Abstract
Zinc oxide nanoparticles (ZnONPs) have become the widely used metal oxide nanoparticles and drawn the interest of global researchers due to their biocompatibility, low toxicity, sustainability and cost-effective properties. Due to their unique optical and chemical properties, it emerges as a potential candidate in the fields of optical, electrical, food packaging and biomedical applications. Biological methods using green or natural routes are more environmentally friendly, simple and less use of hazardous techniques than chemical and/or physical methods in the long run. In addition, ZnONPs are less harmful and biodegradable while having the ability to greatly boost pharmacophore bioactivity. They play an important role in cell apoptosis because they enhance the generation of reactive oxygen species (ROS) and release zinc ions (Zn2+), causing cell death. Furthermore, these ZnONPs work well in conjunction with components that aid in wound healing and biosensing to track minute amounts of biomarkers connected to a variety of illnesses. Overall, the present review discusses the synthesis and most recent developments of ZnONPs from green sources including leaves, stems, bark, roots, fruits, flowers, bacteria, fungi, algae and protein, as well as put lights on their biomedical applications such as antimicrobial, antioxidant, antidiabetic, anticancer, anti-inflammatory, antiviral, wound healing, and drug delivery, and modes of action associated. Finally, the future perspectives of biosynthesized ZnONPs in research and biomedical applications are discussed.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During the last few decades, the interdisciplinary science “Nanotechnology” has revolutionized science and industrial technologies due to the presence of superior physicochemical or biological properties of nanoparticles (NPs) that are not present in their bulk form [1]. The greatest theoretical physicist at Caltech, Richard Feynman 1959, spread the idea of nanotechnology [2]. Nanoparticles have distinct key properties, such as a large surface area-to-volume ratio, enhanced thermal conductivity and electrochemical reactivity, and are regarded as a significant state of matter [3]. Recently, metal and metal oxide nanoparticles gained tremendous attention due to their promising applications in electronics, biosensors, photocatalyst, agriculture and biomedical fields [4]. The widespread use of NPs in biomedicine is due to their ability to interact with biological membranes, receptors, proteins and nucleic acids due to their small size [5]. The nanostructures exhibited targeted and long-term drug delivery with regulated drug-related toxicity. This technology has shown promise in the treatment of cancer, AIDS and an array of other ailments [6].
Our previous studies demonstrated the biological synthesis of selenium, silver, titanium dioxide and zinc oxide nanoparticles (ZnONPs) using cyanobacterial cell free extract [7,8,9,10,11,12,13,14,15]. Over various metal NPs, the inorganic metal oxide NPs exhibited remarkable biological applications even at low concentration, highly stable at high temperature and pressure due to their unique physicochemical properties [16]. Among the various metal oxide NPs like iron oxide (Fe3O4), copper oxide (CuO), titanium dioxide (TiO2) and cerium dioxide (CeO2), zinc oxide nanoparticles (ZnONPs) have gained scientific attention due to their biocompatibility, long shelf life and cost-effective nature [4]. ZnONPs possess distinctive optical and chemical properties with wide bandgap (3.37 eV) and high excitation binding energy (60 meV) that enables its use in various biomedical, pharmaceutical and photocatalytic applications [17]. Moreover, Zn is an essential trace element present in human physiological system that confers its biocompatibility being less toxic and are highly biodegradable due to the soluble nature of Zn+2 ions [18]. It is abundant in many bodily tissues, including the brain, muscle, bone, skin and integral part of many enzyme systems. Zinc participates in the body's metabolic pathways and plays critical roles in protein and nucleic acid synthesis, hematopoiesis and neurogenesis [4]. Another property that makes ZnONPs as the potential candidate for biomedical applications is the presence of –OH group that allows it to dissolve slowly in acidic (e.g., cancer cells) and basic microenvironments [19]. However, due to its small particle size, nano-ZnO facilitates zinc absorption by the body and widely utilized as a food additive. Moreover, the US Food and Drug Administration (FDA) has approved ZnONPs as a generally-recognized-as-safe (GRAS) substance and nonhemolytic against human red blood [20]. In comparison with other metal oxide NPs, ZnONPs are more economical as well as less toxic and exhibit excellent biomedical applications. It has been reported that majority of pharmaceutically active compounds do not interact with zinc [4]. However, ZnONPs, both alone and doped with other metals, appear as a novel candidate used for tissue regeneration, implant coatings, bio-imaging, wound healing, development of cancer therapies, targeted drug delivery, antimicrobials coatings or bandages, biosensors and gene delivery [21,22,23]. Rahman et al. [24, 25] demonstrated that ZnO and Mg/Cu-dual-doped ZnO exhibit good antibacterial activity against Staphylococcus aureus and E. coli, as well as radical scavenging properties when exposed to visible light. Ahmad et al. [26] synthesized gold-decorated hetero-nanostructured Au-ZnONPs utilizing Carya illinoinensis extract, which demonstrated maximal photocatalytic degradation of rhodamine-B dye (95%) within 180 min when compared to bare ZnONPs. Furthermore, Rahman et al. [27] also synthesized ZnONPs from aqueous leaf extract of Ziziphus mauritiana Lam., which demonstrated antibacterial efficacy against Staphylococcus aureus as well as antioxidant characteristics.
Use of ZnONPs in biomedicine is widespread due to their excellent biocompatibility, low toxicity (especially as anticancer and antibacterial), potent ability to trigger excess reactive oxygen species (ROS) production, release of zinc ions and induction of cell apoptosis [19]. Due to excellent luminescent properties, ZnONPs are one of the main candidates for bio-imaging [28]. Numerous studies support ZnONPs as the most advantageous metal NPs, with low toxicity and superior biocompatibility as the structural atom allocation being the most bioactive region highlights its pharmacological potency against a range of diseases [2, 4]. Taken together, the purpose of this review is to examine the processes used to synthesize ZnONPs while thoroughly comprehending their biological function. Even though ZnONPs have been the focus of an increasing number of studies, this review provides a detailed compilation of current advancements with simple examples to help readers better comprehend the significance of ZnONPs in biomedical research.
Approaches for synthesis and characterization of ZnONPs
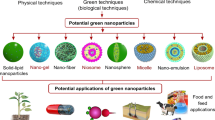
Several synthesis approaches have been developed for ZnONPs and the choice of preparation depends on the specificity of application. Generally, two types of strategies were used—top-down and bottom-up approach, which comprises physical, chemical, and biological (green) synthesis of ZnONPs. The non-conventional methods include micro-fluidic reactor-based synthesis of nanomaterials. The top-down approach involves cutting or physically slicing the bulk materials into nano-sized materials [29]. On the other hand, bottom-up approach uses atoms or molecules for the fabrication of nanomaterials through chemical and biological synthesis. It is less expensive and quicker than the top-down strategy [30]. Different approaches for the synthesis of ZnONPs with their merits and demerits of these synthesis techniques are listed in Table 1. It is crucial to keep in mind that these procedures often require the use of harmful reducing agents and organic solvents, which are extremely reactive and hazardous to the environment [31]. However, green synthesis utilizes a range of biotic resources for the synthesis of ZnONPs, such as plants, bacteria, other biological elements including egg albumin, starch, proteins, gelatin and micro- or macroalgae that are listed in Tables 2 and 3 [32, 33]. Green sources are bio-molecules that act as capping and reducing agents during the synthesis of NPs, further stabilizing and influencing their characteristics [34]. Plants include several secondary metabolites and phytoconstituents that aid in the bio-reduction process during nanoparticle formation. It also played an important role in nanoparticle capping, which was essential for their stability and biocompatibility, and because of these molecules, no additional chemical reducing and capping agents were needed [2]. Microbial-mediated ZnONPs synthesis, on the other hand, offers an advantage over plant-mediated synthesis because microorganisms are easily replicated. The presence of numerous enzymes and biomolecules produced in the suspension or growth medium by microorganisms plays an important role in the bioreduction of NPs and contributes to the development of varied morphologies with mono- and polydispersed NPs [35]. However, there are numerous disadvantages associated with the isolation or screening of potential microorganisms, the usage of chemicals for growth medium, and the cost-effectiveness of synthesis procedures due to their time-consuming nature. Consequently, plants are regarded as the most promising candidates for green synthesis, as they create more stable forms of the same than microbes.
Several biomolecules from plants or other green sources are combined throughout the green synthesis process to create stable and nontoxic ZnONPs. The overall procedure to produce ZnONPs using green sources is summarized schematically in Fig. 1.
The phytochemicals present in the cell extract function both as a stabilizing and as a capping agent for NPs to confer biocompatibility to NPs. High temperatures, high pressures, expensive equipment or hazardous materials are not needed for the process. The current, inexpensive and safe green synthesis of ZnONPs is preferable to the prior, expensive and hazardous techniques [102].
Following synthesis, nanomaterial characterization is necessary to learn about the physicochemical characteristics of the synthesized NPs, including their shape, crystal structure, defects, ionic strength, content and dielectric properties. Numerous studies imply that the form and surface chemistry of nanoparticles affect how safely and effectively they are distributed throughout biological systems (Fig. 2). With this, the polydispersity of materials makes it difficult to characterize NPs size, but it is crucial to understand the morphology because it is believed that the NPs size confers many of the unique properties for their use in nanomedicines [31].
Nanostructures cannot be resolved by optical microscopy; hence, electron microscopy is utilized to describe the nanoparticles. SEM (scanning electron microscopy) and TEM (transmission electron microscopy) are used to determine the morphology and size of NPs [104]. However, TEM is more frequently employed since it employs more potent electrons and provides high-resolution and informative image data about the shape, aggregation state, and distribution on an atomic scale. Energy-dispersive X-ray (EDX) analysis helps to determine the elemental composition and makes it easier to evaluate how synthesized nanoparticles are distributed in living tissues [105]. The 3D topography (height and volume) of NPs can be determined through atomic force microscopy (AFM) analysis [106]. Through the synthesis and capping of NPs, the simple and nondestructive Fourier transform infrared spectroscopy (FTIR) identifies the various metabolites and compounds [107]. The optical properties of colored samples are studied using UV–visible spectroscopy (UV–Vis), where reflectance data are used to investigate the surface plasmon resonance of metals and hypersensitive biological analysis [108]. The effects of the oxidative and reductive environments, phase transitions and thermal stability are determined by thermal gravimetric–differential thermal analysis (TG–DTA) [109]. To obtain size distribution information of molecules and particles majorly in the submicron region, DLS (dynamic light scattering) is very useful [110]. XPS (X-ray photoelectron microscopy) is a technique to analyze the surface chemistry of NPs that measures the empirical formula, chemical state and electronic state of the elements within the NPs. XRD (X-ray diffractometer) provides the information regarding the crystalline structure, phase identification and crystallite size [111].
Biomedical applications and mechanism of action of zinc oxide nanoparticles
ZnONPs have gained a lot of interest in a variety of biological sectors, including anticancer, antibacterial, antioxidant, antidiabetic, anti-inflammatory, drug delivery and many more.
Antibacterial activity
Due to the extensive use of antibiotics in human, animals and food, microorganisms develop antibiotic resistance. It is a worldwide phenomenon that is leading to the public health crisis. World Health Organization in 2001 had declared the antimicrobial resistance as international serious and urgent threats. Biogenic ZnONPs showed the rays of hopes for their use with standard antibiotics against multidrug-resistant bacteria [4]. Antibacterial activity of ZnONPs lies in their ability to induce oxidative stress. When bacterial cells encounter ZnONPs, they absorb Zn+, interact with the thiol group of respiratory enzymes, inhibiting their action, affect the cell membrane and lead to ROS formation that damages the bacterial membranes, DNA and mitochondria, resulting in the death of bacterial cells (Fig. 3) [15]. Biogenic ZnONPs synthesized using different biological material showed varied antibacterial activity, e.g., Nur et al. [112], synthesized spherical-shaped ZnONPs (33 nm) by Punica granatum plant extract and showed a significant antibacterial activity against E. coli and E. faecalis.
ZnONPs derived from cyanobacteria Oscillatoria sp. extract exhibited dose-dependent inhibition in multidrug-resistant (MDR) bacterial strains (S. aureus, B. cereus, E. coli and K. pneumoniae) with lower MIC values of 62.5–125 µg ml−1 in comparison with commercially synthesized ZnONPs [14]. Jayabalan et al. [113] synthesized spherical-shaped ZnONPs (44.5 nm) using Pseudomona putida and presented antimicrobial activity against Pseudomonas otitidis, Pseudomonas oleovorans, Acinetobacter baumannii, Bacillus cereus, and Enterococcus faecalis. Fadwa et al. [114] showed the combination effect of colistin and ZnONPs (2 µg ml−1) against P. aeruginosa. Tyagi et al. [115] showed that ZnONPs chemically conjugated with ciprofloxacin exhibited a 2.9-fold increase in antibacterial activity against E. coli and a 2.8-fold increase for Streptococcus spp. as compared to ciprofloxacin alone. ZnONPs conjugated with ceftriaxone and ampicillin exhibited significant antibacterial activity against gram-positive (Staphylococcus aureus, Streptococcus pneumoniae and Streptococcus pyogenes) and gram-negative (Escherichia coli K1, Serratia marcescens and Pseudomonas aeruginosa) bacteria [116].
ZnONPs synthesized using cyanobacteria Gleocapsa gelatinosa cell extract exhibited enhanced antibiofilm activity against MDR strains (S. aureus, B. cereus, E. coli and K. pneumoniae) with lower minimum biofilm inhibitory concentration (MBIC) values of 46.8 µg ml−1and 93.7 µg ml−1[15]. Flow cytometry analysis and confocal microscopy highlighted the strong interaction of ZnONPs with intracellular components leading to biofilm destruction due to increased permeability, reduced exopolysaccharides secretion, altered bacterial growth and enhanced generation of intracellular ROS [15]. According to Badigera et al. [117] and Shkodenko et al. [118], smaller-sized NPs accumulate on the outer surface of the plasma membrane leading to increased surface tension and membrane depolarization by neutralizing surface potential for easier penetration into the cell. With the increasing concentration of ZnONPs, the penetration rate into the bacterial cell wall increased. ZnONPs after penetration into the cell through electrostatic interactions release Zn+2 ions, causing the conformational changes in enzymes that lead to the distortion of active sites, resulting in the inhibition of membrane proteins that disrupt the bacterial vital functions. In another study, Senthamarai et al. [119] synthesized ZnONPs (19.8 nm) from A. marmelos unripe fruit extract that showed enhanced antibacterial, antibiofilm and antioxidant agent. Furthermore Kappa-carrageenan-wrapped ZnONPs (KC-ZNONPs) exhibited antibacterial activity against MRSA strains, enhanced anti-inflammatory activity and were biocompatible with human RBCs [120]. The findings suggest that ZnONPs have a better antibacterial potential which is influenced by its dosage, treatment time and synthesis approaches. Additionally, increased antibacterial activity is caused by the surface area and size of particle variation, which are notable in biosynthesized ZnONPs. Applications of biogenic ZnONPs in food safety and agriculture have not yet been explored for future use [121].
Antifungal activity
ZnONPs have a promising antifungal activity that depends on their morphology, size, dose as well as source utilized in their biogenic synthesis. The antifungal potential of ZnONPs synthesized from leaf extract of Crinum latifolium inhibited the hyphal development and germ tube formation up to (34%) in Candida albicans [122]. On clinical isolates of Candida sp., the antifungal resistance of a 2% ZnONPs-based cold cream was greater than its antifungal efficacy [123]. ZnONPs from Moringa oleifera were toxic against two plant pathogens, Alternaria saloni and Sclerrotium rolfii [124]. ZnONPs synthesized using Ziziphus nummularia and Prosopis farcta extract exhibited antifungal activity against C. albicans, C. glabrata and C. neoformans with the lowest MIC values of 1.25 mg ml−1[125, 126]. For ZnONPs that can enter fungal (conidial) cells by diffusion and endocytosis, the potential mechanisms of their antifungal activity begin with interference in mitochondrial function, encouragement of ROS generation and release of Zn2+ ions into the cytoplasm. ZnONPs can penetrate the nuclear membrane and produce excessive amounts of ROS and Zn2+ ions, which can lead to permanent DNA damage and cell death [127]. According to Lipovsky et al. [128], free radicals generated by ZnONPs are directly correlated with increased ROS productions, as well as a reduction in cell viability in C. albicans cells. Two mechanisms have been proposed for the antifungal action of ZnONPs. First is that ZnO in aqueous solution generates hydrogen peroxide (H2O2) from its surface, that can penetrate the cell membrane and kill them, inducing oxidative stress. Second is the release of zinc ions in medium with effects on active transport as well as on the amino acid metabolism [129]. Hajar et al. [130] showed the synergistic effect of ZnONPs with fluconazole against C. albicans, and Xue et al. [131] demonstrated the synergistic inhibition of Phytophthora growth by 0.25 g/L ZnONPs with 0.01 g/L thiram.
Antioxidant activity
Cell viability depends critically on oxidative metabolism as the generation of ROS and free radicals during this process may be able to overwhelm some enzymes, such as catalase, peroxidase and superoxide dismutase, leading to deadly effects on cells by damaging proteins, membrane lipids, DNA enzymes, and interfering with cell signaling pathways [132]. Because they can produce lipid peroxides and other harmful free radicals, some oxidized foods are now linked to several serious illnesses, including hepatomegaly and necrosis of epithelial tissues, and a range of natural or synthetic antioxidants are used to combat these harmful free radicals but have disadvantages compared to today's biosynthesized NPs, such as high reactivity and toxicity [133]. According to Das et al. [134], the antioxidant potential of ZnONPs was caused by the transfer of electron density from oxygen to the odd electron situated at the nitrogen atom, in DPPH (2,2-diphenyl-1-picrylhydrazyl), with decreased strength of the n–π* transition at 517 nm. Many plants-derived ZnONPs are studied for antioxidant potential, e.g., Umar et al. [135] showed the hydrogen peroxide scavenging activity of ZnONPs in Albizia lebbeck. Mahendiran et al. [136] showed the (2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) ABTS, 2,2-diphenyl-1-picrylhydrazyl (DPPH), superoxide and hydrogen peroxide scavenging activities of ZnONPs derived from Aloe vera and Hibiscus sabdariffa. In our previous investigation, we observed that Oscillatoria-derived ZnONPs had much higher antioxidant potential than commercially synthesized ZnONPs, with ABTS free radical scavenging activity ranging from 0 to 90% with an IC50 value of 54.2 µg ml−1 in comparison with ascorbic acid at 54.2 µg ml−1 that provides inhibition. It was observed that ZnONPs exhibited dose-dependent inhibition and the order of antioxidant activity was as follows: ABTS > DPPH > SOR > H2O2 [14]. Rehana et al. [137] also reported these scavenging activities in Azadirachta indica and Murraya koenigii. Hafez et al. [138] showed the in vivo antioxidant potential of ZnONPs (39.2 nm) supplemented with meal in broiler chickens at concentrations of 40 and 80 ppm that greatly boosted the activity of enzymes catalase and superoxide dismutase, which in turn decreased the levels of malondialdehyde (MDA).
The ZnONPs synthesized using Azadirachta indica leaf extract were investigated for their antioxidant potential by ferric reduction (FRAP) and DPPH radical scavenging assays. When ZnONPs (20 nm) were administered intravenously for 14 days at a dose of 100 ug, major bodily organs did not exhibit any discernible toxicity [139]. ZnONPs derived from Camellia sinensis leaf extract showed the antioxidant capacity in adipocytes, where ZnONPs exhibited cytoprotective efficacy against H2O2-induced oxidative damage (3T3L1) [140]. Japanese quails (Coturnix japonica) were fed dietary ZnONPs (40 nm) at a dose of 15–60 mg/kg for 60 days to examine its effects. It was observed that ZnONPs supplementation increased the cellular levels of GSH and mRNA levels of several antioxidant enzymes (SOD1, CAT, GPX1 and GPX7) in brain and liver tissues, which in turn reduced the lipid peroxidation. Aspartate aminotransferase and serum alanine aminotransferase activities, as well as the quantities of globulin, albumin and total proteins in the serum, were not significantly altered by the ZnONPs in comparison with the control group [141].
Anti-inflammatory activity
Inflammation is a part of body’s defense mechanism by which the immune system defends body from harmful agents like bacteria, viruses, injuries and toxins to heal itself. An over-reactive inflammatory response is what triggers allergies and some autoimmune disease like arthritis. It has been demonstrated that the ZnONPs have anti-inflammatory properties because they suppress the expression of myeloperoxidase, the NF-pathway, the release of pro-inflammatory cytokines and mast cell degranulation [142]. Ilves et al. [143] investigated that ZnONPs were able to reach into the deep layers of the allergic skin and exerted higher anti-inflammatory properties by decreasing drastically pro-inflammatory cytokines (IL-10, IL-13, IFN-c and Th2 cytokines) in the mouse model of AD. Nagajyothi et al. [144] synthesized ZnONPs using the root extract of P. tenuifolia that exhibited significant anti-inflammatory activity by suppressing nitric oxide (NO) production as well as the related protein expressions of iNOS, COX-2, IL-1β, IL-6 and TNF-α in LPS-stimulated RAW 264.7 macrophages. However, aluminum-doped ZnONPs have been demonstrated to lower mast cell caspase-1 activation and thymic stromal lymphopoietin (TSLP) secretion, which in turn reduces the expression of pro-inflammatory cytokines such IL-1, IL-6 and TNF-α [145]. The capping of flavones such as isoorientin, orientin, isovitexin and vitexin by ZnONPs has been found to have a potent anti-inflammatory response in a myriad of areas, including by inhibiting cyclooxygenase, phospholipase A2 and lipoxygenases (enzymes that produce eicosanoids), which causes a decrease in leukotriene and prostanoids [146]. ZnONPs synthesized using two mangrove plants, Heritiera fomes and Sonneratia apetala, showed higher potential for anti-inflammatory (79%) in comparison with silver nanoparticles (69.1%) [147].
Anti-diabetic activity
Persistent hyperglycemia is a hallmark of the physiological condition known as diabetes. It has been found that zinc plays a significant role in the creation, retention and release of insulin. Additionally, it enhances insulin signaling by enhancing PI3K activity, tyrosine phosphorylation of insulin receptor, and suppression of glycogen synthase kinase [148]. The antidiabetic activity of ZnONPs has been explored because zinc has important role in insulin synthesis, storage, and secretion through mechanisms which increased PI3K activity, insulin receptor tyrosine phosphorylation, and inhibition of glycogen synthase kinase [149]. When compared to other metal nanoparticles, ZnONPs are preferred for their antidiabetic benefits because of their higher cellular penetration, encouragement of glycolysis through hepatic glycogenesis, and increased insulin levels. These variables all work together to boost the expression of the GLUT-4 and INS genes. Additionally, it has synergistic effects on enhanced glucokinase expression and activity as well as IRA and GLUT-2 expression levels [150]. The potential benefits of ZnONPs (40 nm) were studied for 28 days at a dose of 10 mg/kg of ZnONPs orally in STZ-induced type-2 diabetic rats and observed dramatically reduced blood glucose levels while raising plasma insulin and pancreatic interleukin-10 (IL-10) levels. Additionally, ZnONPs reduced oxidative stress-related pancreatic damage by restoring the pancreas' total antioxidant capacity (TAC) and antioxidant defense system [151]. Similarly, in alloxan-induced diabetic rats, Bayrami et al. [152] investigated the anti-diabetic effects of biosynthesized ZnONPs with chemically synthesized ZnONPs utilizing Urtica dioica leaf extract. For 16 days, the diabetic rats were subcutaneously treated either with ZnONPs at a dose of 10 mg/dL or ZnO-extract at a dose of 8 mg/dL. By raising the level of insulin, biogenic ZnONPs exhibited superior antidiabetic potential in comparison with chemically synthesized and showed the reduced total cholesterol levels and elevated high-density lipoprotein cholesterol (HDLC) levels in diabetic rats. Wahba et al. [153] observed that ZnONPs effectively reversed diabetes-induced pancreatic injury by biochemical normalization of blood glucose and serum insulin. Further, El-Gharbawy et al. [154] and Kitture et al. [155] ZnONPs exhibited enhanced efficiency when tested in combination with the antidiabetic drugs, red sandalwood and vildagliptin. Furthermore, the α-amylase inhibitory activity of ZnONPs synthesized from floral extract of Senna auriculata [156] and leaf extract of Andrographis paniculata [157] was investigated for their antidiabetic potential that showed the lower IC50 value of 121.42 µg ml−1&149.65 µg ml−1. In addition, ZnONPs synthesized from M. fragrans and Withanias omnifera extracts were shown to have an excellent α-amylase (73%; 90%) and α-glucosidase (65%; 95%) inhibition, suggesting that they have potential antidiabetic action [79, 158].
Anticancer activity
Cancer is the deadliest disease that develops due to the uncontrolled growth of cells. By 2040, it is anticipated that there will be 29.5 million new instances of cancer diagnosed each year and 16.4 million cancer-related deaths [159]. Cancer persists despite long-run traditional therapeutic approaches and technological developments and has extensive drawbacks, e.g., reduced bioavailability, adverse health effects and high cost make their use limited [2]. It is well renowned that anticancer drugs disrupt the mitochondrial electron transport chain causing increased generation of ROS leading to the impaired mitochondrial and protein activity causing cell death [160]. Due to their capacity to release dissolved zinc ions into the cells, ZnONPs promote ROS generation and activate the apoptotic signaling pathway [161]. Zinc is crucial for the expression of the oncogene p53, which regulates apoptosis by controlling the activity of Caspase-6 enzyme [4]. In addition, the unique electrostatic feature of ZnONPs enables them for selective targeting of cancer cells. An abundance of anionic phospholipids causes electrostatic attraction between ZnONPs and cancer cells, which promotes cancer cells to take up ZnONPs causing cytotoxicity [162]. According to Sana et al. [74], small size of ZnONPs facilitates their permeation and retention inside tumor cells, where they can act. The potential mechanisms underlying the targeted cytotoxicity of ZnONPs against cancer cells are, when exposed to an alkaline intracellular condition, ZnONPs dissolve release Zn2+ ions leading to an increased generation of ROS in cancer cells compared to normal cells initiates the intrinsic mitochondrial apoptotic pathway causing cell death in cancer cells. The molecular mechanism underlying ZnONPs cytotoxicity comprises the generation of ROS to significantly increase oxidative stress, DNA damage and disruptions on cellular lipids and proteins are summarized in Fig. 4. Moghaddam et al. [61] synthesized ZnONPs using Pichia kudriavzevii GY1 that exhibited cytotoxicity against breast cancer MCF-7 cells by apoptosis. ZnONPs-induced apoptosis was mainly through extrinsic/intrinsic apoptotic pathways and down-regulation of antiapoptotic genes of Bcl-2, AKT1, and JERK/2 with up-regulation of proapoptotic genes of p21, p53, JNK, and Bax. ZnONPs synthesized from leaf extract of Raphanus sativus exhibited the higher anticancer potential on treated A549 cell lines [163]. It has been extremely difficult for a drug to be classified as anticancer without being able to distinguish between cancerous and normal cells, as lack of selectivity can cause harmful effects.
Mechanism of action of ZnONPs; a reactive oxygen species (ROS) generation induced by ZnONPs causes DNA damage, apoptogenic substances get released from mitochondrial membrane forming the apoptosomes leading to apoptosis; b at lower pH protons from local environment moved to the surface of negatively charged ZnONPs creating a positively charged surface (ZnOH2+), that enters the cell due to their interaction with negative phospholipids on the cell's outer membrane and after dissolving in acidic lysosomes, ZnONPs release Zn+, that inhibits the respiratory enzymes leading to cell death
Numerous studies have shown ZnONPs preference toward malignant cells. According to Chandrasekaran et al. [164], ZnONPs selectively induce apoptosis in C2C12 myoblastoma cancer cell with increased caspase-3 (CASP3) enzyme activity and ROS generation in comparison with 3T3-L1 adipocytes. In another study, ZnONPs (47.2 nm) synthesized from aqueous leaf extract of Laurus nobilis exhibited the cytotoxicity against human A549 lung cancer cells at concentrations of 80 μg ml−1 and showed no effect on normal murine RAW264.7 macrophage cells [165]. Along this line, Wahab et al. [166] showed that ZnONPs were most toxic against T98G cancer cells, moderately toxic against KB epithermoids cells and least effective against normal human HEK cells.
Even though many of treatments have a poor therapeutic index, several routinely given medications can slow down the rate at which cells divide. Sharma et al. [167] and Hackenberg et al. [168] showed that the cytotoxicity of chemotherapeutic agents like doxorubicin (DOX), cisplatin (CPT) and paclitaxel (PTX) increased significantly in combination with ZnONPs. ZnONPs in combination with sorafenib exhibited significant anticancer activity against Ehrlich carcinoma cells in mice with an effective decrease in tumor weight/tumor cell viability and a significant increase in DNA fragmentation, generation of reactive oxygen species and expression of the apoptotic gene caspase-3 in the tumor tissues [169]. ZnONPs conjugated with L-asparaginase from Aspergillus terreus exhibited significant anticancer activity against MCF-7 cell line that decreased 35% viability of cancer cells [170].
Wound healing activity
The largest organ in the body, the skin, shields us from outside threats and develops wounds when damaged. Healing from a wound takes patience, and microbial infection (P. aeruginosa and S. aureus) may slow down the process [171]. Metal oxide nanoparticles have the potential to get rid of pathogenic species and hasten wound healing. Biogenic ZnONPs showed huge potential as a wound-healing agent for the treatment of normal injuries and open wounds [172]. Owing to the strong antimicrobial properties, ZnONPs have been successfully used in wound dressings. ZnONPs synthesized using Aloe barbadensis leaf extract showed the healing efficiency of ZnONPs/silica gel dressings (30 ppm) in mouse wounds within 11 days in comparison with control [173]. Biosynthesized ZnONPs using Prosophisfracta and coffee can penetrate cotton wound bandages, resulting in patches with a potent antibacterial action. As a result, they may be used to treat and cover wounds that are prone to infection, like burns or diabetic sores [174]. According to Raguvaran et al. [175], ZnONPs-loaded sodium alginate-gum “Acacia hydrogels” (SAGA-ZnONPs) showed the wound healing effects in sheep fibroblast cells. Rayyif et al. [176] demonstrated the fabrication of nano-coated wound dressing containing ZnONPs that impair bacteria viability of chronic wounds after 6 h of contact in a dose-dependent manner and maintained for up to 3 days. Karahaliloglu et al. [177] combined chitosan/ silk sericin scaffolds with lauric acid and ZnONPs. The diameter of zone of inhibition increased from 2 to 7 mm for E. coli, and 2.5 to 6 mm for S. aureus after treatment of ZnONPs. According to Soubhagya et al. [178], bio-nanocomposite-based 3D chitosan/pectin/ZnONPs porous films showed nil cytotoxicity, cell growth and migration, or adverse effects on primary human dermal fibroblast cells (HFCs), referring to a safe biomaterial for accelerating wound healing. In addition, azithromycin-doped ZnONPs impregnated into an HPMC gel by Saddik et al. [179] exhibited the improved bacterial eradication and epidermal repair, which in turn stimulated tissue development and expedited the healing of the infected lesion. Manuja et al. [180] demonstrated similar potential for accelerating healing by ZnONPs hydrogels synthesized from sodium alginate gum acacia that left no scar at the rabbit skin excision wound.
Antiviral activity
Numerous viruses, including hepatitis C and E virus (HCV, HEV), herpes simplex virus (HSV), human papillomavirus (HPV), human immunodeficiency virus (HIV) and severe acute respiratory syndrome coronavirus (SARS-CoV), have been shown to be significantly inhibited by ZnONPs. The antiviral evaluation of ZnONPs synthesized from Plumbago indica leaf extract revealed that they showed remarkable activity against herpes simplex virus type 1 (HSV-1), with CC50 and IC50 values of 43.9 and 23 µg ml−1, respectively, in comparison with acyclovir, which at 1 µg ml−1 offered 100% complete immunity against HSV-1 [181]. A study by Ghaffari et al. [182] reported that PEGylated ZnONPs and ZnONPs exhibited higher inhibition rates of 94.6% and 52.2%, against H1N1 influenza virus. It caused a remarkable reduction in fluorescence emission intensity in PEGylated ZnONPs-treated cells with lower cytotoxicity on MDCK-SIAT1 cells and concluded that PEGylated ZnONPs may be an efficient and promising antiviral treatment for H1N1 influenza virus infection. According to TeVelthuis et al. [183] and Erk et al. [184], the toll-like receptor signaling pathways and proteins downstreaming are the action mechanisms underpinning the antiviral efficacy of ZnONPs, which in turn promote the innate and adaptive immune response leading to the generation of pro-inflammatory cytokines that impede the virus. Zn2+ ions have antiviral capabilities by inhibiting virus proliferation, packaging and expulsion during its life cycle, causing reactive oxygen species generation, and preventing infection. In addition to blocking viral RNA-dependent RNA polymerase activity, zinc also alters the host immune response to prevent viral invasion and inhibits viral replication and the translation of viral polyproteins [183]. However, ZnONPs have the ability to dissolve water molecules, release Zn2+ ions and absorb UV–Vis light, producing ROS like H2O2 and OH− free radicals [185, 186]. ZnONPs have been shown to inhibit both SARS-COV and retrovirus in vitro RNA polymerase activity, and zinc ions inhibit virus replication in cell culture [183]. The molecular mechanism underlying ZnONPs toxicity against SARS-CoV-2 virus involves the generation of ROS that significantly increase oxidative stress; RNA degradation and disruptions on cellular proteolysis are summarized in Fig. 5. ZnONPs impede SARS-CoV-2 RNA-dependent RNA polymerase (RdRp) via altering template binding during the elongation stage of RNA translation. Furthermore, ZnONPs inhibit membrane fusion by interacting with the histidine residue of viral E1 protein, which obstructs the viral polyprotein proteolytic mechanism in turn inhibiting the viral integration [187]. Collectively, ZnONPs serve as a prospective candidate for their use in nanomedical viral-targeting therapy for the SARS-COV virus owing to its inertness to an array of medicines and persistent visible fluorescence. Recently, El-Megharbel et al. [188] observed that ZnONPs had remarkable antiviral activity against SARS-CoV-2 with an IC50 value of 526 ng ml−1and cytotoxic levels with a CC50 value of 292.2 ng ml−1 against VERO-E6 cells. Additionally, it was found that ZnONPs-enhanced generation of ROS causes damage to the SARS-CoV-2 membrane proteins. Jana et al. [189] showed that polysaccharide-encapsulated ZnONPs exhibited remarkable antiviral activity against human cytomegalovirus (HCMV), with cell survival rates of 93.6% and 92.4% at 400 µg ml−1. Gupta et al. [190] investigated the antiviral efficacy of both ZnONPs and tetrapod-shaped ZnO(TP) against hepatitis E and hepatitis C viruses. Both showed significant antiviral activity, but ZnO(TP) were more effective and showed no cytotoxicity even at higher doses as compared to control.
Application of ZnONPs in drug delivery
Drug delivery using ZnONPs has emerged as an incredibly efficient approach for treating various illnesses, such as cancers. Because of their cheap fabrication from inexpensive metal precursors, biocompatibility and efficient cellular absorption via endosomes, ZnONPs have been suggested as a feasible prospect for targeted drug delivery. The pH-dependent release of the targeted drug and ZnONPs into the cytoplasm that occurs through receptor-mediated endocytosis is the underpinning mechanism behind the increased cytotoxic potential of anticancer drug-loaded ZnONPs [191]. Additionally, the excessive generation of ROS and Zn2+ ions from ZnONPs results in the apoptosis of cancer cells [2]. Biologically synthesized doxorubicin-loaded ZnONPs using Borassus flabellifer extract exhibited dose-dependent cytotoxicity against human breast cancer (MCF-7) and colon cancer (HT-29) cell lines with an IC50 value of 0.125 µg ml−1and displayed low cytotoxicity in the murine model system, according to the in vivo toxicity evaluation [192]. Using chitosan-coated ZnONPs synthesized from ethanolic leaf extract of Camellia sinensis, Akbarian et al. [191] fortunately loaded paclitaxel (PTX) and observed the enhanced cytotoxicity against malignant MCF-7 cell lines without being nontoxic on healthy fibroblast cell lines. According to Yuan et al. [193], ZnO quantum dots loaded with chitosan were used to deliver anticancer drug doxorubicin to HeLa cells.
Applications of ZnONPs in bioimaging
The inherent photoluminescence properties of ZnONPs make them a suitable candidate for biosensing applications. Their chemical structure consists of hydroxyl (− OH) groups, which makes it easier to dissolve in a basic and acidic environment especially in a tumor microenvironment for efficient imaging [4]. Jiang et al. [28] used ZnO nanosheets for imaging the leukemia K562 cells and observed the clear yellow orange light emission around or inside the cells under UV excitation, suggesting the successful penetration of the cells by these ZnO nanosheets. Pan et al. [194] used photoluminescent ZnO@polymer core–shell NPs for mouse imaging via intradermal and intravenous injections. According to Sudhagar et al. [195], green, fluorescent ZnONPs conjugated with transferrin were involved in cancer cell imaging with lower cytotoxicity. Singh et al. [196] doped zinc oxide quantum dots (QDs) with Gd and observed an increase in the emission intensity and lower toxicity to HeLa cancer cells, imaged with confocal microscopy. Furthermore, many studies had reported the use of ZnONPs in bioimaging like., use of ZnONPs for skin tissue architecture [197], ZnO nanocrystals in KB cells [198], CdSe(S)/ZnO-QDs in S. oneidensis [199], ZnONPs in human skin and rat liver cells [200], ZnONPs in plant tissue cell implosion [201] and ZnONPs in blood cells of zebrafish and roots/shoots of Arabidopsis [202].
Application of ZnONPs in tissue engineering
Due to their antineoplastic, angiogenic, UV scattering, antioxidant, collagen production, bio-mineralization and wound-healing characteristics, ZnONPs have become alternative biomaterial for tissue engineering and regenerative medicine applications [203]. They can aid in promoting tissue repair while lowering immunogenicity and preventing illness. For tissue engineering and regenerative medicine applications, ZnONPs are reported to stimulate cell growth, proliferation, transformation and metabolic functions in a numerous cell line [204]. As evidenced by in vitro and in vivo approach, the application of biogenic ZnONPs in tissue engineering has grown even more specific due to its proangiogenic features that may be incredibly beneficial in improving the integration of sophisticated biomaterials into host tissue. Yousefi et al. [205] investigated the ZnONPs/chitosan tubular scaffold for tendon restoration in a rabbit model and observed complete absorption of scaffold at repair site after eight weeks of treatment. It also prevented the formation of adhesions and infections around the tendon with enhanced angiogenesis and collagen fibril rearrangement, pointing to its promising use in the treatment of tendon acute injuries. Shubha et al. [206] showed that biosynthesized ZnONPs using gallic acid isolated from the aqueous extract of Phyllanthus emblica exhibited less toxicity than clinically recommended ZnONPs and observed noticeable results in 3T3 fibroblasts from Balb mice, which suggested its use near connective tissue cells because they are benign to cells. Using the stem extract Artemisia annua-derived ZnONPs, Wang et al. [207] showed the impact of ZnONPs on bone regeneration inMG-63 Cells. Without significantly increasing cytotoxicity, biogenic ZnONPs demonstrated improved osteoblast proliferation, differentiation, collagen production and calcium mineralization. According to a study by Shafique et al. [208], Cymbopogon citratus leaf extract derived ZnONPs exhibited the promising callogenesis and regeneration frequency in Panicum virgatum nodes and internodes. It was confidently expected that this exploration would advance tissue culture technology by increasing the rate of plant in vitro regeneration in various species. Heidari et al. [209] and Harikrishnan and Sivasamy [210] reported the efficacy of nanohydroxyapatite/ZnO (HA/ZnO) scaffolds and polycaprolactone/ZnO(PCL-ZnO) scaffolds for bone tissue regeneration in human osteoblast cells. Improved biocompatibility and biosorption properties were demonstrated by the HA/ZnO scaffold, whereas PCL/ZnO scaffolds offer a nano-porous matrix for improved cell adherence and higher cell proliferation. Furthermore, Forero et al. [211] showed the potential efficacy of nano-copper-zinc alloy (nCuZn) containing chitosan/gelatin/nano-hydroxyapatite (Ch/G/nHAp) scaffold in bone tissue regeneration and observed scaffolds-induced osteogenesis and increased mouse embryonic fibroblast proliferation and adhesion (MEFs). The scaffold promoted the growth of the surrounding tissues after in vivo implant, encouraging the formation of granulation tissue.
Future perspectives
ZnONPs demonstrate superior attributes to bulk materials because of its small size and high surface area-to-volume ratio, due to which they are being investigated in a variety of disciplines, including the biosensor, agriculture, cosmetic and food industries. Biologically synthesized ZnONPs have gained significant attention due to their eco-friendly, simple, and economical nature, which allows producing NPs on a big scale. Biomolecules present in cell extract facilitates the reduction and stabilization of synthesized ZnONPs and exhibited enhanced antimicrobial, anticancer, anti-inflammatory, drug delivery, bioimaging along with other biomedical applications. The capacity of the ZnONPs to increase the bioavailability of therapeutic drugs while acting as drug carriers to increase therapeutic efficacy has also long been acknowledged. Many critical variables must be dealt with in the future for the reliable and efficient production of ZnONPs for innovative applications. Modulation of various variables such as pH, temperature, salt precursor and extract concentration should be explored for efficient regulation of particle size distribution and morphology for economically feasible ZnONPs fabrication on a large scale in order to meet future demands. With the growing prevalence of ZnONPs, researchers should examine their buildup in the surroundings and possible long-term impacts on humans and animals. Furthermore, ZnONPs may be modified by biomolecules such as polysaccharides and proteins to boost persistence and their biocompatibility as these organic compounds have properties similar to human tissue.
Conclusion
The ZnONPs are promising candidates for biological applications due to their intrinsic toxicity of ROS generation, activating apoptotic signals within the cells to hinder both microbial pathogens and malignant cells. The present review highlighted the green synthesis of ZnONPs, to get over the limits of traditional chemical and physical techniques and its use in various biomedical applications. It emphasizes how the use of biogenic ZnONPs in drug administration, nanomedicine and cure can open up new avenues for giving more customized, safer and efficient therapeutic options for tumors and HIV/AIDS, as well as noninvasive diagnostics and nutraceutical delivery. Researchers will eventually be able to deliver medications for a greater duration of time with enhanced accuracy and penetration by manipulating size of NPs and surface characteristics. Furthermore, in vitro and in vivo studies are anticipated to explain the cellular level mechanism of action, with significance in numerous biomedical diagnostics and therapeutic disciplines. As a result, rapid progress in the application of biologically synthesized ZnONPs is expected in the future decades.
References
Singh G, Babele PK, Kumar A, Srivastava A, Sinha RP, Tyagi MB (2014) Synthesis of ZnO nanoparticles using the cell extract of the cyanobacterium, Anabaena strain L31 and its conjugation with UV-B absorbing compound shinorine. J Photochem Photobiol B Biol 138:55–62
Murali M, Kalegowda N, Gowtham HG, Ansari MA, Alomary MN et al (2021) Plant-mediated zinc oxide nanoparticles: advances in the new millennium towards understanding their therapeutic role in biomedical applications. Pharmaceutics 13(10):1662
Murali M, Mahendra C, Nagabhushan, Rajashekar N, Sudarshana MS, Raveesha KA, Amruthesh KN (2017) Antibacterial and antioxidant properties of biosynthesized zinc oxide nanoparticles from Ceropegia candelabrum L.—An endemic species. Spectrochim Acta A Mol Biomol Spectrosc 179:104–109
Mishra P, Mishra H, Ekielski A, Talegaonkar S et al (2017) Zinc oxide nanoparticles: a promising nanomaterial for biomedical applications. Drug Discov Today 22(12):1825–1834
Yu Z, Li Q, Wang J, Yu Y, Wang Y, Zhou Q, Li P (2020) Reactive oxygen species-related nanoparticle toxicity in the biomedical field. Nanoscale Res Lett 15:115
Khan AU, Khan M, Cho MH et al (2020) Selected nanotechnologies and nanostructures for drug delivery, nanomedicine and cure. Bioprocess Biosyst Eng 43:1339–1357
Afzal B, Yasin D, Husain S, Zaki A, Srivastava P, Kumar R, Fatma T (2019) Screening of cyanobacterial strains for the selenium nanoparticles synthesis and their antioxidant activity. Biocatal Agric Biotechnol 21:101307
Afzal B, Yasin D, Naaz H et al (2021) Biomedical potential of Anabaena variabilis NCCU-441 based selenium nanoparticles and their comparison with commercial nanoparticles. Sci Rep 11:13507
Husain S, Sardar M, Fatma T (2015) Screening of cyanobacterial extracts for synthesis of silver nanoparticles. World J Microbiol Biotechnol. https://doi.org/10.1007/s11274-015-1869-3
Husain S, Afreen A, Yasin D, Hemlata, Afzal B, Fatma T (2019) Cyanobacteria as a bioreactor for synthesis of silver nanoparticles-an effect of different reaction conditions on the size of nanoparticles and their dye decolorization ability. J Microbiol Methods 162:77–82
Husain S, Verma SK, Yasin D, Hemlata A, Rizvi MM, Fatma T (2021) Facile green bio-fabricated silver nanoparticles from Microchaete infer dose-dependent antioxidant and anti-proliferative activity to mediate cellular apoptosis. Bioorg Chem 107:104535
Zaki A, Aziz MN, Ahmad R, Ahamad I, Ali MS (2022) Synthesis, purification and characterization of Plectonema derived AgNPs with elucidation of the role of protein in nanoparticle stabilization. RSC Adv 12:2497–2510
Siddiqui T, Khan NJ, Asif N, Ahamad I, Yasin D, Fatma T (2022) Screening, characterisation and bioactivities of green fabricated TiO2 NP via cyanobacterial extract. Environ Sci Pollut Res Int 29(26):39052–39066
Asif N, Fatima S, Aziz MA, Shehzadi ZA, Fatma T (2021) Biofabrication and characterization of cyanobacteria derived ZnO NPs for their bioactivity comparison with commercial chemically synthesized nanoparticles. Bioorg Chem 113:104999
Asif N, Fatima S, Siddiqui T, Fatma T (2022) Investigation of morphological and biochemical changes of zinc oxide nanoparticles induced toxicity against multi drug resistance bacteria. J Trace Elem Med Biol 74:127069
Singh TA, Das J, Sil PC (2020) Zinc oxide nanoparticles: a comprehensive review on its synthesis, anticancer and drug delivery applications as well as health risks. Adv Colloid Interf Sci 286:102317
Shaba EY, Jacob JO, Tijani JO, Suleiman MAT (2021) A critical review of synthesis parameters affecting the properties of zinc oxide nanoparticle and its application in wastewater treatment. Appl Water Sci 11:48
Kielbik P, Kaszewski J, Rosowska J, Wolska E, Witkowski B, Gralak M, Gajewski Z, Godlewski M, Godlewski MM (2017) Biodegradation of the ZnO: Eu nanoparticles in the tissues of adult mouse after alimentary application. Nanomed Nanotechnol Biol Med 13:843–852
Kalpana VN, Rajeswari VD (2018) A review on green synthesis, biomedical applications, and toxicity studies of ZnO NPs. Bioinorg Chem Appl 2018:3569758
Souza VGL, Rodrigues C, Valente S, Pimenta C, Pires JRA, Alves MM, Santos CF, Coelhoso IM, Fernando AL (2020) Eco-Friendly ZnO/Chitosan bionanocomposites films for packaging of fresh poultry meat. Coatings 10:110
Iqbal J, Abbasi BA, Mahmood T, Kanwal S, Ahmad R, Ashraf M (2019) Plant extract mediated green approach for the synthesis of ZnONPs: characterization and evaluation of cytotoxic, antimicrobial and antioxidant potentials. J Mol Struct 1189:315–327
Khatami M, Alijani HQ, Heli H, Sharifi I (2018) Rectangular shaped zinc oxide nanoparticles: green synthesis by Stevia and its biomedical efficiency. Ceram Int 44:15596–15602
Puspasari V, Ridhova A, Hermawan A et al (2022) ZnO-based antimicrobial coatings for biomedical applications. Bioprocess Biosyst Eng 45:1421–1445
Rahman A, Harunsani MH, Tan AL, Ahmad N, Min B, Khan MM (2021) Influence of Mg and Cu dual-doping on phytogenic synthesized ZnO for light induced antibacterial and radical scavenging activities. Mater Sci Semicond Process 128:105761
Rahman A, Harunsani MH, Tan AL et al (2021) Effect of Mg doping on ZnO fabricated using aqueous leaf extract of Ziziphus mauritiana Lam. for antioxidant and antibacterial studies. Bioprocess Biosyst Eng 44:875–889
Ahmad M, Rehman W, Khan MM, Qureshi MT, Gul A, Haq S, Ullah R, Rab A, Menaa F (2021) Phytogenic fabrication of ZnO and gold decorated ZnO nanoparticles for photocatalytic degradation of Rhodamine B. J Environ Chem Eng 9(1):104725
Rahman A, Harunsani MH, Tan AL et al (2021) Antioxidant and antibacterial studies of phytogenic fabricated ZnO using aqueous leaf extract of Ziziphus mauritiana Lam. Chem Pap 75:3295–3308
Jiang H et al (2011) Facile and mild preparation of fluorescent ZnO nanosheets and their bioimaging applications. Appl Surface Sci 257:6991–6995
Malfatti L, Pinna A, Enzo S, Falcaro P, Marmiroli B, Innocenzi P (2015) Tuning the phase transition of ZnO thin films through lithography: An integrated bottom-up and top-down processing. J Synchrotron Radiat 22:165–171
Krupinski P, Kornowicz A, Sokołowski K, Cie’slak AM, Lewinski J (2016) Applying mechanochemistry forbottom-up synthesis and host–guest surface modification of semiconducting nanocrystals: a case ofwater-soluble cyclodextrin-coated zinc oxide. Chem Eur J 22:7817–7823
Mandal AK, Katuwal S, Tettey F, Gupta A, Bhattarai S, Jaisi S, Bhandari DP, Shah AK, Bhattarai N, Parajuli N (2022) Current research on zinc oxide nanoparticles: synthesis, characterization, and biomedical applications. Nanomaterials 12(17):3066
Azizi S, Ahmad MB, Namvar F, Mohamad R (2014) Green biosynthesis and characterization of zinc oxide nanoparticles usingbrown marine macroalga Sargassum muticum aqueous extract. Mater Lett 116:275–277
Lu R, Yang D, Cui D, Wang Z, Guo L (2012) Egg white-mediated green synthesis of silver nanoparticles with excellent biocompatibility and enhanced radiation effects on cancer cells. Int J Nanomed 7:2101–2107
Rahman A, Harunsani MH, Tan AL et al (2021) Zinc oxide and zinc oxide-based nanostructures: biogenic and phytogenic synthesis, properties and applications. Bioprocess Biosyst Eng 44:1333–1372
Mohd Yusof H, Mohamad R, Zaidan UH, Abdul Rahman NA (2019) Microbial synthesis of zinc oxide nanoparticles and their potential application as an antimicrobial agent and a feed supplement in animal industry: a review. J Anim Sci Biotechnol 10:1–22
Saravanan M, Gopinath V, Chaurasia MK, Syed A, Ameen F, Purushothaman N (2018) Green synthesis of anisotropic zinc oxide nanoparticles with antibacterial and cytofriendly properties. MicrobPathog 115:57–63
Taran M, Rad M, Alavi M (2018) Biosynthesis of TiO2and ZnO nanoparticles by Halomonas elongata IBRC-M 10214 in different conditions of medium. BioImpacts 8:81–89
Al-Zahrani H, El-Waseif A, El-Ghwas D (2018) Biosynthesis and evaluation of TiO2 and ZnO nanoparticles from in vitro stimulation of Lactobacillus johnsonii. J Innov Pharm Biol Sci 5:6–20
Król A, Railean-Plugaru V, Pomastowski P, Złoch M, Buszewski B (2018) Mechanism study of intracellular zinc oxide nanocomposites formation. Colloids Surf A Physicochem Eng Asp 553:349–358
Rajabairavi N, Raju CS, Karthikeyan C, Varutharaju K, Nethaji S, Hameed ASH et al (2017) Biosynthesis of novel zinc oxide nanoparticles (ZnONPs) using endophytic bacteria Sphingobacterium thalpophilum. Springer Proc Phys 189:245–254
Rauf MA, Owais M, Rajpoot R, Ahmad F, Khan N, Zubair S (2017) Biomimetically synthesized ZnO nanoparticles attain potent antibacterial activity against less susceptible: S. aureus skin infection in experimental animals. RSC Adv 7:36361–36373
Balraj B, Senthilkumar N, Siva C, Krithikadevi R, Julie A, Potheher IV et al (2017) Synthesis and characterization of zinc oxide nanoparticles using marine Streptomyces sp. with its investigations on anticancer and antibacterial activity. Res Chem Intermed 3:2367–2376
Busi S, Rajkumari J, Pattnaik S, Parasuraman P, Hnamte S (2016) Extracellular synthesis of zinc oxide nanoparticles using Acinetobacter schindleri SIZ7 and its antimicrobial property against foodborne pathogens. J Microbiol Biotechnol Food Sci 5:407–411
Tripathi RM, Bhadwal AS, Gupta RK, Singh P, Shrivastav A, Shrivastav BR (2014) ZnO nanoflowers: novel biogenic synthesis and enhanced photocatalytic activity. J PhotochemPhotobiol B Biol 141:288–295
Singh BN, Rawat AKS, Khan W, Naqvi AH, Singh BR (2014) Biosynthesis of stable antioxidant ZnO nanoparticles by Pseudomonas aeruginosa rhamnolipids. PLoS One 9:e106937
Dhadapani P, Siddarth AS, Kamalasekaran S, Maruthamuthu S, Rajagopal G (2014) Bio-approach: ureolytic bacteria mediated synthesis of ZnO nanocrystals on cotton fabric and evaluation of their antibacterial properties. Carbohydr Polym 103:448–455
Selvarajan E, Mohanasrinivasan V (2013) Biosynthesis and characterization of ZnO nanoparticles using lactobacillus plantarum VITES07. Mater Lett 112:180–182
Mishra M, Paliwal JS, Singh SK, Selvarajan E, Subathradevi C, Mohanasrinivasan V (2013) Studies on the inhibitory activity of biologically synthesized and characterized zinc oxide nanoparticles using lactobacillus sporogens against Staphylococcus aureus. J Pure Appl Microbiol 7:1263–1268
Jayaseelan C, Rahuman AA, Kirthi AV et al (2012) Novel microbial route to synthesize ZnO nanoparticles using Aeromonas hydrophila and their activity against pathogenic bacteria and fungi. Spectrochim Acta A Mol Biomol Spectrosc 90:78–84
Barani M, Masoudi M, Mashreghi M, Makhdoumi A, Eshghi H (2021) Cell-free extract assisted synthesis of ZnO nanoparticles using aquatic bacterial strains: biological activities and toxicological evaluation. Int J Pharm 606:120878
Shamim A, Abid MA, Tariq MT (2019) Biogenic synthesis of zinc oxide (ZnO) nanoparticles using a fungus (Aspargillusniger) and their characterization. Int J Chem 11:2
Sharma JL, Dhayal V, Sharma RK (2021) White-rot fungus mediated green synthesis of zinc oxide nanoparticles and their impregnation on cellulose to develop environmental friendly antimicrobial fibers. 3 Biotech 11(6):269
Kumar RV, Vinoth S, Baskar V, Arun M, Gurusaravanan P (2022) Synthesis of zinc oxide nanoparticles mediated by Dictyotadichotoma endophytic fungi and its photocatalytic degradation of fast green dye and antibacterial applications. S Afr J Bot 151:337–344
Shamsuzzaman MA, Khanam H, Aljawfi RN (2017) Biological synthesis of ZnO nanoparticles using C. albicans and studying their catalytic performance in the synthesis of steroidal pyrazolines. Arab J Chem 10:S1530–S1536
Rajan A, Cherian E, Baskar G (2016) Biosynthesis of zinc oxide nanoparticles using Aspergillus fumigatus JCF and its antibacterial activity. Int J Mod Sci Technol 1:52–57
Sarkar J, Ghosh M, Mukherjee A, Chattopadhyay D, Acharya K (2014) Biosynthesis and safety evaluation of ZnO nanoparticles. Bioprocess Biosyst Eng 37:165–171
Sumanth B, Lakshmeesha TR, Ansari MA, Alzohairy MA, Udayashankar AC, Shobha B, Niranjana SR, Srinivas C, Almatroudi A (2020) Mycogenic synthesis of extracellular zinc oxide nanoparticles from Xylaria acuta and its nanoantibiotic potential. Int J Nanomed 2(15):8519–8536
Raliya R, Tarafdar JC (2013) ZnO nanoparticle biosynthesis and its effect on phosphorous-mobilizing enzyme secretion and gum contents in cluster bean (Cyamopsis tetragonoloba L.). Agric Res 2:48–57
Baskar G, Chandhuru J, Fahad KS, Praveen AS (2013) Mycological synthesis, characterization and antifungal activity of zinc oxide nanoparticles. Asian J Pharm Technol 3:142–146
Velmurugan P, Shim J, You Y, Choi S, Kamala-Kannan S, Lee KJ et al (2010) Removal of zinc by live, dead, and dried biomass of Fusarium spp. isolated from the abandoned-metal mine in South Korea and its perspective of producing nanocrystals. J Hazard Mater 182:317–324
Moghaddam AB, Moniri M, Azizi S, Rahim RA, Ariff AB, Saad WZ et al (2017) Biosynthesis of ZnO nanoparticles by a new Pichia kudriavzevii yeast strain and evaluation of their antimicrobial and antioxidant activities. Molecules 22:1–18
Chauhan R, Reddy A, Abraham J (2015) Biosynthesis of silver and zinc oxide nanoparticles using Pichia fermentans JA2 and their antimicrobial property. Appl Nanosci 5:63–71
Selim YA, Azb MA, Ragab I, El-Azim MH (2020) Green synthesis of zinc oxide nanoparticles using aqueous extract of Deverratortuosa and their cytotoxic activities. Scientific Rep 10:3445
Vinayagama R, Selvaraja R, Arivalagan P, Varadavenkatesan T (2020) Synthesis, characterization and photocatalytic dye degradation capability of Calliandrahaematocephala-mediated zinc oxide nanoflowers. J Photochem Photobiol B Biol 203:111760
Naseer M, Aslam U, Khalid B, Chen B (2020) Green route to synthesize Zinc Oxide Nanoparticles using leaf extracts of Cassia fistula and Melia azadarach and their antibacterial potential. Sci Rep 10:9055
Salem MSE-D, Mahfouz AY, Fathy RM (2020) The antibacterial and antihemolytic activities assessment of zinc oxide nanoparticles synthesized using plant extracts and gamma irradiation against the uro-pathogenic multidrug resistant Proteus vulgaris, Biometals Int. J Role Met Ions Biol Biochem Med 34:175–196
Sathish KG, Rajkuberan C, Manikandan K, Prabukumar S et al (2017) Facile biosynthesis of antimicrobial zinc oxide (ZnO) nanoflakes using leaf extract of Couroupitaguianensis. Aubl. Mater Lett 188:383–386
Dhandapani KV, Anbumani D, Gandhi AD, Annamalai P et al (2020) Green route for the synthesis of zinc oxide nanoparticles from Melia azedarach leaf extract and evaluation of their antioxidant and antibacterial activities. Biocatal Agric Biotechnol 24:101517
Colak H, Karaköse E (2017) Green synthesis and characterization of nanostructured ZnO thin films using Citrus aurantifolia (lemon) peel extract by spin-coating method. J Alloys Compd 690:658–662
Malaikozhundan B, Vaseeharan B, Vijayakumar S et al (2017) Biological therapeutics of Pongamia pinnata coated zinc oxide nanoparticles against clinically important pathogenic bacteria, fungi and MCF-7 breast cancer cells. Microb Pathog 104:268–277
Jafarirad S, Mehrabi M, Divband B, Kosari-Nasab M (2016) Biofabrication of zinc oxide nanoparticles using fruit extract of Rosa canina and their toxic potential against bacteria: a mechanistic approach. Mater Sci Eng C 59:296–302
Sutradhar P, Saha M (2017) Green synthesis of zinc oxide nanoparticles using tomato (Lycopersicon esculentum) extract and its photovoltaic application. J Exp Nanosci 11:314–327
Singh AK, Pal P, Gupta V, Yadav TP, Gupta V, Singh SP (2018) Green synthesis, characterization and antimicrobial activity of zinc oxide quantum dots using Eclipta alba. Mater Chem Phys 203:40–48
Sana SS, Kumbhakar DV, Pasha A, Pawar SC, Grace AN, Singh RP, Nguyen VH, Le QV, Peng W (2020) Crotalaria verrucosa leaf extract mediated synthesis of Zinc oxide nanoparticles: assessment of antimicrobial and anticancer activity. Molecules 25:4896
Shahriyari Rad S, Sani AM, Mohseni S (2019) Biosynthesis, characterization and antimicrobial activities of zinc oxide nanoparticles from leaf extract of Mentha pulegium (L.). Microb Pathog 131:239–245
Matinise N, Fuku XG, Kaviyarasu K, Mayedwa N, Maaza M (2017) ZnO nanoparticles via Moringa oleifera green synthesis: physical properties & mechanism of formation. Appl Surf Sci 406:339–347
Sorbiun M, ShayeganMehr E, Ramazani A, TaghaviFardood S (2018) Green synthesis of zinc oxide and copper oxide nanoparticles using aqueous extract of oak fruit hull (jaft) and comparing their photocatalytic degradation of basic violet 3. Int J Environ Res 12:29–37
Singh K, Singh J, Rawat M (2019) Green synthesis of zinc oxide nanoparticles using Punica Granatum leaf extract and its application towards photocatalytic degradation of Coomassie brilliant blue R-250 dye. SN Appl Sci 1:1–8
Faisal S, Jan H, Shah SA, Shah S, Khan A, Akbar MT, Rizwan M, Jan F, Wajidullah, Akhtar N et al (2021) Green synthesis of Zinc oxide (ZnO) nanoparticles using aqueous fruit extracts of Myristica fragrans: Their characterizations and biological and environmental applications. ACS Omega 6:9709–9722
Kumar HKN, Mohana NC, Nuthan BR, Rameshal KP et al (2019) Phyto-mediated synthesis of zinc oxide nanoparticles using aqueous plant extract of Ocimum americanum and evaluation of its bioactivity. SN Appl Sci 1:651
Gharagozlou M, Baradaran Z, Bayati R (2015) A green chemical method for synthesis of ZnO nanoparticles from solid-state decomposition of Schiff-bases derived from amino acid alanine complexes. Ceram Int 41:8382–8387
Mukhtar SS, Hassan AS, Morsy NM, Hafez TS, Hassaneen HM, Saleh FM (2021) Overview on synthesis, reactions, applications, and biological activities of schiff bases. Egypt J Chem 64:6541–6554
Ambika S, Sundrarajan M (2015) Green biosynthesis of ZnO nanoparticles using Vitex negundo L. extract: spectroscopic investigation of interaction between ZnO nanoparticles and human serum albumin. J Photochem Photobiol B Biol 149:143–148
Anita S, Ramachandran T, Koushik CV, Rajendran R, Mahalakshmi M (2010) Preparation and characterization of zinc oxide nanoparticles and a study of the anti-microbial property of cotton fabric treated with the particles. JTATM 6:1–2
Shehabeldine AM, Hashem AH, Wassel AR, Hasanin M (2021) Antimicrobial and antiviral activities of durable cotton fabric treated with nanocomposite based on zinc oxide nanoparticles, acyclovir, nanochitosan, and clove oil. Appl Biochem Biotechnol 194:783–800
Nie L, Gao L, Feng P, Zhang J, Fu X, Liu Y, Yan X, Wang T (2006) Three-dimensional functionalized tetrapod-like ZnO nanostructures for plasmid DNA delivery. Small 2:621–625
Ramani M, Ponnusamy S, Muthamizhchelvan C, Marsili E (2014) Amino acid-mediated synthesis of zinc oxide nanostructures and evaluation of their facet-dependent antimicrobial activity. Colloids Surf B 117:233–239
Ebadi M, Zolfaghari MR, Aghaei SS, Zargar M, Shafiei M, Zahiri HS, Noghabi KA (2019) A bio-inspired strategy for the synthesis of zinc oxide nanoparticles (ZnONPs) using the cell extract of cyanobacterium Nostoc sp. EA03: from biological function to toxicity evaluation. RSC Adv 9:23508–23525
Ebadi M, Zolfaghari MR, Aghaei SS, Zargar M, Noghabi KA (2021) Desertifilum sp. EAZ03 cell extract as a novel natural source for the biosynthesis of zinc oxide nanoparticles and antibacterial, anticancer and antibiofilm characteristics of synthesized zinc oxide nanoparticles. J Appl Microbiol 132:221–236
El-Belely EF, Farag MMS, Said HA, Amin AS, Azab E, Gobouri AA, Fouda A (2021) Green synthesis of zinc oxide nanoparticles (ZnO-NPs) using Arthrospira platensis (class: cyanophyceae) and evaluation of their biomedical activities. Nanomaterials 11:95
Khalafi T, Buazar F, Ghanemi K (2019) Phycosynthesis and enhanced photocatalytic activity of /czinc oxide nanoparticles toward organosulfur pollutants. Sci Rep 9:6866
Ishwarya R, Vaseeharana B, Kalyania S, Banumathia B et al (2018) Facile green synthesis of zinc oxide nanoparticles using Ulva lactuca seaweed extract and evaluation of their photocatalytic, antibiofilm and insecticidal activity. J Photochem Photobiol B Biol 178:249–258
Rao MD, Gautam P (2016) Synthesis and characterization of ZnO nanoflowers using Chlamydomonas reinhardtii: A green approach. Environ Progr Sustain Energy 35(4):1020–1026
Gurusamy S, Kulanthaisamya MR, Haria DG et al (2019) Environmental friendly synthesis of TiO2-ZnO nanocomposite catalyst and silver nanomaterials for the enhanced production of biodiesel from Ulva lactuca seaweed and potential antimicrobial properties against the microbial pathogens. J Photochem Photobiol B Biol 193:118–130
Pandimurugan R, Thambidurai S (2016) Novel seaweed capped ZnO nanoparticles for effective dye photodegradation and antibacterial activity. Adv Powder Technol 27(4):1062–1072
Basha SK, Lakshmi V, Kumar VS (2016) Ammonia sensor and antibacterial activities of green zinc oxide nanoparticles. Sens Bio-Sens Res 10:34–40
Sanaeimehr Z, IrajJavadi I, Namvar F (2018) Antiangiogenic and antiapoptotic effects of green-synthesized zinc oxide nanoparticles using Sargassum muticum algae extraction. Cancer Nano. https://doi.org/10.1186/s12645-018-0037-5
Murugan K, Roni M, Panneerselvam C, Aziz T et al (2018) Sargassum wightii-synthesized ZnO nanoparticles reduce the fitness and reproduction of the malaria vector Anopheles stephensi and cotton bollworm Helicoverpaarmigera. Physiol Mol Plant Pathol 101:202–213
Nagarajan S, Kuppusamy AK (2013) Extracellular synthesis of zinc oxide nanoparticle using seaweeds of gulf of Mannar, India. J Nanobiotechnol 11:39
Pandimurugan R, Thambidurai S (2017) UV protection and antibacterial properties of seaweed capped ZnO nanoparticles coated cotton fabrics. Int J Biol Macromol 105(1):788–795
Priyadharshini RI, Prasannaraj G, Geetha N, Venkatachalam P (2014) Microwave-mediated extracellular synthesis of metallic silver and zinc oxide nanoparticles using macro-algae (Gracilaria edulis) extracts and its anticancer activity against human PC3 cell lines. Appl BiochemBiotechnol 174:2777–2790
Parthiban C, Sundaramurthy N (2015) Biosynthesis, characterization of ZnO nanoparticles by using Pyrus pyrifolia leaf extract and their photocatalytic activity. Int J Innov Res Sci Eng Technol 4:9710–9718
Ali A, Phull A-R, Zia M (2018) Elemental zinc to zinc nanoparticles: is ZnO NPs crucial for life? Synthesis, toxicological, and environmental concerns. Nanotechnol Rev 7:413–441
Kumar SS, Venkateswarlu P, Rao VR, Rao GN (2013) Synthesis, characterization and optical properties of zinc oxide nanoparticles. Int Nano Lett. https://doi.org/10.1186/2228-5326-3-30
Nagaonkar D, Rai M (2015) Sequentially reduced biogenic silver-gold nanoparticles with enhanced antimicrobial potential over silver and gold monometallic nanoparticles. Adv Mater Lett 6:334–341
Femi V, Prabha PH, Sudha P, Devibala B, Jerald AL (2011) Antibacterial effect of ZnO-Au nanocomposites. Int J Biotechnol Eng 1:1–8
Mishra V, Sharma R (2015) Green synthesis of zinc oxide nanoparticles using fresh peels extract of Punica granatum and its antimicrobial activities. Int J Pharma Res Health Sci 3:694–699
Talam SN, Karumuri SR, Gunnam N. (2012) Synthesis, characterization, and spectroscopic properties of ZnO nanoparticles. Int Scholarly Res Network 6
Chauhan KK, Mahajan JR (2017) Synthesis and characterization of nano zinc oxide emeraldine salt composite. Int J Sci Res Phys Appl Sci 5:6–11
Omar FM, Aziz HA, Stoll S (2014) Stability of ZnO nanoparticles in solution. influence of pH, dissolution, aggregation and disaggregation effects. J Coll Sci Biotechnol 3:75–84
Joshi M, Bhattacharyya A, Ali SW (2008) Characterization techniques for nanotechnology applications in textiles. Indian J Fibre Text Res 33:304–317
Nur S, Shameli K, Mei-Theng WM, Teow SY, Chew J, Ismail NA (2019) Cytotoxicity and antibacterial activities of plant-mediated synthesized zinc oxide (ZnO) nanoparticles using Punica granatum (pomegranate) fruit peels extract. J Mol Struct J 1189:57–65
Jayabalan J, Mani G, Krishnan N, Pernabas J, Devadoss JM, Jang HT (2019) Green biogenic synthesis of zinc oxide nanoparticles using Pseudomonas putida culture and its In vitro antibacterial and anti-biofilm activity. Biocatal Agric Biotechnol 21:101327
Fadwa A, Alkoblan DK, Mateen A, Albarag AM (2021) Synergistic effects of zinc oxide nanoparticles and various antibiotics combination against Pseudomonas aeruginosa clinically isolated bacterial strains. Saudi J Biol Sci 28(1):928–935
Tyagi PK, Gola D, Tyagi S et al (2020) Synthesis of zinc oxide nanoparticles and its conjugation with antibiotic: antibacterial and morphological characterization. Environ Nanotechnol Monit Manag 14:100391
Akbar N, Aslam Z, Siddiqui R, Shah MR, Khan NA (2021) Zinc oxide nanoparticles conjugated with clinically-approved medicines as potential antibacterial molecules. AMB Express 11:104
Badigera PM, Patil PM, Badiger MV, Patel KPR, Gadgil BST, Pandit A, Bohara RA (2020) Biofilm formation to inhibition: role of zinc oxide-based nanoparticles. Mater Sci Eng C 108:110319
Shkodenko L, Kassirov I, Koshel E (2020) Metal oxide nanoparticles against bacterial biofilms: perspectives and limitations. Microorganisms 8:1545
Senthamarai MD, Malaikozhundan B (2022) Synergistic action of zinc oxide nanoparticle using the unripe fruit extract of Aegle marmelos (L.)-antibacterial, antibiofilm, radical scavenging and ecotoxicological effects. Mater Today Commun 30:103228
Vijayakumar S, Saravanakumar K, Malaikozhundan B, Divya M, Vaseeharan B, Lara EF, Wang MH (2020) Biopolymer K-carrageenan wrapped ZnO nanoparticles as drug delivery vehicles for anti MRSA therapy. Int J Biol Macromol 144:9–18
Sangeetha G, Rajeshwari S, Venckatesh R (2011) Green synthesis of zinc oxide nanoparticles by Aloe barbadensis miller leaf extract: Structure and optical properties. Mat Res Bull 46(12):2560–2566
Jalal M, Ansari MA, Ali SG, Khana HM, Rehman S (2018) Anticandidal activity of bioinspired ZnONPs: effect on growth, cell morphology and key virulence attributes of Candida species. Artif Cells Nanomed Biotechnol 46(1):912–925
Sonia S, Ruckmani K, Sivakumar M (2017) Antimicrobial and antioxidant potentials of biosynthesized colloidal zinc oxide nanoparticles for a fortified cold cream formulation: a potent nanocosmeceutical application. Mater Sci Eng C 79:581–589
Surendra TV et al (2016) Vegetable peel waste for the production ofZnO nanoparticles and its toxicological efficiency, antifungal, hemolytic, and antibacterial activities. Nanoscale Res Lett 11:546
Padalia H, Chanda S (2017) Characterization, antifungal and cytotoxic evaluation of green synthesized zinc oxide nanoparticles using Ziziphus nummularia leaf extract. Artif Cells Nanomed Biotechnol 45(8):1751–1761
Miri A, Mahdinejad N, Ebrahimy O, Khatamid M, Saranif M (2019) Zinc oxide nanoparticles: Biosynthesis, characterization, antifungal and cytotoxic activity. Mater Sci Eng, C 104:109981
Sun Q, Li J, Le T (2018) Zinc oxide nanoparticle as a novel class of antifungal agents: current advances and future perspectives. J Agric Food Chem 66:11209–11220
Lipovsky A, Nitzan Y, Gedanken A, Lubart R (2011) Antifungal activity of ZnO nanoparticles-the role of ROS mediated cell injury. Nanotechnology 22:912–925
Ficociello G, Caris MG, Trillò G, Cavallini D, Sarto MS, Uccelletti D, Mancini P (2018) Anti-candidal activity and in vitro cytotoxicity assessment of graphene nanoplatelets decorated with zinc oxide nanorods. Nanomaterials 8:752
Hajar AA, Dakhili M, Saghazadeh M, Aghaei SS, Nazari R (2020) Synergistic antifungal effect of fluconazole combined with ZnO nanoparticles against Candida albicans strains from vaginal candidiasis. Med Lab J 14(3):26–32
Xue J, Luo Z, Li P, Ding Y, Cui Y, Wu Q (2014) A residue-free green synergistic antifungal nanotechnology for pesticide thiram by ZnO nanoparticles. Sci Rep 4:5408
Maensiri S, Laokul P, Klinkaewnarong J, Phokha S, Promarak V, Seraphin S (2008) Indium oxide (In2O3) nanoparticles using Aloe vera plant extract: Synthesis and optical properties. J Optoelectron Adv Mater 10:161–165
Ryu CS, Kim CH, Lee SY, Lee KS, Choung KJ, Song GY, Kim B-H, Ryu SY, Lee HS, Kim SK (2012) Evaluation of the total oxidant scavenging capacity of saponins isolated from Platycodon grandiflorum. Food Chem 132:333–337
Das D, Nath BC, Phukon P, Kalita A, Dolui SK (2013) Synthesis of ZnO nanoparticles and evaluation of antioxidant and cytotoxic activity. Colloids Surf B Biointerfaces 111:556–560
Umar H, Kavaz D, Rizaner N (2019) Biosynthesis of zinc oxide nanoparticles using Albizia lebbeck stem bark, and evaluation of its antimicrobial, antioxidant, and cytotoxic activities on human breast cancer cell lines. Int J Nanomed 14:87–100
Mahendiran D, Subash G, Selvan DA, Rehana D, Kumar S, Rahiman AK (2017) Biosynthesis of zinc oxide nanoparticles using plant extracts of Aloe vera and Hibiscus sabdariffa: phytochemical, antibacterial, antioxidant and anti-proliferative studies. BioNanoScience 7:530–545
Rehana D, Mahendiran D, Kumar RS, Rahiman AK (2017) In vitro antioxidant and antidiabetic activities of zinc oxide nanoparticles synthesized using different plant extracts. Bioprocess Biosyst Eng 40:943–957
Hafez A, Nassef E, Fahmy M, Elsabagh M, Bakr A, Hegazi E (2020) Impact of dietary nano-zinc oxide on immune response and antioxidant defense of broiler chickens. Environ Sci Pollut Res Int 27(16):19108–19114
Sohail MF, Rehman M, Hussain SZ, Huma ZE, Shahnaz G, Qureshi OS et al (2020) Green synthesis of zinc oxide nanoparticles by Neem extract as multi-facet therapeutic agents. J Drug Deliv Sci Technol 59:101911
Biswas P, Adhikari A, Mondal S, Das M, Bhattacharya SS, Pal D et al (2021) Synthesis and spectroscopic characterization of a zinc oxide-polyphenol nanohybrid from natural resources for enhanced antioxidant activity with less cytotoxicity. Mater Today Proceed 43(6):3481–3486
El-Bahr SM, Shousha S, Albokhadaim I, Shehab A, Khattab W, Ahmed-Farid O et al (2020) Impact of dietary zinc oxide nanoparticles on selected serum biomarkers, lipid peroxidation and tissue gene expression of antioxidant enzymes and cytokines in Japanese quail. BMC Vet Res 16:1–2
Agarwal H, Shanmugam V (2019) A review on anti-inflammatory activity of green synthesized zinc oxide nanoparticle: mechanism based approach. Bioorg Chem 94:103423
Ilves M et al (2014) Topically applied ZnO nanoparticles suppress allergen induced skin inflammation but induce vigorous IgE production in the atopic dermatitis mouse model. Part FibreToxicol 11:38
Nagajyothi PC, Cha SJ, Yang IJ, Sreekanth TVM, Kim KJ, Shin HM (2015) Antioxidant and anti-inflammatory activities of zinc oxide nanoparticles synthesized using Polygala tenuifolia root extract. J Photochem Photobiol B Biol 146:10–17
Kim M-H, Seo J-H, Kim H-M, Jeong H-J (2016) Aluminum-doped zinc oxide nanoparticles attenuate the TSLP levels via suppressing caspase-1 in activated mast cells. J Biomater Appl 30:1407–1416
Jan H, Shah M, Andleeb A, Faisal S, Khattak A, Rizwan M, Drouet S, Hano C, Abbasi BH (2021) Plant-based synthesis of zinc oxide nanoparticles (ZnONPs) using aqueous leaf extract of Aquilegia pubiflora: their antiproliferative activity against HepG2 cells inducing reactive oxygen species and other in vitro properties. Oxid Med Cell Longev 2021:e4786227
Thatoi P, Kerry RG, Gouda S, Das G et al (2016) Photo-mediated green synthesis of silver and zinc oxide nanoparticles using aqueous extracts of two mangrove plant species Heritiera fomes and Sonneratia apetala and investigation of their biomedical applications. J PhotochemPhotobiol B Biol 163:311–318
Chausmer AB (1998) Zinc, insulin and diabetes. J Am Coll Nutr 17:109–115
Jansen J, Karges W, Rink L (2009) Zinc and diabetes—Clinical links and molecular mechanisms. J Nutr Biochem 20:399–417
Bayrami A, Parvinroo S, Habibi-Yangjeh A, Pouran SR (2017) Bio-extract-mediated ZnO nanoparticles: microwave-assisted synthesis, characterization and antidiabetic activity evaluation. Artif Cells Nanomed Biotechnol 46:730–739
Ali KA (2020) Therapeutic efficacy of bone marrow- derived mesenchymal stem cells plus zinc oxide nanoparticles against streptozotocin- induced type 2 diabetes in albino rats. Sys Rev Pharm 11(5):40–51
Bayrami A, Haghgooie S, Pouran SR, Arvanag FM, Habibi-Yangjeh A (2020) Synergistic antidiabetic activity of ZnO nanoparticles encompassed by Urtica dioica extract. Adv Powder Technol 31:2110–2118
Wahba NS et al (2016) Efficacy of zinc oxide nanoparticles in attenuating pancreatic damage in a rat model of streptozotocin-induced diabetes. Ultrastruct Pathol 40:358–373
El-Gharbawy RM et al (2016) Zinc oxide nanoparticles and a standard antidiabetic drug restore the function and structure of beta cells in Type-2 diabetes. Biomed Pharmacother 84:810–820
Kitture R et al (2015) ZnO nanoparticles-red sandalwood conjugate: a promising anti-diabetic agent. J Nanosci Nanotechnol 15:4046–4051
Chandrasekaran S, Anbazhagan V, Anusuya S (2022) Green route synthesis of ZnO nanoparticles using Senna auriculata aqueous flower extract as reducing agent and evaluation of its antimicrobial, antidiabetic and cytotoxic activity. Appl Biochem Biotechnol. https://doi.org/10.1007/s12010-022-03900-0
Rajakumar G, Thiruvengadam M, Mydhili G, Gomathi T, Chung I (2017) Green approach for synthesis of zinc oxide nanoparticles from Andrographis paniculata leaf extract and evaluation of their antioxidant, anti-diabetic, and anti-inflammatory activities. Bioprocess Biosyst Eng 41:21–30
Malaikozhundan B, Vinodhini J, Kalanjiam MAR, Vinotha V, Palanisamy S, Vijayakumar S, Vaseeharan B, Mariyappan A (2020) High synergistic antibacterial, antibiofilm, antidiabetic and antimetabolic activity of Withania somnifera leaf extract-assisted zinc oxide nanoparticles. Bioprocess Biosyst Eng 43:1533–1547
Bray F, Laversanne M, Weiderpass E, Soerjomataram I (2021) The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer 127:2864–2866
Ott M, Gogvadze V, Orrenius S, Zhivotovsky B (2007) Mitochondria, oxidative stress and cell death. Apoptosis 12:913–922
Bisht G, Rayamajhi S (2016) ZnO nanoparticles: a promising anticancer agent. Nanobiomedicine 3:3–9
Zhang H, Liang Z, Zhang J, Wang WP, Zhang H, Lu Q (2020) Zinc oxide nanoparticle synthesized from Euphorbia fischeriana root inhibits the cancer cell growth through modulation of apoptotic signaling pathways in lung cancer cells. Arab J Chem 13:6174–6183
Umamaheswari A, Prabu SL, John SA, Puratchikody A (2021) Green synthesis of zinc oxide nanoparticles using leaf extracts of Raphanus sativus var. longipinnatus and evaluation of their anticancer property in A549 cell lines. Biotechnol Rep 29:e00595
Chandrasekaran M, Pandurangan M (2016) In vitro selective anti-proliferative effect of zinc oxide nanoparticles against co-cultured C2C12 myoblastoma cancer and 3T3-L1 normal cells. Biol Trace Elem Res 172:148–154
Vijayakumar S, Vaseeharan B, Malaikozhundan B, Shobiya M (2016) Laurus nobilis leaf extract mediated green synthesis of ZnO nanoparticles: Characterization and biomedical applications. Biomed Pharmacother 84:1213–1222
Wahab R et al (2013) ZnO nanoparticles induces cell death in malignant humanT98G gliomas, KB and non-malignant HEK cells. J Biomed Nanotechnol 9:1181–1189
Sharma H et al (2016) Development and characterization of metal oxide nanoparticles for the delivery of anticancer drug. Artif Cells Nanomed Biotechnol 44:672–679
Hackenberg S et al (2012) Antitumor activity of photo-stimulated zinc oxide nanoparticles combined with paclitaxel or cisplatin in HNSCC cell lines. J Photochem Photobiol B 114:87–93
Nabil A, Elshemy M, Asem M, Motaal MA et al (2020) Zinc oxide nanoparticle synergizes sorafenib anticancer efficacy with minimizing its cytotoxicity. Oxid Med Cell Longev 2020:1362104
Baskar G, Chandhuru J, Fahad KS, Praveen AS et al (2015) Anticancer activity of fungal L-asparaginase conjugated with zinc oxide nanoparticles. J Mater Sci Mater Med 26(1):5380
Ezealisiji KM, Siwe-Noundou X, Maduelosi B, Nwachukwu N, Krause RWM (2019) Green synthesis of zinc oxide nanoparticles using Solanum torvum (L) leaf extract and evaluation of the toxicological profile of the ZnO nanoparticles–hydrogel composite in Wistar albino rats. Int Nano Lett 9:99–107
Shao F, Yang A, Yu DM, Wang J, Gong X, Tian HX (2018) Bio-synthesis of Barleriagibsoni leaf extract mediated zinc oxide nanoparticles and their formulation gel for wound therapy in nursing care of infants and children. J Photochem Photobiol B Biol 189:267–273
Batool M, Khurshid S, Qureshi Z, Daoush WM (2021) Adsorption, antimicrobial and wound healing activities of biosynthesised zinc oxide nanoparticles. Chem Pap 75:893–907
Khatami M, Varma RS, Zafarnia N, Yaghoobi H, Sarani M, Kumar VG (2018) Applications of green synthesized Ag, ZnO and Ag/ZnO nanoparticles for making clinical antimicrobial wound-healing bandages. Sustain Chem Pharm 10:9–15
Raguvaran R et al (2017) Sodium alginate and gum acacia hydrogels of ZnO nanoparticles show wound healing effect on fibroblast cells. Int J Biol Macromol 96:185–191
Rayyif SM, Mohammed HB, Curuțiu C, Bîrcă AC et al (2021) ZnO Nanoparticles-modified dressings to inhibit wound pathogens. Materials 14(11):3084
Karahaliloglu Z et al (2016) Antibacterial chitosan/silk sericin 3D porous scaffolds as a wound dressing material. Artif Cells Nanomed Biotechnol 45:1–14
Soubhagya A, Moorthi A, Prabaharan M (2020) Preparation and characterization of chitosan/pectin/ZnO porous films for wound healing. Int J Biol Macromol 157:135–145
Saddik MS, Elsayed MMA, El-Mokhtar MA, Sedky H, Abdel-Aleem JA, Abu-Dief AM, Al-Hakkani MF, Hussein HL, Al-Shelkamy SA, Meligy FY et al (2022) Tailoring of novel azithromycin-loaded zinc oxide nanoparticles forwound healing. Pharmaceutics 14:111
Manuja A, Raguvaran R, Kumar B, Kalia A, Tripathi B (2020) Accelerated healing of full thickness excised skin wound in rabbits using single application of alginate/acacia based nanocomposites of ZnO nanoparticles. Int J Biol Macromol 155:823–833
Melk MM, El-Hawary SS, Melek FR, Saleh DO, Ali OM, El Raey MA, Selim NM (2021) Antiviral activity of zinc oxide nanoparticles mediated by Plumbago indica L. extract against Herpes Simplex Virus Type 1 (HSV-1). Int J Nanomed 16:8221–8233
Ghaffari H, Tavakoli A, Moradi A et al (2019) Inhibition of H1N1 influenza virus infection by zinc oxide nanoparticles: another emerging application of nanomedicine. J Biomed Sci 26:70
Te Velthuis AJW, van denWorm SHE, Sims AC, Baric RS, Snijder EJ, Van Hemert MJ (2010) Zn2+ inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog 6:e1001176
Erk I, Huet J-C, Duarte M, Duquerroy S, Rey F, Cohen J, Lepault J (2003) A zinc ion controls assembly and stability of the major capsid protein of rotavirus. J Virol 77:3595–3601
Haase H, Ober-Blöbaum JL, Engelhardt G, Hebel S, Heit A, Heine H, Rink L (2008) Zinc signals are essential for lipopolysaccharide-induced signal transduction in monocytes. J Immunol 181:6491–6502
Lishchynskyi O, Shymborska Y, Stetsyshyn Y, Raczkowska J, Skirtach AG, Peretiatko T, Budkowski A (2022) Passive antifoulingand active self-disinfecting antiviral surfaces. Chem Eng J 446:137048
Hamdi H, Abdel-Bar HM, Elmowafy E, El-khouly A, Mansour M, Awad GAS (2021) Investigating the internalization and COVID-19 antiviral computational analysis of optimized nanoscale zinc oxide. ACS Omega 6(10):6848–6860
El-Megharbel S, Alsawat M, Al-Salmi F, Hamza R (2021) Utilizing of (Zinc Oxide Nano-Spray) for disinfection against “SARS-CoV-2” and testing its biological effectiveness on some biochemical parameters during (COVID-19 pandemic)—”zno nanoparticles have antiviral activity against (SARS-CoV-2)”. Coatings 11:388
Jana B, Chatterjee A, Roy D, Ghorai S, Pan D, Pramanik SK, Chakraborty N, Ganguly J (2021) Chitosan/benzyloxybenzaldehyde modified ZnO nano template having optimized and distinct antiviral potency to human cytomegalovirus. Carbohydr Polym 278:118965
Gupta J, Irfan M, Ramgir N, Muthe KP, Debnath AK, Ansari S, Gandhi J, Ranjith-Kumar CT, Surjit M (2022) Antiviral activity of zinc oxide nanoparticles and tetrapods against the Hepatitis E and Hepatitis C viruses. Front Microbiol 23(13):881595
Akbarian M, Mahjoub S, Elahi SM, Zabihi E, Tashakkorian H (2020) Green synthesis, formulation and biological evaluation of anovel ZnO nanocarrier loaded with paclitaxel as drug delivery system on MCF-7 cell line. Colloids Surf B Biointerfaces 186:110686
Vimala K, Sundarraj S, Paulpandi M, Vengatesan S, Kannan S (2014) Green synthesized doxorubicin loaded zinc oxide nanoparticles regulates the Bax and Bcl-2 expression in breast and colon carcinoma. Process Biochem 49:160–172
Yuan Q, Hein S, Misra RDK (2010) New generation of chitosan-encapsulated ZnO quantum dots loaded with drug: synthesis, characterization and in vitro drug delivery response. Acta Biomater 6:2732–2739
Pan ZY et al (2011) The application of ZnO luminescent nanoparticles in labeling mice. Contrast Media Mol Imaging 6:328–330
Sudhagar S, Sathya S, Pandian K, Lakshmi BS (2011) Targeting and sensing cancer cells with ZnO nanoprobes in vitro. Biotechnol Lett 33:1891–1896
Singh SP (2011) Multi-functional magnetic quantum dots for cancer theranostics. J Biomed Nanotechnol 7:95
Mohammed YH, Barkauskas DS, Holmes A, Grice J, Roberts MS (2020) Noninvasive in vivo human multiphoton microscopy: a key method in proving nanoparticulate zinc oxide sunscreen safety. J Biomed Opt 25:1–19
Jaatinen EA, Fernando JFS, Shortell MP, Walden SL (2020) Accurate determination of nonlinear refraction in ZnO and Au composite nanostructures. Opt Mater Express 10:653–661
Prasanna APS, Venkataprasanna KS, Pannerselvam B, Asokan V, Jeniffer RS, Venkatasubbu GD (2020) MultifunctionalZnO/SiO2 Core/Shell Nanoparticles for bioimaging and drug delivery application. J Fluoresc 30:1075–1083
Couto N, Newton JRA, Russo C, Karunakaran E, Achour B et al (2021) Label-Free quantitative proteomics and substrate-based mass spectrometry imaging of xenobiotic metabolizing enzymes in ex vivo human skin and a human living skin equivalent model, Drug Metab. Dispos 49:39–52
Kilin V, Campargue G, Fureraj I, Sakong S, Sabri T, Riporto F et al (2020) Wavelength-selective nonlinear imaging and photo-induced cell damage by dielectric harmonic nanoparticles. ACS Nano 14:4087–4095
Chattopadhyay S, Kumawat A, Misra KP, Halder N, Bandyopadhyay A et al (2021) Micro-strain administered SHG intensity enhancement by heavy Ce doping in co-precipitated ZnO nanoparticles. Mater Sci Eng B Solid-State Mater Adv Technol 266:115041
Ghazali NAB, Mani MP, Jaganathan SK (2018) Green-synthesized Zinc oxide nanoparticles decorated nanofibrous polyurethane mesh loaded with virgin coconut oil for tissue engineering application. Curr Nanosci 14:280–289
Cruz DM, Mostafavi E, Vernet-Crua A, Barabadi H, Shah V, Cholula-Díaz J-L, Guisbiers G, Webster TJ (2020) Green nanotechnology-based zinc oxide (ZnO) nanomaterials for biomedical applications: a review. J Phys Mater 3:034005
Yousefi A, Sarrafzadeh-Rezaei F, Asri-Rezaei S, Farshid AA, Behfar M (2018) Fabrication of novel tubular scaffold for tendon repair from chitosan in combination with zinc oxide nanoparticles. Vet Res Forum 9(2):105–111
Shubha P, Likhith Gowda M, Namratha K, Manjunatha HB, Byrappa K (2019) In vitro and in vivo evaluation of green hydrothermal synthesized ZnO nanoparticles. J Drug Deliv Sci Technol 49:692–699
Wang D, Cui L, Chang X, Guan D (2020) Biosynthesis and characterization of zinc oxide nanoparticles from Artemisia annua and investigate their effect on proliferation, osteogenic differentiation and mineralization in human osteoblast-like MG-63 Cells. J PhotochemPhotobiol B 202:111652
Shafique S, Jabeen N, Ahmad KS, Irum S, Anwaar S, Ahmad N, Alam S, Ilyas M, Khan TF, Hussain SZ (2020) Green fabricated zinc oxide nanoformulated media enhanced callus induction and regeneration dynamics of Panicum virgatum L. PLoS One 15:e0230464
Heidari F, Bazargan-Lari R, Razavi M, Fahimipour F, Vashaee D, Tayebi L (2020) Nano-hydroxyapatite and nano-hydroxyapatite/zinc oxide scaffold for bone tissue engineering application. Int J Appl Ceram Technol 17:2752–2761
Harikrishnan P, Sivasamy A (2020) Preparation, characterization of electrospun polycaprolactone-nano zinc oxide composite scaffolds for osteogenic applications. Nano-Struct Nano-Objects 23:100518
Forero JC, Roa E, Reyes JG, Acevedo C, Osses N (2017) Development of useful biomaterial for bone tissue engineering by incorporating nano-copper-zinc alloy (nCuZn) in Chitosan/Gelatin/Nano-Hydroxyapatite (Ch/G/nHAp) scaffold. Materials 10(10):1177
Acknowledgements
The authors are grateful to Jamia Millia Islamia, India, for their support.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Asif, N., Amir, M. & Fatma, T. Recent advances in the synthesis, characterization and biomedical applications of zinc oxide nanoparticles. Bioprocess Biosyst Eng 46, 1377–1398 (2023). https://doi.org/10.1007/s00449-023-02886-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-023-02886-1