Abstract
Key message
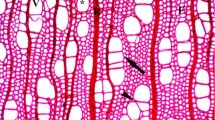
In some circumstances vessel elements, both broad and narrow, enlarge only symplastically in tangential direction. Rays play a special role in regulating intrusive enlargement of vessel elements.
Abstract
The aim of this study was to analyse relations occurring between vessel elements and surrounding cells, i.e. susceptibility to separation of walls of cells occurring in the vicinity of vessel elements, with regard to their type—cells of the axial/radial system. On the basis of separation/lack of separation of cell walls, and thus change in cell contacts/lack of change in cell contacts, we have estimated the contribution of particular types of growth—intrusive and symplastic—in the formation of vessel elements in: Acer pseudoplatanus, Betula pendula, Catalpa bignonioides, Quercus robur and Robinia pseudoacacia. Striking differences in susceptibility to separation of periclinal walls of axial and ray system cells were noticed. Periclinal walls of axial system cells were easily separated, even in cells which were not directly adjacent to vessel elements. Continuity of ray cells was maintained even in cases when the vessel element enlarged directly between two rays. It seems that the direct vicinity of ray increases the contribution, as well as the range of symplastic growth in a tangential direction during vessel element formation. Growth of a vessel element in a tangential direction has been described as intrusive so far. A vessel element seems to enlarge only symplastically, when derived from a mother cell located directly between two rays. Observed differences in susceptibility to separation of cell walls indicate the important role of rays in regulating intrusive expansion of vessel elements.





Similar content being viewed by others
References
Aloni R (1980) Role of auxin and sucrose in the differentiation of sieve and tracheary elements in plant tissue cultures. Planta 150:255–263
Aloni R (2015) Ecophysiological implications of vascular differentiation and plant evolution. Trees-Struct Funct 29:1–16
Aloni R, Zimmermann MH (1983) The control of vessel size and density along the plant axis: a new hypothesis. Differentiation 24:203–208
Barnett JR (1992) Reactivation of the cambium in Aesculus hippocastanum L.: a transmission electron microscope study. Ann Bot 70:169–177
Beck CB (2010) An introduction to plant structure and development. Cambridge University Press, Cambridge
Bollhöner B, Prestele J, Touminen H (2012) Xylem cell death: emerging understanding of regulation and function. J Exp Bot 63:1081–1094
Butterfield BG, Meylan BA (1982) Cell wall hydrolysis in the tracheary elements of the secondary xylem. In: Baas P (ed) New perspectives in wood anatomy. Martinus Nijhoff/ Dr W. Junk Publishers, The Hague, pp 71–84
Dié A, Kitin P, N’Guessan Kouamé F, Van den Bulcke J, Van Acker J, Beeckman H (2012) Fluctuations of cambial activity in relation to precipitation result in annual rings and intra-annual growth zones of xylem and phloem in teak (Tectona grandis) in Ivory Coast. Ann Bot 110:861–873
Escamez S, Tuominen H (2014) Programmes of cell death and autolysis in tracheary elements: when a suicidal cell arranges its own corpse removal. J Exp Bot 65:1313–1321
Evert RF (2006) Esau’s plant anatomy: meristems, cells, and tissues of the plant body: their structure, function, and development. Wiley, New Jersey
Fukuda H (1997) Tracheary element differentiation. Plant Cell 9:1147–1156
Gärtner H, Nievergelt D (2010) The core-microtome: A new tool for surface preparation on cores and time series analysis of varying cell parameters. Dendrochronologia 28:85–92
Gärtner H, Schweingruber FH (2013) Microscopic preparation techniques for plant stem analysis. Verlag Dr. Kessel, Remagen-Oberwinter
Gizińska A (2018) Formowanie pierścieniowej naczyniowości drewna Quercus robur L. w pierwszych latach aktywności kambium. Dissertation, University of Opole
Hejnowicz Z (1980) Tensional stress in the cambium and its developmental significance. Am J Bot 67:1–5
Hejnowicz Z (1997) Graviresponses in herbs and trees: a major role for the redistribution of tissue and growth stresses. Planta 203:S136–S146
Hejnowicz Z (2012) Anatomia i histogeneza roślin naczyniowych. Organy wegetatywne. PWN, Warszawa
Im K-H, Cosgrove DJ, Jones AM (2000) Subcellular localization of expansin mRNA in xylem cells. Plant Physiol 123:463–470
Itoh T, Hayashi S, Kishima T (1968) Cambial activity and radial growth in SUGI trees (Japanese cryptomeria). Wood Res-Slovakia 45:23–35
Ivanova A, Dolezal J, Gärtner H, Schweingruber F (2015) Forty centimeter long transverse and radial sections cut from fresh increment cores. IAWA J 36:460–463
Jura J, Kojs P, Iqbal M, Szymanowska-Pułka J, Włoch W (2006) Apical intrusive growth of cambial fusiform initials along the tangential walls of adjacent fusiform initials: evidence for a new concept. Aust J Bot 54:493–504
Karczewska D, Karczewski J, Włoch W et al (2009) Mathematical modeling of intrusive growth of fusiform initials in relation to radial growth and expanding cambial circumference in Pinus sylvestris L. Acta Biotheor 57:331–348
Kitin P, Sano Y, Funada R (2003) Three-dimensional imaging and analysis of differentiating secondary xylem by confocal microscopy. IAWA J 24:211–222
Kojs P (2012) A qualitative model of symplastic and intrusive growth of the vascular cambium of broadleaved trees—a biomechanical perspective. In: 7th Plant Biomechanics International Conference. Clermont-Ferrand, pp 344–345
Kojs P (2013) Ogólna hipoteza przyrostu wtórnego drzew liściastych. In: Streszczenia referatów i plakatów. Biologia i ekologia roślin drzewiastych. Kórnik-Poznań, pp 164–166
Kojs P, Rusin T (2011) Diurnal strains in plants. In: Gliński J, Horabik J, Lipiec J (eds) Encyclopedia of agrophysic. Springer, Dordrecht, pp 220–224
Kojs P, Rusin A, Iqbal M, Włoch W, Jura J (2004a) Readjustments of cambial initials in Wisteria floribunda (Willd.) DC. for development of storeyed structure. New Phytol 163:287–297
Kojs P, Włoch W, Rusin A (2004b) Rearrangement of cells in storeyed cambium of Lonchocarpus sericeus (Poir.) DC connected with formation of interlocked grain in the xylem. Trees-Struct Funct 18:136–144
Kudo K, Nabeshima E, Begum S, Yamagishi Y, Nakaba S, Oribe Y, Yasue K, Funada R (2014) The effects of localized heating and disbudding on cambial reactivation and formation of earlywood vessels in seedlings of the deciduous ring-porous hardwood, Quercus serrata. Ann Bot 113:1021–1027
Kwiatkowska D, Nakielski J (2011) Mechanics of the meristems. In: Wojtaszek P (ed) Mechanical integration of plant cells and plants. Springer, Berlin, pp 133–172
Larson PR (1994) The vascular cambium. Development and structure. Springer-Verlag, Berlin
Marcati CR, Longo LR, Wiedenhoeft A, Barros CF (2014) Comparative wood anatomy of root and stem of Citharexylum myrianthum (Verbenaceae). Rodriguésia 65:567–576
Mellerowicz EJ (2006) Xylem cell expansion—lessons from poplar. In: Hayashi T (ed) The science and lore of the plant cell wall: biosynthesis, structure and function. BrownWalker Press, Boca Raton, pp 267–275
Mellerowicz EJ, Baucher M, Sundberg B, Boerjan W (2001) Unravelling cell wall formation in the woody dicot stem. Plant Mol Biol 47:239–274
Miodek A, Gizińska A, Klisz M, Wojda T, Ukalski K, Kojs P (2020) Direct exposure to solar radiation causes radial growth eccentricity at the beginning of the growing season in Robinia pseudoacacia. IAWA J 41:61–84
Miodek A, Gizińska A, Wilczek A, Włoch W (2013) Growth of wood fibers in circular-symmetrical annual xylem increment. In: Biedunkiewicz A, Dynowska M (eds) Interdyscyplinarne i aplikacyjne znaczenie nauk botanicznych. Streszczenia wystąpień ustnych i plakatów 56. Zjazdu Polskiego Towarzystwa Botanicznego. Mantis, Olsztyn, pp 114–115
Piermattei A, Crivellaro A, Carrer M, Urbinati C (2015) The „blue ring”: anatomy and formation hypothesis of a new tree-ring anomaly in conifers. Trees-Struct Funct 29:613–620
Rao KS, Kim JS, Kim YS (2011) Early changes in the radial walls of storied fusiform cambial cells during fiber differentiation. IAWA J 32:333–340
Richter HG, Dallwitz MJ (2000 onwards) Commercial timbers: descriptions, illustrations, identification, and information retrieval. In English, French, German, Portuguese, and Spanish. Version: 9th April 2019. https://www.delta-intkey.com/wood/en/index.htm. Accessed 21 Feb 2020
Sachs T (1991) Cell polarity and tissue pattering in plants. Development 113:83–93
Sass-Klaassen U, Sabajo CR, den Ouden J (2011) Vessel formation in relation to leaf phenology in pedunculate oak and European ash. Dendrochronologia 29:171–175
Siedlecka A, Wiklund S, Péronne M-A, Micheli F, Leśniewska J, Sethson I, Edlund U, Richard L, Sundberg B, Mellerowicz EJ (2008) Pectin methyl esterase inhibits intrusive and symplastic cell growth in developing wood cells of Populus. Plant Physiol 146:554–565
Sinnott EW, Bloch R (1939) Changes in intercellular relationships during the growth and differentiation of living plant tissues. Am J Bot 26:625–634
Wenham MW, Cusick F (1975) The growth of secondary wood fibres. New Phytol 74:247–261
Wilczek A (2013) The mechanism of intrusive growth of vessel element mother cells according to tigmo-osmotic hypothesis of broad-leaved trees’ radial increment. In: Biedunkiewicz A, Dynowska M (eds) Interdyscyplinarne i aplikacyjne znaczenie nauk botanicznych. Streszczenia wystąpień ustnych i plakatów 56. Zjazdu Polskiego Towarzystwa Botanicznego. Mantis, Olsztyn, pp 176–177
Wilczek A, Gizińska A, Miodek A, Włoch W (2014) Nowa hipoteza wzrostu promieniowego i przebudowy kambium waskularnego roślin drzewiastych. Kosmos 63:591–601
Wilczek A, Jura-Morawiec J, Kojs P, Iqbal M, Włoch W (2011a) Correlation of intrusive growth of cambial initials to rearrangement of rays in the vascular cambium. IAWA J 32:313–331
Wilczek A, Włoch W, Iqbal M, Kojs P (2011b) Position of rays and lateral deviation of vessel elements in the stem wood of some dicotyledonous species with storeyed, double-storeyed, and nonstoreyed cambia. Botany 89:849–860
Wilczek AB, Iqbal M, Włoch W, Klisz M (2018) Geometric analysis of intrusive growth of wood fibres in Robinia pseudoacacia. IAWA J 39:191–208
Włoch W, Mazur E, Bełtowski M (2002) Formation of spiral grain in the wood of Pinus sylvestris L. Trees 16:306–312
Włoch W, Wilczek A, Jura-Morawiec J, Kojs P, Iqbal M (2013) Modelling for rearrangement of fusiform initials during radial growth of the vascular cambium in Pinus sylvestris L. Trees-Struct Funct 27:879–893
Włoch W, Zagórska-Marek B (1982) Reconstruction of storeyed cambium in the linden. Acta Soc Bot Pol 51:215–228
Wodzicki TJ, Brown CL (1973) Cellular differentiation of the cambium in the Pinaceae. Bot Gaz 134:139–146
Yahya R, Koze K, Sugiyama J (2011) Fibre length in relation to the distance from vessels and contact with rays in Acacia mangium. IAWA J 32:341–350
Yahya R, Sundaryono A, Imai T, Sugiyama J (2015) Distance from vessels changes fiber morphology in Acacia mangium. IAWA J 36:36–43
Zagórska-Marek B (1991) Ontogeneza kambium. Wiad Bot 25:89–110
Zakrzewski J (1983) Hormonal control of cambial activity and vessel differentiation in Quercus robur. Physiol Plant 57:537–542
Zakrzewski J (1991) Effect of indole-3-acetic acid (IAA) and sucrose on vessel size and density in isolated stem segments of oak (Quercus robur). Physiol Plant 81:234–238
Zasada JC, Zahner R (1969) Vessel element development in the earlywood of red oak (Quercus rubra). Can J Bot 47:1965–1971
Acknowledgements
We thank David Oldroyd for linguistic corrections of the text.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by V. De Micco.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gizińska, A., Miodek, A. & Kojs, P. Rays hamper intrusive growth of vessel elements. Trees 35, 749–760 (2021). https://doi.org/10.1007/s00468-020-02071-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-020-02071-x