Abstract
Symbioses with fungi are important and ubiquitous on dry land but underexplored in the sea. As yet only one seagrass has been shown to form a specific root-fungus symbiosis that resembles those occurring in terrestrial plants, namely the dominant long-lived Mediterranean species Posidonia oceanica (Alismatales: Posidoniaceae) forming a dark septate (DS) endophytic association with the ascomycete Posidoniomyces atricolor (Pleosporales: Aigialaceae). Using stereomicroscopy, light and scanning electron microscopy, and DNA cloning, here we describe a novel root-fungus symbiosis in the Indo-Pacific seagrass Thalassodendron ciliatum (Alismatales: Cymodoceaceae) from a site in the Gulf of Aqaba in the Red Sea. Similarly to P. oceanica, the mycobiont of T. ciliatum occurs more frequently in thinner roots that engage in nutrient uptake from the seabed and forms extensive hyphal mantles composed of DS hyphae on the root surface. Contrary to P. oceanica, the mycobiont occurs on the roots with root hairs and does not colonize its host intraradically. While the cloning revealed a relatively rich spectrum of fungi, they were mostly parasites or saprobes of uncertain origin and the identity of the mycobiont thus remains unknown. Symbioses of seagrasses with fungi are probably more frequent than previously thought, but their functioning and significance are unknown. Melanin present in DS hyphae slows down their decomposition and so is true for the colonized roots. DS fungi may in this way conserve organic detritus in the seagrasses’ rhizosphere, thus contributing to blue carbon sequestration in seagrass meadows.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Seagrasses, “the whales of the plant world”, are the only vascular plants that have returned to a fully submerged life in the marine environment. Their ancestors were among basal lineages of the brackish Alismatales that appeared more than 100 Mya, during the Cretaceous (Larkum et al. 2018). With ca. 70 described species, seagrasses represent a minor part of the total vascular plants’ diversity, yet they play indispensable roles in nutrient cycling, maintaining coastal ecosystems’ biodiversity and integrity, and blue carbon (= organic carbon sequestered in coastal and marine ecosystems) storage. They occur in coastal areas of all continents except Antarctica and their underwater meadows are among the most productive ecosystems on Earth, storing as much organic carbon per unit area as terrestrial forests (Fourqurean et al. 2012). In forests, most plants depend on the nutrient uptake through fungal hyphae (i.e., through mycorrhizal symbioses), plant necromass decomposition is to a large extent governed by fungi (Osono 2007; Boddy and Watkinson 1995; Read et al. 2004), and plants invest significant amounts of the photosynthetically fixed carbon to the underground mycelium of their mycorrhizal mycobionts (Hawkins et al. 2023). However, despite many reports on the diversity of marine fungi associating with seagrasses (e.g., Sakayaroj et al. 2010; Mata and Cebrián 2013; Ettinger and Eisen 2019; Vohník 2022), next to nothing is known about their functioning and how they contribute to the ecosystem services provided by seagrasses, including blue carbon storage.
Fungi are a diverse clade of eukaryotic organisms inhabiting almost all terrestrial, freshwater, and marine ecosystems. They represent a substantial part of the microbial diversity on Earth and play a key role in global biomass turnover and major food webs, comprising important predators, parasites, pathogens, and beneficial symbionts of many organisms. Fungi have interacted with plants long before terrestrialization and the resulting symbioses are among the oldest and most important associations on our planet (Naranjo-Ortiz and Gabaldón 2019). Arguably the most significant and widespread terrestrial plant-fungus symbioses are mycorrhizae, lichens, and various foliar and root endophytic associations. Symbioses with fungal endophytes (= fungi colonizing plant tissues without causing damage) evolved as a part of the defense system against herbivores, pathogens, and drought stress, thus enhancing plant growth (Saikkonen et al. 1998; Arnold 2007) and occur in most vascular plants. Mycorrhizae evolved as means of nutrient uptake, occur in most vascular land plants, and their predecessors probably facilitated plant terrestrialization (Selosse and Le Tacon 1998; Brundrett 2002). The most ancient and by far the most common mycorrhizal type is arbuscular mycorrhiza (AM), “the mother of plant root endosymbioses” (Parniske 2008) occurring in ca. 74% of vascular plant species, incl. many freshwater, salt marsh, and mangrove plants (van der Heijden et al. 2015). On the other hand, while extant Alismatales comprise a mixture of non-mycorrhizal and AM families, the four seagrass families (Cymodoceaceae, Hydrocharitaceae, Posidoniaceae, and Zosteraceae) are regarded as non-mycorrhizal (Brundrett 2017).
The absence of mycorrhizae in seagrasses is not surprising, as they can take up nutrients from the water column through the leaves and from the seabed through the non-mycorrhizal roots (Short and McRoy 1984; Terrados and Williams 1997). However, many marine ecosystems are oligotrophic, resulting in the leaf uptake being unable to cover all nutritional needs, and several seagrasses form organic seabed sediments storing large amounts of nutrients that are not directly accessible to non-mycorrhizal roots. The prime example is Posidonia oceanica (Posidoniaceae) dominating the mostly oligotrophic Mediterranean Sea (Powley et al. 2017) that forms “matte”, an organo-mineral seabed sediment which can be several meters thick and hundreds to thousands of years old (Serrano et al. 2012), sometimes being referred to as soil (Piñeiro-Juncal et al. 2020). Indeed, matte in a way resembles peat and peatland ecosystems gave rise to a specific type of mycorrhizal symbiosis that is formed by fungal symbionts (= mycobionts) phylogenetically close to saprobic fungi (Fehrer et al. 2019; Rice and Currah 2006). Intriguingly, also P. oceanica hosts a specific root-fungus symbiosis (Vohník et al. 2015) that is morphologically similar to the association formed by the dark septate endophytes (DSE) in the roots of most terrestrial plants (Jumpponen and Trappe 1998). DSE are a miscellaneous group of mostly sterile ascomycetous mycobionts that in some cases facilitate host’s nutrient uptake (Usuki and Narisawa 2007; Newsham 1999), hence some authors include them among mycorrhizal fungi (Jumpponen 2001). The symbiosis is formed by Posidoniomyces atricolor that represents an independent marine biotrophic lineage in the Aigialaceae family (Pleosporales), its closest relatives being plant-associated saprobes from marine, terrestrial, and freshwater habitats in Southeast Asia and Central America (Vohník et al. 2019).
Except P. oceanica, no other seagrass is known to form any similar root-fungus symbiosis. On the other hand, only a small fraction of seagrasses has been thoroughly examined for colonization by fungi, so one may wonder how many novel symbioses, perhaps formed by hitherto undescribed mycobiont lineages, await discovery. The factors favoring seagrass symbioses with fungi that improve nutrient uptake include seagrass species that produce high amounts of biomass (i.e., with high nutrient demands) and oligotrophic conditions with most mineral nutrients bound in recalcitrant (typically organic) substrates. In other words, they would be more likely to evolve in highly productive seagrasses occurring in oligotrophic waters with a seabed containing organic detritus (e.g., seagrass necromass). Thus, it may come as no surprise that we recently discovered an association formed by a dark septate (DS) mycobiont in the roots of Thalassodendron ciliatum (Cymodoceaceae), a highly productive seagrass in the mostly oligotrophic Red Sea that produces dense root mats accumulating organic matter (Lipkin 1979). In this paper we describe its anatomy and morphology using stereomicroscopy, and light and scanning electron microscopy. In addition, we used DNA cloning followed by Sanger sequencing to search for the mycobiont forming this novel marine symbiosis.
Materials and methods
Sampling
Thalassodendron ciliatum (Forsk.) den Hartog was sampled on February 25, 2019, at a site on the eastern coast of the Sinai Peninsula, between Nuweiba and Taba, close to Ras Shitan (= R. El Shetan, R. Shaitan, R. Shaitani, R. Shattein, etc.), Egypt (N29.1234, E34.6855; Fig. 1), where it grows in coralligenous white sand enriched with (broken) shells of various marine organisms (Foraminifera, Mollusca, etc.). In a dense monospecific meadow (Fig. 2A), intact samples (roots + rhizomes + shoots + leaves; Fig. 2B) of healthy-looking specimens were collected using scuba diving in ca. 6 m depth at three points ca. 3 m apart. The sampling depth was measured with a Freedom dive computer (Divesoft, Czechia), the underwater photos were taken with a Canon S100 camera in an underwater housing (Ikelite, USA). Upon delivery to the surface, the roots were separated, pooled, stored in 50% ethanol in seawater and transported to the laboratory where they were kept in a fridge at ca. 6 °C until used. A representative specimen (roots in 50% ethanol) was deposited in the Herbarium of the Institute of Botany, Czech Academy of Sciences, Průhonice, Czechia (PRA) under the accession number PRA-21596.
The investigated Thalassodendron ciliatum meadow (A) showing a patch of seagrass necromass in the foreground (not sampled). Next to nothing is known about the role of marine fungi in nutrient cycling in seagrass meadows. (B) Morphology of T. ciliatum, note its dense root system. Also note the coralligenous white sand in the background that forms the seabed at the investigated locality. Bar represents 5 cm
Microscopy
All roots were initially screened with an Olympus SZX12 stereomicroscope and divided into two categories, namely 1/ without any visible fungal colonization and 2/ with visible fungal colonization on the root surface. To find out whether the root hairs’ presence is correlated with fungal colonization, fifty random root segments from both categories, each ca. 1 cm in length, were scored for the absence/presence of the root hairs. To find out whether the fungal colonization preferentially occurred in thinner (= terminal, younger) roots, the diameter of each root segment was measured at ten different points using QuickPHOTO MICRO ver. 3.2 (Promicra, Czechia), the average value was calculated in MS Excel, and the two categories were compared using the Kolmogorov-Smirnov two-sample test in STATISTICA 64 (Dell, USA). To screen possible intraradical fungal colonization, handmade longitudinal and transversal semithin sections of the roots from both categories were examined at high magnification (400×, 600×, and 1000×) with an Olympus BX60 microscope equipped with differential interference contrast. Microphotographs were taken with an Olympus DP70 camera and QuickPHOTO MICRO. In parallel, they were examined with a FEI Quanta 200 ESEM scanning electron microscope in the environmental mode at 275 Pa and − 12.5 to − 10 °C. Photo-documentation was adjusted for clarity and contrast as needed and assembled into figures using Paint.net ver. 4.3.12 (dotPDN LLC, Rick Brewster, and contributors).
DNA isolation, amplification, cloning, and sequencing
150 mg (fresh weight) of roots with visible superficial fungal colonization (Fig. 3A, B) were sonicated 1 × 5 min in 50% ethanol and 10 × 5 min in sterile Water for Molecular Biology (BioConcept, Switzerland) using an EMMI-05P ultrasonic bath (EMAG Technologies, Germany). Total DNA was extracted from the treated roots using a DNeasy Plant Mini Kit (Qiagen, Germany) according to the manufacturer’s instructions. The ITS rDNA region was amplified using the ITS1F + ITS4 primer pair (Gardes and Bruns 1993; White et al. 1990). The PCRs were performed in 25 µl reactions and contained 1x TopBio Plain PP Master Mix (TopBio, Czechia), each primer at 0.2 mM, 20 µg BSA, 1 mM MgCl2, and 25 ng of the isolated DNA. The cycling conditions were as follows: 5 min at 95 °C followed by 35 cycles of 95 °C for 30 s, 55 °C for 30 s and 72 °C for 1 min, and a final extension at 72 °C for 10 min.
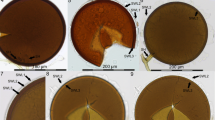
Morphology of Thalassodendron ciliatum roots and their superficial fungal colonization. (A) A random sample of roots differing in color, diameter, and presence/absence of the root hairs. Stereomicroscopy (SM), bar = 200 μm. (B) Selected roots displaying the characteristic fungal colonization on the root surface (arrows). SM, bar = 1000 μm. (C) A magnified view of a root colonized by dark mycelium (arrows), note the numerous root hairs. SM, bar = 500 μm. (D) A detail of the characteristic fungal colonization on the root surface (arrows). Light microscopy with differential interference contrast, bar = 100 μm
Prior to cloning, the PCR products were excised from 1% agarose gels and purified with a Zymoclean Gel DNA Recovery kit (Zymoresearch, USA). The gel-purified fragments were cloned using the TOPO TA cloning kit (Invitrogen, USA) following the manufacturer’s instructions, but downscaled to half reactions. The colonies were transferred into 20 µl ddH20 and denatured at 95 °C for 10 min. They served as templates for subsequent PCR amplifications by the M13 forward and reverse primers (Invitrogen).
85 clones were sequenced at SEQme (Czechia) using the M13R primer (CAGGAAACAGCTATGACC). The obtained sequences were screened in Finch TV v. 1.4.0 (Geospiza, USA) and high-quality sequences were manually edited in the same software.
Identification of the clones and trophic modes of the detected fungi
The edited sequences were aligned in Bioedit v. 7.1.8 (Hall 1999) and clustered at 99% similarity into operational taxonomic units (OTU) in TOPALi (Biomathematiscs & Statistics Scotland). When more than one, sequences within an OTU were aligned and a representative sequence was chosen based on quality and length. Representative sequences of each OTU were subjected to BLASTn searches (Zhang et al. 2000) in GenBank at NCBI (Sayers et al. 2019) as described in Vohník (2020). Their phylogenetic background was checked in Blast Tree View (https://www.ncbi.nlm.nih.gov/blast/treeview/treeview.cgi). Each OTU was assigned a species hypothesis (SH) in UNITE (Nilsson et al. 2019) and its tentative trophic mode was searched in The Faces of Fungi (Jayasiri et al. 2015), FUNGuild (Nguyen et al. 2016), and BioLib.cz (https://www.biolib.cz/cz/main/) databases. Representative sequences of each OTU were deposited in GenBank under the accession numbers OR392720-53. Fungal taxonomy followed MycoBank (https://www.mycobank.org).
Results
Microscopy
The root color varied from yellow/ochre (the youngest roots) to dark brown/black (the oldest roots) (Figs. 2B and 3A). The youngest roots typically possessed vigorous root hairs and these gradually disappeared with the root’s age (Fig. 3A). The root surface of darker/older roots was often densely colonized by DS fungal hyphae that formed discontinuous hyphal mantles resembling the pseudoparenchymatous nets formed by the terrestrial DSE or “mycélium en palmettes” (Figs. 3B–D and 4A and B) (Ducomet 1907; Renard et al. 2021). These typically originated from individual hyphae growing more or less linearly on the roots surface but later starting to produce shorter isodiametric cells that radially spread around (Fig. 4A, B). The mantles often covered the basal parts of the root hairs (Fig. 4C–F).
Typical features of the novel fungal symbiosis in the roots of Thalassodendron ciliatum (A) A discontinuous hyphal mantle (pseudoparenchymatous net) on the root surface. The cavity in the right side of the photo is due to a detached root hair. Light microscopy (LM) with differential interference contrast (DIC), bar = 20 μm. (B) Dark septate hyphae growing on the root surface either individually or parallelly attached to each other (resembling prosenchyma), eventually giving rise to the pseudoparenchymatous tissue (arrows). The cavities (asterisks) are after detached root hairs, also note a root hair in the upper part of the photo. LM with DIC, bar = 50 μm. (C) A hyphal mantle (asterisk) extending to the basal parts of the root hairs (arrows). LM with DIC, bar = 50 μm. (D) As in C. Scanning electron microscopy (SEM), bar = 100 μm. (E) A transversal section through a root with the root hairs and the characteristic fungal colonization on the root surface (arrows). Note no apparent intraradical fungal colonization. SEM, bar = 200 μm. (F) A detail from E. SEM, bar = 20 μm. (G) A transversal section through a root with rudiments of the hyphal mantles (arrows) accompanied by an unidentified substance, possibly of fungal origin (arrowheads). Note air lacunae (a) and the rhizodermal cells probably filled with phenolic compounds (some indicated by asterisks). LM with DIC, bar = 50 μm. (H) The hyphal mantle (asterisk) covering the root surface is detached in the left part of the photo, leaving imprints in the unidentified substance (arrow). Note some hyphae with visible septa on the mantle’s surface (arrowheads) and the cavity left after a detached root hair surrounded by fungal hyphae in the upper right corner of the photo. SEM, bar = 50 μm. (K) As in H. LM with DIC, bar = 50 μm
Transversal sections through the roots revealed no intraradical hyphal colonization (Fig. 4E–G). The mantles typically consisted of a single hyphal layer and were often accompanied by an unidentified substance (possibly of fungal origin) occurring between their abaxial surface and the root’s surface (Fig. 4G). When the mantle detached from the root, it left an imprint in the substance (Fig. 4H&K). The rhizodermal cells below the mantles were filled with a brownish substance (Fig. 4G), resembling the tannin cells formed in many ectomycorrhizae (Agerer 1987).
Individual surface DS hyphae not producing mantles could be seen irrespectively of the roots age and the presence/absence of the root hairs (Fig. 5A). Some older roots, typically without visible DS fungal colonization and already without root hairs, had some of their rhizodermal cells filled with light- to dark brown structures of varied shapes (Fig. 5B–G) and these are interpreted here as polyphenolic substances occurring in the vacuoles of the tannin cells, similar to those occurring in the Mediterranean endemic seagrass P. oceanica (Lefebvre et al. 2023).
Some features of Thalassodendron ciliatum roots free of the novel fungal symbiosis. (A) Fungal hyphae (arrows) occurred on the root surface irrespective of the root age, absence/presence of the root hairs, and absence of the novel fungal symbiosis. Stereomicroscopy (SM), bar = 200 μm. (B) Older roots typically had a proportion of their rhizodermal cells filled with light- to dark-brown structures of varied shapes and these cells are interpreted here as the tannin cells (arrows). Light microscopy with differential interference contrast (DIC), bar = 200 μm. (C) Upon closer look, most rhizodermal cells were filled with a brownish substance (probably polyphenolic compound(-s), see Fig. 4G), resembling the tannin cells formed in many ectomycorrhizae (asterisks). It seemed like a transformation of this substance(-s) gives rise to the light- to dark-brown structures (arrows) also depicted in Fig. 5B. DIC, bar = 100 μm. (D, E, F) Details of the light- to dark-brown structures. DIC, bars = 20 μm. (G) The tannin cells (arrows). Scanning electron microscopy (SEM), bar = 20 μm. (H) The tips of some root hairs had a globular shape (arrows), remotely resembling the terminal swellings previously reported in the adhesive root hairs of the dominant Mediterranean seagrass Posidonia oceanica. SM, bar = 200 μm
The root hairs’ presence was not correlated with visible DS fungal colonization as all screened roots in both categories (colonized vs. non-colonized) possessed root hairs, either intact or broken at their bases. The colonized roots (349.5 ± 54.2 μm, mean ± SD) had significantly smaller diameter (p < 0.001) than the non-colonized roots (505 ± 266.5 μm).
The tips of some root hairs had a globular shape (Fig. 5H) and in rare cases, they remotely resembled undeveloped terminal swellings previously reported in the adhesive root hairs of P. oceanica (Badalamenti et al. 2015; Kolátková and Vohník 2019). Alternatively, they might represent developing galls of an unidentified phytomyxid (Elliott et al. 2019; Kolátková et al. 2023).
Identification of the clones and trophic modes of the detected fungi
The sequencing yielded 80 high-quality sequences and after editing, they were clustered into 34 OTU (Table 1). These belonged to Ascomycota (20 OTU/39 sequences), Basidiomycota (13/40), and Spermatophyta (1/1). In Ascomycota, Helotiales comprised 5 OTU/8 sequences, followed by Pleosporales (4/8), Hypocreales (3/12), Cladosporiales (3/5), Dothideales (2/3), Eurotiales (1/1), and Serinales (1/1). In Basidiomycota, Malasseziales comprised 5 OTU/26 sequences, followed by Polyporales (3/4), Tremellales (1/2), Russulales (1/2), and Agaricales (1/2). One ascomycetous and two basidiomycetous OTU could not be identified below the phylum level. The Spermatophyta OTU represented T. ciliatum (Table 1).
Because of their low taxonomic resolution, a trophic mode could not be attributed to 11 fungal OTU (Table 1). Most of the remaining fungal OTU were either saprotrophs (incl. wood saprobes) or pathotrophs (animal, human, or plant pathogens). None of the detected fungi were related to known mycorrhizal or DSE fungi, including Pos. atricolor, the dominant DSE of P. oceanica.
Discussion
Prior to this study, only one seagrass has been reported to form a specific root-fungus symbiosis resembling those commonly occurring on dry land, and our observations thus extend the distribution and host taxonomic range of these associations for the NE Red Sea and another species in another seagrass family, respectively. However, unlike P. oceanica that is endemic to the Mediterranean, T. ciliatum is distributed across the Indo-Pacific (Green and Short 2003), making its symbiosis a potentially widespread phenomenon. The same is true for the more speciose Cymodoceaceae vs. Posidoniaceae that occur in the Caribbean, NW Africa, the Mediterranean, and most of the Indo-Pacific vs. being limited to the Mediterranean and SW to SE Australia (Angiosperm Phylogeny Website 2023). On the other hand, it is not known whether other members of Cymodoceaceae and Posidoniaceae form similar root-fungus symbioses. For example, despite that Cymodocea nodosa often co-occurs with P. oceanica and belongs to the same family as T. ciliatum, it does not seem to form any specific root-fungus symbiosis (Vohník et al. 2015).
Root-fungus symbioses in T. ciliatum and P. oceanica
In addition to the differences in their distribution and taxonomy as well as anatomy and morphology of their roots, T. ciliatum and P. oceanica to some extent differ in anatomy and morphology of their root-fungus symbioses (Table 2). The most surprising difference is the absence of any visible intraradical hyphae in T. ciliatum, because in P. oceanica fungal hyphae often vigorously develop within the hypodermis (Vohník et al. 2015, 2019), forming the intracellular microsclerotia characteristic of DSE (e.g., Lukešová et al. 2015; Yu et al. 2001). In addition, while in P. oceanica fungal hyphae infrequently colonize the rhizodermal cells, these are fungus-free and filled with what appears as polyphenolic substances in T. ciliatum (cf. Cariello et al. 1979; McMillan 1984). Lastly, in T. ciliatum the DS fungal mantles cover the basal parts of the root hairs, a trait to our knowledge unknown in terrestrial roots, while these are typically absent in P. oceanica roots colonized by Pos. atricolor (Borovec and Vohník 2018). On the other hand, the mycobionts of both seagrasses form extensive hyphal mantles on the root surface (Vohník 2022; Vohník et al. 2017; this study) that are morphologically identical to those formed by DSE and certain ectomycorrhizal (EcM) fungi on the roots of compatible terrestrial plants (e.g., Kaldorf et al. 2004). Intriguingly, similar structures called mycélium en palmettes (Ducomet 1907) are formed by some foliicolous Dothideomycetes on the leaf and twig cuticle where the respective mycobionts eventually form thyriothecia (Renard et al. 2021). However, these have not been detected on the roots investigated here.
Fungal partners in T. ciliatum and P. oceanica
It has been repeatedly shown that Pos. atricolor mycelium develops from the intracellular microsclerotia occurring in the hypodermis of P. oceanica (Vohník et al. 2016, 2019; Vohník 2021, 2022) and Pos. atricolor has been detected in the terminal roots of P. oceanica adults at every sampled locality in the whole N Mediterranean (M. Vohník, unpublished data). At the same time, Pos. atricolor has not been detected in any other host or substrate nor by any other research team. In addition, the mycobiota of P. oceanica roots typically comprises lulworthioid fungi (Lulworthiales) (Torta et al. 2022; Poli et al. 2021; Vohník et al. 2016, 2017) but their functioning is unclear (Vohník 2022). To our surprise, none of these fungi nor their relatives were detected in the investigated T. ciliatum roots. This might be due to their genuine absence, the different detection methods used in this (cloning) and the previous (culturing and high-throughput sequencing) studies, or incompatibility with the primers used in this study (cf. (Vohník et al. 2012).
To our knowledge, this is the first report on the mycobiota associated with the roots of T. ciliatum. In general, the most surprising results were the relatively high incidence of basidiomycetes and the dominance of saprotrophs and pathotrophs, both to a large extent due to the high incidence of Malassezia spp. (Table 1). Malassezia are ecologically versatile yeasts known from both terrestrial and marine environments and they occur on such diverse substrates as corals, deep-sea vents, and mammal skin (e.g., Amend 2014). They are commensals, pathogens, and saprobes and only rarely form hyphae (e.g., Saadatzadeh et al. 2001). It is thus not probable that they form the DS hyphal mantles characteristic of the novel root-fungus symbiosis reported here. Similarly, none of the six non-Malassezia OTU with ≥ 3 sequences seem like probable candidates for the observed colonization pattern. For example, Fusarium poae is a known plant pathogen (e.g., Stenglein 2009), Trichoderma are mycoparasites, saprobes, and pathogens (e.g., Williams et al. 2003) and none of them typically produce melanized hyphae (Podgórska-Kryszczuk et al. 2022; Wang et al. 2016).
Four OTU belonged to Pleosporales but none to Aigialaceae, i.e., the same family as Pos. atricolor. OTU-6/Pleosporales sp. grouped with Stagonospora sp. (GenBank OM337558, Massarinaceae), Phaeosphaeriopsis sp. (HQ630983, Phaeosphaeriaceae) obtained from Miscanthus giganteus (Poales: Poaceae) from Illinois, USA (Shrestha et al. 2011), and Didymocyrtis cladoniicola (LT796877, Phaeosphaeriaceae) from USA, all with > 99% sequence similarity. Stagonospora are probable plant pathogens (e.g., Solomon et al. 2006), Phaeosphaeriaceae are pathogenic, saprobic, or hyperparasitic mostly on monocotyledons and especially Poaceae (Hyde et al. 2013), and D. cladoniicola is a probable lichen parasite (Lawrey and Diederich 2018). While no GenBank entry displayed > 90% sequence similarity with OTU-7/Pleosporales sp., OTU 25 belongs to Stagonospora sp. and OTU 26 to Pyrenochaetopsis sp. (Pyrenochaetopsidaceae), displaying 99.4% sequence similarity with Pyrenochaetopsis sp. PG293 (AB916515) from a bird feather from Svalbard (Singh et al. 2016). Pyrenochaetopsis comprises commensals, plant endophytes and pathogens, and saprobes occurring in animals, humans, plants, soil, and water (e.g., Špetík et al. 2021).
When searching for the mycobiont forming the novel symbiosis one should not discriminate fungi related to known saprobes and/or pathogens. For example, Pos. atricolor represents the only biotrophic lineage within the otherwise saprobic Aigialaceae (Vohník et al. 2019; Suetrong et al. 2009), certain mycorrhizal fungi also inhabit the soil and wood as saprobes (Rice and Currah 2006; Fehrer et al. 2019; Kolařík and Vohník 2018; Vohník and Réblová 2023), etc. Likewise, not all fungi belonging to genera, families, and orders comprising widespread plant endophytes necessarily share this trait, an excellent example being Helotiales (e.g., Zijlstra et al. 2005). In our study, five OTU belonged to Helotiales: OTU 5 and 18 displayed affinities to Crocicreas gramineum (Helotiaceae) which is a saprobe on plant debris and leaves, especially on Poaceae (Domínguez 2017). OTU 20 clustered with several Lemonniera sp. (Discinellaceae) that are saprotrophs on dead plant material (Ekanayaka et al. 2019). Finally, OTU 21 and 22 belonged to Tetracladium (Helotiales inc. sed.) which comprises aquatic hyphomycetes sometimes colonizing plant roots as endophytes (Selosse et al. 2008). Under these circumstances, we cannot be sure if we detected the mycobiont forming the novel symbiosis nor what is its taxonomy. Nevertheless, despite the limited sampling our study reveals a relatively high fungal diversity associated with the roots of a common Indo-Pacific seagrass that begs further investigation, a situation similar to many freshwater plants (e.g., Kohout et al. 2012).
Functioning of DS fungal associations in seagrasses
There is an ongoing debate about the role of DSE in plant ecology and physiology and it seems that they can be beneficial, neutral, or detrimental associates of terrestrial plants, depending on the phytobiont and mycobiont taxonomy and ontogeny as well as a wide array of environmental conditions (Newsham 2011; Reininger and Sieber 2012; Usuki and Narisawa 2007; Vohník et al. 2003; Mayerhofer et al. 2013). On the other hand, virtually nothing is known about the functioning of DSE/DS mycobionts in seagrasses and changing this will require manipulative monoxenic inoculation experiments, isotopic studies, and genome analyses. In P. oceanica, there is an ontogenetic shift from the seedlings whose roots possess dense root hairs but lack the DSE symbiosis to the adults mostly without root hairs but regularly forming the DSE symbiosis, which is similar to non-mycorrhizal vs. EcM roots (Borovec and Vohník 2018). However, it is unknown whether this shift is directly related to Pos. atricolor and in T. ciliatum, the mycobiont’s presence does not seem to be in any relationship with the presence of the root hairs.
Although indirect, this study provides two important hints on the functioning of the novel symbiosis in T. ciliatum. First, the observation that the hyphal mantles stay on the root surface without visible intraradical colonization suggests that the mycobiont lives as a fungal epiphyte. Epiphytism in fungi is an ancient widespread trait that has evolved independently in several ascomycetous lineages (Hongsanan et al. 2016) but typically concerns plant aboveground organs, especially the leaves, and to our knowledge has never been reported from the roots. While it is unclear whether any parallels can be drawn between terrestrial leaf and marine root fungal epiphytes, they might protect the roots from bacterial, fungal, and viral pathogens, damage caused by herbivores, osmotic stress, etc. In this context, it is interesting to note that older roots typically without fungal colonization had their rhizodermal cells filled with light- to dark brown structures of varied shapes, possibly formed by polyphenolic substances that protect the roots from the stresses listed above (Kumar et al. 2020). Since these were less intense in the colonized roots, one might hypothesize that the hyphal mantles take over their protection role, eventually saving the seagrass the energy and metabolites necessary to produce these substances. Second, the DS fungal colonization was more frequent in thinner terminal roots that are typically the sites of nutrient uptake, indicating a possible role of the mycobiont in the seagrass nutrition, as already hypothesized for Pos. atricolor in the dominant Mediterranean seagrass P. oceanica (Vohník et al. 2015). However, the apparent epiphytic nature of the novel symbiosis hints against a direct nutrient transfer between the mycobiont and its host seagrass. On the other hand, some fungi may benefit their plant partners without forming intraradical mycorrhizal structures, as experimentally demonstrated by Kariman et al. (2014). In any case, further research is needed to test these hypotheses.
Conclusions
Our results indicate that specific root-fungus symbioses in seagrasses might be more frequent than previously thought, being so far confirmed in two highly productive seagrasses from two different families inhabiting two different regions. While their functioning and significance are currently unknown, they appear in healthy-looking terminal roots (i.e., the sites of the nutrient uptake from the seabed) of healthy-looking hosts. The two so far known symbioses are formed by mycobionts with relatively thick melanized hyphae that produce mantles on the root surface that might confer protection against herbivores and pathogens. Melanin slows down decomposition of the fungal mycelium and hence also the colonized terrestrial roots (Langley et al. 2006). If similar is true for some seagrasses (e.g., P. oceanica and T. ciliatum), their root-symbiotic fungi would significantly contribute to the accumulation and stabilization of blue carbon buried in the seabed below the respective seagrass meadows.
Data availability
Sequences generated in this study are available in GenBank at NCBI under the accession numbers OR392720-53. A Thalassodendron ciliatum root specimen is deposited in the Herbarium of the Institute of Botany, Czech Academy of Sciences, Průhonice, Czechia (PRA) under the accession number PRA-21596.
References
Agerer R (1987) Colour atlas of ectomycorrhizae. Einhorn, Schwäbisch Gmünd
Amend A (2014) From dandruff to deep-sea vents: Malassezia-like fungi are ecologically hyper-diverse. PLoS Pathog 10:e1004277. https://doi.org/10.1371/journal.ppat.1004277
Angiosperm Phylogeny Website (2023) http://www.mobot.org/MOBOT/research/APweb/welcome.html, accessed 30/8/2023
Arnold AE (2007) Understanding the diversity of foliar endophytic fungi: progress, challenges, and frontiers. Fungal Biol Rev 21:51–66. https://doi.org/10.1016/j.fbr.2007.05.003
Badalamenti F, Alagna A, Fici S (2015) Evidences of adaptive traits to rocky substrates undermine paradigm of habitat preference of the Mediterranean seagrass Posidonia oceanica. Sci Rep 5:8804. https://doi.org/10.1038/srep08804
Boddy L, Watkinson SC (1995) Wood decomposition, higher fungi, and their role in nutrient redistribution. Can J Bot 73:1377–1383. https://doi.org/10.1139/b95-400
Borovec O, Vohník M (2018) Ontogenetic transition from specialized root hairs to specific root-fungus symbiosis in the dominant Mediterranean seagrass Posidonia oceanica. Sci Rep 8:1–11. https://doi.org/10.1038/s41598-018-28989-4
Brundrett MC (2002) Coevolution of roots and mycorrhizas of land plants. New Phytol 154:275–304. https://doi.org/10.1046/j.1469-8137.2002.00397.x
Brundrett MC (2017) Global diversity and importance of mycorrhizal and nonmycorrhizal plants, in: Tedersoo L (ed) Biogeography of mycorrhizal symbiosis. Springer International Publishing. https://doi.org/10.1007/978-3-319-56363-3_21
Cariello L, Zanetti L, De Stefano S (1979) Posidonia ecosystem – V. Phenolic compounds from marine phanerogames, Cymodocea nodosa and Posidonia oceanica. Comp Biochem Physiol B Biochem Mol Biol 62B:159–161. https://doi.org/10.1016/0305-0491(79)90304-3
Domínguez ER (2017) Crocicreas gramineum var. gramineum. https://www.centrodeestudiosmicologicosasturianos.org/?p=725, accessed 2/9/2023
Ducomet V (1907) Recherches sur le développement de quelques champignons parasites à Thalle subcuticulaire. Thèse Doctorale. Faculté des sciences de Paris, Rennes
Ekanayaka A, Hyde K, Gentekaki E, McKenzie E, Zhao Q et al (2019) Preliminary classification of Leotiomycetes. Mycosphere 10:310–489. https://doi.org/10.5943/mycosphere/10/1/7
Elliott JK, Simpson H, Teesdale A, Replogle A, Elliott MG et al (2019) A novel phagomyxid parasite produces sporangia in root hair galls of eelgrass (Zostera marina). Protist 170:64–81. https://doi.org/10.1016/j.protis.2018.12.001
Ettinger CL, Eisen JA (2019) Characterization of the mycobiome of the seagrass, Zostera marina, reveals putative associations with marine chytrids. Front Microbiol 10:2476. https://doi.org/10.3389/fmicb.2019.02476
Fehrer J, Réblová M, Bambasová V, Vohník M (2019) The root-symbiotic Rhizoscyphus ericae aggregate and Hyaloscypha (Leotiomycetes) are congeneric: phylogenetic and experimental evidence. Stud Mycol 92:195–225. https://doi.org/10.1016/j.simyco.2018.10.004
Fourqurean JW, Duarte CM, Kennedy H, Marbà N, Holmer M et al (2012) Seagrass ecosystems as a globally significant carbon stock. Nat Geosci 5:505–509. https://doi.org/10.1038/ngeo1477
Gardes M, Bruns TD (1993) ITS primers with enhanced specificity for basidiomycetes - application to the identification of mycorrhizae and rusts. Mol Ecol 2:113–118. https://doi.org/10.1111/j.1365-294X.1993.tb00005.x
Green EP, Short FT (2003) World atlas of seagrasses. University of California Press, USA
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41:95–98
Hawkins H-J, Cargill RIM, van Nuland ME, Hagen SC, Field KJ et al (2023) Mycorrhizal mycelium as a global carbon pool. Curr Biol 33:R560–R573. https://doi.org/10.1016/j.cub.2023.02.027
Hongsanan S, Sánchez-Ramírez S, Crous PW, Ariyawansa HA et al (2016) The evolution of fungal epiphytes. Mycosphere 7:1690–1712. https://doi.org/10.5943/mycosphere/7/11/6
Hyde KD, Jones EBG, Liu J-K, Ariyawansa H, Boehm E et al (2013) Families of Dothideomycetes. Fungal Divers 63:1–313. https://doi.org/10.1007/s13225-013-0263-4
Jayasiri SC, Hyde KD, Ariyawansa HA, Bhat J, Buyck B et al (2015) The Faces of Fungi database: fungal names linked with morphology, phylogeny and human impacts. Fungal Divers 74:3–18. https://doi.org/10.1007/s13225-015-0351-8
Jumpponen A (2001) Dark septate endophytes – are they mycorrhizal? Mycorrhiza 11:207–211. https://doi.org/10.1007/s005720100112
Jumpponen A, Trappe JM (1998) Dark septate endophytes: a review of facultative biotrophic root-colonizing fungi. New Phytol 140:295–310
Kaldorf M, Renker C, Fladung M, Buscot F (2004) Characterization and spatial distribution of ectomycorrhizas colonizing aspen clones released in an experimental field. Mycorrhiza 14:295–306. https://doi.org/10.1007/s00572-003-0266-1
Kariman K, Barker SJ, Jost R, Finnegan PM, Tibbett M (2014) A novel plant–fungus symbiosis benefits the host without forming mycorrhizal structures. New Phytol 201:1413–1422. https://doi.org/10.1111/nph.12600
Kohout P, Sýkorová Z, Čtvrtlíková M, Rydlová J, Suda J et al (2012) Surprising spectra of root-associated fungi in submerged aquatic plants. FEMS Microbiol Ecol 80:216–235. https://doi.org/10.1111/j.1574-6941.2011.01291.x
Kolařík M, Vohník M (2018) When the ribosomal DNA does not tell the truth: the case of the taxonomic position of Kurtia Argillacea, an ericoid mycorrhizal fungus residing among Hymenochaetales. Fungal Biol 122:1–18. https://doi.org/10.1016/j.funbio.2017.09.006
Kolátková V, Vohník M (2019) Adaptive traits in the seagrass Posidonia oceanica: Root hairs with spiral cell walls, not spiral root hairs. Aquat Bot 155:52–53. https://doi.org/10.1016/j.aquabot.2018.11.013
Kolátková V, Mooney M, Kelly K, Hineva E, Gawryluk RMR et al. (2023) Eelgrass (Zostera spp.) associated phytomyxids are host-specific congeneric parasites and predominant eukaryotes in the eelgrass rhizosphere on a global scale. Environ Microbiol 25:1522–1537. https://doi.org/10.1111/1462-2920.16376
Kumar S, Abedin MM, Singh AK, Das S (2020) Role of phenolic compounds in plant-defensive mechanisms. In: Lone R, Shuab R, Kamili A (eds) Plant phenolics in sustainable agriculture. Springer, Singapore. https://doi.org/10.1007/978-981-15-4890-1_22
Langley JA, Chapman SK, Hungate BA (2006) Ectomycorrhizal colonization slows root decomposition: the post-mortem fungal legacy. Ecol Lett 9:955–959. https://doi.org/10.1111/j.1461-0248.2006.00948.x
Larkum AWD, Waycott M, Conran JG (2018) Evolution and biogeography of seagrasses. in: Larkum AWD, Kendrick GA, Ralph PJ (2018) Seagrasses of Australia. Springer, Cham. https://doi.org/10.1007/978-3-319-71354-0_1
Lawrey JD, Diederich P (2018) Lichenicolous fungi: worldwide checklist, including isolated cultures and sequences available. http://www.lichenicolous.net, accessed 1/9/2023
Le Renard L, Stockey RA, Upchurch GR, Berbee ML (2021) Extending the fossil record for foliicolous Dothideomycetes: Bleximothyrium ostiolatum gen. et sp. nov., a unique fly-speck fungus from the Lower Cretaceous of Virginia, USA. Am J Bot 108:129–144. https://doi.org/10.1002/ajb2.1602
Lefebvre L, Compère P, Gobert S (2023) The formation of aegagropiles from the Mediterranean seagrass Posidonia oceanica (L.) Delile (1813): plant tissue sources and colonisation by melanised fungal mycelium. Mar Biol 170:19. https://doi.org/10.1007/s00227-022-04166-0
Lipkin Y (1979) Quantitative aspects of seagrass communities, particularly of those dominated by Halophila stipulacea, in Sinai (Northern Red Sea). Aquat Bot 7:119–128. https://doi.org/10.1016/0304-3770(79)90016-0
Lukešová T, Kohout P, Větrovský T, Vohník M (2015) The potential of dark septate endophytes to form root symbioses with ectomycorrhizal and ericoid mycorrhizal middle European forest plants. PLoS ONE 10:e0124752. https://doi.org/10.1371/journal.pone.0124752
Mata JL, Cebrián J (2013) Fungal endophytes of the seagrasses Halodule wrightii and Thalassia testudinum in the northcentral Gulf of Mexico. Bot Mar 56:541–545. https://doi.org/10.1515/bot-2013-0047
Mayerhofer MS, Kernaghan G, Harper KA (2013) The effects of fungal root endophytes on plant growth: a meta-analysis. Mycorrhiza 23:119–128. https://doi.org/10.1007/s00572-012-0456-9
McMillan C (1984) The condensed tannins (proanthocyanidins) in seagrasses. Aquat Bot 20:351–357. https://doi.org/10.1016/0304-3770(84)90099-8
Naranjo-Ortiz MA, Gabaldón T (2019) Fungal evolution: major ecological adaptations and evolutionary transitions. Biol Rev 94:1443–1476. https://doi.org/10.1111/brv.12510
Newsham KK (1999) Phialophora graminicola, a dark septate fungus, is a beneficial associate of the grass Vulpia ciliata ssp. ambigua. New Phytol 144:517–524. https://doi.org/10.1046/j.1469-8137.1999.00537.x
Newsham KK (2011) A meta-analysis of plant responses to dark septate root endophytes. New Phytol 190:783–793. https://doi.org/10.1111/j.1469-8137.2010.03611.x
Nguyen NH, Song Z, Bates ST, Branco S, Tedersoo L et al (2016) FUNGuild: an open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol 20:241–248. https://doi.org/10.1016/j.funeco.2015.06.006
Nilsson RH, Larsson K-H, Taylor AFS, Bengtsson-Palme J, Jeppesen TS et al (2019) The UNITE database for molecular identification of fungi: handling dark taxa and parallel taxonomic classifications. Nucleic Acids Res 47:D259–D264. https://doi.org/10.1093/nar/gky1022
Osono T (2007) Ecology of ligninolytic fungi associated with leaf litter decomposition. Ecol Res 22:955–974. https://doi.org/10.1007/s11284-007-0390-z
Parniske M (2008) Arbuscular mycorrhiza: the mother of plant root endosymbioses. Nat Rev Microbiol 6:763–775. https://doi.org/10.1038/nrmicro1987
Piñeiro-Juncal N, Leiva-Dueñas C, Serrano O, Mateo MÁ, Martínez-Cortízas A (2020) Pedogenic processes in a Posidonia oceanica mat. Soil Syst 4:18. https://doi.org/10.3390/soilsystems4020018
Podgórska-Kryszczuk I, Solarska E, Kordowska-Wiater M (2022) Biological control of Fusarium culmorum, Fusarium graminearum and Fusarium poae by antagonistic yeasts. Pathogens 11:86. https://doi.org/10.3390/pathogens11010086
Poli A, Prigione V, Bovio E, Perugini I, Varese GC (2021) Insights on Lulworthiales inhabiting the Mediterranean Sea and description of three novel species of the genus Paralulworthia. J Fungi 7:940. https://doi.org/10.3390/jof7110940
Powley HR, Cappellen PV, Krom MD (2017) Nutrient cycling in the Mediterranean Sea: The key to understanding how the unique marine ecosystem functions and responds to anthropogenic pressures. in: Mediterranean Identities - Environment, Society, Culture. InTech. https://doi.org/10.5772/intechopen.70878
Read DJ, Leake JR, Perez-Moreno J (2004) Mycorrhizal fungi as drivers of ecosystem processes in heathland and boreal forest biomes. Can J Bot 82:1243–1263. https://doi.org/10.1139/B04-123
Reininger V, Sieber TN Mycorrhiza reduces adverse effects of dark septate endophytes (DSE) on growth of conifers. PLoS ONE 7:e0042865. https://doi.org/10.1371/journal.pone.0042865
Rice AV, Currah RS (2006) Oidiodendron maius: Saprobe in Sphagnum peat, mutualist in ericaceous roots? In: Schulz BJE, Boyle CJC, Sieber TN (eds) Microbial Root endophytes, vol 9. Springer, Berlin. https://doi.org/10.1007/3-540-33526-9_13
Saadatzadeh MR, Ashbee HR, Holland KT, Ingham E (2001) Production of the mycelial phase of Malassezia in vitro. Med Mycol 39:487–493. https://doi.org/10.1080/714031063
Saikkonen K, Faeth SH, Helander M, Sullivan TJ (1998) Fungal endophytes: a continuum of interactions with host plants. Annu Rev Ecol Syst 29:319–343. https://doi.org/10.1146/annurev.ecolsys.29.1.319
Sakayaroj J, Preedanon S, Supaphon Jones EBG, Phongpaichit S (2010) Phylogenetic diversity of endophyte assemblages associated with the tropical seagrass Enhalus acoroides in Thailand. Fungal Divers 42:27–45. https://doi.org/10.1007/s13225-009-0013-9
Sayers EW, Cavanaugh M, Clark K, Ostell J, Pruitt KD, Karsch-Mizrachi I (2019) GenBank. Nucleic Acids Res 47:D94–D99. https://doi.org/10.1093/nar/gky989
Selosse M-A, Le Tacon F (1998) The land flora: a phototroph–fungus partnership? Trends Ecol Evol 13:15–20. https://doi.org/10.1016/S0169-5347(97)01230-5
Selosse M, Vohník M, Chauvet E (2008) Out of the rivers: are some aquatic hyphomycetes plant endophytes? New Phytol 178:3–7. https://doi.org/10.1111/j.1469-8137.2008.02390.x
Serrano O, Mateo MA, Renom P, Julià R (2012) Characterization of soils beneath a Posidonia oceanica meadow. Geoderma 185–186:26–36. https://doi.org/10.1016/j.geoderma.2012.03.020
Short FT, McRoy CP (1984) Nitrogen uptake by leaves and roots of the seagrass Zostera marina L. Bot Mar 27:547–555. https://doi.org/10.1515/botm.1984.27.12.547
Shrestha P, Szaro TM, Bruns TD, Taylor JW (2011) Systematic search for cultivatable fungi that best deconstruct cell walls of Miscanthus and sugarcane in the field. Appl Environ Microbiol 77:5490–5504. https://doi.org/10.1128/AEM.02996-10
Singh SM, Tsuji M, Gawas-Sakhalker P, Loonen MJJE, Hoshino T (2016) Bird feather fungi from Svalbard Arctic. Polar Biol 39:523–532. https://doi.org/10.1007/s00300-015-1804-y
Solomon PS, Lowe RGT, Tan K-C, Waters ODC, Oliver RP (2006) Stagonospora nodorum: cause of Stagonospora nodorum blotch of wheat. Mol Plant Pathol 7:147–156. https://doi.org/10.1111/j.1364-3703.2006.00326.x
Špetík M, Berraf-Tebbal A, Pokluda R, Eichmeier A (2021) Pyrenochaetopsis kuksensis (Pyrenochaetopsidaceae), a new species associated with an ornamental boxwood in the Czech Republic. Phytotaxa 498:177–185. https://doi.org/10.11646/phytotaxa.498.3.3
Stenglein SA (2009) Fusarium poae: a pathogen that needs more attention. J Plant Pathol 91:25–36. https://doi.org/10.4454/jpp.v91i1.621
Suetrong S, Schoch CL, Spatafora JW, Kohlmeyer J, Volkmann-Kohlmeyer B et al (2009) Molecular systematics of the marine Dothideomycetes. Stud Mycol 64:155–173. https://doi.org/10.3114/sim.2009.64.09
Terrados J, Williams SL (1997) Leaf versus root nitrogen uptake by the surfgrass Phyllospadix torreyi. Mar Ecol Prog Ser 149:267–277. https://www.int-res.com/abstracts/meps/v149/p267-277
Torta L, Burruano S, Giambra S, Conigliaro G, Piazza G et al. (2022) Cultivable fungal endophytes in roots, rhizomes and leaves of Posidonia oceanica (L.) Delile along the coast of Sicily, Italy. Plants 11:1139. https://doi.org/10.3390/plants11091139
Usuki F, Narisawa K (2007) A mutualistic symbiosis between a dark septate endophytic fungus, Heteroconium chaetospira, and a nonmycorrhizal plant, Chinese cabbage. Mycologia 99:175–184. https://doi.org/10.3852/mycologia.99.2.175
van der Heijden MGA, Martin FM, Selosse MA, Sanders IR (2015) Mycorrhizal ecology and evolution: the past, the present, and the future. New Phytol 205:1406–1423. https://doi.org/10.1111/nph.13288
Vohník M (2020) Ericoid mycorrhizal symbiosis: theoretical background and methods for its comprehensive investigation. Mycorrhiza 30:671–695. https://doi.org/10.1007/s00572-020-00989-1
Vohník M (2021) Bioerosion and fungal colonization of the invasive foraminiferan Amphistegina lobifera in a Mediterranean seagrass meadow. Biogeosciences 18:2777–2790. https://doi.org/10.5194/bg-18-2777-2021
Vohník M (2022) Are lulworthioid fungi dark septate endophytes of the dominant Mediterranean seagrass Posidonia oceanica? Plant Biol 24:127–133. https://doi.org/10.1111/plb.13353
Vohník M, Réblová M (2023) Fungi in hair roots of Vaccinium spp. (Ericaceae) growing on decomposing wood: colonization patterns, identity, and in vitro symbiotic potential. Mycorrhiza 33:69–86. https://doi.org/10.1007/s00572-023-01101-z
Vohník M, Lukančič S, Bahor E, Regvar M, Vosátka M et al (2003) Inoculation of Rhododendron cv. Belle-Heller with two strains of Phialocephala fortinii in two different substrates. Folia Geobot 38:191–200. https://doi.org/10.1007/BF02803151
Vohník M, Sadowsky JJ, Kohout P, Lhotáková Z, Nestby R, Kolařík M (2012) Novel root-fungus symbiosis in Ericaceae: sheathed ericoid mycorrhiza formed by a hitherto undescribed basidiomycete with affinities to Trechisporales. PLoS ONE 7:e39524. https://doi.org/10.1371/journal.pone.0039524
Vohník M, Borovec O, Župan I, Vondrášek D, Petrtýl M et al (2015) Anatomically and morphologically unique dark septate endophytic association in the roots of the Mediterranean endemic seagrass Posidonia oceanica. Mycorrhiza 25:663–672. https://doi.org/10.1007/s00572-015-0642-7
Vohník M, Borovec O, Kolařík M (2016) Communities of cultivable root mycobionts of the seagrass Posidonia oceanica in the northwest Mediterranean Sea are dominated by a hitherto undescribed pleosporalean dark septate endophyte. Microb Ecol 71:442–451. https://doi.org/10.1007/s00248-015-0640-5
Vohník M, Borovec O, Župan I, Kolařík M, Sudová R (2017) Fungal root symbionts of the seagrass Posidonia oceanica in the central Adriatic Sea revealed by microscopy, culturing and 454-pyrosequencing. Mar Ecol Prog Ser 583:107–120. https://doi.org/10.3354/meps12337
Vohník M, Borovec O, Kolaříková Z, Sudová R, Réblová M (2019) Extensive sampling and high-throughput sequencing reveal Posidoniomyces atricolor gen. et sp. nov. (Aigialaceae, Pleosporales) as the dominant root mycobiont of the dominant Mediterranean seagrass Posidonia oceanica. MycoKeys 55:59–86. https://doi.org/10.3897/mycokeys.55.35682
Wang G, Cao X, Ma X, Guo M, Liu C et al (2016) Diversity and effect of Trichoderma spp. associated with green mold disease on Lentinula edodes in China. Microbiologyopen 5:709–718. https://doi.org/10.1002/mbo3.364
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. in: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR Protocols. Elsevier. https://doi.org/10.1016/B978-0-12-372180-8.50042-1
Williams J, Clarkson JM, Mills PR, Cooper RM (2003) Saprotrophic and mycoparasitic components of aggressiveness of Trichoderma harzianum groups toward the commercial mushroom Agaricus bisporus. Appl Environ Microbiol 69:4192–4199. https://doi.org/10.1128/AEM.69.7.4192-4199.2003
Yu T, Nassuth A, Peterson RL (2001) Characterization of the interaction between the dark septate fungus Phialocephala fortinii and Asparagus officinalis roots. Can J Microbiol 47:741–753. https://doi.org/10.1139/cjm-47-8-741
Zhang Z, Schwartz S, Wagner L, Miller W (2000) A greedy algorithm for aligning DNA sequences. J Comput Biol 7:203–214. https://doi.org/10.1089/10665270050081478
Zijlstra JD, Van’t Hof P, Baar J, Verkley GJM, Summerbell RC et al (2005) Diversity of symbiotic root endophytes of the Helotiales in ericaceous plants and the grass, Deschampsia flexuosa. Stud Mycol 53:147–162. https://doi.org/10.3114/sim.53.1.147
Acknowledgements
The authors wish to thank (chronologically) Hamdi Kassem and African Divers Nuweiba for organizing the sampling dive, Viktorie Kolátková for the help with the sampling in the Red Sea and the consultation about seagrass phytomyxids, Jiří Machač for taking the SEM photos, John Kuo for the advice on some morphological aspects of Thalassodendron ciliatum roots, Luciana Migliore for the constructive comments on an earlier version of our manuscript, Ludovic Le Renard for bringing our attention to the mycélium en palmettes, and an anonymous reviewer and Jan Colpaert (editor) for kindly investing their time to review our manuscript.
Funding
Open access publishing supported by the National Technical Library in Prague. Despite repeated attempts, no specific funding has been obtained for this study. The sampling was done during an expedition supported by the Grant Agency of Charles University (GAUK 1308218) and the laboratory work was partly covered by the first author’s own money. This study constitutes a part of the long-term research project of the Czech Academy of Sciences, Institute of Botany (RVO 67985939).
Open access publishing supported by the National Technical Library in Prague.
Author information
Authors and Affiliations
Contributions
MV designed the study, obtained funding, collected and processed the samples, performed the microscopic evaluation, edited sequences, assigned fungal taxonomy and traits, wrote the main body of the paper, and reviewed the manuscript. JJ performed the cloning, contributed to the writing, and reviewed the manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vohník, M., Josefiová, J. Novel epiphytic root-fungus symbiosis in the Indo-Pacific seagrass Thalassodendron ciliatum from the Red Sea. Mycorrhiza (2024). https://doi.org/10.1007/s00572-024-01161-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00572-024-01161-9