Abstract
Purpose
To investigate oral and dental late effects in survivors of childhood brain tumors medulloblastoma (MB) and central nervous system supratentorial primitive neuroectodermal tumor (CNS-PNET).
Methods
This cross-sectional study assessed oral and dental late effects in MB/CNS-PNET survivors treated before 20 years of age, and with a minimum of 2 years since treatment. Participants went through an oral and radiographic examination. We assessed oral status using the decayed-missing-filled index (DMFT), oral dryness, maximum mouth opening (MMO), fungal infection, and registration of dental developmental disturbances (DDD) in the form of hypodontia, microdontia, and enamel hypoplasia.
Results
The 46 participants’ mean age at enrolment was 27 ± 12.8 years and at treatment 8.5 ± 5.2 years, and the mean time since treatment was 18.9 ± 12 years. Over a third (35%) of survivors had reduced mouth opening (mean 29.3 ± 5.6 mm (range 16–35)). A significantly lower MMO was found in individuals treated ≤ 5 years compared to survivors treated > 5 years (p = 0.021). One or more DDD were registered in 30.4% of the survivors, with a significantly higher prevalence in individuals treated ≤ 5 years (p < 0.001). Hypodontia was the most prevalent type of DDD. There was no difference in DMFT score in relation to age at treatment. Oral dryness was not frequently reported or observed in these survivors.
Conclusion
Survivors of childhood MB/CNS-PNET are at risk of oral and dental late effects including reduced mouth opening and DDD. The risk is highest in survivors treated before the age of 5.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Medulloblastoma (MB) and central nervous system supratentorial primitive neuroectodermal tumor (CNS-PNET) are malignant brain tumors [1] accounting for 15–20% and 2.5% of all pediatric brain tumors respectively [2, 3]. Although survival rates have improved in the last decades [3], the multimodal treatment including surgery, chemotherapy, and/or radiotherapy (RT) [2, 4] entails a high risk of severe late effects [3,4,5]. RT to the CNS axis is necessary for cure and is administered to all patients except individuals under the age of 3–5 years who are, despite a consequently lower cure potential, most often treated without RT due to its high risk of late neurocognitive impairment [2, 4]. Chemotherapy often consists of combinations of different chemotherapeutic agents including vincristine and alkylating agents such as cyclophosphamide [2].
Survivors of childhood cancer are at risk of oral and dental late effects [6, 7]. Young age at treatment and certain treatment modalities seem to increase prevalence and/or severity of these late effects [6,7,8]. Treatment before the age of 5 years is associated with a higher risk of dental developmental disturbances (DDD) than treatment at a later age [8, 9]. Additionally, the risk of DDD may increase further if the treatment includes cranial irradiation [10] and/or exposure to high doses of chemotherapeutic agents [9, 11]. Dental caries is reported to be higher in survivors of childhood cancers compared to healthy age- and gender-matched controls [12, 13]. The risk of dental caries may be related to age at treatment, as its prevalence is reported to be higher in patients treated after the age of 5 years compared to patients treated before 5 years of age [9, 13]. Pediatric cancer survivors treated with high doses of the chemotherapeutic drug cyclophosphamide may experience reduced salivary flow [11]. Additionally, patients receiving craniospinal irradiation (CSI) are at risk of developing xerostomia and secondary malignancies in the parotid glands [14].
Although there are several studies on oral and dental late effects in survivors of childhood cancer treatment [8,9,10,11,12, 15,16,17], only a few studies included small numbers of pediatric brain tumor survivors in their study population [14, 18,19,20]. No studies have assessed oral and dental late effects in a homogenous group of MB/CNS-PNET survivors. Considering the risk of irreversible treatment-related damage to oral tissues, more knowledge on the type and frequency of oral late effects is needed. Thus, the aim of this study was to investigate oral and dental late effects in long-term survivors of childhood MB/CNS-PNET.
Methods
Study design and participants
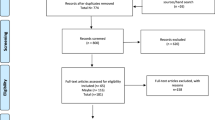
This cross-sectional descriptive study of oral and dental late effects was a sub-study of a large multidisciplinary study at Oslo University Hospital (OUH) [2, 5, 21, 22]. Survivors of pediatric MB/CNS-PNET were identified using the archives of Department of Pathology, surgical protocols at Department of Neurosurgery, and data from the Cancer Registry of Norway [5]. To be included in the study, the participants had to (1) be treated ≤ the age of 20 years at OUH between January 1, 1974, and December 31, 2013, (2) have a histophatologically confirmed MB/CNS-PNET, and (3) have a minimum of 2 years since treatment. During the selected study period, 157 patients treated for MB/CNS-PNET were identified, of which 63 subjects were alive, and invited to participate. Recruitment of participants is described in Fig. 1.
An oral examination was performed by a dentist in a dental office at OUH between September 2016 and October 2018. Clinical photographs of the oral cavity including oral mucosa and teeth were taken from all survivors. In addition, panoramic radiographs were taken with Planmeca PM 2002 CC Proøine Pan/Ceph (Planmeca Oy, Helsinki, Finland). Survivors who could not complete oral and/or radiographical examination due to severe neurocognitive and/or physical disabilities were excluded from the study.
Oral health parameters
Dental caries
Dental caries was evaluated in each participant using the decayed-missing-filled teeth index for permanent teeth (DMFT), or for deciduous teeth (dmft), according to the World Health Organization criteria [23]. The total DMFT and dmft scores ranged from 0 to 28 and from 0 to 20, respectively. Third molars were excluded.
Oral dryness
To assess patient-reported oral dryness, the participants were asked to fill in the Norwegian translation of the Summated Xerostomia Inventory-Dutch Version (SXI-D) questionnaire [24]. This SXI-D is a validated patient-reported questionnaire and consists of five statements: (1) my mouth feels dry when eating a meal, (2) my mouth feels dry, (3) I have difficulty eating dry food, (4) I have difficulties swallowing dry food, and (5) my lips feel dry. Participants were asked to choose from three possible response categories: “never” (score 1), “occasionally” (score 2), or “often” (score 3), with a summated score ranging from 5 to 15, where a higher score indicated a severe problem related to oral dryness [24].
Observer-rated oral dryness was assessed using the Clinical Oral Dryness Score (CODS) index [25] and the dental mirror friction test; the latter is a screening method for saliva lubrication of mucous membranes [26]. CODS index is a grading measure consisting of 10 features of oral dryness; each positive feature scores 1 point [25]. A total score was given for each participant: 1–3 mild dryness, 4–6 moderate dryness, and 7–10 severe dryness [25, 27]. The dental mirror friction test was performed by sliding a dental mirror along the buccal mucosa, and the presence of friction was assessed as follows: 0 = no friction and 1 = friction [26].
Maximum mouth opening (MMO) measurement
MMO was measured with a metallic ruler from the incisal edge of upper frontal teeth to incisal edge of lower frontal teeth, three times in each participant. The mean score was calculated, and MMO ≤ 35 mm was used as a cut-off value for reduced mouth opening [28].
Oral fungal infection
The presence of oral fungal infection was assessed by clinical examination supplemented by a microbiological test. Separate sterile cotton swabs were rubbed against two oral sites: (1) the anterior part of the tongue and (2) the buccal mucosa. Swab samples were inoculated onto CHROMagar (CHROMagar™ Candida, Paris/France) culture plates and incubated at 37 °C for 48 h. A diagnosis of oral candidiasis was made only in cases where clinical as well as microbiological findings were positive [29].
Dental developmental disturbances
DDD were assessed by a clinical and radiographic examination. Since age at diagnosis and treatment may impact DDD [8, 9], the survivors were separated in 2 age groups when DDD were addressed: (1) survivors treated ≤ 5 years of age, (2) survivors treated > 5 years of age. Third molars were excluded.
Disturbances were only evaluated in permanent teeth. Hypodontia, missing development of a tooth, was registered if the participant was past the age of expected normal dental development [30]. Registration of DDD was done as follows (Fig. 2) [9, 15, 16]:
-
1)
Hypodontia, when the survivor had no history of tooth extraction, or a missing tooth was obvious on radiographs (Fig. 2a)
-
2)
Microdontia, tooth ≤ 50% of expected size (Fig. 2b)
-
3)
Obvious enamel hypoplasia of the teeth (Fig. 2c)
Radiograph and photos illustrating dental development disturbances recorded in survivors of childhood MB/CNS-PNET. a Hypodontia (missing development of a tooth) of permanent teeth. b Microdontia (tooth ≤ 50% of expected size). c Enamel hypoplasia. Abbreviations: MB medulloblastoma CNS-PNET central nervous system supratentorial primitive neuroectodermal tumor
Statistical analyses
Patient characteristics were reported by descriptive statistics. Categorical variables were presented as frequencies with proportion, while continuous variables were presented as mean with standard deviation (SD) and range. Continuous variables that were not normally distributed were presented with median and interquartile range (IQR) in addition to mean (SD). The chi-square test and Fisher’s Mid-p test were used for comparison of proportions, as appropriate, while comparison of means was performed by an independent t-test. In case of non-normally distributed outcomes, comparison of median was performed using the median test. A p value < 0.05 was considered statistically significant. All analyses were performed using SPSS (IBM SPSS Statistics 27.0 for Windows, IBM Corp., Armonk, NY) and Stata (StataCorp. 2019. Stata Statistical Software: Release 16. College Station, TX: StataCorp LLC.).
Results
Participants
Of all 63 invited survivors, 50 (79%) consented to participate (Fig. 1). Four survivors were not able to go through an oral and radiographic examination due to severe cognitive and/or physical impairment [21]. These four individuals were excluded from the study. Ultimately, 46 (73%) survivors were included in our sub-study on oral and dental late effects.
Survivor and treatment characteristics are presented in Table 1. The mean age at treatment was 8.5 years (range 0.2–19.2 years), and the mean age at study entry was 27 years (range 5.5–51.9 years). The mean time since treatment was 18.9 years (range 3.2–40.4). In total, 40 (87%) of the 46 survivors received CSI, with a mean dose of 31.6 Gy (range 23.4–36 Gy). One patient received focal proton therapy without CSI. Five participants did not receive RT due to young age (< 3–5 years) at treatment. Thirty-eight (82.6%) of the 46 survivors received chemotherapy including alkylating agents in 18 (47.4%) and vincristine in 37 (97.4%) patients.
Oral health parameters
Dental caries
Six (13%) survivors had deciduous teeth present; all of them had dmft/DMFT score 0. The mean DMFT score in all participants was 5.2 ± 6 (range 0–21). There was no significant difference in DMFT score between survivors treated at age > 5 years compared to survivors treated at age ≤ 5 years (0 vs 4, p = 0.139) (Table 2).
Oral dryness
Patient-reported and observed oral dryness outcomes are presented in Table 3. The mean SXI sum score, which indicates survivor` subjective evaluation of oral dryness, was 6.8 ± 1.8 (range 5–13). The mean CODS was 0.5 ± 1.1 (range 0–4). Eight (17.4%) survivors showed mild and one (2.2%) had moderate signs of oral dryness. The CODS features “mirror sticking to buccal mucosa and/or tongue” and “frothy saliva” were the most commonly registered. Most participants, 37 (80.4%), showed no clinical signs of oral dryness according to the CODS index. Among the nine survivors with positive oral dryness features according to the CODS index, the mean sum score of SXI was 8.4 ± 2.7 (range 5–13). Presence of oral dryness, evaluated by the sliding mirror friction test, was found in five (11%) survivors; the mean CODS score among these was 3 (range 2–4).
Maximum mouth opening measurement
The mean MMO among all participants was 38.5 ± 8.4 (range 16–55). A significantly lower MMO (p = 0.021) was found in survivors treated ≤ 5 years of age compared to survivors treated at an age > 5 years (Table 2). Reduced mouth opening, measured as MMO ≤ 35 mm, was found in 16 (35%) of 46 survivors. The mean score in these 16 was 29.3 ± 5.6 mm (range 16–35) (Table 2). A survivor with MMO of 16 mm is shown in Fig. 3.
Oral fungal infection
None of the participating survivors had clinical signs and/or a positive microbiology test of oral candidiasis.
Dental developmental disturbances
Prevalence of DDD in survivors is listed in Table 2. Almost one-third (30.4%) of all participants had one or more DDD. Hypodontia was the most prevalent and found in 10 (21.7%) of the 46 survivors. Significant differences were found in the prevalence of total DDD, hypodontia, microdontia, and enamel hypoplasia between survivors treated ≤ 5 years of age and individuals treated > 5 years of age (Table 2).
Discussion
This cross-sectional study is the first to investigate long-term (mean 18.9 years) oral and dental late effects in a homogenous group of childhood MB/CNS-PNET survivors. Of 46 study participants, 30.4% survivors showed DDD, and more than one-third of survivors (35%) had reduced mouth opening (≤ 35 mm) at the time of examination. Both parameters were more prevalent in survivors treated before the age of 5.
A reduced mouth opening may affect oral function, nutritional status, oral hygiene, dental care, and health-related quality of life [7, 31]. RT-induced trismus, MMO ≤ 35 mm, in adult head and neck cancer (HNC) survivors is well known [28, 31, 32], while the reported prevalence of reduced mouth opening in childhood brain cancer survivors may vary [7, 17, 33,34,35]. Cetnier and coworkers found no limited mouth opening in survivors of different childhood cancers [17], while the prevalence in survivors of childhood nasopharyngeal cancer has been reported to range from 7 to 27%, with a higher risk in individuals receiving RT doses above 50 Gy [7, 33,34,35]. As RT-induced craniofacial growth disturbance may occur in survivors of childhood cancers [7, 10, 20, 36], it is not unlikely that this may affect the range of mouth opening. Additionally, trismus may be associated with effects of RT to the temporomandibular joint, mandible, or muscles of mastication [7].
The criteria of reduced MMO used in the present study is validated for head and neck cancer patients at the age > 18 years [28]. It should be noted that our study sample included survivors younger than 18 years at the time of study examination. However, previous studies assessing trismus in childhood nasopharyngeal cancer survivors have included those below the age of 18, the youngest being 7 years of age [33,34,35].
The prevalence of hypodontia, the absence of a tooth development, has been reported to be 4.5–6.5% in the Norwegian population (third molar excluded) [37, 38]. In our study, a higher prevalence of hypodontia (21.7%) was found, but this corresponds well with reported hypodontia frequencies in other childhood cancer populations (5 to 31%) [9, 12, 16,17,18,19, 39, 40]. However, it should be noted that cancer diagnosis, treatment modality, and criteria of hypodontia registration differ among the studies [9, 12, 16,17,18,19, 39, 40].
Hypodontia was the most common DDD in our study, in 21.7% of survivors, microdontia the second most common in 15.2%, and enamel hypoplasia the less common in 8.7%. In contrast, the Norwegian study by Wilberg and coworkers (2016) on 111 survivors of childhood leukemia, treated with chemotherapy at an early age, hypodontia was found in 5%, microdontia in 28%, and enamel hypoplasia in 46% [9]. The higher prevalence of hypodontia in our study population compared to childhood leukemia survivors treated with chemotherapy [9] may be due to treatment modalities combining CSI with chemotherapy [40] in MB/CNS-PNET patients.
Since most survivors in our study were treated with the combination of CSI and chemotherapy, it is difficult to evaluate the independent impact of different treatment modalities on dental development [6]. Several previous studies reported that vincristine and alkylating substances such as cyclophosphamide may be associated with DDD in childhood cancer survivors [6, 11, 41, 42]. In contrast, in a recent published study no association between DDD and specific type of chemotherapy agent was reported [40]. Even though brain tumor patients are not directly irradiated towards the jaws and teeth, scattering to these surrounding tissues may occur [43]. Sonis and coworkers (1990) compared survivors of childhood acute lymphoblastic leukemia (ALL) treated with chemotherapy alone with ALL patients treated with chemotherapy in combination with either 18 Gy or 24 Gy cranial irradiation. They found an association between severity of DDD and treatment including cranial irradiation, especially in patients receiving 24 Gy compared to 18 Gy [10]. The mean CSI dose was higher in our study population (31.6 Gy, range 23.4–36 Gy), which may indicate even greater risk of DDD.
In concurrence with previous studies [8,9,10, 16, 18, 41], we found the risk of DDD to be significantly associated with cancer treatment performed before the age of 5 [8,9,10, 16, 18, 41].
Participants’ self-evaluation of oral dryness (mean SXI score 6.8 ± 1.8) indicated that the overall group of survivors did not have a problem with dry mouth. However, the range of SXI score (5–13) among participants showed a wide variation within the group of MB/CNS-PNET survivors. It should be noted that neurocognitive function in long-term survivors of MB/CNS-PNET may also vary [21]. The ability to reflect on their subjective feeling of oral dryness may thus be compromised. Most of the survivors, 80.4%, had no clinical signs of oral dryness. The rest (19.6%) showed 2–4 clinical oral dryness signs where “mirror sticking to buccal mucosa and/or tongue” and “frothy saliva” were the most frequent signs. In comparison, a Norwegian study using the same methods on survivors of adult HNC reported a mean SXI score of 11.9 ± 2.5, and 45% of 29 participants had clinical signs of severe oral dryness (CODS score > 6) [44].
There are conflicting reports on oral dryness in survivors of different childhood cancers [7, 9, 34, 45]. Wilberg and coworkers (2016) reported xerostomia in 23% of 111 long-term survivors of childhood leukemia (mean age of 29.1 years at examination) [9], while as many as 88% of 17 pediatric survivors of nasopharyngeal cancer treated with chemo-radiation reported xerostomia in the study of Sahai and coworkers (2016) [45]. In contrast, Küpeli and coworkers reported xerostomia in only three (1.2%) of 84 survivors of childhood nasopharyngeal carcinoma [34]. Reported xerostomia in survivors of childhood cancer may vary due to different factors like how xerostomia was addressed, type of study population and treatment modality, age at treatment, and time since treatment [9, 34, 45].
Several studies have examined the risk of dental caries in childhood cancer survivors [9, 12, 13, 17]. Cetiner and coworkers (2019) found no significant difference in mean dmft/DMFT score between survivors of different childhood cancers and healthy controls [17]. In contrast, other studies have shown an increased risk of dental caries in childhood cancer survivors [12, 13]. In the Danish study by Wogelius and coworkers (2008), an increased risk of dental caries was found in survivors treated after the age of 5 years, compared to survivors treated before the age of 5 [13]. Similar results were reported by Wilberg and coworkers (2016) in survivors of childhood leukemia, where treatment after the age of 5 years was associated with a significantly higher mean DMFT score compared to survivors younger than 5 years at treatment [9]. Our findings on DMFT in MB/CNS-PNET survivors are in discordance with these studies, as we found no significant difference in DMFT score between participants treated ≤ 5 years or after the age of 5 years. Why there are no differences between the groups in our study is not clear. One theory is that childhood survivors of malign brain tumors often have multiple severe sequela after treatment [3], and therefor oral hygiene are more closely monitored by a parent/guardian and the health system.
The relatively large homogenous study population of long-term survivors of childhood malignant brain tumors and the long median time since treatment are strengths of this study. A possible limitation is the survivors who were not able to participate due to severe late effects. Additionally, the lack of a matched control group is a limitation. Furthermore, oral and dental late effects may change over time during cancer treatment, and we did not have a baseline oral and dental clinical and/or radiographical examination of participants.
This study revealed oral and dental late effects including a reduced mouth opening and DDD in long-term survivors of childhood MB/CNS- PNET. The risk of developing a reduced mouth opening and DDD seems to be related to treatment at a young age ≤ 5 years. Our findings emphasize the importance of patient information and a close and careful follow-up of these patients by health personnel with knowledge on monitoring oral and dental health years after treatment.
Data availability
Can be available from the corresponding author if requested.
References
King AA, Seidel K, Di C, Leisenring WM, Perkins SM, Krull KR, Sklar CA, Green DM, Armstrong GT, Zeltzer LK, Wells E, Stovall M, Ullrich NJ, Oeffinger KC, Robison LL, Packer RJ (2017) Long-term neurologic health and psychosocial function of adult survivors of childhood medulloblastoma/PNET: a report from the childhood cancer survivor study. Neuro Oncol 19(5):689–698
Stensvold E (2020) Paediatric and adolescent medulloblastoma and CNS-PNET in Norway 1974–2013: survival, regional differences, and late effects. [Dissertation]. University of Oslo, Oslo, p 25–28
Salloum R, Chen Y, Yasui Y, Packer R, Leisenring W, Wells E, King A, Howell R, Gibson TM, Krull KR, Robinson LL, Oeffinger Kobel C, Foulandi M, Armstrong GT (2019) Late morbidity and mortality among medulloblastoma survivors diagnosed across three decades: a report from the childhood cancer survivor study. J Clin Oncol 37(9):731–740
Millard NE, De Branganca KC (2016) Medulloblastoma. J Child Neurol 31(12):1341–1353
Stensvold E, Krossnes BK, Lundar T, Due-Tønnessen BJ, Fric R, Due-Tønnessen P, Bechensteen AG, Myklebust TÅ, Johannesen TB, Brandal P (2017) Outcome for children treated for medulloblastoma and supratentorial primitive neuroectodermal tumor (CNS-PNET)- a retrospective analysis spanning 40 years of treatment. Acta Oncol 56:698–705
Gawade PL, Hudson MM, Kaste SC, Neglia JP, Constine LS, Robison LL, Ness KK (2014) A systematic review of dental late effects in survivors of childhood cancer. Pediatr Blood Cancer 61:407–416
Effinger KE, Migliorati CA, Hudson MM, McMullen KP, Kaste SC, Ruble K, Guilcher GMT, Shah AJ, Castellino SM (2014) Oral and dental late effects in survivors of childhood cancer: a Children’s Oncology Group report. Support Care Cancer 22:2009–2019
Pajari U, Lanning M (1995) Developmental defects of teeth in survivors of childhood ALL are related to the therapy and age at diagnosis. Med Pediatr Oncol 24:310–314
Wilberg P, Kanellopoulos A, Ruud E, Hjermstad MJ, Fosså SD, Herlofson BB (2016) Dental abnormalities after chemotherapy in long-term survivors of childhood acute lymphoblastic leukemia 7–40 years after diagnosis. Support Care Cancer 24:1497–1506
Sonis AL, Tarbell N, Valachovic RW, Gelber R, Schwenn M, Sallan S (1990) Dentofacial development in long-term survivors of acute lymphoblastic leukemia. Cancer 66:2645–2652
Hsieh SG, Hibbert S, Shaw P, Ahern V, Arora M (2011) Association of cyclophosphamide use with dental developmental defects and salivary gland dysfunction in recipients of childhood antineoplastic therapy. Cancer 117:2219–2227
Avșar A, Elli M, Darka Ö, Pinarli G (2007) Long-term effects of chemotherapy on caries formation, dental development, and salivary factors in childhood cancer survivors. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 104:781–789
Wogelius P, Dahllöf G, Gorst-Rasmussen A, Sørensen HT, Rosthøj S, Poulsen S (2008) A population-based observational study of dental caries among survivors of childhood cancer. Pediatr Blood Cancer 50:1220–1226
King MT, Modlin L, Millin L, Donaldson SS, Gibbs IC, Choi CYH, Soltys SG (2016) The parotid gland is an under recognized organ at risk for craniospinal irradiation. Technol Cancer Res Treat 15:472–479
Dahllöf G, Barr M, Bolme P, Modéer, Lönnqvist B, Ringdén O, Heimdahl A (1988) Disturbances in dental development after total body irradiation in bone marrow transplant recipients. Oral Surrg Oral Med Oral Pathol 65:41–4
Hölttä P, Alaluusua S, Saarinen-Pihkala UM, Peltola J, Hovi L (2005) Agenesis and microdontia of permanent teeth as late adverse effects after stem cell transplantation in young children. Cancer 103:181–190
Çetiner D, Çetiner S, Uraz A, Alpaslan GH, Alpaslan C, Memikoğlu TUT, Karadeniz C (2019) Oral and dental alterations and growth disruption following chemotherapy in long-term survivors of childhood malignancies. Support Care Cancer 27:1891–1899
Kang CM, Hahn SM, Kim HS, Lyu CJ, Lee JH, Lee J, Han JW (2018) Clinical risk factors influencing dental developmental disturbances in childhood cancer survivors. Cancer Res Treat 50:926–935
Cubukcu CE, Sevinir B, Ercan I (2012) Disturbed dental development of permanent teeth in children with solid tumors and lymphomas. Pediatr Blood Cancer 58:80–84
Karsila-Tenovuo S, Jahnukainen K, Peltomäki T, Minn H, Kulumala J, Salmi TT, Rönning (2001) Disturbances in craniofacial morphology in children treated for solid tumors. Oral Oncol 37:586–592
Stadskleiv K, Stensvold E, Stokka K, Bechensteen AG, Brandal P (2020) Neuropsychological functioning in survivors of childhood medulloblastoma/CNS-PNET: The role of secondary medical complications. Clin Neuropsychol 30:1–26
Tanem KE, Stensvold E, Wilberg P, Skaare AB, Singh PB, Brandal P, Herlofson BB (2022) Taste and smell function in long-term survivors after childhood medulloblastoma/CNS-PNET. Support Care Cancer 30(7):6155–6162. https://doi.org/10.1007/s00520-022-07048-9
World Health Organization oral health surveys (1997) basic methods, 4th edn. World Health Organization, Geneva
Thomson WM, Van der Putten GJ, De Baat C, Ikebe K, Matsuda KI, Enoki K, Hopcraft MS, Ling GY (2011) Shortening the Xerostomia Inventory. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 112(3):322–327
Osailan S, Pramanik R, Shirlaw P, Proctor B, Challcombe SJ (2012) Clinical assessment of oral dryness: development of a scoring system related to salivary flow and mucosal wetness. Oral Surg Oral Med Oral Pathol Oral Radiol 114(5):597–603
Henricsson V, Svensson A, Axéll T (1990) Evaluation of some electrical methods for objective assessment of oral mucosal dryness. Scand J Dent Res 98:520–528
Challacombe SJ, Osailan SM, Proctor GB (2015) Clinical scoring scales for assessment of dry mouth. In: Carpenter G (ed) dry mouth. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-55154-3_8
Van der Geer S, Van Rijn PV, Kamstra JI, Roodenburg JLN, Dijkstra PU (2019) Criterion for trismus in head and neck cancer patients: a verification study. Support Care Cancer 27:1129–1137
Raju SB, Rajappa S (2011) Isolation and identification of candida from the oral cavity. ISRN Dent 2011:487921
Koch G, Thesleff I (2001) Developmental disturbances and shape of teeth and their treatment. In: Koch G, Poilsen S (eds) Pediatric dentistry. A clinical approach, 1st edn. Munksgaard, Copnhagen, pp 253–271
Pauli N, Johnson J, Finizia C et al (2013) The incidence of trismus and long-term impact on health-related quality of life in patients with head and neck cancer. Acta Oncol 52(6):1137–1145
Saghafi E, Tuomi L, Kjeller G (2021) The prevalence and symptoms of temporomandibular disorders in head and neck cancer patients. Acta Odontol Scand 15:1–6
Daoud J, Toumi N, Bouaziz M, Ghorbel A, Jlidi R, Drira MM, Frikha M (2003) Nasopharyngeal carcinoma in childhood and adolescence: analysis of a series of 32 patients treated with combined chemotherapy and radiotherapy. Eur J Cancer 39:2349–2354
Küpeli S, Varan A, Ozyar E, Atahan IL, Yalçin B, Kutluk T, Akyüz C, Büyükpamukç M (2006) Treatment results of 84 patients with nasopharyngeal carcinoma in childhood. Pediatr Blood Cancer 46:454–458
Zubizarreta PA, D’Antonio G, Raslawski E, Gallo G, Preciado MV, Casak SJ, Scopinaro M, Morales G, Sackmann-Muriel F (2000) Nasopharyngeal carcinoma in childhood and adolescence. Cancer 89:690–695
Gevorgyan A, La Scala GC, Neligan PC, Pang CY, Forrest CR (2007) Radiation-induced craniofacial bone growth disturbances. J Caniofac Surg 18:1001–1007
Aasheim B, Ögaard B (1993) Hypodontia in 9 year-old Norwegians related to need of orthodontic treatment. Scand J Dent Res 101:257–260
Nordgarden H, Jensen JL, Storhaug K (2002) Reported prevalence of congenitally missing teeth in two Norwegian counties. Community Dent Health 19:258–261
Marec-Berard P, Azzi D, Chaux-Bodard AG, Lagrange H, Gourmet R, Bergeron C (2005) Long-term effects on chemotherapy on dental status in children treated for nephroblastoma. Pediatr Hematol Oncol 22:581–588
Halperson E, Matalon V, Goldstein G, Spilberg SS, Herzog K, Fux.-Noy A, Shmueli A, Ram D, Moskovitz M (2022) The prevalence of dental developmental anomalies among childhood cancer survivors according to types of anticancer treatment. Sci Reports 22:4485
Stolze J, Vlaanderen KCE, Holtbach FCED, Teepen JC, Kremer LCM, Loonen JJ, Broeder EVD, van den Heiden-van der Loo M, Louwerens M, Raber-Durlacher JE, Bresters D, Brand HS (2021) Long-term effects of childhood cancer treatment on dentition and oral health: a dentist survey study from the DCCSS LATER 2 study. Cancers 13:5264
Macleod RI, Wlbury RR, Soames JV (1987) Effects of cytotoxic chemotherapy on dental development. J R Soc Med 80:207–209
Lee CT, Bilton SD, Famiglietti RM, Riley BA, Mahajan A, Chang EL, Maor MH, Woo SY, Cox JD, Smith AR (2005) Treatment planning with protons for pediatric retinoblastoma, medulloblastoma, and pelvic sarcoma: how do protons compare with other conformal techniques? Int J Radiat Oncol Biol Phys 63:362–372
Westgaard KL, Hynne H, Amdal CD, Young A, Singh PB, Chen X, Rykke M, Hove LH, Aqrawi LA, Utheim TP, Herlofson BB, Jensen JL (2021) Oral and ocular late effects in head and neck cancer patients treated with radiotherapy. Sci rep 11:4026
Sahai P, Mohanti BK, Sharma A, Thakar A, Bhasker S, Kakkar A, Sharma MC, Upadhyay AD (2017) Clinical outcome and morbidity in pediatric patients with nasopharyngeal cancer treated with chemoradiotherapy. Pediatr Blood Cancer 64(2):259–266. https://doi.org/10.1002/pbc.26240
Acknowledgements
The authors would like to thank the survivors and their families for their time, patience, and positive spirit when participating in this study. Thanks to Ragnhild Sørum Falk for assisting with statistical analyses. In addition, we would like to thank the nurses Elna Hamilton Larsen and Karin Sylte Hammeren for their much-appreciated assistance during the study.
Funding
Open access funding provided by University of Oslo (incl Oslo University Hospital) This study was financially supported by the Pediatric Research Foundation at OUH.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study design. The draft of the manuscript, tables, and figures was done by the corresponding author. All co-authors contributed with comments throughout the process of finalizing the article. The final manuscript was read and approved by all authors.
Corresponding author
Ethics declarations
Ethics approval
The Regional Committee for Medical Research Ethics (2015/2362REK sør-øst B), Health Region South-Eastern Norway, approved this study. It was registered at ClinicalTrials.gov (NCT02851355), and ethical standards of Declaration of Helsinki were followed.
Consent to participate
Informed consent was obtained from all participants. For participants < 16 years and for adults with severe neurocognitive disabilities, their parents/guardians gave consent.
Consent for publication
Informed consent was obtained for publications of data. For adult individuals with severe neurocognitive disabilities or participants < 16 years, their parents/guardians gave consent.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tanem, K.E., Stensvold, E., Wilberg, P. et al. Oral and dental late effects in long-term survivors of childhood embryonal brain tumors. Support Care Cancer 30, 10233–10241 (2022). https://doi.org/10.1007/s00520-022-07405-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-022-07405-8