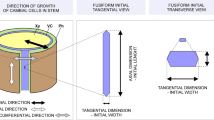
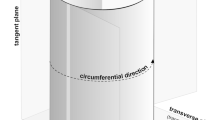
Abstract
This study on the cambium of Pinus sylvestris L. examines the intrusive growth of fusiform cambial initials and its possible contribution to the tangential and radial expansions of the cambial cylinder. The location and extent of intrusive growth of the fusiform initials were determined by microscopic observations and by mathematical modeling. In order to meet the required circumferential expansion of the cambial cylinder, the fusiform initials grow in groups by means of a symplastic rather than intrusive growth, leaving no room for the assumption that intrusive growth of the initials takes place between radial walls and has a direct role in the increase of the cambial circumference. Therefore, it is postulated that the fusiform initials grow intrusively between the tangential walls of the neighboring initials and their immediate derivatives and not between the radial walls of the adjacent initials as per common belief.







Similar content being viewed by others
Abbreviations
- N r :
-
Number of periclinal divisions
- L :
-
Circumference of cambial cylinder
- ΔL :
-
Increase in circumference of cambial cylinder
- r :
-
Radius of cambial cylinder
- Δr :
-
Increase in the cambial radius
- l r :
-
Radial width of the fusiform initial
- l c :
-
Tangential width of the fusiform initial
- Δl r :
-
Increase in radial width of the fusiform initial
- Δl c :
-
Increase in tangential width of the fusiform initial
- u r :
-
Radial displacement of the initial cell
- du r :
-
Changes in radial displacement
- dr :
-
Radial width of the intrusively growing cell
- dl :
-
Tangential width of the intrusively growing cell
- ε r :
-
Strain in radial direction
- ε l :
-
Strain in tangential direction
- T :
-
Time span between two successive periclinal divisions
- Δt :
-
Progress of time between two successive periclinal divisions
- a :
-
Factor covering the phases of advancement towards a periclinal division and ranging in value from zero to one
- t 0 − t 9 :
-
Ten consecutive time intervals during the growth of a fusiform initial
References
Ajmal S, Iqbal M (1987a) Seasonal rhythms of structure and behavior of vascular cambium in Ficus rumphii. Ann Bot (Lond) 60:949–956
Ajmal S, Iqbal M (1987b) Annual rhythm of cambial activity in Streblus asper. IAWA Bull (n. s) 8:275–283
Bailey IW (1923) The cambium and its derivative tissues IV. The increase in girth of the cambium. Am J Bot 10:499–509. doi:10.2307/2446389
Bannan MW, Bayley IL (1956) Cell size and survival in conifer cambium. Can J Bot 34:769–776. doi:10.1139/b56-058
Barlow PW, Brain P, Powers SJ (2002) Estimation of directional division frequencies of vascular cambium and in marginal meristematic cells of plants. Cell Prolif 35:49–68. doi:10.1046/j.1365-2184.2002.00225.x
Butterfield BG (1972) Developmental changes in the vascular cambium of Aschynomene hispida Willd. N Z J Bot 10:373–386
Catesson AM (1964) Origine, fonctionnement et variations cytologiques saisonnieres du cambium de l’Acer pseudoplatanus L. (Aceracees). Ann Sci Nat Bot Ser 12(5):229–498
Catteson AM (1984) La dynamique cambiale. Ann Sci Nat Bot Ser 13(6):23–43
Cottrell AH (1964) The mechanical properties of matter. John Wiley & Sons, New York
Dickison WC (2000) Integrative plant anatomy. Harcourt/Academic Press, San Diego
Erickson RO (1986) Symplastic growth and symplasmic transport. Plant Physiol 82:1153
Esau K (1965) Plant anatomy. John Wiley & Sons, New York
Evert RF (1961) Some aspects of cambial development in Pyrus communis. Am J Bot 48:479–488. doi:10.2307/2439451
Evert RF (2006) Esau’s plant anatomy; Meristems, cells and tissues of the plant body; Their structure, function and development, 3rd edn. John Wiley & Sons, Hoboken
Fahn A (1967) Plant Anatomy. Pergamon Press, Oxford
Forest L, Martin JS, Padilla F, Chassat F, Giroud F, Demongeot J (2004) Morphogenetic processes: application to cambial growth dynamics. Acta Biotheor 52:415–438. doi:10.1023/B:ACBI.0000046607.17817.20
Forest L, Padilla F, Martinez S, Demongeot J, San Martin J (2006) Modeling of auxin transport affected by gravity and differential radial growth. J Theor Biol 241:241–251. doi:10.1016/j.jtbi.2005.11.029
Funada R, Catesson AM (1991) Partial cell wall lysis and the resumption of meristematic activity in Fraxinus excelsior cambium. IAWA Bull (n. s) 12:439–444
Gandar PW (1983) Growth in root apices II. Deformation and rate of deformation. Bot Gaz 144:11–19. doi:10.1086/337338
Guitard D, Masse H, Yamamoto H, Okuyama T (1999) Growth stress generation: a new mechanical model of the dimensional change of wood cells during maturation. J Wood Sci 45:384–391. doi:10.1007/BF01177910
Hejnowicz Z (1961) Anticlinal divisions, intrusive growth, and loss of fusiform initials in nonstoreyed cambium. Acta Soc Bot Pol 30:729–758
Hejnowicz Z (1968) The structural mechanism involved in the changes of grain in timber. Acta Soc Bot Pol 37:347–365
Hejnowicz Z, Brański S (1966) Quantitative analysis of cambium growth in Thuja. Acta Soc Bot Pol 35:395–400
Hejnowicz Z, Romberger JA (1984) Growth tensor of plant organs. J Theor Biol 110:93–114. doi:10.1016/S0022-5193(84)80017-X
Hejnowicz Z, Zagórska–Marek B (1974) Mechanism of changes in grain inclination in wood produced by storeyed cambium. Acta Soc Bot Pol 43:381–398
Iqbal M (1990) The vascular cambium. Research Studies Press, Taunton
Iqbal M (1995) Structure and behaviour of vascular cambium and the mechanism and control of cambial growth. In: Iqbal M (ed) The cambial derivatives. Stuttgart, Germany, pp 1–67
Jean RV (1984) Mathematical approach to pattern and form in plant growth. John Wiley & Sons, New York
Jura J (2005) Formation of storeyed structure in the cambium of Wisteria floribunda DC. Ph. D thesis, Department of Biophysics and Cell Biology, Silesian University, Poland (in Polish)
Jura J, Kojs P, Iqbal M, Szymanowska-Pułka J, Włoch W (2006) Apical intrusive growth of cambial fusiform initial along the tangential walls of adjacent fusiform initials: evidence for a new concept. Aust J Bot 54:493–504. doi:10.1071/BT05130
Kojs P, Włoch W, Rusin A (2004a) Rearrangement of cells in storeyed cambium of Lonchocarpus sericeus (Poir.) DC. connected with formation of interlocked in the xylem. Trees (Berl) 18:136–144. doi:10.1007/s00468-003-0292-9
Kojs P, Włoch W, Iqbal M, Rusin A, Jura J (2004b) Readjustment of cambial initials in Wisteria floribunda (Willd.) DC to ensure the development of storeyed structure. New Phytol 163:287–297. doi:10.1111/j.1469-8137.2004.01120.x
Kramer EM (2002) A Mathematical model of pattern formation in the vascular cambium of trees. J Theor Biol 216:147–158. doi:10.1006/jtbi.2002.2551
Kramer EM, Groves JV (2003) Defect coarsening in a biological system: the vascular cambium of cottonwood trees. Phys Rev E 67:041914-1–041914-4
Krawczyszyn J (1977) The transition from non–storeyed to storeyed cambium in Fraxinus excelsior. 1. The occurrence of radial anticlinal divisions. Can J Bot 55:3034–3041. doi:10.1139/b77-342
Krawczyszyn J, Romberger JA (1979) Cyclical cell length changes in wood in relation to storied structure and interlocked grain. Can J Bot 57:787–794. doi:10.1139/b79-100
Larson PR (1994) The vascular cambium: development and structure. In: Timell TE (ed) Springer Series in Wood Science. Springer-Verlag, Berlin
Meek GA (1976) Practical electron microscopy for biologists. John Wiley & Sons, London
Nägeli C (1864) Dickenwachstum des Stengels und Anordnung der Gefässstränge bei den Sapindaceen. Beitr Wiss Bot 4:1–72
Philipson WR, Ward JM, Butterfield BG (1971) The vascular cambium its development and activity. Chapman and Hall Ltd, London
Prusinkiewicz P (2003) Modeling plant growth and development. Curr Opin Plant Biol 7:79–83. doi:10.1016/j.pbi.2003.11.007
Włoch W, Połap E (1994) The intrusive growth of initial cells in re-arrangement of cells in cambium of Tilia cordata Mill. Acta Soc Bot Pol 63:109–116
Włoch W, Mazur E, Kojs P (2001) Intensive change of inclination of cambial initials in Picea abies (L.) Karst. tumours. Trees (Berl) 15:498–502. doi:10.1007/s00468-001-0127-5
Włoch W, Mazur E, Bełtowski M (2002) Formation of spiral grain in the wood of Pinus sylvestris L. Trees (Berl) 16:306–312. doi:10.1007/s00468-002-0174-6
Włoch W, Jura-Morawiec J, Kojs P, Iqbal M, Krawczyszyn J (2009) Does intrusive growth of fusiform initials really contribute to circumferential growth of vascular cambium? Botany 87:1–10. doi:10.1139/B08-122
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Karczewska, D., Karczewski, J., Włoch, W. et al. Mathematical Modeling of Intrusive Growth of Fusiform Initials in Relation to Radial Growth and Expanding Cambial Circumference in Pinus sylvestris L.. Acta Biotheor 57, 331–348 (2009). https://doi.org/10.1007/s10441-009-9068-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10441-009-9068-y