Abstract
Purpose
To analyze the effect of endplate weakness prior to PLIF or TLIF cage implantation and compare it to the opposite intact endplate of the same vertebral body. In addition, the influence of bone quality on endplate resistance was investigated.
Methods
Twenty-two human lumbar vertebrae were tested in a ramp-to-failure test. One endplate of each vertebral body was tested intact and the other after weakening with a rasp (over an area of 200 mm2). Either a TLIF or PLIF cage was then placed and the compression load was applied across the cage until failure of the endplate. Failure was defined as the first local maximum of the force measurement. Bone quality was assessed by determining the Hounsfield units (HU) on CT images.
Results
With an intact endplate and a TLIF cage, the median force to failure was 1276.3N (693.1–1980.6N). Endplate weakening reduced axial endplate resistance to failure by 15% (0–23%). With an intact endplate and a PLIF cage, the median force to failure was 1057.2N (701.2–1735.5N). Endplate weakening reduced axial endplate resistance to failure by 36.6% (7–47.9%). Bone quality correlated linearly with the force at which endplate failure occurred. Intact and weakened endplates showed a strong positive correlation: intact-TLIF: r = 0.964, slope of the regression line (slope) = 11.8, p < 0.001; intact-PLIF: r = 0.909, slope = 11.2, p = 5.5E−05; weakened-TLIF: r = 0.973, slope = 12.5, p < 0.001; weakened-PLIF: r = 0.836, slope = 6, p = 0.003.
Conclusion
Weakening of the endplate during cage bed preparation significantly reduces the resistance of the endplate to subsidence to failure: endplate load capacity is reduced by 15% with TLIF and 37% with PLIF. Bone quality correlates with the force at which endplate failure occurs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The use of intervertebral cages as part of spinal fusion surgery has increased rapidly in recent years. In the United States alone, the rate of lumbar fusion has increased by 322.6% between 2011 and 2019 [1]. This is because they offer biological and mechanical advantages: the insertion of bone grafts improves and accelerates bony fusion [2], they expand the intervertebral space and facilitate lordosis correction [3], and absorb axial compression forces, thereby reducing the forces on pedicle screws and rods, which should reduce the risk of implant failure [4]. Posterior lumbar interbody fusion (PLIF) is used for various degenerative spinal conditions including degenerative disc disease, spondylolisthesis, spinal stenosis, and transforaminal lumbar interbody fusion (TLIF), which is preferred for foraminal stenosis [5].
As the number of fusion surgeries has increased, so have the associated complications [6]. Cage subsidence can cause pain due to loss of intervertebral height with foraminal narrowing and subsequent nerve root impingement, instrumentation failure, pseudoarthrosis, early screw loosening, kyphotic deformity, and adjacent segment degeneration [7]. Known risk factors include low bone density, posterior cage positioning, limited implant contact area and poor endplate condition [8]. Weakening of the endplate during cage bed preparation can also cause subsidence [9]. Typically, the cage bed is carefully prepared with a rasp to achieve bleeding and eventual bony fusion, but if this is done too aggressively, the endplate may be unknowingly opened [10].
Biomechanical studies have shown that endplate morphology plays an important role: If only the edge of the cage rests on the endplate due to the concave shape of the lower and upper endplate geometry, subsidence may occur due to peak loads [11]; the center of the endplate should be avoided as an isolated contact area, as this region is the weakest while the posterolateral region is the strongest [12].
Despite knowledge of the importance of cage positioning and the influence of endplate weakening, the biomechanical effect of endplate weakening has not been quantified yet.
The aim of this study was to analyze the effect of endplate weakening by PLIF and TLIF cages and to compare it to the other intact endplate of the same vertebral body. In addition, the influence of bone quality on endplate resistance was investigated. The hypotheses of the present study are that (1) endplate weakening significantly affects endplate load capacity and that (2) bone quality affects endplate resistance.
Materials and methods
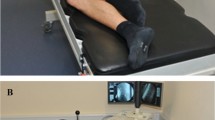
The study was approved by the local ethics committee. Twenty-two lumbar vertebrae (three L1, five L2, five L3, five L4, four L5) obtained from five fresh-frozen human specimens (Science Care, Phoenix, AZ, USA) were tested in this study. The median age was 55.4 years (range 36–75, two males and three females). Computed tomography (CT) scans (SOMATOM Edge Plus, Siemens Healthcare GmbH) showed no bony defects or deformities. The lumbar vertebrae were isolated, taking care not to damage the endplates. Any remnants of the disc were sharply removed. First, one of the two endplates was biomechanically tested by immobilizing the vertebral bodies in customized 3D-printed clamps in a testing machine (Fig. 1A) [13]. The vertebrae were then released from the clamps and inverted to test the other endplate. To ensure a stable fixation, the vertebral bodies were cast in appropriate boxes using polymethylmethacrylate (PMMA; Fig. 1B, E) to test the second endplates. The boxes were made of polylactic acid (PLA). Two different fixation techniques (clamps and potting) were used for practical and financial reasons.
Biomechanical experiments
One endplate of each vertebral body was tested in the intact state and the other in the weakened state. The endplates were weakened with a shaver commonly used to prepare the disc space prior to cage insertion. Cortical bone was removed over an area of 200 mm2, corresponding to the head of the shaver, until cancellous bone was visible in the area where the cage was to be placed. Either a PLIF (PLIF; MectaLIF Posterior; Fig. 1B) or a TLIF cage (TLIF; MectaLIF Transforaminal; Fig. 1C) was then placed on the intact or weakened endplate. Allocation was randomised. Care was taken to ensure that there were equal numbers of vertebrates in the PLIF and TLIF groups. The rationale for using a unilateral PLIF (uPLIF) cage in our study was to be able to draw more appropriate conclusions regarding bilateral PLIF (bPLIF) cages (than vice versa) without underestimating the force acting on the endplate when using a single cage. Subsequently, uniaxial compression load was applied to the cage using a static testing machine (Zwick/Roell Allroundline 10kN and testXpert III software; Fig. 1A). The test setup was performed in accordance with the ASTM F2077-18 (Test Methods for Intervertebral Body Fusion Devices) standard for compression test configuration [14]. The endplate was loaded through an open spherical joint to ensure that the force was applied uniformly across the cage. In addition, the vertebra was mounted on an x-y stage that allowed translational motion orthogonal to the loading direction (Fig. 1A). Prior to biomechanical testing, the posterior bony structures were removed using an oscillating saw to ensure that the compression force was applied to the cage in isolation. A Xforce HP 10kN load cell with an accuracy of ± 0.5% for force measurements above 100N, manufactured by the same company as the testing machine, was used.
Biomechanical testing protocol
Each vertebral body was tested twice in a ramp-to-failure test: (1) the intact endplate on one side, and (2) the weakened endplate on the other side. Eleven of the 22 lumbar vertebrae were assigned to the PLIF group and eleven to the TLIF group.
The cages were preloaded with ± 20N in the compression plane. Compression was applied at a velocity of 5 mm/min according to the specifications of the ASTM 2077–18 test standard. The force was recorded until 5 mm displacement were reached (Fig. 1D–E).
Data evaluation and statistical analysis
The force at which failure occurred, indicating sintering of the cage and therefore fracture of the endplate, was defined as the first local maximum of the force measurement (Fig. 2). The maximum compressive force that resulted in failure of the intact endplate was compared with the force that resulted in failure of the weakened endplate using paired nonparametric comparisons (Wilcoxon signed-rank test). Probability density functions were fitted onto the failure force results for different cage configurations (uPLIF, bPLIF, and TLIF) in order to compare the distributions of forces measured for the intact and the weakened endplates. In addition, bone quality was assessed for each vertebra from the CT images in Hounsfield units (HU) according to Schreiber et al. [15]. The average HU value resulting from three elliptical regions of interest within the vertebral body on axial slices (below the superior endplate, in the middle of the vertebral body, and above the inferior endplate) was set as the reference value for the status of the vertebral (trabecular) bone. Variable tube voltage used during CT acquisition was taken into account by adapting the HU according to the results of Afifi et al. [16]. Intraclass correlation coefficient (ICC) (Model: two-way mixed effect; Definition: absolute agreement, Type: single measurement) was: 0.996 [0.991,0.998]. Linear regression analysis was then used to investigate the resulting average HU value as a factor influencing endplate resistance to force through linear regression analysis. The significance level α was set to 0.05 and the values are specified as median (25th–75th percentile).
Results
Failure force in intact and weakened endplates
With a TLIF cage, the median force at failure was 1276.3N (693.1–1980.6N) with an intact endplate. With a weakened endplate, the force at which failure occurred was significantly reduced by a median of 15% (0–23%, p = 0.04; Fig. 3). The weakened endplates resisted a median force of 1149N for TLIF cages.
With a PLIF cage, the median force at failure was 1057.2N (701.2–1735.5N) with an intact endplate. With a weakened endplate, the force at which failure occurred was significantly reduced by a median of 36.6% (7–47.9%, p = 0.007; Fig. 3). The weakened endplates resisted a median force of 1101.7N for PLIF cages.
Bone quality correlated linearly with the force at which endplate failure occurred (Fig. 4). Both, intact and weakened endplates showed a strong positive correlation: intact—TLIF: r = 0.964, slope of the regression line (slope) = 11.8, p < 0.001; intact—PLIF: r = 0.909, slope = 11.2, p = 5.5E − 05; weakened—TLIF: r = 0.973, slope = 12.5, p < 0.001; weakened—PLIF: r = 0.836, slope = 6, p = 0.003.
Discussion
The most important finding of the study is that endplate weakening significantly reduced endplate resistance to subsidence of the endplates, which may promote cage migration or subsidence. The effect was even more pronounced when a uPLIF cage was used (70% (median)–36.6%) compared to TLIF cage (83.1% (median)–15%).
Lumbar interbody fusion techniques have evolved rapidly over the past few decades, leading to an expansion of indications [17]. Among these, PLIF and TLIF are the most commonly used techniques [18, 19]. They provide solid fusion and allow for neural decompression, provide an additional fusion interface with a higher fusion rate than bone graft alone [20], restore the intervertebral height and help restore alignment [21]. From a biomechanical point of view, the interbody fusion device is designed to support the anterior column and transmit compressive forces [22, 23]. However, failure to achieve bony fusion may result in cage migration or subsidence. Cage subsidence rates reported in the literature range from 0 to 65% [21, 24,25,26,27], with implant contact area, endplate condition, and bone mineral density appearing to play an important role [8, 28].
The contact area between the cage and the endplate depends on the morphology of the endplate (i.e., highly concave or irregularly shaped endplates reduce the contact area), and on the shape and size of the cage. The smaller the surface contact area, the higher the stress on the endplate [29,30,31]. Banana-shaped and anatomically contoured cages increase the contact surface compared to rectangular cages and therefore theoretically have a lower tendency to subside [11]. This may explain why the failure load of the uPLIF was slightly lower than that of the TLIF in the present study. Due to the concavity of the endplates, the PLIF cage often only came into contact with the bone at the edges, causing force peaks in these areas and resulting in fractures.
Violation of the endplates during cage bed preparation appears to have a relevant biomechanical effect: the exposure of cancellous bone reduced the failure force by 15% for the TLIF and 37% for the PLIF, respectively. To compare the distributions of forces measured for the intact and the weakened endplates, and to better assess the potential clinical implications of the experimental results, probability density functions were fitted to the failure force results for each cage configuration (uPLIF, bPLIF, and TLIF; Fig. 5).
Illustration of the probability density functions of the intact and weakened endplate for three different cage configurations. For all three cage configurations, the cage loads in the upright position and during physical activity (such as lifting an object) are evident, as are the areas under the probability density function curves: the probability of endplate fracture is higher in the weakened state than in the intact state for all configurations
Preload and percentage of external load transfer through the cage were obtained from previously published biomechanical experiments involving axially compressed spinal segments with load cell instrumented vertebral cages [32]. Specifically, an average preload of up to 328N was measured (uPLIF 224N; bPLIF 328N; TLIF 317N), and up to 50% (uPLIF 40%; bPLIF 50%; TLIF 44%) of the external compressive load was transmitted through the cage to the ventral column of the spine, thus affecting the endplates. It was therefore possible to determine the likely loading of each cage configuration during upright standing (about 1000N acting on the spine) and during more vigorous daily activities (about 3000N on the spine) and to link it to the cage-specific failure forces of intact and weakened endplates [33, 34]. During upright standing, likely 624N, 828N, and 757N are going through the uPLIF, the bPLIF, and the TLIF, respectively. During more vigorous activities, the uPLIF, bPLIF and TLIF are subjected to 1424N, 1828N, and 1637N, respectively. Consequently, 12% (bPLIF) to 23% (TLIF) of intact endplates would fail during upright standing, whereas up to 39% (uPLIF) of the weakened endplates could fail under such loads (Fig. 5).
Considering the subsidence rates from clinical studies, the mean incidence of subsidence with bPLIF is 15.8% (10–65.1%) [21, 25, 35, 36], whereas the mean incidence of subsidence with TLIF is approximately 25.3% (0–51.2%) [8, 9, 21, 27, 37,38,39,40,41,42,43,44]. This clinically reported difference between TLIF and bPLIF subsidence is consistent with the experimentally determined distributions of endplate failure: both indicate a higher propability of failure with TLIF than with bPLIF (Fig. 5). In addition, the average clinically observed subsidence is within the range of endplates predicted to fail during standing (12–24% for bPLIF and 23–35% TLIF cages). This suggests that subsidence occurring in the early postoperative period may be induced by exposure to comparatively low spinal loads experienced during upright posture or non-impact activities.
Biomechanically, bone quality correlated positively with the absolute resistance force of the endplate: the higher the mean intracorporal HU values, the more axial compression force had to be applied until a fracture occurred in both the intact and weakened states. An increase of 10 HU in the mean intracorporal HU of an intact vertebral body resulted in an increase of 100N in the failure force (slope of the regression lines is approximately 10, Fig. 4). While this appears to be unchanged in a weakened vertebral body with the use of a TLIF cage, endplate weakening appears to be more detrimental with the use of a PLIF cage (Fig. 4). Reasons for this phenomenon could be the different positioning area for TLIF and PLIF, with a more “peripheral” placement of TLIF and the slightly larger contact area of TLIF.
This biomechanical understanding can guide clinical practice: in osteoporotic spines, where there is a higher risk of screw loosening and cage subsidence [9], bone density should be optimized preoperatively whenever possible [45], cages with large footprints should be preferred over small cages, and great care should be taken to preserve the endplates.
Some authors claim that neither fusion rates nor clinical outcomes are affected by cage subsidence [46], however, many of the advantages that come with a cage, as mentioned above, are lost with subsidence.
Overall, we believe that endplate preparation should be performed with caution, as cortical bone compromise is associated with significant loss of resistance to axial compression (Fig. 6). In addition, ventral force transmission is further compromised by the previous disc dissection required for cage insertion [47]. This results in increased stress at the screw-bone interface. Park et al. [9] demonstrated a correlation between cage positioning, endplate injury, single cage use, and cage migration. Cage migration did not result in subsidence in all cases. However, the rate of non-fusion and screw loosening was significantly higher in patients with cage subsidence. Understanding these biomechanical relationships can be used to improve clinical decision-making.
Illustrative visualization of lumbar vertebral body segments without and with a cage, with cage subsidence and after successful bony fusion. Compared to the intervertebral disc, an inserted cage increases the stiffness of the construct and thus the force transmission to the underlying screws. With bony fusion, the stiffness is further increased. If cage subsidence is present, the cage cannot transmit interbody forces and the stiffness of the anterior column decreases
This in vitro biomechanical study has several limitations. Firstly, an isolated axial compression force was applied to the vertebral body. This is a simplified model that does not represent the complex movement of the spine. However, we know that the axial compression force is the main loading direction for all loading cases, therefore it is an appropriate study scenario. Secondly, the endplates were weakened with a shaver until cancellous bone was visible. It is possible that in some cases a little more substance was removed than in others. However, since the endplate in the cage area was completely removed during shaving, the effect of a little more or less depth should be negligible compared to the effect of a missing endplate. Thirdly, the degrees of freedom in our setup always allowed the cage to align perfectly with the endplate. In vivo, it is possible that a cage is loaded more unilaterally and collapses more quickly. However, in another study, it was found that under high loads, the cage is mostly loaded relatively evenly (anterior vs posterior load distribution) [32]. Fourthly, HU are widely used to assess bone quality, but the absolute values vary between scanners and they are notoriously difficult to compare between studies. However, within this study only relative comparisons between the samples were drawn, which are justifiable from our point of view, as the imaging parameters were the same. Lastly, we have tried to position the cages in the same area of the endplate, but small variations may have been unavoidable.
Conclusion
Weakening of the endplate during cage bed preparation significantly reduces the resistance of the endplate to subsidence to failure: endplate load capacity is reduced by 15% with TLIF and 37% with PLIF. Bone quality correlates with the force at which endplate failure occurs.
Data availability
None.
References
Lambrechts MJ, Siegel N, Heard JC et al (2022) Trends in single-level lumbar fusions over the past decade using a national database. World Neurosurg 167:e61–e69. https://doi.org/10.1016/j.wneu.2022.07.092
Blumenthal SL, Ohnmeiss DD (2003) Intervertebral cages for degenerative spinal diseases. Spine J 3:301–309. https://doi.org/10.1016/s1529-9430(03)00004-4
EVANS JH, (1985) Biomechanics of Lumbar Fusion. Clin Orthop Relat Res 193:38–46. https://doi.org/10.1097/00003086-198503000-00005
Kim DH, Hwang RW, Lee G-H et al (2020) Comparing rates of early pedicle screw loosening in posterolateral lumbar fusion with and without transforaminal lumbar interbody fusion. Spine J 20:1438–1445. https://doi.org/10.1016/j.spinee.2020.04.021
Fujibayashi S, Neo M, Takemoto M et al (2010) Paraspinal-approach transforaminal lumbar interbody fusion for the treatment of lumbar foraminal stenosis. J Neurosurg Spine 13:500–508. https://doi.org/10.3171/2010.4.spine09691
Tempel ZJ, McDowell MM, Panczykowski DM et al (2017) Graft subsidence as a predictor of revision surgery following stand-alone lateral lumbar interbody fusion. J Neurosurg Spine 28:50–56. https://doi.org/10.3171/2017.5.spine16427
Chen Y, Chen D, Guo Y et al (2008) Subsidence of titanium mesh cage: a study based on 300 cases. J Spinal Disord Tech 21:489–492. https://doi.org/10.1097/bsd.0b013e318158de22
Zhou Q, Chen X, Xu L et al (2019) Does vertebral end plate morphology affect cage subsidence after transforaminal lumbar interbody fusion? World Neurosurg 130:e694–e701. https://doi.org/10.1016/j.wneu.2019.06.195
Park M-K, Kim K-T, Bang W-S et al (2019) Risk factors for cage migration and cage retropulsion following transforaminal lumbar interbody fusion. Spine J 19:437–447. https://doi.org/10.1016/j.spinee.2018.08.007
Steffen T, Tsantrizos A, Aebi M (2000) Effect of implant design and endplate preparation on the compressive strength of interbody fusion constructs. Spine 25:1077–1084. https://doi.org/10.1097/00007632-200005010-00007
Schmoelz W, Keiler A (2015) Intervertebral cages from a biomechanical point of view. Orthopade 44:132–137. https://doi.org/10.1007/s00132-014-3071-y
Kim KS, Yang TK, Lee JC (2005) Radiological changes in the bone fusion site after posterior lumbar interbody fusion using carbon cages impacted with laminar bone chips: follow-up study over more than 4 years. Spine 30:655–660. https://doi.org/10.1097/01.brs.0000155421.07796.7f
Cornaz F, Fasser M-R, Spirig JM et al (2019) 3D printed clamps improve spine specimen fixation in biomechanical testing. J Biomech 98:109467. https://doi.org/10.1016/j.jbiomech.2019.109467
Committee F (2014) Test methods for intervertebral body fusion devices. https://doi.org/10.1520/f2077-14
Schreiber JJ, Anderson PA, Rosas HG et al (2011) Hounsfield units for assessing bone mineral density and strength. J Bone Joint Surg 93:1057–1063. https://doi.org/10.2106/jbjs.j.00160
Afifi MB, Abdelrazek A, Deiab NA et al (2019) The effects of CT x-ray tube voltage and current variations on the relative electron density (RED) and CT number conversion curves. J Radiat Res Appl Sci 13:1–11. https://doi.org/10.1080/16878507.2019.1693176
Reisener M-J, Pumberger M, Shue J et al (2020) Trends in lumbar spinal fusion—a literature review. J Spine Surg 6:752–761
Cole CD, McCall TD, Schmidt MH, Dailey AT (2009) Comparison of low back fusion techniques: transforaminal lumbar interbody fusion (TLIF) or posterior lumbar interbody fusion (PLIF) approaches. Curr Rev Musculoskelet Med 2:118–126. https://doi.org/10.1007/s12178-009-9053-8
Mobbs RJ, Phan K, Malham G et al (2015) Lumbar interbody fusion: techniques, indications and comparison of interbody fusion options including PLIF, TLIF, MI-TLIF, OLIF/ATP, LLIF and ALIF. J Spine Surg Hong Kong 1:2–18. https://doi.org/10.3978/j.issn.2414-469x.2015.10.05
Brantigan JW, Steffee AD (1993) A carbon fiber implant to aid interbody lumbar fusion. Spine 18:2106–2117. https://doi.org/10.1097/00007632-199310001-00030
Amorim-Barbosa T, Pereira C, Catelas D et al (2022) Risk factors for cage subsidence and clinical outcomes after transforaminal and posterior lumbar interbody fusion. Eur J Orthop Surg Traumatol 32:1291–1299. https://doi.org/10.1007/s00590-021-03103-z
Lund T, Oxland TR, Jost B et al (1998) Interbody cage stabilisation in the lumbar spine: biomechanical evaluation of cage design, posterior instrumentation and bone density. J Bone Joint Surg 80:351–359. https://doi.org/10.1302/0301-620x.80b2.7693
PC McAfee (1999) Current concepts review - interbody fusion cages in reconstructive operations on the spine. J Bone Joint Surg 81:859–880. https://doi.org/10.2106/00004623-199906000-00014
Le TV, Baaj AA, Dakwar E et al (2012) Subsidence of polyetheretherketone intervertebral cages in minimally invasive lateral retroperitoneal transpsoas lumbar interbody fusion. Spine 37:1268–1273. https://doi.org/10.1097/brs.0b013e3182458b2f
Lee N, Kim KN, Yi S et al (2017) Comparison of outcomes of anterior, posterior, and transforaminal lumbar interbody fusion surgery at a single lumbar level with degenerative spinal disease. World Neurosurg 101:216–226. https://doi.org/10.1016/j.wneu.2017.01.114
Malham GM, Parker RM, Blecher CM, Seex KA (2015) Assessment and classification of subsidence after lateral interbody fusion using serial computed tomography. J Neurosurg Spine 23:589–597. https://doi.org/10.3171/2015.1.spine14566
Kuang L, Wang B, Lü G (2017) Transforaminal lumbar interbody fusion versus mini-open anterior lumbar interbody fusion with oblique self-anchored stand-alone cages for the treatment of lumbar disc herniation. Spine 42:E1259–E1265. https://doi.org/10.1097/brs.0000000000002145
Polikeit A, Ferguson SJ, Nolte LP, Orr TE (2003) The importance of the endplate for interbody cages in the lumbar spine. Eur Spine J 12:556–561. https://doi.org/10.1007/s00586-003-0556-5
Goh JCH, Wong H-K, Thambyah A, Yu C-S (2000) Influence of PLIF cage size on lumbar spine stability. Spine 25:35. https://doi.org/10.1097/00007632-200001010-00008
Grant JP, Oxland TR, Dvorak MF (2001) Mapping the structural properties of the lumbosacral vertebral endplates. Spine 26:889–896. https://doi.org/10.1097/00007632-200104150-00012
Lim T-H, Kwon H, Jeon C-H et al (2001) Effect of endplate conditions and bone mineral density on the compressive strength of the graft-endplate interface in anterior cervical spine fusion. Spine 26:951–956. https://doi.org/10.1097/00007632-200104150-00021
Calek A-K, Cornaz F, Suter M et al (2023) Load distribution on intervertebral cages with and without posterior instrumentation. Spine J. https://doi.org/10.1016/j.spinee.2023.10.017
Jost B, Cripton PA, Lund T et al (1998) Compressive strength of interbody cages in the lumbar spine: the effect of cage shape, posterior instrumentation and bone density. Eur Spine J 7:132–141. https://doi.org/10.1007/s005860050043
Wilke H, Neef P, Caimi M et al (1999) New in vivo measurements of pressures in the intervertebral disc in daily life. Spine 24:755–762. https://doi.org/10.1097/00007632-199904150-00005
Oh KW, Lee JH, Lee J-H et al (2017) The correlation between cage subsidence, bone mineral density, and clinical results in posterior lumbar interbody fusion. Clin Spine Surg 30:E683–E689. https://doi.org/10.1097/bsd.0000000000000315
Suzuki T, Abe E, Miyakoshi N et al (2013) Posterior-approach vertebral replacement with rectangular parallelepiped cages (PAVREC) for the treatment of osteoporotic vertebral collapse with neurological deficits. J Spinal Disord Tech 26:E170–E176. https://doi.org/10.1097/bsd.0b013e318286fc18
Mun HY, Ko MJ, Kim YB, Park SW (2019) Usefulness of oblique lateral interbody fusion at L5–S1 level compared to transforaminal lumbar interbody fusion. J Korean Neurosurg S 63:723–729. https://doi.org/10.3340/jkns.2018.0215
Kim M-C, Chung H-T, Cho J-L et al (2013) Subsidence of polyetheretherketone cage after minimally invasive transforaminal lumbar interbody fusion. J Spinal Disord Tech 26:87–92. https://doi.org/10.1097/bsd.0b013e318237b9b1
Isaacs RE, Sembrano JN, Tohmeh AG, Group SDS (2016) Two-year comparative outcomes of MIS lateral and MIS transforaminal Interbody fusion in the treatment of degenerative spondylolisthesis. Spine 41:S133–S144. https://doi.org/10.1097/brs.0000000000001472
Choi W-S, Kim J-S, Ryu K-S et al (2016) Minimally invasive transforaminal lumbar interbody fusion at L5–S1 through a unilateral approach: technical feasibility and outcomes. Biomed Res Int 2016:2518394. https://doi.org/10.1155/2016/2518394
Lin G-X, Quillo-Olvera J, Jo H-J et al (2017) Minimally invasive transforaminal lumbar interbody fusion: a comparison study based on end plate subsidence and cystic change in individuals older and younger than 65 years. World Neurosurg 106:174–184. https://doi.org/10.1016/j.wneu.2017.06.136
Pereira C, Silva PS, Cunha M et al (2018) How does minimally invasive transforaminal lumbar interbody fusion influence lumbar radiologic parameters? World Neurosurg 116:e895–e902. https://doi.org/10.1016/j.wneu.2018.05.125
Ko MJ, Park SW, Kim YB (2019) Correction of spondylolisthesis by lateral lumbar interbody fusion compared with transforaminal lumbar interbody fusion at L4–5. J Korean Neurosurg S 62:422–431. https://doi.org/10.3340/jkns.2018.0143
Zhao Y, Jia J, Liu W et al (2020) Influence of contoured versus straight rod on clinical outcomes and sagittal parameters in minimally invasive transforaminal lumbar interbody fusion (MIS-TLIF) at L4/5 level-more than 5 years follow-up. J Orthop Sci 25:89–95. https://doi.org/10.1016/j.jos.2019.03.008
Yolcu YU, Zreik J, Alvi MA et al (2020) Use of teriparatide prior to lumbar fusion surgery lowers two-year complications for patients with poor bone health. Clin Neurol Neurosurg 198:106244. https://doi.org/10.1016/j.clineuro.2020.106244
Schiffman M, Brau SA, Henderson R, Gimmestad G (2003) Bilateral implantation of low-profile interbody fusion cages: subsidence, lordosis, and fusion analysis. Spine J 3:377–387. https://doi.org/10.1016/s1529-9430(03)00145-1
Burkhard MD, Spirig JM, Wanivenhaus F et al (2023) Residual motion of different posterior instrumentation and interbody fusion constructs. Eur Spine J 32:1411–1420. https://doi.org/10.1007/s00586-023-07597-5
Acknowledgements
Imaging was performed with support of the Swiss Center for Musculoskeletal Imaging, SCMI, Balgrist Campus AG, Zurich.
Funding
Open access funding provided by University of Zurich. The authors, their immediate families, and any research foundations with which they are affiliated have not received any financial payments or other benefits from any commercial entity related to the subject of this article.
Author information
Authors and Affiliations
Contributions
AKC: data collection, design of the study, results interpretation, manuscript writing and editing; FC: conception and manuscript editing; MS: technical support and data collection; MRF: data analysis and manuscript editing; MF: manuscript editing; JW: conception, design of the study, results interpretation and manuscript editing;
Corresponding author
Ethics declarations
Conflict of interest
MF reports being a Consultant for Incremed (Balgrist University Startup), Zimmer Biomet, Medacta, and 25 Segments (Balgrist Startup). All the other authors report no conflicts of interest.
Ethical approval
Kantonale Ethikkommission Zurich had given the approval for the study. (Basec No. KEK-ZH-Nr. 2022–00104).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Calek, AK., Cornaz, F., Suter, M. et al. Endplate weakening during cage bed preparation significantly reduces endplate load capacity. Eur Spine J (2024). https://doi.org/10.1007/s00586-024-08289-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00586-024-08289-4