Abdel MP, Harmsen WS, Berry DJ (2015) Cemented bipolar hemiarthroplasty provides definitive treatment for femoral neck fractures at 20 years and beyond. Clin Orthop Relat Res 473:3595–3599. https://doi.org/10.1007/s11999-015-4462-z
Lavernia CJ, Villa JM (2015) Does race affect outcomes in total joint arthroplasty? Clin Orthop Relat Res 473:3535–3541. https://doi.org/10.1007/s11999-015-4481-9
Wagner ER, Van Demark R III, Kor DJ et al (2015) Intraoperative periprosthetic fractures in proximal interphalangeal joint arthroplasty. J Hand Surg Am 40:2149–2154. https://doi.org/10.1016/j.jhsa.2015.06.101
Garcia GH, Taylor SA, DePalma BJ et al (2015) Patient activity levels after reverse total shoulder arthroplasty: what are patients doing? Am J Sports Med 43:2816–2821. https://doi.org/10.1177/0363546515597673
Jacobs CA, Christensen CP, Karthikeyan T (2016) Subchondral bone marrow edema had greater effect on postoperative pain after medial unicompartmental knee arthroplasty than total knee arthroplasty. J Arthroplasty 31:491–494. https://doi.org/10.1016/j.arth.2015.09.023
Fujishiro T, Hiranaka T, Hashimoto S et al (2016) The effect of acetabular and femoral component version on dislocation in primary total hip arthroplasty. Int Orthop 40:697–702. https://doi.org/10.1007/s00264-015-2924-2
Lombardi AVJ, Berend KR, Adams JB, Satterwhite KL (2016) Adverse reactions to metal on metal are not exclusive to large heads in total hip arthroplasty. Clin Orthop Relat Res 474:432–440. https://doi.org/10.1007/s11999-015-4539-8
Ouanezar H, Franck F, Jacquel A et al (2016) Does computer-assisted surgery influence survivorship of cementless total knee arthroplasty in patients with primary osteoarthritis? A 10-year follow-up study. Knee Surg Sports Traumatol Arthrosc 24:3448–3456. https://doi.org/10.1007/s00167-016-4112-3
Brooks PJ (2016) Hip resurfacing: a large, US single-surgeon series. Bone Joint J 98-B:10–13. https://doi.org/10.1302/0301-620X.98B1.36360
Fehring KA, Howe BM, Martin JR et al (2016) Preoperative evaluation for pelvic discontinuity using a new reformatted computed tomography scan protocol. J Arthroplasty 31:2247–2251. https://doi.org/10.1016/j.arth.2016.02.028
Chambers S, Ramaskandhan J, Siddique M (2016) Radiographic severity of arthritis affects functional outcome in total ankle replacement (TAR). Foot Ankle Int 37:351–354. https://doi.org/10.1177/1071100716638021
SooHoo NF, Li Z, Chan V et al (2016) The importance of risk adjustment in reporting total joint arthroplasty outcomes. J Arthroplasty 31:590–595. https://doi.org/10.1016/j.arth.2015.09.041
Singh J, Politis A, Loucks L et al (2016) Trends in revision hip and knee arthroplasty observations after implementation of a regional joint replacement registry. Can J Surg 59:304–310. https://doi.org/10.1503/cjs.002916
Chan VW, Chan PK, Chiu KY et al (2016) Why do Hong Kong patients need total hip arthroplasty? An analysis of 512 hips from 1998 to 2010. Hong Kong Med J 22:11–15. https://doi.org/10.12809/hkmj144483
Albayrak I, Apiliogullari S, Dal CN et al (2017) Efficacy of pulsed radiofrequency therapy to dorsal root ganglion adding to tens and exercise for persistent pain after total knee arthroplasty. J Knee Surg 30:134–142. https://doi.org/10.1055/s-0036-1583268
Zarling BJ, Sikora-Klak J, Bergum C, Markel DC (2017) How do preoperative medications influence outcomes after total joint arthroplasty? J Arthroplasty 32:S259–S262. https://doi.org/10.1016/j.arth.2017.04.031
Feng B, Lin J, Jin J et al (2017) Thirty-day postoperative complications following primary total knee arthroplasty: a retrospective study of incidence and Risk Factors at a Single Center in China. Chin Med J 130:2551–2556. https://doi.org/10.4103/0366-6999.213071
Abdel MP, Ledford CK, Kobic A et al (2017) Contemporary failure aetiologies of the primary, posterior-stabilised total knee arthroplasty. Bone Joint J 99-B:647–652. https://doi.org/10.1302/0301-620X.99B5.BJJ-2016-0617.R3
Ricciardi BF, Henderson PW, McLawhorn AS et al (2017) Gluteus maximus advancement flap procedure for reconstruction of posterior soft tissue deficiency in revision total hip arthroplasty. Orthopedics 40:e495–e500. https://doi.org/10.3928/01477447-20170308-06
Markel DC, Allen MW, Hughes RE et al (2017) Quality initiative programs can decrease total joint arthroplasty transfusion rates—a multicenter study using the MARCQI Total Joint Registry Database. J Arthroplasty 32:3292–3297. https://doi.org/10.1016/j.arth.2017.06.009
Statz JM, Schoch BS, Sanchez-Sotelo J et al (2017) Shoulder arthroplasty for locked anterior shoulder dislocation: a role for the reversed design. Int Orthop 41:1227–1234. https://doi.org/10.1007/s00264-017-3450-1
Statz JM, Chalmers BP, Ledford CK et al (2017) Outcomes of shoulder arthroplasty in haematopoietic stem cell transplant patients. Int Orthop 41:2555–2564. https://doi.org/10.1007/s00264-017-3553-8
Rojas J, Bautista M, Bonilla G et al (2018) A retrospective study on the relationship between altered native acetabular angle and vertical implant malpositioning. Int Orthop 42:769–775. https://doi.org/10.1007/s00264-017-3584-1
Postler A, Lützner C, Beyer F et al (2018) Analysis of total knee arthroplasty revision causes. BMC Musculoskelet Disord 19:55. https://doi.org/10.1186/s12891-018-1977-y
Torre M, Luzi I, Mirabella F et al (2018) Cross-cultural adaptation and validation of the Italian version of the Hip disability and Osteoarthritis Outcome Score (HOOS). Health Qual Life Outcomes 16:115. https://doi.org/10.1186/s12955-018-0935-6
Hurst JM, Ranieri R, Berend KR et al (2018) Outcomes after arthroscopic evaluation of patients with painful medial unicompartmental knee arthroplasty. J Arthroplasty 33:3268–3272. https://doi.org/10.1016/j.arth.2018.05.031
Werthel J-D, Schoch B, Frankle M et al (2018) Shoulder arthroplasty for sequelae of obstetrical brachial plexus injury. J Hand Surg Am 43:871.e1-871.e7. https://doi.org/10.1016/j.jhsa.2018.02.006
Hughes RE, Zheng H, Igrisan RM et al (2018) The Michigan Arthroplasty Registry Collaborative Quality Initiative Experience: improving the quality of care in Michigan. J Bone Joint Surg Am 100:e143. https://doi.org/10.2106/JBJS.18.00239
Kolling C, Borovac M, Audigé L et al (2018) Return to sports after reverse shoulder arthroplasty-the Swiss perspective. Int Orthop 42:1129–1135. https://doi.org/10.1007/s00264-017-3715-8
Boe C, Wagner E, Rizzo M (2018) Long-term outcomes of silicone metacarpophalangeal arthroplasty: a longitudinal analysis of 325 cases. J Hand Surg Eur 43:1076–1082. https://doi.org/10.1177/1753193418778461
Lampropoulou-Adamidou K, Hartofilakidis G (2019) Comparison of the long-term outcome of cemented Charnley low-friction arthroplasty with hybrid arthroplasty in patients with congenital hip disease. Bone Joint J 101-B:1050–1057. https://doi.org/10.1302/0301-620X.101B9.BJJ-2018-1208.R1
Hernandez NM, Sierra RJ, Trousdale RT (2019) Constrained liner revision is less effective with each subsequent constrained liner revision at preventing instability. J Arthroplasty 34:S282–S286. https://doi.org/10.1016/j.arth.2019.01.061
Singleton N, Nicholas B, Gormack N, Stokes A (2019) Differences in outcome after cruciate retaining and posterior stabilized total knee arthroplasty. J Orthop Surg 27:2309499019848154. https://doi.org/10.1177/2309499019848154
Audigé L, Graf L, Flury M et al (2019) Functional improvement is sustained following anatomical and reverse shoulder arthroplasty for fracture sequelae: a registry-based analysis. Arch Orthop Trauma Surg 139:1561–1569. https://doi.org/10.1007/s00402-019-03224-5
Raddaoui K, Khedhri W, Zoghlami K et al (2019) Perioperative morbidity in total knee arthroplasty. Pan Afr Med J 33:233. https://doi.org/10.11604/pamj.2019.33.233.19095
Bass AR, Mehta B, Szymonifka J et al (2019) Racial disparities in total knee replacement failure as related to poverty. Arthritis Care Res 71:1488–1494. https://doi.org/10.1002/acr.24028
Kang JR, Logli AL, Tagliero AJ, Sperling JW (2019) The router bit extraction technique for removing a well-fixed humeral stem in revision shoulder arthroplasty. Bone Joint J 101-B:1280–1284. https://doi.org/10.1302/0301-620X.101B10.BJJ-2018-1592.R1
Toft F, Moro F (2019) Does ORIF of rare scapular spine fractures sustained after reverse shoulder arthroplasty benefit elderly patients? A case-series appraisal. Orthop Traumatol Surg Res 105:1521–1528. https://doi.org/10.1016/j.otsr.2019.07.023
Shah RF, Bini S, Vail T (2020) Data for registry and quality review can be retrospectively collected using natural language processing from unstructured charts of arthroplasty patients. Bone Joint J 102-B:99–104. https://doi.org/10.1302/0301-620X.102B7.BJJ-2019-1574.R1
Florissi I, Galea VP, Sauder N et al (2020) Development and early findings of a semiautomated arthroplasty registry in a multi-institutional healthcare network. Bone Joint J 102-B:90–98. https://doi.org/10.1302/0301-620X.102B7.BJJ-2019-1622.R1
Wegrzyn J, Malatray M, Pibarot V et al (2020) Is isolated mobile component exchange an option in the management of intraprosthetic dislocation of a dual mobility cup? Clin Orthop Relat Res 478:279–287. https://doi.org/10.1097/CORR.0000000000001055
Ulivi M, Meroni V, Orlandini L et al (2020) Opportunities to improve feasibility, effectiveness and costs associated with a total joint replacements high-volume hospital registry. Comput Biol Med 121:103775. https://doi.org/10.1016/j.compbiomed.2020.103775
Liebensteiner M, Köglberger P, Ruzicka A et al (2020) Unicondylar vs. total knee arthroplasty in medial osteoarthritis: a retrospective analysis of registry data and functional outcome. Arch Orthop Trauma Surg 140:545–549. https://doi.org/10.1007/s00402-020-03377-8
Song JH, Kwon WH, Oh S-B, Moon KH (2020) Use of a constrained acetabular liner to prevent and treat recurrent dislocation after total hip replacement arthroplasty. Orthop Surg 12:2004–2012. https://doi.org/10.1111/os.12811
Gauci M-O, Cavalier M, Gonzalez J-F et al (2020) Revision of failed shoulder arthroplasty: epidemiology, etiology, and surgical options. J Shoulder Elbow Surg 29:541–549. https://doi.org/10.1016/j.jse.2019.07.034
Gill SD, Hill-Buxton L-M, Gwini SM et al (2020) Simultaneous (two-surgeon) versus staged bilateral knee arthroplasty: an observational study of intraoperative and post-operative outcomes. ANZ J Surg 90:826–832. https://doi.org/10.1111/ans.15766
Barrack TN, Abu-Amer W, Schwabe MT et al (2020) The burden and utility of routine follow-up at one year after primary arthroplasty. Bone Joint J 102-B:85–89. https://doi.org/10.1302/0301-620X.102B7.BJJ-2019-1632.R1
LaHaise KM, Vargo DV, Barrazueta GA et al (2021) Range of motion at discharge predicts need for manipulation following total knee arthroplasty. J Knee Surg 34:187–191. https://doi.org/10.1055/s-0039-1694024
Borton ZM, Prasad G, Konstantopoulos G et al (2021) Mid- to long-term survivorship of the cemented, semiconstrained discovery total elbow arthroplasty. J Shoulder Elbow Surg 30:1662–1669. https://doi.org/10.1016/j.jse.2020.12.007
Zhang S, Lau BPH, Ng YH et al (2022) Machine learning algorithms do not outperform preoperative thresholds in predicting clinically meaningful improvements after total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc 30:2624–2630. https://doi.org/10.1007/s00167-021-06642-4
Markel JF, Driscoll JA, Zheng TH et al (2022) Causes of early hip revision vary by age and gender: analysis of data from a statewide quality registry. J Arthroplasty 37:S616–S621. https://doi.org/10.1016/j.arth.2022.03.014
Mathew JI, Nicholson AD, Finocchiaro A et al (2022) Outcomes of shoulder arthroplasty by year of index procedure: Are we getting better? J Shoulder Elbow Surg 31:245–251. https://doi.org/10.1016/j.jse.2021.08.024
Tsiridis E, Kenanidis E, Potoupnis M, Sayegh FE (2020) Direct superior approach with standard instrumentation for total hip arthroplasty: safety and efficacy in a prospective 200-case series. Hip Int 30:552–558. https://doi.org/10.1177/1120700019843120
Sierra RJ, Cabanela ME (2002) Conversion of failed hip hemiarthroplasties after femoral neck fractures. Clin Orthop Relat Res 66:129–139. https://doi.org/10.1097/00003086-200206000-00015
Lavernia C, D’apuzzo M, Hernandez VH, Lee DJ (2005) Patient-perceived outcomes in thigh pain after primary arthroplasty of the hip. Clin Orthop Relat Res 441:268–273. https://doi.org/10.1097/00003086-200512000-00041
Schneiderbauer MM, Sierra RJ, Schleck C et al (2005) Dislocation rate after hip hemiarthroplasty in patients with tumor-related conditions. J Bone Joint Surg Am 87:1810–1815. https://doi.org/10.2106/JBJS.D.02830
Geiger F, Mau H, Krüger M, Thomsen M (2008) Comparison of a new mobile-bearing total knee prosthesis with a fixed-bearing prosthesis: a matched pair analysis. Arch Orthop Trauma Surg 128:285–291. https://doi.org/10.1007/s00402-007-0552-z
Gandhi R, Razak F, Tso P et al (2009) Greater perceived helplessness in osteoarthritis predicts outcome of joint replacement surgery. J Rheumatol 36:1507–1511. https://doi.org/10.3899/jrheum.080466
Ghomrawi HMK, Dolan MM, Rutledge J, Alexiades MM (2011) Recovery expectations of hip resurfacing compared to total hip arthroplasty: a matched pairs study. Arthritis Care Res 63:1753–1757. https://doi.org/10.1002/acr.20626
Elson LC, Barr CJ, Chandran SE et al (2013) Are morbidly obese patients undergoing total hip arthroplasty at an increased risk for component malpositioning? J Arthroplasty 28:41–44. https://doi.org/10.1016/j.arth.2013.05.035
Huang D-CT, Tatman P, Mehle S, Gioe TJ (2013) Cumulative revision rate is higher in metal-on-metal THA than metal-on-polyethylene THA: analysis of survival in a community registry. Clin Orthop Relat Res 471:1920–1925. https://doi.org/10.1007/s11999-013-2821-1
Wiater BP, Boone CR, Koueiter DM, Wiater JM (2013) Early outcomes of staged bilateral reverse total shoulder arthroplasty: a case-control study. Bone Joint J 95-B:1232–1238. https://doi.org/10.1302/0301-620X.95B9.31445
Stryker LS, Abdel MP, Morrey ME et al (2013) Elevated postoperative blood glucose and preoperative hemoglobin A1C are associated with increased wound complications following total joint arthroplasty. J Bone Joint Surg Am 95(808–14):S1-2. https://doi.org/10.2106/JBJS.L.00494
Boyle MJ, Singleton N, Frampton CMA, Muir D (2013) Functional response to total hip arthroplasty in patients with hip dysplasia. ANZ J Surg 83:554–558. https://doi.org/10.1111/j.1445-2197.2012.06198.x
Berend KR, Lombardi AVJ, Morris MJ et al (2013) Two-stage treatment of hip periprosthetic joint infection is associated with a high rate of infection control but high mortality. Clin Orthop Relat Res 471:510–518. https://doi.org/10.1007/s11999-012-2595-x
Pappou I, Virani NA, Clark R et al (2014) Outcomes and costs of reverse shoulder arthroplasty in the morbidly obese: a case control study. J Bone Joint Surg Am 96:1169–1176. https://doi.org/10.2106/JBJS.M.00735
Goodman SM, Zhu R, Figgie MP et al (2014) Short-term total hip replacement outcomes in ankylosing spondylitis. J Clin Rheumatol 20:363–368. https://doi.org/10.1097/RHU.0000000000000138
Adelani MA, Crook K, Barrack RL et al (2014) What is the prognosis of revision total hip arthroplasty in patients 55 years and younger? Clin Orthop Relat Res 472:1518–1525. https://doi.org/10.1007/s11999-013-3377-9
Chang C-H, Chang Y, Chen DW et al (2014) Topical tranexamic acid reduces blood loss and transfusion rates associated with primary total hip arthroplasty. Clin Orthop Relat Res 472:1552–1557. https://doi.org/10.1007/s11999-013-3446-0
Krismer M, Nogler M, Huber D, Oberaigner W (2015) Cemented ABG-II prosthesis: 5-year results. Hip Int 25:56–60. https://doi.org/10.5301/hipint.5000185
Morris BJ, Haigler RE, O’Connor DP et al (2015) Outcomes of staged bilateral reverse shoulder arthroplasties for rotator cuff tear arthropathy. J Shoulder Elbow Surg 24:474–481. https://doi.org/10.1016/j.jse.2014.08.008
Shah UH, Mandl LA, Mertelsmann-Voss C et al (2015) Systemic lupus erythematosus is not a risk factor for poor outcomes after total hip and total knee arthroplasty. Lupus 24:900–908. https://doi.org/10.1177/0961203314566635
Lavernia CJ, Villa JM, Iacobelli DA (2015) What is the role of mental health in primary total knee arthroplasty? Clin Orthop Relat Res 473:159–163. https://doi.org/10.1007/s11999-014-3769-5
LoVerde ZJ, Mandl LA, Johnson BK et al (2015) Rheumatoid arthritis does not increase risk of short-term adverse events after total knee arthroplasty: a retrospective case-control study. J Rheumatol 42:1123–1130. https://doi.org/10.3899/jrheum.141251
Langen S, Gaber S, Zdravkovic V et al (2016) Lateral subvastus approach with tibial tubercle osteotomy for primary total knee arthroplasty: clinical outcome and complications compared to medial parapatellar approach. Eur J Orthop Surg Traumatol 26:215–222. https://doi.org/10.1007/s00590-015-1718-y
Mandl LA, Zhu R, Huang W-T et al (2016) Short-term total hip arthroplasty outcomes in patients with psoriatic arthritis or psoriatic skin disease compared to patients with osteoarthritis. Arthritis Rheumatol 68:410–417. https://doi.org/10.1002/art.39431
Fein AW, Figgie CA, Dodds TR et al (2016) Systemic lupus erythematosus does not increase risk of adverse events in the first 6 months after total knee arthroplasty. J Clin Rheumatol 22:355–359. https://doi.org/10.1097/RHU.0000000000000435
Rowan FE, Gorenchtein M, Aslam S et al (2016) A comparison of acetabular impaction grafting and trabecular metal for revision arthroplasty. Hip Int 26:350–354. https://doi.org/10.5301/hipint.5000362
Roberts JE, Mandl LA, Su EP et al (2016) Patients with systemic lupus erythematosus have increased risk of short-term adverse events after total hip arthroplasty. J Rheumatol 43:1498–1502. https://doi.org/10.3899/jrheum.151373
Morison Z, Moojen DJF, Nauth A et al (2016) Total hip arthroplasty after acetabular fracture is associated with lower survivorship and more complications. Clin Orthop Relat Res 474:392–398. https://doi.org/10.1007/s11999-015-4509-1
Lim JBT, Bin Abd Razak HR, Zainul-Abidin S et al (2017) What are the preoperative outcome measures that predispose to periprosthetic fractures after primary total knee arthroplasty? J Arthroplasty 32:2531–2534. https://doi.org/10.1016/j.arth.2017.03.013
Redmond JM, Gupta A, Dunne K et al (2017) What factors predict conversion to THA after arthroscopy? Clin Orthop Relat Res 475:2538–2545. https://doi.org/10.1007/s11999-017-5437-z
Tay KS, Cher EWL, Zhang K et al (2017) Comorbidities have a greater impact than age alone in the outcomes of octogenarian total knee arthroplasty. J Arthroplasty 32:3373–3378. https://doi.org/10.1016/j.arth.2017.05.041
Charpentier PM, Srivastava AK, Zheng H et al (2018) Readmission rates for one versus two-midnight length of stay for primary total knee arthroplasty: analysis of the Michigan Arthroplasty Registry Collaborative Quality Initiative (MARCQI) Database. J Bone Joint Surg Am 100:1757–1764. https://doi.org/10.2106/JBJS.18.00166
Song SY, Goodman SB, Suh G et al (2018) Surgery before subspecialty referral for periprosthetic knee infection reduces the likelihood of infection control. Clin Orthop Relat Res 476:1995–2002. https://doi.org/10.1097/CORR.0000000000000423
Behrend H, Zdravkovic V, Bösch M, Hochreiter B (2019) No difference in joint awareness after TKA: a matched-pair analysis of a classic implant and its evolutional design. Knee Surg Sports Traumatol Arthrosc 27:2124–2129. https://doi.org/10.1007/s00167-019-05407-4
Lim JBT, Pang HN, Tay KJD et al (2019) Clinical outcomes and patient satisfaction following revision of failed unicompartmental knee arthroplasty to total knee arthroplasty are as good as a primary total knee arthroplasty. Knee 26:847–852. https://doi.org/10.1016/j.knee.2019.04.016
Jochimsen KN, Jacobs CA, Duncan ST (2019) Femoroacetabular impingement is more common in military veterans with end-stage hip osteoarthritis than civilian patients: a retrospective case control study. Mil Med Res 6:27. https://doi.org/10.1186/s40779-019-0218-5
Benelli G, Maritato M, Cerulli Mariani P, Sasso F (2019) Revision of ASR hip arthroplasty: analysis of two hundred and ninety six recalled patients at seven years. Int Orthop 43:97–101. https://doi.org/10.1007/s00264-018-4128-z
Lim JBT, Pang HN, Tay KJD et al (2020) Increased constraint of rotating hinge knee prosthesis is associated with poorer clinical outcomes as compared to constrained condylar knee prosthesis in total knee arthroplasty. Eur J Orthop Surg Traumatol 30:529–535. https://doi.org/10.1007/s00590-019-02598-x
Nyffeler RW, Altioklar B, Bissig P (2020) Causes of acromion and scapular spine fractures following reverse shoulder arthroplasty: a retrospective analysis and literature review. Int Orthop 44:2673–2681. https://doi.org/10.1007/s00264-020-04813-5
Lall AC, Secretov E, Battaglia MR et al (2020) Effect of alcohol consumption on patient-reported outcomes in hip arthroscopy: a matched controlled study with minimum 2-year follow-up. Hip Int 30:457–468. https://doi.org/10.1177/1120700019853554
White BJ, Patterson J, Scoles AM et al (2020) Hip arthroscopy in patients aged 40 years and older: greater success with labral reconstruction compared with labral repair. Arthroscopy 36:2137–2144. https://doi.org/10.1016/j.arthro.2020.04.031
Jacob I, Benson J, Shanaghan K, Gonzalez Della Valle A (2020) Acetabular positioning is more consistent with the use of a novel miniature computer-assisted device. Int Orthop 44:429–435. https://doi.org/10.1007/s00264-020-04484-2
Schafer KA, Clohisy JC, Nepple JJ (2020) Rapidly progressive arthritis in femoroacetabular impingement: patient characteristics and risk factors for total hip arthroplasty by the age of forty. Iowa Orthop J 40:129–134
Dugdale EM, Siljander MP, Trousdale RT (2021) Factors associated with early return to driving following total joint arthroplasty. J Arthroplasty 36:3392–3400. https://doi.org/10.1016/j.arth.2021.05.028
Rainer WG, Abdel MP, Freedman BA et al (2021) Pelvic tilt and the pubic symphysis to sacrococcygeal junction distance: risk factors for hip dislocation observed on anteroposterior pelvis radiographs. J Arthroplasty 36:S367–S373. https://doi.org/10.1016/j.arth.2021.02.079
Loppini M, Pisano A, Gandolfi CE et al (2021) Complications, readmission and reoperation rates in one-stage bilateral versus unilateral total hip arthroplasty: a high-volume single center case-control study. Sci Rep 11:6299. https://doi.org/10.1038/s41598-021-85839-6
Damsgaard CW, Gad BV, Bono OJ et al (2021) Intraoperative proximal tibia periprosthetic fractures in primary total knee arthroplasty. J Knee Surg 34:1269–1274. https://doi.org/10.1055/s-0040-1708037
Kriechling P, Hodel S, Paszicsnyek A et al (2022) Incidence, radiographic predictors, and clinical outcome of acromial stress reaction and acromial fractures in reverse total shoulder arthroplasty. J Shoulder Elbow Surg 31:1143–1153. https://doi.org/10.1016/j.jse.2021.11.012
Forlizzi JM, Puzzitiello RN, Hart P-A et al (2022) Predictors of poor and excellent outcomes after reverse total shoulder arthroplasty. J Shoulder Elbow Surg 31:294–301. https://doi.org/10.1016/j.jse.2021.07.009
Kenanidis E, Paparoidamis G, Pegios VF et al (2022) Earlier functional recovery and discharge from hospital for THA patients operated on via direct superior compared to standard posterior approach: a retrospective frequency-matched case-control study. Hip Int. https://doi.org/10.1177/11207000221086506
Gandhi R, Wasserstein D, Razak F et al (2010) BMI independently predicts younger age at hip and knee replacement. Obesity 18:2362–2366. https://doi.org/10.1038/oby.2010.72
Philippon MJ, Briggs KK, Carlisle JC, Patterson DC (2013) Joint space predicts THA after hip arthroscopy in patients 50 years and older. Clin Orthop Relat Res 471:2492–2496. https://doi.org/10.1007/s11999-012-2779-4
Lübbeke A, Zimmermann-Sloutskis D, Stern R et al (2014) Physical activity before and after primary total hip arthroplasty: a registry-based study. Arthritis Care Res 66:277–284. https://doi.org/10.1002/acr.22101
Heffler MA, Barlow R, Xi Y et al (2018) Multi-parametric muscle and fat correlation of computed tomography parameters to outcomes in a total hip arthroplasty population. BMC Musculoskelet Disord 19:4. https://doi.org/10.1186/s12891-017-1926-1
Klem N-R, Smith A, O’Sullivan P et al (2020) What influences patient satisfaction after TKA? A qualitative investigation. Clin Orthop Relat Res 478:1850–1866. https://doi.org/10.1097/CORR.0000000000001284
Baryeh K, Maillot C, Gummaraju A, Rivière C (2021) Disappointing relationship between functional performance and patient satisfaction of UKA patients: a cross sectional study. Orthop Traumatol Surg Res 107:102865. https://doi.org/10.1016/j.otsr.2021.102865
Gummaraju A, Maillot C, Baryeh K et al (2021) Oxford Knee Score and EQ-5d poorly predict patient’s satisfaction following mechanically aligned total knee replacement: a cross-sectional study. Orthop Traumatol Surg Res 107:102867. https://doi.org/10.1016/j.otsr.2021.102867
Maillot C, Harman C, Al-Zibari M et al (2022) Moderate relationship between function and satisfaction of total hip arthroplasty patients: a cross sectional study. Hip Int 32:25–31. https://doi.org/10.1177/1120700020921110
Moverman MA, Puzzitiello RN, Menendez ME et al (2022) Rotator cuff fatty infiltration and muscle atrophy: relation to glenoid deformity in primary glenohumeral osteoarthritis. J Shoulder Elbow Surg 31:286–293. https://doi.org/10.1016/j.jse.2021.07.007
Dowsey MM, Scott A, Nelson EA et al (2016) Using discrete choice experiments as a decision aid in total knee arthroplasty: study protocol for a randomised controlled trial. Trials 17:416. https://doi.org/10.1186/s13063-016-1536-5
Page RS, Williams S, Selvaratnam A et al (2018) Protocol for a single-centre, parallel-arm, double-blind randomised trial evaluating the effects of tourniquet use in total knee arthroplasty on intra-operative and post-operative outcomes. BMC Musculoskelet Disord 19:435. https://doi.org/10.1186/s12891-018-2352-8
Abdullah HR, Tan SR, Lee SJ et al (2018) Protocol for a single-centre prospective observational study of postoperative delirium following total joint arthroplasties among South East Asians. BMJ Open 8:e019426. https://doi.org/10.1136/bmjopen-2017-019426
Grassi A, Golinelli D, Tedesco D et al (2019) Patient-reported outcome measures (PROMs) after elective hip, knee and shoulder arthroplasty: protocol for a prospective cohort study. BMC Musculoskelet Disord 20:374. https://doi.org/10.1186/s12891-019-2745-3
Arias-de la Torre J, Domingo L, Martínez O et al (2019) Evaluation of the effectiveness of hip and knee implant models used in Catalonia: a protocol for a prospective registry-based study. J Orthop Surg Res 14:61. https://doi.org/10.1186/s13018-019-1087-z
Gould D, Dowsey M, Spelman T et al (2019) Patient-related risk factors for unplanned 30-day readmission following total knee arthroplasty: a protocol for a systematic review and meta-analysis. Syst Rev 8:215. https://doi.org/10.1186/s13643-019-1140-3
Lo CWT, Brodie MA, Tsang WWN et al (2021) Acceptability and feasibility of a community-based strength, balance, and Tai Chi rehabilitation program in improving physical function and balance of patients after total knee arthroplasty: study protocol for a pilot randomized controlled trial. Trials 22:129. https://doi.org/10.1186/s13063-021-05055-5
Zhou Y, Weeden C, Patten L et al (2022) Evaluating willingness for surgery using the SMART Choice (Knee) patient prognostic tool for total knee arthroplasty: study protocol for a pragmatic randomised controlled trial. BMC Musculoskelet Disord 23:179. https://doi.org/10.1186/s12891-022-05123-0
Gausden EB, Garner MR, Warner SJ et al (2016) Tranexamic acid in hip fracture patients: a protocol for a randomised, placebo controlled trial on the efficacy of tranexamic acid in reducing blood loss in hip fracture patients. BMJ Open 6:e010676. https://doi.org/10.1136/bmjopen-2015-010676
Turan A, Babazade R, Elsharkawy H et al (2017) Novel needle guide reduces time to perform ultrasound-guided femoral nerve catheter placement: a randomised controlled trial. Eur J Anaesthesiol 34:135–140. https://doi.org/10.1097/EJA.0000000000000584
Lin S-J, Chang F-C, Huang T-W et al (2018) Temporal change of interleukin-6, C-reactive protein, and skin temperature after total knee arthroplasty using triclosan-coated sutures. Biomed Res Int 2018:9136208. https://doi.org/10.1155/2018/9136208
Alleva R, Tognù A, Tomasetti M et al (2019) Effect of different anaesthetic techniques on gene expression profiles in patients who underwent hip arthroplasty. PLoS ONE 14:e0219113. https://doi.org/10.1371/journal.pone.0219113
Turgeon TR, Cameron B, Burnell CD et al (2019) A double-blind randomized controlled trial of total knee replacement using patient-specific cutting block instrumentation versus standard instrumentation. Can J Surg 62:460–467. https://doi.org/10.1503/cjs.018318
Dowsey MM, Brown WA, Cochrane A et al (2022) Effect of bariatric surgery on risk of complications after total knee arthroplasty: a randomized clinical trial. JAMA Netw Open 5:e226722. https://doi.org/10.1001/jamanetworkopen.2022.6722
James EW, Blevins JL, Gausden EB et al (2019) Increased utilization of constraint in total knee arthroplasty following anterior cruciate ligament and multiligament knee reconstruction. Bone Joint J 101-B:77–83. https://doi.org/10.1302/0301-620X.101B7.BJJ-2018-1492.R1
Hart A, Wyles CC, Abdel MP et al (2019) Thirty-day major and minor complications following total hip arthroplasty—a comparison of the direct anterior, lateral, and posterior approaches. J Arthroplasty 34:2681–2685. https://doi.org/10.1016/j.arth.2019.06.046
Rojanasopondist P, Galea VP, Connelly JW et al (2019) What preoperative factors are associated with not achieving a minimum clinically important difference after THA? Findings from an international multicenter study. Clin Orthop Relat Res 477:1301–1312. https://doi.org/10.1097/CORR.0000000000000667
Mazzotti A, Perna F, Golinelli D et al (2019) Preoperative valgus deformity has twice the risk of failure as compared to varus deformity after total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc 27:3041–3047. https://doi.org/10.1007/s00167-018-5331-6
Pitta M, Khoshbin A, Lalani A et al (2019) Age-related functional decline following total knee arthroplasty: risk adjustment is mandatory. J Arthroplasty 34:228–234. https://doi.org/10.1016/j.arth.2018.09.046
Fontana MA, Lyman S, Sarker GK et al (2019) Can machine learning algorithms predict which patients will achieve minimally clinically important differences from total joint arthroplasty? Clin Orthop Relat Res 477:1267–1279. https://doi.org/10.1097/CORR.0000000000000787
Kahlenberg CA, Lyman S, Joseph AD et al (2019) Comparison of patient-reported outcomes based on implant brand in total knee arthroplasty: a prospective cohort study. Bone Joint J 101-B:48–54. https://doi.org/10.1302/0301-620X.101B7.BJJ-2018-1382.R1
Abdel MP, Miller LE, Hanssen AD, Pagnano MW (2019) Cost analysis of dual-mobility versus large femoral head constructs in revision total hip arthroplasty. J Arthroplasty 34:260–264. https://doi.org/10.1016/j.arth.2018.09.085
Yadegari I, Bohm E, Ayilara OF et al (2019) Differential item functioning of the SF-12 in a population-based regional joint replacement registry. Health Qual Life Outcomes 17:114. https://doi.org/10.1186/s12955-019-1166-1
Stambough JB, Curtin BM, Odum SM et al (2019) Does change in ESR and CRP guide the timing of two-stage arthroplasty reimplantation? Clin Orthop Relat Res 477:364–371. https://doi.org/10.1097/01.blo.0000533618.31937.45
Kim S-C, Lim Y-W, Jo W-L et al (2019) Fourth-generation ceramic-on-ceramic THA results in improvements in midterm outcomes compared to third-generation THA but does not resolve noise problems: a cohort study of a single-hip system. BMC Musculoskelet Disord 20:263. https://doi.org/10.1186/s12891-019-2641-x
Lombardo DJ, Siljander MP, Gehrke CK et al (2019) Fretting and corrosion damage of retrieved dual-mobility total hip arthroplasty systems. J Arthroplasty 34:1273–1278. https://doi.org/10.1016/j.arth.2019.02.008
Castagnini F, Bordini B, Stea S et al (2019) Highly porous titanium cup in cementless total hip arthroplasty: registry results at eight years. Int Orthop 43:1815–1821. https://doi.org/10.1007/s00264-018-4102-9
Ayilara OF, Zhang L, Sajobi TT et al (2019) Impact of missing data on bias and precision when estimating change in patient-reported outcomes from a clinical registry. Health Qual Life Outcomes 17:106. https://doi.org/10.1186/s12955-019-1181-2
Gungor S, Fields K, Aiyer R et al (2019) Incidence and risk factors for development of persistent postsurgical pain following total knee arthroplasty: a retrospective cohort study. Medicine 98:e16450. https://doi.org/10.1097/MD.0000000000016450
Mehta B, Szymonifka J, Dey S et al (2019) Living in immigrant communities does not impact total knee arthroplasty outcomes: experience from a high-volume center in the United States. BMC Musculoskelet Disord 20:67. https://doi.org/10.1186/s12891-019-2446-y
Hart A, Janz V, Trousdale RT et al (2019) Long-term survivorship of total hip arthroplasty with highly cross-linked polyethylene for osteonecrosis. J Bone Joint Surg Am 101:1563–1568. https://doi.org/10.2106/JBJS.18.01218
Marks M, Hensler S, Wehrli M et al (2019) Minimal important change and patient acceptable symptom state for patients after proximal interphalangeal joint arthroplasty. J Hand Surg Eur 44:175–180. https://doi.org/10.1177/1753193418799568
Statz JM, Duethman NC, Trousdale RT, Taunton MJ (2019) Outcome of direct anterior total hip arthroplasty complicated by superficial wound dehiscence requiring irrigation and debridement. J Arthroplasty 34:1492–1497. https://doi.org/10.1016/j.arth.2019.03.020
Crawford DA, Adams JB, Morris MJ et al (2019) Partial 2-stage exchange for infected total hip arthroplasty: an updated report. J Arthroplasty 34:3048–3053. https://doi.org/10.1016/j.arth.2019.07.001
Khoshbin A, Stavrakis A, Sharma A et al (2019) Patient-reported outcome measures of total knee arthroplasties for post-traumatic arthritis versus osteoarthritis: a short-term (5- to 10-year) retrospective matched cohort study. J Arthroplasty 34:872-876.e1. https://doi.org/10.1016/j.arth.2019.01.022
Hart A, Hernandez NM, Abdel MP et al (2019) Povidone-iodine wound lavage to prevent infection after revision total hip and knee arthroplasty: an analysis of 2,884 cases. J Bone Joint Surg Am 101:1151–1159. https://doi.org/10.2106/JBJS.18.01152
Fu MC, Chang B, Wong AC et al (2019) PROMIS physical function underperforms psychometrically relative to American Shoulder and Elbow Surgeons score in patients undergoing anatomic total shoulder arthroplasty. J Shoulder Elbow Surg 28:1809–1815. https://doi.org/10.1016/j.jse.2019.02.011
Affatato S, Cosentino M, Castagnini F, Bordini B (2019) Registry study on failure incidence in 1,127 revised hip implants with stem trunnion re-use after 10 years of follow-up: limited influence of an adapter sleeve. Acta Orthop 90:417–420. https://doi.org/10.1080/17453674.2019.1618649
Crawford DA, Adams JB, Morris MJ et al (2019) Revision of failed metal-on-metal total hip arthroplasty: midterm outcomes of 203 consecutive cases. J Arthroplasty 34:1755–1760. https://doi.org/10.1016/j.arth.2019.04.019
Jennings JM, White S, Martin JR et al (2019) Revisions of modular metal-on-metal THA have a high risk of early complications. Clin Orthop Relat Res 477:344–350. https://doi.org/10.1097/CORR.0000000000000363
Crawford DA, Adams JB, Hurst JM et al (2019) Ten-year minimum outcomes and survivorship with a high flexion knee system. J Arthroplasty 34:1975–1979. https://doi.org/10.1016/j.arth.2019.04.039
Busanelli L, Castagnini F, Bordini B et al (2019) The biological acetabular reconstruction with bone allografts in hip revision arthroplasty. Musculoskelet Surg 103:173–179. https://doi.org/10.1007/s12306-018-0573-5
Goldman AH, Sierra RJ, Trousdale RT et al (2019) The Lawrence D. Dorr Surgical Techniques & Technologies Award: Why are contemporary revision total hip arthroplasties failing? An analysis of 2500 cases. J Arthroplasty 34:S11–S16. https://doi.org/10.1016/j.arth.2019.01.031
Boyer B, Bordini B, Caputo D et al (2019) Unilateral versus bilateral total knee arthroplasty: a registry study on survival and risk factors. Orthop Traumatol Surg Res 105:627–631. https://doi.org/10.1016/j.otsr.2019.01.023
Hernandez NM, Hart A, Taunton MJ et al (2019) Use of Povidone-Iodine Irrigation Prior to wound closure in primary total hip and knee arthroplasty: an analysis of 11,738 cases. J Bone Joint Surg Am 101:1144–1150. https://doi.org/10.2106/JBJS.18.01285
Ledford CK, Perry KI, Hanssen AD, Abdel MP (2019) What are the contemporary etiologies for revision surgery and revision after primary, noncemented total hip arthroplasty? J Am Acad Orthop Surg 27:933–938. https://doi.org/10.5435/JAAOS-D-17-00842
Brown TS, McLaughlin RJ, Berry DJ et al (2019) What is the survivorship of revision surgery performed for the chronically dislocated THA? Clin Orthop Relat Res 477:374–379. https://doi.org/10.1097/CORR.0000000000000392
Lalani A, Lee Y-Y, Pitta M et al (2019) Age-related decline in patient-reported outcomes 2 and 5 years following total hip arthroplasty. J Arthroplasty 34:1999–2005. https://doi.org/10.1016/j.arth.2019.02.023
Lee WC, Bin Abd Razak HR, Allen JC et al (2019) Achieving Minimum Clinically Important Difference in Oxford Knee Score and Short Form-36 physical component summary is less likely with single-radius compared with multiradius total knee arthroplasty in Asians. J Knee Surg 32:227–232. https://doi.org/10.1055/s-0038-1641139
Lampart M, Behrend H, Moser LB, Hirschmann MT (2019) Due to great variability fixed HKS angle for alignment of the distal cut leads to a significant error in coronal TKA orientation. Knee Surg Sports Traumatol Arthrosc 27:1434–1441. https://doi.org/10.1007/s00167-018-5041-0
Abdel MP, Petis SM, Taunton MJ et al (2019) Long-term results of patellar bone-grafting for severe patellar bone loss during revision total knee arthroplasty. J Bone Joint Surg Am 101:1636–1644. https://doi.org/10.2106/JBJS.19.00519
Menendez ME, Shaker J, Lawler SM et al (2019) Negative patient-experience comments after total shoulder arthroplasty. J Bone Joint Surg Am 101:330–337. https://doi.org/10.2106/JBJS.18.00695
Burger JA, Kleeblad LJ, Laas N, Pearle AD (2019) The influence of preoperative radiographic patellofemoral degenerative changes and malalignment on patellofemoral-specific outcome scores following fixed-bearing medial unicompartmental knee arthroplasty. J Bone Joint Surg Am 101:1662–1669. https://doi.org/10.2106/JBJS.18.01385
Hernandez NM, Petis SM, Hanssen AD et al (2019) Infection after unicompartmental knee arthroplasty: a high risk of subsequent complications. Clin Orthop Relat Res 477:70–77. https://doi.org/10.1097/CORR.0000000000000372
Chalmers BP, Limberg AK, Athey AG et al (2019) Total knee arthroplasty after distal femoral osteotomy long-term survivorship and clinical outcomes. Bone Joint J 101-B:660–666. https://doi.org/10.1302/0301-620X.101B6.BJJ-2018-1334.R2
Hood BR, Cowen ME, Zheng HT et al (2019) Association of aspirin with prevention of venous thromboembolism in patients after total knee arthroplasty compared with other anticoagulants: a noninferiority analysis. JAMA Surg 154:65–72. https://doi.org/10.1001/jamasurg.2018.3858
Blevins JL, Chiu Y-F, Lyman S et al (2019) Comparison of expectations and outcomes in rheumatoid arthritis versus osteoarthritis patients undergoing total knee arthroplasty. J Arthroplasty 34:1946-1952.e2. https://doi.org/10.1016/j.arth.2019.04.034
Kim E, Talmo CT, Anderson MC et al (2019) Incidence and risk factors for posterior cruciate ligament avulsion during cruciate retaining total knee arthroplasty. J Knee Surg 32:1138–1142. https://doi.org/10.1055/s-0038-1676068
Carducci MP, Zimmer ZR, Jawa A (2019) Predictors of unsatisfactory patient outcomes in primary reverse total shoulder arthroplasty. J Shoulder Elbow Surg 28:2113–2120. https://doi.org/10.1016/j.jse.2019.04.009
Chalmers BP, Limberg AK, Tibbo ME et al (2019) Total knee arthroplasty after high tibial osteotomy results in excellent long-term survivorship and clinical outcomes. J Bone Joint Surg Am 101:970–978. https://doi.org/10.2106/JBJS.18.01060
Bunzli S, O’Brien P, Klem N et al (2020) Misconceived expectations: Patient reflections on the total knee replacement journey. Musculoskelet Care 18:415–424. https://doi.org/10.1002/msc.1475
Sheth MM, Morris BJ, Laughlin MS et al (2020) Outcomes of anatomic shoulder arthroplasty performed on B2 vs. A1 type glenoids. J Shoulder Elbow Surg 29:2571–2577. https://doi.org/10.1016/j.jse.2020.03.050
Castagnini F, Bordini B, Biondi F et al (2020) Mixed ceramic combinations in primary total hip arthroplasty achieved reassuring mid-to-longterm outcomes. J Mater Sci Mater Med 31:56. https://doi.org/10.1007/s10856-020-06393-7
Giesinger JM, Giesinger K, Federico B et al (2020) Differences in case mix and outcomes between Swiss and Scottish total knee arthroplasty patients. Knee Surg Sports Traumatol Arthrosc 28:1797–1804. https://doi.org/10.1007/s00167-019-05597-x
Goldman AH, Osmon DR, Hanssen AD et al (2020) Aseptic reoperations within 1 year of primary total knee arthroplasty markedly increase the risk of later periprosthetic joint infection. J Arthroplasty 35:3668–3672. https://doi.org/10.1016/j.arth.2020.06.054
Goodman SM, Mehta BY, Kahlenberg CA et al (2020) Assessment of a satisfaction measure for use after primary total joint arthroplasty. J Arthroplasty 35:1792-1799.e4. https://doi.org/10.1016/j.arth.2020.02.039
Sachdeva S, Baker JF, Bauwens JE et al (2020) Can revision TKA patients achieve similar clinical functional improvement compared to primaries? J Knee Surg 33:1219–1224. https://doi.org/10.1055/s-0039-1693415
Owen AR, Markos JR, Mabry TM et al (2020) Contemporary primary total knee arthroplasty is durable in patients diagnosed with ankylosing spondylitis. J Arthroplasty 35:3161–3165. https://doi.org/10.1016/j.arth.2020.06.033
Polascik BA, Hidaka C, Thompson MC et al (2020) Crosswalks between knee and hip arthroplasty short forms: HOOS/KOOS JR and Oxford. J Bone Joint Surg Am 102:983–990. https://doi.org/10.2106/JBJS.19.00916
Abdulla I, Mahdavi S, Khong H et al (2020) Does body mass index affect the rate of adverse outcomes in total hip and knee arthroplasty? A retrospective review of a total joint replacement database. Can J Surg 63:E142–E149. https://doi.org/10.1503/cjs.006719
Bigart KC, Nahhas CR, Ruzich GP et al (2020) Does femoral morphology predict the risk of periprosthetic fracture after cementless total hip arthroplasty? J Arthroplasty 35:S359–S363. https://doi.org/10.1016/j.arth.2020.02.048
Tew M, Dalziel K, Dowsey M et al (2020) Exploring the impact of quality of life on survival: a case study in total knee replacement surgery. Med Decis Mak 40:302–313. https://doi.org/10.1177/0272989X20913266
Johnson NR, Trofa DP, Saltzman BM et al (2020) Healing rate and clinical outcomes of lesser tuberosity osteotomy for anatomic shoulder arthroplasty. J Am Acad Orthop Surg Glob Res Rev. https://doi.org/10.5435/JAAOSGlobal-D-19-00119
Pua Y-H, Kang H, Thumboo J et al (2020) Machine learning methods are comparable to logistic regression techniques in predicting severe walking limitation following total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc 28:3207–3216. https://doi.org/10.1007/s00167-019-05822-7
Li J, Ji Q, Ni M et al (2020) Management of intraoperative acetabular fracture in primary total hip arthroplasty. BMC Musculoskelet Disord 21:383. https://doi.org/10.1186/s12891-020-03356-5
Maniscalco P, Quattrini F, Ciatti C et al (2020) Neck modularity in total hip arthroplasty: a retrospective study of nine hundred twenty-eight titanium neck implants with a maximum follow-up of eighteen years. Int Orthop 44:2261–2266. https://doi.org/10.1007/s00264-020-04686-8
Blevins JL, Rao V, Chiu Y-F et al (2020) Predicting implant size in total knee arthroplasty using demographic variables. Bone Joint J 102-B:85–90. https://doi.org/10.1302/0301-620X.102B6.BJJ-2019-1620.R1
Ghomrawi HMK, Lee LY-Y, Nwachukwu BU et al (2020) Preoperative expectations associated with postoperative dissatisfaction after total knee arthroplasty: a cohort study. J Am Acad Orthop Surg 28:e145–e150. https://doi.org/10.5435/JAAOS-D-18-00785
Pallante GD, Statz JM, Milbrandt TA, Trousdale RT (2020) Primary total hip arthroplasty in patients 20 years old and younger. J Bone Joint Surg Am 102:519–525. https://doi.org/10.2106/JBJS.19.00699
Buller LT, Rao V, Chiu Y-F et al (2020) Primary total knee arthroplasty performed using high-viscosity cement is associated with higher odds of revision for aseptic loosening. J Arthroplasty 35:S182–S189. https://doi.org/10.1016/j.arth.2019.08.023
Glanzmann MC, Audigé L, Schwyzer H-K, Kolling C (2020) Re-intervention and revision rates following primary reverse total shoulder arthroplasty—review of a local shoulder arthroplasty registry. Int Orthop 44:2365–2370. https://doi.org/10.1007/s00264-020-04721-8
Bin Abd Razak HR, Lee JHM, Tan SM et al (2020) Satisfaction rates are low following revision total knee arthroplasty in Asians Despite improvements in patient-reported outcome measures. J Knee Surg 33:1041–1046. https://doi.org/10.1055/s-0039-1692629
Pachter CS, Garfinkel JH, Cochrane NH et al (2020) Smoking is a risk factor for wound complications after direct anterior hip arthroplasty with mesh tape closure. Adv Skin Wound Care 33:43–46. https://doi.org/10.1097/01.ASW.0000613544.11947.8b
Desai VS, Pareek A, DeDeugd CM et al (2020) Smoking, unemployment, female sex, obesity, and medication use yield worse outcomes in patellofemoral arthroplasty. Knee Surg Sports Traumatol Arthrosc 28:2962–2969. https://doi.org/10.1007/s00167-019-05704-y
Domb BG, Chen SL, Shapira J et al (2020) The evolution of hip arthroscopy: what has changed since 2008-a single surgeon’s experience. Arthroscopy 36:761–772. https://doi.org/10.1016/j.arthro.2019.10.009
Goldman AH, Osmon DR, Hanssen AD et al (2020) The Lawrence D. Dorr Surgical Techniques & Technologies Award: aseptic reoperations within one year of primary total hip arthroplasty markedly increase the risk of later periprosthetic joint infection. J Arthroplasty 35:S10–S14. https://doi.org/10.1016/j.arth.2020.02.054
Carlson SW, Sierra RJ, Trousdale RT (2020) Total hip arthroplasty in patients with osteogenesis imperfecta. J Arthroplasty 35:2131–2135. https://doi.org/10.1016/j.arth.2020.03.023
Greenberg A, Kandel L, Liebergall M et al (2020) Total knee arthroplasty for Valgus Deformity via a lateral approach: clinical results, comparison to medial approach, and review of recent literature. J Arthroplasty 35:2076–2083. https://doi.org/10.1016/j.arth.2020.03.037
Rosinsky PJ, Annin S, Maldonado DR et al (2020) Arthroscopic Ligamentum Teres reconstruction: minimum 2-year patient-reported outcomes with subanalysis of patients with Ehlers–Danlos syndrome. Arthroscopy 36:2170–2182. https://doi.org/10.1016/j.arthro.2020.04.028
Chan PK, Hwang YY, Cheung A et al (2020) Blood transfusions in total knee arthroplasty: a retrospective analysis of a multimodal patient blood management programme. Hong Kong Med J 26:201–207. https://doi.org/10.12809/hkmj198289
Zhang C-F, He L, Fang X-Y et al (2020) Debridement, antibiotics, and implant retention for acute periprosthetic joint infection. Orthop Surg 12:463–470. https://doi.org/10.1111/os.12641
Arias-de la Torre J, Smith K, Dregan A et al (2020) Impact of comorbidity on the short- and medium-term risk of revision in total hip and knee arthroplasty. BMC Musculoskelet Disord 21:447. https://doi.org/10.1186/s12891-020-03455-3
Lee S-H, Shih H-N, Chang C-H et al (2020) Influence of extension stem length and diameter on clinical and radiographic outcomes of revision total knee arthroplasty. BMC Musculoskelet Disord 21:15. https://doi.org/10.1186/s12891-019-3030-1
Hoskins W, Dowsey MM, Spelman T, Choong PFM (2020) Early surgical complications of total hip arthroplasty related to surgical approach. ANZ J Surg 90:2050–2055. https://doi.org/10.1111/ans.16149
Hatta T, Statz JM, Itoi E et al (2020) Shoulder arthroplasty in patients with immunosuppression following solid organ transplantation. J Shoulder Elbow Surg 29:44–49. https://doi.org/10.1016/j.jse.2019.05.042
Moverman MA, Menendez ME, Mahendraraj KA et al (2021) Patient risk factors for acromial stress fractures after reverse shoulder arthroplasty: a multicenter study. J Shoulder Elbow Surg 30:1619–1625. https://doi.org/10.1016/j.jse.2020.09.012
Helder O, Marks M, Schweizer A et al (2021) Complications after surface replacing and silicone PIP arthroplasty: an analysis of 703 implants. Arch Orthop Trauma Surg 141:173–181. https://doi.org/10.1007/s00402-020-03663-5
Montalti M, Bordini B, Natali S et al (2021) Revisions for periprosthetic hip infections do not fail more than revisions for aseptic loosening, but mortality is higher. J Arthroplasty 36:1074–1079. https://doi.org/10.1016/j.arth.2020.09.038
Di Martino A, Castagnini F, Stefanini N et al (2021) Survival rates and reasons for revision of different stem designs in total hip arthroplasty for developmental dysplasia: a regional registry study. J Orthop Traumatol 22:29. https://doi.org/10.1186/s10195-021-00590-y
Ponzio DY, Rothermel SD, Chiu Y-F et al (2021) Does physical activity level influence total hip arthroplasty expectations, satisfaction, and outcomes? J Arthroplasty 36:2850–2857. https://doi.org/10.1016/j.arth.2021.03.052
Drager J, Polce EM, Fu M et al (2021) Patients undergoing anatomic total shoulder arthroplasty achieve clinically significant outcomes faster than those undergoing reverse shoulder arthroplasty. J Shoulder Elbow Surg 30:2523–2532. https://doi.org/10.1016/j.jse.2021.02.015
Poondla RK, Sheth MM, Heldt BL et al (2021) Anatomic and reverse shoulder arthroplasty in patients 70 years of age and older: a comparison cohort at early to midterm follow-up. J Shoulder Elbow Surg 30:1336–1343. https://doi.org/10.1016/j.jse.2020.08.030
Sheth MM, Morris BJ, Laughlin MS et al (2021) Early to midterm outcomes of anatomic shoulder arthroplasty performed on dysplastic glenoids. J Shoulder Elbow Surg 30:S77–S83. https://doi.org/10.1016/j.jse.2020.08.017
Owen AR, Tibbo ME, van Wijnen AJ et al (2021) Acquired idiopathic stiffness after contemporary total knee arthroplasty: incidence, risk factors, and results over 25 years. J Arthroplasty 36:2980–2985. https://doi.org/10.1016/j.arth.2021.03.051
Di Martino A, Coppola MAR, Bordini B et al (2021) Clinical and radiological outcomes of total hip arthroplasty in patients affected by Paget’s disease: a combined registry and single-institution retrospective observational study. J Orthop Traumatol 22:13. https://doi.org/10.1186/s10195-021-00574-y
Russo A, Cavagnaro L, Chiarlone F et al (2021) Clinical outcomes and survivorship of two-stage total hip or knee arthroplasty in septic arthritis: a retrospective analysis with a minimum five-year follow-up. Int Orthop 45:1683–1691. https://doi.org/10.1007/s00264-021-05013-5
Gould D, Thuraisingam S, Shadbolt C et al (2021) Cohort profile: the St Vincent’s Melbourne Arthroplasty Outcomes (SMART) Registry, a pragmatic prospective database defining outcomes in total hip and knee replacement patients. BMJ Open 11:e040408. https://doi.org/10.1136/bmjopen-2020-040408
Shah NS, Foote AM, Steele CA et al (2021) Does preoperative disease severity influence outcomes in reverse shoulder arthroplasty for cuff tear arthropathy? J Shoulder Elbow Surg 30:2745–2752. https://doi.org/10.1016/j.jse.2021.04.035
Schwarz JS, Lygrisse KA, Roof MA et al (2021) Early, mid-term, and late-term aseptic femoral revisions after THA: comparing causes, complications, and resource utilization. J Arthroplasty 36:3551–3555. https://doi.org/10.1016/j.arth.2021.05.041
Katakam A, Bragdon CR, Chen AF et al (2021) Elevated body mass index is a risk factor for failure to achieve the knee disability and osteoarthritis outcome score-physical function short form minimal clinically important difference following total knee arthroplasty. J Arthroplasty 36:1626–1632. https://doi.org/10.1016/j.arth.2020.12.019
Giesinger K, Giesinger JM, Hamilton DF et al (2021) Higher body mass index is associated with larger postoperative improvement in patient-reported outcomes following total knee arthroplasty. BMC Musculoskelet Disord 22:635. https://doi.org/10.1186/s12891-021-04512-1
Harmer JR, Wyles CC, Mara KC et al (2021) Impact of perioperative pain control on knee range of motion and development of arthrofibrosis following primary total knee arthroplasty. J Arthroplasty 36:532–536. https://doi.org/10.1016/j.arth.2020.08.037
Katakam A, Melnic CM, Bragdon CR et al (2021) Low body mass index is a predictor for mortality and increased length of stay following total joint arthroplasty. J Arthroplasty 36:72–77. https://doi.org/10.1016/j.arth.2020.07.055
Illgen RL, Lewallen DG, Yep PJ et al (2021) Migration patterns for revision total hip arthroplasty in the United States as reported in the American Joint Replacement Registry. J Arthroplasty 36:1401–1406. https://doi.org/10.1016/j.arth.2020.10.030
Goh GS, Khow YZ, Tay DK et al (2021) Preoperative mental health influences patient-reported outcome measures and satisfaction after revision total knee arthroplasty. J Arthroplasty 36:2878–2886. https://doi.org/10.1016/j.arth.2021.03.026
Kahlenberg CA, Baral EC, Lieberman LW et al (2021) Retrieval analysis of polyethylene components in rotating hinge knee arthroplasty implants. J Arthroplasty 36:2998–3003. https://doi.org/10.1016/j.arth.2021.04.003
Grau LC, Ong AC, Restrepo S et al (2021) Survivorship, clinical and radiographic outcomes of a novel cementless metal-backed patella design. J Arthroplasty 36:S221–S226. https://doi.org/10.1016/j.arth.2021.02.032
Gausden EB, Cross WW III, Mabry TM et al (2021) Total hip arthroplasty for femoral neck fracture: What are the contemporary reasons for failure? J Arthroplasty 36:S272–S276. https://doi.org/10.1016/j.arth.2021.02.008
Siljander MP, Trousdale RT, Perry KI et al (2021) Total hip arthroplasty in patients with osteopetrosis. J Arthroplasty 36:1367–1372. https://doi.org/10.1016/j.arth.2020.10.018
Palazzuolo M, Antoniadis A, Mahlouly J, Wegrzyn J (2021) Total knee arthroplasty improves the quality-adjusted life years in patients who exceeded their estimated life expectancy. Int Orthop 45:635–641. https://doi.org/10.1007/s00264-020-04917-y
Di Martino A, Bordini B, Barile F et al (2021) Unicompartmental knee arthroplasty has higher revisions than total knee arthroplasty at long term follow-up: a registry study on 6453 prostheses. Knee Surg Sports Traumatol Arthrosc 29:3323–3329. https://doi.org/10.1007/s00167-020-06184-1
Sagheb E, Ramazanian T, Tafti AP et al (2021) Use of natural language processing algorithms to identify common data elements in operative notes for knee arthroplasty. J Arthroplasty 36:922–926. https://doi.org/10.1016/j.arth.2020.09.029
Ingelsrud LH, Terluin B, Gromov K et al (2021) Which Oxford Knee Score level represents a satisfactory symptom state after undergoing a total knee replacement? Acta Orthop 92:85–90. https://doi.org/10.1080/17453674.2020.1832304
Tracey RW, Akram F, Della Valle CJ et al (2021) Clinical outcomes in isolated tibial revision with cruciate retaining total knee arthroplasty. J Arthroplasty 36:2536–2540. https://doi.org/10.1016/j.arth.2021.02.013
Ling DI, Finocchiaro A, Schneider B et al (2021) CORR Insights®: what factors are associated with patient-reported outcome measure questionnaire completion for an electronic shoulder arthroplasty registry? Clin Orthop Relat Res 479:148–150. https://doi.org/10.1097/CORR.0000000000001424
Visvanathan A, Wilson C, Jackman E et al (2021) Design, construction, and early results of a formal local revision knee arthroplasty registry. J Knee Surg 34:1284–1295. https://doi.org/10.1055/s-0040-1708040
Teo BJX, Koh JSB, Jiang L et al (2019) Association of the 36-Item Short Form Health Survey Physical Component Summary Score with patient satisfaction and improvement 2 years after total knee arthroplasty. JAMA Netw Open 2:e190062. https://doi.org/10.1001/jamanetworkopen.2019.0062
Castagnini F, Bordini B, Tassinari E et al (2019) Delta-on-delta ceramic bearing surfaces in revision hip arthroplasty. J Arthroplasty 34:2065–2071. https://doi.org/10.1016/j.arth.2019.04.068
Johnson RL, Abdel MP, Frank RD et al (2019) Impact of frailty on outcomes after primary and revision total hip arthroplasty. J Arthroplasty 34:56-64.e5. https://doi.org/10.1016/j.arth.2018.09.078
Nguyen U-SDT, Perneger T, Franklin PD et al (2019) Improvement in mental health following total hip arthroplasty: the role of pain and function. BMC Musculoskelet Disord 20:307. https://doi.org/10.1186/s12891-019-2669-y
Chua JL, Goh GS-H, Liow MHL et al (2019) Modern TKA implants are equivalent to traditional TKA implants in functional and patellofemoral joint-related outcomes. Knee Surg Sports Traumatol Arthrosc 27:1116–1123. https://doi.org/10.1007/s00167-018-5161-6
Wyles CC, Vargas-Hernandez JS, Carlson SW et al (2019) Single-dose perioperative antibiotics do not increase the risk of surgical site infection in unicompartmental knee arthroplasty. J Arthroplasty 34:S327–S330. https://doi.org/10.1016/j.arth.2019.02.041
Bordini B, Stea S, Castagnini F et al (2019) The influence of bearing surfaces on periprosthetic hip infections: analysis of thirty nine thousand, two hundred and six cementless total hip arthroplasties. Int Orthop 43:103–109. https://doi.org/10.1007/s00264-018-4097-2
Wyles CC, Tibbo ME, Fu S et al (2019) Use of natural language processing algorithms to identify common data elements in operative notes for total hip arthroplasty. J Bone Joint Surg Am 101:1931–1938. https://doi.org/10.2106/JBJS.19.00071
Boyer B, Bordini B, Caputo D et al (2019) What are the influencing factors on hip and knee arthroplasty survival? Prospective cohort study on 63619 arthroplasties. Orthop Traumatol Surg Res 105:1251–1256. https://doi.org/10.1016/j.otsr.2019.07.020
Giordano BD, Kuhns BD, Perets I et al (2020) Acetabular morphologic characteristics predict early conversion to arthroplasty after isolated hip arthroscopy for femoroacetabular impingement. Am J Sports Med 48:188–196. https://doi.org/10.1177/0363546519888894
Steinhaus ME, Buller LT, Romero JA et al (2020) Body mass index classification is independently associated with health-related quality of life after primary total knee arthroplasty: an institutional registry-based study. J Knee Surg 33:399–409. https://doi.org/10.1055/s-0039-1677811
Goh GS, Liow MHL, Chen JY et al (2020) Can octogenarians undergoing total knee arthroplasty experience similar functional outcomes, quality of life, and satisfaction rates as their younger counterparts? A propensity score matched analysis of 1188 patients. J Arthroplasty 35:1833–1839. https://doi.org/10.1016/j.arth.2020.02.033
Chaudhry H, MacDonald SJ, Howard JL et al (2020) Clinical outcomes and midterm survivorship of an uncemented primary total hip arthroplasty system. J Arthroplasty 35:1662–1666. https://doi.org/10.1016/j.arth.2020.01.063
Sheth MM, Morris BJ, Laughlin MS et al (2020) Lower socioeconomic status is associated with worse preoperative function, pain, and increased opioid use in patients with primary glenohumeral osteoarthritis. J Am Acad Orthop Surg 28:287–292. https://doi.org/10.5435/JAAOS-D-19-00490
Rosinsky PJ, Chen SL, Yelton MJ et al (2020) Outpatient vs. inpatient hip arthroplasty: a matched case-control study on a 90-day complication rate and 2-year patient-reported outcomes. J Orthop Surg Res 15:367. https://doi.org/10.1186/s13018-020-01871-8
Galea VP, Ingelsrud LH, Florissi I et al (2020) Patient-acceptable symptom state for the Oxford Hip Score and Forgotten Joint Score at 3 months, 1 year, and 2 years following total hip arthroplasty: a registry-based study of 597 cases. Acta Orthop 91:372–377. https://doi.org/10.1080/17453674.2020.1750877
Tew M, Dalziel K, Clarke P et al (2020) Patient-reported outcome measures (PROMs): can they be used to guide patient-centered care and optimize outcomes in total knee replacement? Qual Life Res 29:3273–3283. https://doi.org/10.1007/s11136-020-02577-4
Liow MHL, Goh GS, Pang H-N et al (2020) Should patients aged 75 years or older undergo medial unicompartmental knee arthroplasty? A propensity score-matched study. Arch Orthop Trauma Surg 140:949–956. https://doi.org/10.1007/s00402-020-03440-4
Galea VP, Florissi I, Rojanasopondist P et al (2020) The patient acceptable symptom state for the harris hip score following total hip arthroplasty: validated thresholds at 3-month, 1-, 3-, 5-, and 7-year follow-up. J Arthroplasty 35:145-152.e2. https://doi.org/10.1016/j.arth.2019.08.037
Marzel A, Schwyzer H-K, Kolling C et al (2020) The Schulthess local Shoulder Arthroplasty Registry (SAR): cohort profile. BMJ Open 10:e040591. https://doi.org/10.1136/bmjopen-2020-040591
Thompson Z, Khoshbin A, Ward S et al (2020) The early-to medium-term results of a hemispherical, porous coated acetabular shell with multiple different bearing combinations are excellent with the exception of metal-on-metal. Int Orthop 44:2537–2543. https://doi.org/10.1007/s00264-020-04817-1
Yeo MGH, Goh GS, Chen JY et al (2020) Are Oxford Hip Score and Western Ontario and McMaster Universities Osteoarthritis Index useful predictors of clinical meaningful improvement and satisfaction after total hip arthroplasty? J Arthroplasty 35:2458–2464. https://doi.org/10.1016/j.arth.2020.04.034
Ramaskandhan J, Rashid A, Kometa S, Siddique MS (2020) Comparison of 5-year patient-reported outcomes (PROMs) of total ankle replacement (TAR) to total knee replacement (TKR) and total hip replacement (THR). Foot Ankle Int 41:767–774. https://doi.org/10.1177/1071100720918880
Holtz N, Hamilton DF, Giesinger JM et al (2020) Minimal important differences for the WOMAC osteoarthritis index and the Forgotten Joint Score-12 in total knee arthroplasty patients. BMC Musculoskelet Disord 21:401. https://doi.org/10.1186/s12891-020-03415-x
Bonilla GA, Montoya BE, Restrepo VE et al (2021) Institutional arthroplasty registry: what is the minimum acceptable dataset to be included in your hospital? Recommendations from a single-country national consensus using the Delphi method. Int Orthop 45:5–12. https://doi.org/10.1007/s00264-020-04866-6
Khow YZ, Goh GS, Chen JY et al (2021) Change in body mass index after simultaneous bilateral total knee arthroplasty: risk factors and its influence on functional outcomes. J Arthroplasty 36:1974–1979. https://doi.org/10.1016/j.arth.2021.01.059
Ho KK-W, Lau LC-M, Chau W-W et al (2021) End-stage knee osteoarthritis with and without sarcopenia and the effect of knee arthroplasty—a prospective cohort study. BMC Geriatr 21:2. https://doi.org/10.1186/s12877-020-01929-6
Vigdorchik JM, Sculco PK, Inglis AE et al (2021) Evaluating alternate registration planes for imageless, computer-assisted navigation during total hip arthroplasty. J Arthroplasty 36:3527–3533. https://doi.org/10.1016/j.arth.2021.05.037
Lützner J, Beyer F, Günther K-P, Huber J (2021) Higher treatment effect after total knee arthroplasty is associated with higher patient satisfaction. Knee Surg Sports Traumatol Arthrosc 29:3426–3432. https://doi.org/10.1007/s00167-020-06272-2
Miozzari HH, Barea C, Hannouche D, Lübbeke A (2021) History of previous surgery is associated with higher risk of revision after primary total knee arthroplasty: a cohort study from the Geneva Arthroplasty Registry. Acta Orthop 92:709–715. https://doi.org/10.1080/17453674.2021.1970322
Woo BJ, Chen JY, Lai YM et al (2021) Improvements in functional outcome and quality of life are not sustainable for patients ≥ 68 years old 10 years after total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc 29:3330–3336. https://doi.org/10.1007/s00167-020-06200-4
Ho KW, Pong G, Poon WC et al (2021) Progression of health-related quality of life of patients waiting for total knee arthroplasty. J Eval Clin Pract 27:69–74. https://doi.org/10.1111/jep.13388
Khow YZ, Liow MHL, Lee M et al (2021) Coronal alignment of fixed-bearing unicompartmental knee arthroplasty femoral component may affect long-term clinical outcomes. J Arthroplasty 36:478–487. https://doi.org/10.1016/j.arth.2020.07.070
Castagnini F, Bordini B, Cosentino M et al (2021) The influence of bearing surfaces on revisions due to dislocations in total hip arthroplasty. J Mater Sci Mater Med 32:123. https://doi.org/10.1007/s10856-021-06598-4
Gonzalez AI, Nguyen U-SDT, Franklin P et al (2022) 1-year trajectories of patients undergoing primary total hip arthroplasty: Patient reported outcomes and resource needs according to education level. BMC Musculoskelet Disord 23:84. https://doi.org/10.1186/s12891-022-05004-6
Bonnefoy-Mazure A, Poncet A, Gonzalez A et al (2022) Limping and patient satisfaction after primary total hip arthroplasty: a registry-based cohort study. Acta Orthop 93:602–608. https://doi.org/10.2340/17453674.2022.3489
Baker CM, Restrepo C, Hozack WJ (2022) Minimum five-year outcomes of modular dual mobility in primary total hip arthroplasty. J Arthroplasty 37:S566–S570. https://doi.org/10.1016/j.arth.2022.02.118
Wik TS, Klaksvik J, Husby OS et al (2022) Patient-reported outcome after primary and aseptic revision hip arthroplasty: 1-year follow-up of 3,559 primary and 406 revision THAs in an institutional registry. Acta Orthop 93:132–137. https://doi.org/10.2340/17453674.2021.852
Gifstad T, Nordskar JJ, Egeberg T et al (2022) Cementless unicompartmental knee arthroplasty results in higher pain levels compared to the cemented technique: a prospective register study. Knee Surg Sports Traumatol Arthrosc 30:2738–2743. https://doi.org/10.1007/s00167-021-06617-5
Castiello E, Affatato S (2020) Progression of osteoarthritis and reoperation in unicompartmental knee arthroplasty: a comparison of national joint registries. Int J Artif Organs 43:203–207. https://doi.org/10.1177/0391398819879697
Hannon CP, Kruckeberg BM, Lewallen DG et al (2022) Treatment of flexion instability after primary total knee arthroplasty: operative and nonoperative management of 218 cases. J Arthroplasty 37:S333–S341. https://doi.org/10.1016/j.arth.2022.02.069
Yakkanti RR, Ocksrider JL, Patel AA et al (2022) Unexpected wear of a uniquely designed moderately cross-linked polyethylene in total hip arthroplasty. J Arthroplasty 37:1130–1135. https://doi.org/10.1016/j.arth.2022.01.093
Moverman MA, Puzzitiello RN, Pagani NR et al (2022) Functional somatic syndromes are associated with suboptimal outcomes and high cost after shoulder arthroplasty. J Shoulder Elbow Surg 31:48–55. https://doi.org/10.1016/j.jse.2021.05.015
Katakam A, Florissi IS, Colon Iban YE et al (2021) Class III obesity increases risk of failure to achieve the 1-year hip disability and Osteoarthritis Outcome Score-Physical Function Short Form minimal clinically important difference following total hip arthroplasty. J Arthroplasty 36:187–192. https://doi.org/10.1016/j.arth.2020.07.035
Soh S-E, Harris IA, Cashman K et al (2022) Minimal clinically important changes in HOOS-12 and KOOS-12 scores following joint replacement. J Bone Joint Surg Am 104:980–987. https://doi.org/10.2106/JBJS.21.00741
Singh JA, Lewallen DG (2014) Depression in primary TKA and higher medical comorbidities in revision TKA are associated with suboptimal subjective improvement in knee function. BMC Musculoskelet Disord 15:127. https://doi.org/10.1186/1471-2474-15-127
Muscatelli SR, Zheng H, Hughes RE et al (2021) Non-inferiority of aspirin for venous thromboembolism prophylaxis after hip arthroplasty in a statewide registry. J Arthroplasty 36:2068-2075.e2. https://doi.org/10.1016/j.arth.2021.01.025
Canetti R, Malatray M, Pibarot V, Wegrzyn J (2022) Dual mobility cups associated with proximal femoral replacement in nontumoral indications: results and complications. Orthop Traumatol Surg Res 108:103029. https://doi.org/10.1016/j.otsr.2021.103029
Johnson RL, Frank RD, Abdel MP et al (2022) Frailty transitions one year after total joint arthroplasty: a cohort study. J Arthroplasty 37:10-18.e2. https://doi.org/10.1016/j.arth.2021.08.022
Koh DTS, Woo YL, Yew AKS, Yeo S-J (2021) Kinematic aligned femoral rotation leads to greater patella tilt but similar clinical outcomes when compared to traditional femoral component rotation in total knee arthroplasty. A propensity score matched study. Knee Surg Sports Traumatol Arthrosc 29:1059–1066. https://doi.org/10.1007/s00167-020-06081-7
Goh GS, Bin Abd Razak HR, Tay DK-J et al (2021) Early post-operative oxford knee score and knee society score predict patient satisfaction 2 years after total knee arthroplasty. Arch Orthop Trauma Surg 141:129–137. https://doi.org/10.1007/s00402-020-03612-2
Allport J, Ramaskandhan J, Alkhreisat M, Siddique MS (2021) Patient-reported outcome measures and radiological outcomes in mobile-bearing total ankle arthroplasty with varus or valgus deformity. Foot Ankle Int 42:176–182. https://doi.org/10.1177/1071100720949852
Balazs GC, Dooley M, Wang D et al (2022) Clinical outcomes of open hip abductor tendon repair with minimum two-year follow-up. Hip Int 32:516–522. https://doi.org/10.1177/1120700020965487
Cherian NJ, Ohnoutka C, Peissig EJ et al (2022) Cemented patellar implant malposition: a non-issue for the painful total knee arthroplasty. J Arthroplasty 37:S859–S863. https://doi.org/10.1016/j.arth.2022.02.020
Lucchini S, Baleani M, Giardina F et al (2022) A case-driven hypothesis for multi-stage crack growth mechanism in fourth-generation ceramic head fracture. J Orthop Surg Res 17:293. https://doi.org/10.1186/s13018-022-03190-6
Macheras GA, Lepetsos P, Galanakos SP et al (2022) Early failure of an uncemented femoral stem, as compared to two other stems with similar design, following primary total hip arthroplasty performed with direct anterior approach. Hip Int 32:166–173. https://doi.org/10.1177/1120700020940671
Kenanidis E, Kakoulidis P, Leonidou A et al (2021) Survival of monoblock RM vitamys compared with modular PINNACLE cups: mid-term outcomes of 200 hips performed by a single surgeon. Hip Int 31:465–471. https://doi.org/10.1177/1120700019885619
Claxton MR, Wagner ER, Rizzo M (2022) Long-term outcomes of MCP surface replacement arthroplasty in patients with rheumatoid arthritis. Hand 17:271–277. https://doi.org/10.1177/1558944720926631
Goh GS, Zeng GJ, Khow YZ et al (2021) No difference in long-term outcomes between men and women undergoing medial fixed-bearing cemented unicompartmental knee arthroplasty: a retrospective cohort study with minimum 10-year follow up. Knee 30:26–34. https://doi.org/10.1016/j.knee.2021.03.006
Harmer JR, Wyles CC, Larson DR et al (2022) Changing surgical approach from primary to revision total hip arthroplasty is not associated with increased risk of dislocation or re-revisions. J Arthroplasty 37:S622–S627. https://doi.org/10.1016/j.arth.2022.03.007
Shaw JH, Rahman TM, Wesemann LD et al (2022) Comparison of postoperative instability and acetabular cup positioning in robotic-assisted versus traditional total hip arthroplasty. J Arthroplasty 37:S881–S889. https://doi.org/10.1016/j.arth.2022.02.002
Dagneaux L, Amundson AW, Larson DR et al (2021) Contemporary mortality rate and outcomes in nonagenarians after primary total knee arthroplasty. J Arthroplasty 36:3456–3462. https://doi.org/10.1016/j.arth.2021.05.015
Dagneaux L, Amundson AW, Larson DR et al (2021) Contemporary mortality rate and outcomes in nonagenarians undergoing primary total hip arthroplasty. J Arthroplasty 36:1373–1379. https://doi.org/10.1016/j.arth.2020.10.040
Hughes RE, Batra A, Hallstrom BR (2017) Arthroplasty registries around the world: valuable sources of hip implant revision risk data. Curr Rev Musculoskelet Med 10:240–252. https://doi.org/10.1007/s12178-017-9408-5
Stickles B, Phillips L, Brox WT et al (2001) Defining the relationship between obesity and total joint arthroplasty. Obes Res 9:219–223. https://doi.org/10.1038/oby.2001.24
Guo S-J, Shao H-Y, Huang Y et al (2020) Retrospective cohort study comparing complications, readmission, transfusion, and length of stay of patients undergoing simultaneous and staged bilateral total hip arthroplasty. Orthop Surg 12:233–240. https://doi.org/10.1111/os.12617
Kostuj T, Preis M, Walther M et al (2014) German Total Ankle Replacement Register of the German Foot and Ankle Society (D. A. F.)—presentation of design and reliability of the data as well as first results. Z Orthop Unfall 152:446–454. https://doi.org/10.1055/s-0034-1382933
Arias-de la Torre J, Puigdomenech E, Valderas JM et al (2019) Availability of specific tools to assess patient reported outcomes in hip arthroplasty in Spain. Identifying the best candidates to incorporate in an arthroplasty register. A systematic review and standardized assessment. PLoS One 14(4): https://doi.org/10.1371/journal.pone.0214746
Ranawat CS, Meftah M, Windsor EN, Ranawat AS (2012) Cementless fixation in total knee arthroplasty: down the boulevard of broken dreams - affirms. J Bone Joint Surg Br 94(11 Suppl A):82–84: https://doi.org/10.1302/0301-620X.94B11.30826
van der List JP, McDonald LS, Pearle AD (2015) Systematic review of medial versus lateral survivorship in unicompartmental knee arthroplasty. Knee 22(6):454–460. https://doi.org/10.1016/j.knee.2015.09.011
Park CN, White PB, Meftah M, Ranawat AS, Ranawat CS (2016) Diagnostic algorithm for residual pain after total knee arthroplasty. Orthopedics 39(2):e246–e252. https://doi.org/10.3928/01477447-20160119-06
van der List JP, Zuiderbaan HA, Pearle AD (2016) Why do medial unicompartmental knee arthroplasties fail today? J Arthroplasty 31(5):1016–1021. https://doi.org/10.1016/j.arth.2015.11.030
van der List JP, Chawla H, Joskowicz L, Pearle AD (2016) Current state of computer navigation and robotics in unicompartmental and total knee arthroplasty: a systematic review with meta-analysis. Knee Surg Sports Traumatol Arthrosc 24(11):3482–3495. https://doi.org/10.1007/s00167-016-4305-9
van der List JP, Chawla H, Villa JC, Pearle AD (2017) Why do patellofemoral arthroplasties fail today? A systematic review. Knee 24(1):2–8. https://doi.org/10.1016/j.knee.2015.11.002
van der List JP, Chawla H, Zuiderbaan HA, Pearle AD (2017) Survivorship and functional outcomes of patellofemoral arthroplasty: a systematic review. Knee Surg Sports Traumatol Arthrosc 25(8):2622–2631. https://doi.org/10.1007/s00167-015-3878-z
Gu A, Michalak AJ, Cohen JS, Almeida ND, McLawhorn AS, Sculco PK (2018) Efficacy of manipulation under anesthesia for stiffness following total knee arthroplasty: a systematic review. J Arthroplasty 33(5):1598–1605. https://doi.org/10.1016/j.arth.2017.11.054
Stryker LS, Abdel MP, Hanssen AD (2013) Predictive value of inflammatory markers for irrigation and debridement of acute TKA infection. Orthopedics 36(6):765–770. https://doi.org/10.3928/01477447-20130523-22
Oviedo Baena AM, Moeschler SM, Smith HM, Duncan CM, Schroeder DR, Kopp SL (2015) Perioperative comorbidities and complications among patients undergoing primary total knee arthroplasty: a retrospective analysis and prospective survey. J Clin Anesth 27(7):558–565. https://doi.org/10.1016/j.jclinane.2015.07.011
Amanatullah DF, Stryker L, Schoenecker P et al (2015) Similar clinical outcomes for THAs with and without prior periacetabular osteotomy. Clin Orthop Relat Res 473(2):685–691. https://doi.org/10.1007/s11999-014-4026-7
Werthel JD, Lonjon G, Jo S, Cofield R, Sperling JW, Elhassan BT (2017) Long-term outcomes of cemented versus cementless humeral components in arthroplasty of the shoulder: a propensity score-matched analysis. Bone Joint J 99-B(5):666–673. https://doi.org/10.1302/0301-620X.99B5.BJJ-2016-0910.R1
Brown TS, Van Citters DW, Berry DJ, Abdel MP (2017) The use of highly crosslinked polyethylene in total knee arthroplasty. Bone Joint J 99-B(8):996–1002. https://doi.org/10.1302/0301-620X.99B8.BJJ-2017-0028.R1
Shi GG, Huh J, Gross CE et al (2015) Total ankle arthroplasty following prior infection about the ankle. Foot Ankle Int 36(12):1425–1429. https://doi.org/10.1177/1071100715597430
Mirza AJ, Lombardi AV Jr, Morris MJ, Berend KR (2014) A mini-anterior approach to the hip for total joint replacement: optimising results: improving hip joint replacement outcomes. Bone Joint J 96(11 Supple A):32–35. https://doi.org/10.1302/0301-620X.96B11.34348
Funding
Open access funding provided by HEAL-Link Greece. The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
Conceptualisation: EK; Literature Search and data Analysis: AZ, EK; Writing—original draft preparation: AZ, EK; Writing—review and editing: EK, ET; Supervision: ET, MP.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
This is a scoping review study. No ethical approval was required. The authors submitted the study to PROSPERO (registration number: 356482); however, it was automatically rejected as a scoping review (scoping reviews do not meet the requirements of PROSPERO).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zgouridou, A., Kenanidis, E., Potoupnis, M. et al. Global mapping of institutional and hospital-based (Level II–IV) arthroplasty registries: a scoping review. Eur J Orthop Surg Traumatol 34, 1219–1251 (2024). https://doi.org/10.1007/s00590-023-03691-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00590-023-03691-y