Abstract
Nanomaterials have been rapidly developed during the last decades, yet many nanoparticles synthesized by classical methods are toxic and their synthesis procedure is not sustainable. Here we review the green synthesis of nanoparticles from biomass and waste with a focus on synthetic mechanisms and applications in energy production and storage, medicine, environmental remediation, and agriculture and food. Biomass use for synthesis include microorganisms, fungi, plants, and agro-industrial bio-waste. Compared to conventional synthesis, green synthesis allows a 30% reduction in energy consumption, cost savings of up to 40%, and a 50% increase in production output. Biomedical applications comprise antibacterials, anticancers, antioxidants, and drug delivery mechanisms. Carbon quantum dots and photovoltaics are discussed in the energy section. Agricultural and food applications focus on nanofertilization, pest control, and food quality. Environmental remediation includes water and soil purification.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, nanoparticle synthesis has witnessed a remarkable shift toward sustainable and environmentally friendly approaches. Conventional nanoparticle synthesis methods frequently involve using hazardous chemicals and high-energy processes, raising environmental concerns, and producing toxic by-products. On the other hand, green synthesis methods provide a viable solution by utilizing bio-based materials such as microorganisms, plants, and agricultural waste as environmentally friendly sources for nanoparticle synthesis (Karim et al. 2023; Xu et al. 2023). Numerous studies have shown that green synthesis methods effectively produce nanoparticles with desirable properties. Microbial-mediated synthesis has shown great promise because microorganisms can reduce metal ions and form nanoparticles. Furthermore, fungal and algal-mediated synthesis have emerged as viable alternatives, offering sustainable and scalable methods for nanoparticle production (Subramaniyam et al. 2015; Wang et al. 2021). Plant-mediated synthesis has received significant attention because of plant species abundance and diversity, which provide a rich source of bioactive compounds for nanoparticle synthesis (Monga et al. 2020; Rashwan et al. 2023a). Plant parts such as leaves, roots, and seeds have been studied for their ability to reduce metal ions and facilitate the formation of nanoparticles. Furthermore, the valorization of agro-industrial bio-waste has pioneered a novel method for converting agricultural residues and industrial byproducts into bio-nanosorbents, bio-nanocatalysts, and bio-nanodisinfectants (Bishnoi et al. 2018; Omran 2020; Tavker et al. 2021).
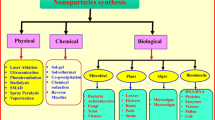
Green-synthesized nanoparticles exhibit vast applications due to their biocompatibility and ability to release substances in a controlled manner (Rashwan et al. 2022a, b). In the realm of biomedicine, these nanoparticles hold great promise for applications in drug delivery systems (Fang et al. 2019; Owoseni-Fagbenro et al. 2019), cancer treatment (Devanesan et al. 2021; Yusefi et al. 2021), and diagnostic imaging. Moreover, their utilization extends to agriculture, where they play a pivotal role in safeguarding crops, delivering nutrients, and bolstering plant growth. These applications enhance agricultural practices and uphold sustainability, minimizing adverse environmental effects. Green nanoparticles have also been found used in environmental remediation, such as water and soil purification, antimicrobial agents, and air pollution control (Debnath et al. 2020; Fahimmunisha et al. 2020; Jain et al. 2021; Kalaba et al. 2021; Uddin et al. 2021). Their ability to remove contaminants and improve remediation processes helps to make the environment greener and cleaner. Furthermore, green nanoparticles have shown promise in photovoltaics and energy storage applications, improving the efficiency and performance of solar cells and storage devices, and thus contributing to developing sustainable energy solutions. Furthermore, these nanoparticles are versatile in fields other than those mentioned above. They provide opportunities in the cosmetics and food industries for improved formulations and safer products (Kumar et al. 2019; Gao et al. 2020). They also function as sensors, enabling the precise detection and monitoring of various substances. Furthermore, in the domains of textiles and electronics, they elevate functional attributes and open doors to inventive applications (Barzinjy et al. 2020). Green nanoparticles offer broader utility and innovation potential, spanning fields like nanoelectronics, nanosorbents, and catalysis, as shown in Fig. 1.
Nanoparticle synthesis and applications. Numerous techniques are employed to synthesize nanoparticles, including physical, chemical, and biological methods. Green nanoparticles could result from sources such as plant extracts, organisms, enzymes, agricultural waste, and ultrasound- and microwave-assisted synthesis. A spectrum of green synthesis techniques yields nanoparticles like liposomes, niosomes, nanoemulsions, and nanogels. These eco-friendly nanoparticles find applications in fields such as agriculture, biomedicine, environment, food, feed, and energy
Therefore, the novelty of this review lies in its comprehensive exploration of green synthesis methods and their diverse applications. This review highlights the use of green-synthesized nanoparticles in medicine, agriculture, environmental remediation, energy, and various other fields, demonstrating their potential to revolutionize industries and contribute to a more sustainable future. Green synthesis methods appeal to researchers and enterprises looking for greener alternatives because of their eco-friendliness, biocompatibility, and cost-effectiveness. However, addressing issues such as reproducibility, stability, and control over nanoparticle size and shape is critical to advance their practical application.
Resources for green synthesis of nanomaterials
Microbial, fungal, and algal-mediated synthesis
Traditional physical and chemical methods are recognized for their high energy and time costs and the use of environmentally harmful chemicals. Chemical synthesis techniques require a metal precursor, reducing agents, and a stabilizing agent, with various processes suggested, such as reducing silver nitrate in the presence of a stabilizing and reducing agent in an aqueous solution. Different reducing substances like citrate, ascorbate, borohydride, hydrogen gas, surfactants, and ligands, or polymers such as polyvinylpyrrolidone and polyethylene glycol are stabilizing agents. Factors like the starting concentration of the silver nitrate solution, the stabilizing agent's concentration, and the molar ratio of silver nitrate to the reducing agent significantly influence the size of the resulting nanoparticles (Gudikandula and Charya Maringanti 2016). Therefore, chemical approaches have limited advantages and lack environmentally friendly synthesis techniques.
Research has shifted toward cleaner and more eco-friendly synthesis processes. Using bio-based routes for synthesizing nanomaterials is widely recognized as a more advantageous alternative to conventional methods, offering significant potential in nanoparticle production. Numerous studies have highlighted the ability of microorganisms to synthesize nanomaterials. Certain bacterial species can accumulate these particles intracellularly for navigational purposes. Moreover, living organisms such as plants, algae, bacteria, fungi, and even animals play a crucial role in creating nanoparticles by utilizing specific biomolecules, including enzymes, for in situ reduction of metal ions, such as silver ions, to produce silver nanoparticles (Gupta et al. 2023; Kulkarni et al. 2023). Biological synthesis offers advantages in terms of the purity and safety of the resulting nanoparticles and the production of uniformly sized and well-defined nanoparticles, often surpassing physicochemical methods. These features of biological synthesis align with several core principles of green chemistry, making the biosynthesis process environmentally friendly. Additionally, using biomolecules as capping and stabilizing agents enhances the microbial activity of nanoparticles made through biological processes.
Due to the synergistic interaction among microorganisms, several advantageous traits can be observed, such as accelerated multiplication rates, the production of diverse secondary metabolites, rapid growth in confined spaces, and the capacity to synergistically deactivate pollutants. Consequently, microorganisms serve as a suitable medium for synthesizing nanozerovalent iron particles (Monga et al. 2020). Different acidophilic microbe strains show different capabilities for reducing iron oxide; moreover, the presence of electron donors and the choice of growth conditions can further modify the characteristics mentioned above (Wang et al. 2016; Xie et al. 2017). For instance, the reduction of uranium (VI) by zerovalent iron nanoparticles is improved by Bacillus subtilis, according to Ding et al. (2015). Furthermore, zerovalent iron nanoparticles containing Dehalobacter demonstrated an increase in the decomposition of trichloroethane by 14-fold (Lee et al. 2015). Microorganisms, with their advantageous traits and varying capabilities, serve as a suitable medium for synthesizing nanozerovalent iron particles, enhancing reduction processes and pollutant decomposition.
In addition, Chlorococcum sp. algae have been used to synthesize spherical-shaped nanoiron nanoparticles from iron chloride precursor, producing 20–50-nm nanoiron particles (Subramaniyam et al. 2015). Transmission electron microscopy analysis revealed that the synthesized nanoiron particles were predominantly found on the surface of the microalgal cells rather than being localized inside the cells. This observation suggests that the presence of biomolecules, specifically carbonyl and amine compounds derived from polysaccharides and glycoproteins within the algal cells, played a crucial role in synthesizing nanoiron. The Fourier transform infrared analysis further confirmed the involvement of these biomolecules in nanoiron synthesis. The microorganism route for synthesizing nanozerovalent nanoparticles is more advantageous than conventional methods.
Green nanoparticle synthesis mediated by fungi has numerous advantages, including simplicity and ease of amplification, ease of processing, economic feasibility, biomass processing, significant surface distance recovery, and optimal mycelium growth. As a result, fungi have enormous promise in the synthesis of nanoparticles. Wang et al. (2021) used Aspergillus sydowii culture supernatant to synthesize silver nanoparticles in vitro. Mohamed et al. (2021) employed Penicillium chrysogenum to develop in vitro microbial synthesis of medical zinc oxide and copper oxide nanoparticles. Overall, the synthesis of nanoparticles by living organisms has enormous promise (Kaur and Gupta 2009), as shown in Fig. 2.
Green synthesis of nanozerovalent iron using microorganisms, i.e., M and plants, i.e., P. Microbial culture is mixed with iron salt and incubated to produce nanoparticles. Aqueous plant extract produces nanoparticles with iron salt in situ. The obtained nanoparticles are centrifuged to get the solid nanoparticles from the suspension. The drying step and/or annealing is the final step to obtain the green synthesized nanoparticles. nZVI refers to nanozerovalent iron
Microorganisms play crucial roles in green nanoparticle synthesis, either directly or indirectly. However, the synthesis of nanoparticles through microbe-mediated processes often displays slow reaction rates, presenting difficulties in managing the diverse array of involved species. Furthermore, these particles typically exhibit heterogeneity in size distribution, necessitating the expertise of skilled personnel during production, consequently leading to substantial cost increments in scaling up the process (Saif et al. 2016). The following characteristics should be addressed while producing extremely stable and well-characterized iron nanoparticles:
-
When choosing an organism for synthesis, focus on its traits like enzyme activity and metabolic pathways, prioritizing those with high detoxification and metal accumulation capabilities.
-
It is critical to adjust the conditions for optimal development and activity conditions to increase enzyme activity, including pH, light, temperature, inoculum size, and buffer.
-
Ensuring the attainment of ideal reaction conditions, including incorporating sustainable alternatives such as microwaves or visible light, becomes crucial when scaling up procedures to a substantial level.
Green synthesesusing plant parts
Green nanoparticle synthesis utilizing different plant components has emerged as a sustainable and eco-friendly approach. This innovative method harnesses the inherent properties of plant extracts, such as leaves, stems, roots, and even fruits, to reduce metal salts and form nanoparticles. By avoiding harsh chemicals and energy-intensive processes, this technique minimizes environmental impact and produces nanoparticles with unique attributes. Interestingly, plant-mediated route synthesis of nanozerovalent iron is one of the most prominent biosynthetic techniques for assembling metal nanoparticles (Machado et al. 2015), as shown in Fig. 2.
Many phytochemical compounds with various functional groups are participating in reducing metal ions to iron nanoparticles. These compounds include polyphenols, metallothioneins, ascorbates, and glutathione, which can chelate metal ions and facilitate superoxide-driven processes, thereby promoting the formation of stable nanoparticles. A typical synthesis combines metal ions, e.g., ferric chloride or ferrous chloride, in an aqueous solution with a plant extract. The phenolic hydroxide groups present in the biomolecules of the extract form complexes with the ferric or ferrous ions, leading to their reduction to iron nanoparticles within a period ranging from a few minutes to several hours. These biomolecules also function as effective stabilizers, thus preventing the aggregation of nanoparticles (Monga et al. 2020); therefore, these molecules play a double role as they reduce the metal ions and act as capping and stabilizing agents.
Plant extracts offer several advantages in the synthesis of nanozerovalent iron. For instance, they are less toxic and exhibit improved water solubility. Additionally, plant extracts enable the production of a larger quantity of nanoparticles, which possess a longer lifespan and exhibit diverse shapes and structures, including round, square, and irregular forms (Wu et al. 2017). Using extracts from various plant components, such as leaves, stems, seeds, roots, and fruits, involves the reduction of metal ions mediated by a range of biomolecules. These plant components are rich in secondary metabolites, including enzymes, proteins, amino acids, vitamins, polysaccharides, alkaloids, polyphenols, flavonoids, and organic acids. These biomolecules, which are biodegradable and non-toxic in most cases, serve as reducing and capping agents, which promote the formation of nanoparticles while effectively inhibiting their agglomeration (Iravani 2011).
Several cases of plant extracts-mediated synthesis of nanozerovalent iron have been documented. For instance, Hoag et al. (2009) used a one-step, environmentally friendly biosynthetic process to effectively synthesize nanozerovalent iron. Green tea extract was mixed with an aqueous ferric chloride solution at room temperature for a few minutes, yielding stable nanoparticles. The dual role of polyphenols found in tea as reducing agents and stabilizers has been a notable discovery in this field. Huang et al. (2014a) conducted a study where they successfully synthesized nanozerovalent iron utilizing extracts from three distinct types of tea: green, oolong, and black tea. These synthesized nanoparticles were subsequently assessed for their efficiency in breaking down malachite green pollutants. Another study by Wang et al. (2014b) showcased the synthesis of polydisperse iron particles using extracts from green tea and Eucalyptus leaves. This process resulted in the formation of nanozerovalent iron characterized by quasi-spherical particles spanning a size range of 20–80 nm. Similarly, Machado et al. (2014) utilized natural extracts from various tree leaves to reduce ferric iron in an aqueous solution and generate nanozerovalent iron particles. In this study, the antioxidant capacity of the extract was evaluated to determine its purity and synthetic suitability. These findings demonstrate the potential of using plant extracts as reducing agents to produce nanozerovalent iron particles.
Furthermore, Machado et al. (2013a) investigated the suitability of twenty-six different plant leaves as prospective bio-reducers. Their investigation revealed that oak, pomegranate, and green tea had the highest antioxidant content. Notably, these extracts were effectively employed in synthesizing nanozerovalent iron particles. Furthermore, using various plant leaves to create nanozerovalent iron particles extended beyond tea extracts. This includes grapes, coffee, roses, gardenia, henna, and fruit trees like cherry, avocado, passionflower, and peach, which were harnessed in an environmentally conscious, one-step synthesis approach (Mondal et al. 2020). It is worthy of mention by many studies that the bio-based reductant agent should be carefully selected because of the incomplete reduction of ferric ions and the lower degradation capacity of the biogenic synthesized nanoparticles (Machado et al. 2013b). Numerous plant-derived constituents have been utilized to achieve in situ synthesis and surface modification of nanozerovalent iron, facilitating the stabilization of the iron particles. These components include guar and xanthan gums, cellulosic materials, riboflavin, starch, and others. For instance, grape seed extract stabilization produced nanozerovalent iron with a greater surface area and a substantially better degrading efficiency than nanozerovalent iron without a stabilizer (Gao et al. 2016). Using plant-mediated processes for nanoparticle synthesis offers a more environmentally friendly approach, resulting in the production of nanoparticles that are not only stabilized but also exhibit enhanced properties compared to conventionally synthesized counterparts. Through these studies, it can be found that plant extracts represent a promising and environmentally friendly approach for synthesizing nanozerovalent iron, facilitated by diverse biomolecules that contribute to the efficient formation and stabilization of nanoparticles.
Bio-fabrication of nanoparticles from agro-industrial bio-waste
A notable portion of municipal solid waste comprises agro-industrial bio-waste remnants, originating from both agricultural activities and industrial processes, particularly prevalent in developing nations (Omran 2020; Rashwan et al. 2023b). Addressing the issue of agro-industrial bio-waste and its detrimental effects through circular practices has become a critical concern, given the significant contributions of the agricultural and industrial sectors to global bio-waste emissions (Mohan and Katakojwala 2021). Additionally, these bio-wastes possess substantial untapped potential and can be reclaimed, repurposed, or transformed into valuable resources. Therefore, leveraging the inherent value of this waste to manufacture economically viable, high-value products plays a crucial role in establishing a sustainable closed-loop economy (Vickers 2017).
Unfortunately, gigantic quantities of agro-industrial bio-waste are annually outputted attained millions of tons, severely influencing the environment, air/water pollution, global warming, and harmful gas formation, among others (Jovanov et al. 2018). The commonly used method is to burn agricultural waste in farms after harvest, which releases harmful gases comprising carbon dioxide, methane, and nitrous oxide. In addition to the generated contaminants like carbon monoxide, ammonia, sulfur dioxide, volatile organic pollutants, and particulate matter, which decline the air quality and result in several health issues (Tyagi et al. 2016). At the same time, green treatment approaches of agro-industrial bio-waste intend to convert them to potential products, such as biodiesel and biochar, and extract bountiful vital components like proteins, antioxidants, carbohydrates, and functional lipids (Sagar et al. 2018). The bio-fabrication of nanomaterials utilizing agro-industrial bio-wastes has cost-effectiveness, low toxicity, and energy-saving features, surpassing the physical and chemical preparation ways (Omran 2020). Bio-wastes comprise natural biomolecules that could act as capping/stabilizing agents and stabilizers (Omran et al. 2021). Consequently, the bountiful and plentiful agro-industrial bio-wastes have been widely utilized to fabricate nanomaterials as follows:
Bio-nanosorbents
The heightened exacerbation of the wastewater issue complicates the scientific community’s search for an effective method to efficiently eliminate well-known pollutants from water (Eltaweil et al. 2023). Numerous remediation approaches have been advocated; nonetheless, adsorption has displayed many features in various manners (Abd El-Monaem et al. 2023). Hence, creating sorbents from abundant and cost-free sources such as agro-industrial materials emerges as an economical and effective solution to the wastewater challenge. In this context, Tavker et al. (2021) prepared silver nanoparticles and cellulose nanofiber from citrus sinensis to adsorb cadmium and chromium from wastewater. The transmission electron microscope images clarified that the average sizes of silver nanoparticles and cellulose nanofiber were 32 nm and 47 nm, respectively. The composite material comprising silver and cellulose nanofibers preferred removing chromium ions over cadmium ions. Precisely, the efficiency of eliminating chromium was measured at 83.5%, while the removal efficiency for cadmium was 32.2%.
In another attempt, Abdelghaffar (2021) developed a bio-sorbent from cellulosic banana peel to eliminate the reactive Orange 5 dye. A composite material of silver, cellulosic banana peel, and chitosan was carefully produced and extensively characterized to achieve this. This comprehensive analysis confirmed the composite's successful synthesis and a clear understanding of its physicochemical properties. Through experimentation, it was determined that the most effective conditions for adsorbing reactive Orange 5 were a pH value of three, an adsorption duration of approximately 90 min, a concentration of 8 g/L of the bio-synthesized composite, and a concentration of 50 mg/L of reactive Orange 5 dye. Notably, the cycling test demonstrated the remarkable regenerative capacity of the bio-fabricated composite. After the fourth cycle, the composite could still remove 95% of reactive Orange 5, indicating its sustained effectiveness over multiple uses.
Bio-nanocatalysts
Bio-nanocatalysts derived from agro-industrial wastes have displayed significant catalytic activity, making them promising for various applications due to their attributes, such as non-toxicity, high efficiency, simple processing, cost-effectiveness, and renewability. As a result, recent research efforts have been directed toward harnessing these bio-wastes to develop environmentally friendly nanocatalysts. An illustrative example is the work of Bishnoi et al. (2018), who utilized the inedible Cynometra ramiflora to synthesize iron oxide nanoparticles. These nanoparticles were employed for the photocatalytic degradation of methylene blue, a common dye pollutant. Experimental findings indicated that methylene blue underwent complete degradation within 110 min under sunlight exposure. Impressively, a reusability assessment demonstrated the sustained performance of the catalyst, with the degradation efficiency only declining to 4% after undergoing five consecutive cycles. This underscores the potential of such eco-friendly nanocatalysts in sustainable environmental applications.
Furthermore, Skiba and Vorobyova (2019) prepared silver nanoparticle-derived orange peel for fast-degrading methylene blue. Characterization analyses, comprising X-ray diffraction, implied the cubic structure of the bio-synthesized silver. Additionally, zeta potential depicted that the surface of silver was rich with negative charges of − 21.7 mV. Notably, the methylene blue degradation efficacy attained 99% during only 35 min, reflecting the superior catalytic property of this costless bio-synthesized catalyst. In another investigation, Narasaiah and Mandal (2020) exploited the cotton boll peels bio-wastes to prepare palladium bio-nanocatalyst for reducing tartrazine, methyl orange, sunset yellow, and Congo red in the presence of sodium boron hydride. Surprisingly, the palladium bio-nanocatalyst showed superior catalytic activity toward the dyes since the degradation rates of tartrazine, methyl orange, sunset yellow, and Congo red reached 96.1%, 96.4%, 97.2%, and 95.3%, respectively. Moreover, Kadam et al. (2020) highlighted the bio-preparation of silver photo-catalysts utilizing cauliflower wastes for methylene blue degradation. The transmission electron microscope image implied that the particles of silver nanocatalysts have a spherical shape in a nanosize of 5–50 nm. Furthermore, the bio-prepared silver exhibited a surface area of 19.2 m2/g and an average pore size of approximately 7.1 nm. Silver bio-nanocatalyst revealed high catalytic degradation toward methylene blue, reaching 97.6% with no metal leaching during the photocatalytic reaction.
To summarize, various agricultural and industrial biological waste has been effectively utilized as a valuable resource for the production of various nanoparticles through biological manufacturing. These nanoparticles derived from agricultural industrial waste have shown significant characteristics, including a considerable surface area and pore size. In addition, nanoparticles synthesized through biological methods exhibit extraordinary capabilities as efficient biological nanoadsorbents, biological nanocatalysts, and others. This trend not only helps to solve the problem of pollutants in wastewater but also provides new avenues for environmentally friendly and sustainable solutions. Extracting useful nanomaterials from waste not only aids in waste management but also facilitates resource reuse, thus contributing an important part to the environmental protection industry. These studies have shown the broad application prospects of biologically prepared nanoparticles, emphasizing their potential in sustainable environmental applications.
Mechanisms and principles
Green biosynthesis of nanoparticles
The green biosynthesis of nanoparticles involves utilizing biological systems, such as plants, bacteria, fungi, or other microorganisms, to facilitate the reduction of metal ions into nanoparticles. The inherent properties of biomolecules like enzymes, proteins, and metabolites in these organisms typically mediate this process. As enzymes can participate in catalytic reactions and complex synthesis, they have enormous potential in biotechnology research and applications, and additional research has shown that attaching enzymes or their substrates to the surface of nanoparticles can enhance catalysis (Bilal et al. 2022, 2023; Jin et al. 2023). In addition, enzymes play a crucial role in cellular activity by catalyzing metabolic reactions (Chapman and Stenzel 2019). The remarkable catalytic efficiency can be attributed mainly to the close interaction of the reaction components in the active site, which have been screened and ordered by nature. The chemical complementarity between folded nucleic acid and peptide enables them to successfully organize the necessary materials in the active site and activate heme (Liu et al. 2017). These findings have significant implications for the design of supramolecular enzyme mimics.
The distinctive catalytic properties of enzymes arise from the spatial organization of crucial functional groups within their active site, a consequence of their intricate three-dimensional folding (Sun et al. 2022). The obvious limitation in catalyst design is the difficulty of recreating the extraordinarily complex three-dimensional structure of the enzyme’s active site. Sun et al. (2022) designed a catalytic nanomaterial that mimics peroxidase. Components of polylysine and deoxyribonucleic acid have been found to exhibit synergistic effects that can enhance heme-catalyzed reactions. Additionally, Zhang et al. (2023) described a chimeric peptide deoxyribose system and introduced a novel design strategy for artificial metalloenzymes. The covalent bond between the g-quadruplex, heme, and an amino acid or oligopeptide determines the internal structure. Enzymes can be immobilized by attaching them to an active substrate or employing the appropriate chemical modifiers and linkers. This approach improves the heat and pH stability of enzymes while simultaneously lowering production costs. Vranish et al. (2017) used semiconductor quantum dots as model nanoparticle materials to link with prototype glucose peroxidase and horseradish peroxidase enzymes. The affinity of the semiconductor quantum dot surface to the substrate significantly affects enzyme activity when peroxidase binds to semiconductor quantum dots. Gu et al. (2022) prepared a co-immobilized dual enzyme biocatalyst with covalent binding. It has been determined that the dual enzyme biocatalyst has superior catalytic activity for the degradation of acridine in wastewater.
By combining enzymatic and chemical catalytic technologies, it is possible to efficiently eliminate pollutants from wastewater. However, their high cost constrains the industrial utilization of pure enzyme preparations. Ji et al. (2017) extracted a crucial enzyme from Pleurotus ostreatus intestines and immobilized it onto functionalized titanium dioxide nanoparticles. Notably, diluting the initial enzyme extract enhanced adsorption efficiency. In a separate study, Yuan et al. (2023) linked laccase to copper oxide@metal–organic frameworks, creating a stable composite through in situ microwave-assisted assembly and demonstrating synergistic catalytic capabilities. Introducing a novel approach, Chen et al. (2022) proposed an amino-functionalized enzyme–nanoparticle conjugate biocatalyst as an economically viable and environmentally benign solution for chlorophenol biodegradation. Notably, ferroferric oxide@silicon dioxide-amino showcased superior performance compared to free laccase across various aspects, encompassing temperature and storage stability, organic solvent resistance, and compatibility with metal ions.
The immobilization of enzymes on solid substrates can enhance enzymatic stability, versatility, and catalytic activity, thereby enhancing the cost-effectiveness of biocatalysis. Although the immobilization of enzymes has led to advancements in various fields, the design of immobilized platforms for biocatalysis involving large-sized substrates remains problematic because protein damage can occur during the generally severe reactions required for these processes. Farmakes et al. (2020) found that the coexistence of enzymes, metals, and compounds on the surface of graphite oxide can improve enzyme immobilization. The zero-curvature graphite oxide surface has a greater enzyme loading capacity than carbon nanotubes. Restricting enzymes to well-defined metal–organic framework compartments is feasible for simulating enzyme cellular environments and identifying structure–function correlations. Pan et al. (2023) used lysozyme as the nucleus of the metal–organic frameworks crystal scaffold, resulting in an unstructured space near the enzyme's outline and determining its relative direction and kinetics.
Microorganisms can generate nanoparticles through the adsorption or reduction of metal ions. This microbial synthesis can be categorized into two types, internal and external synthesis. The production of nanoparticles by different biological agents based on their reaction with diverse metal solutions. Several bacteria can generate a range of inorganic compounds, both within and outside their cells, exhibiting distinct intracellular and extracellular processes (Saravanan et al. 2021). Microbial synthesis notably diminishes the reliance on chemical reagents in nanoparticle production. A selection of instances illustrating extracellular and intracellular nanoparticle synthesis is provided in Table 1. The initial stage of nanoparticle synthesis within microorganisms involves the accumulation of metal particles either outside or inside the bacterial cells. Subsequently, enzymes catalyze the reduction of these metal particles into nanoparticles. Extracellular synthesis is more prevalent in comparison to intracellular synthesis. This preference is likely due to the easier purification and recycling of nanoparticles produced through extracellular synthesis. In contrast, nanoparticles synthesized within cells necessitate cell destruction using methods such as ultrasound for collection, which can complicate purification due to broken cells (Owaid and Ibraheem 2017).
The precise mechanism of microbial intracellular synthesis of nanoparticles is still unknown. An idea of the nanoparticle intracellular synthesis method is that the cell wall surface of microorganisms, negatively charged enzymes, or the cytoplasm of protein groups capture positively charged metal ions. The trapped metal ions are reduced to metal cores, and then different forms of nanoparticles are formed (Patil and Kim 2018). One advantage of using microalgae to generate nanoparticles is that it does not require any pretreatment of microalgae. This is because the formation process of nanoparticles in microalgae depends on metabolic pathways that may be responsible for syntheses, such as photosynthesis, respiration, and nitrogen fixation (Khanna et al. 2019). In addition, microalgae can be harvested year-round compared to other microorganisms, which makes them highly cost-effective and resource-efficient. When external conditions change, microalgae can regulate their metabolic pathways to adapt to environmental changes, and some components in the microalgae body also undergo corresponding changes during this process (Li et al. 2022). For the manufacture of nanoparticles, such properties have broad application prospects. Li et al. (2022) generated 20-nm gold nanospheres and gold hyperbranched nanostructures in Chromochloris zoffiensis.
Fungi and bacteria show the same tolerance and metal bioaccumulation ability, so they are often used to synthesize metal nanoparticles. Similar to the synthesis of nanoparticles by bacteria and microalgae, the fungal synthesis of nanoparticles can also be divided into two methods: extracellular and intracellular synthesis (Rajeshkumar and Sivapriya 2020). The consistency in the well-defined and square plate morphologies observed during the mycosynthesis process, in contrast to non-biologically formed microcrystals, suggests the involvement of a crucial ligand present in the fungal exudate in the controlled nucleation and growth of these particles. In other words, the biological components within the fungal exudate alter the chemical characteristics related to nanoparticle nucleation and development, ultimately leading to the formation of a uniform structure (Brady et al. 2023).
Given the flexibility and affordability of fungal growth on a large scale, extracellular or internal fungi extracts are attractive candidates for manufacturing metal nanoparticles. Several techniques and technologies are available for producing nanoparticles of gold from fungal components. Molnar et al. (2018) demonstrated and compared the outcomes of three different methods for producing gold nanoparticles using the external portion, autolysate, or intracellular components of 29 thermophilic fungi. Nanoparticles with diameters that range from 6 to 40 nm and standard deviations varying from 30 to 70% were produced. Zhang et al. (2019) employed Mariannaea sp. to produce selenium nanoparticles and discovered extracellular selenium nanoparticles in alkaline environments. Due to the difficulty of purification through intracellular culture, fungal-mediated cultivation of nanoparticles is still the majority through extracellular culture.
Ocean endophytic fungi represent an underexplored resource for nanoparticle synthesis and the production of secondary compounds. Manjunath et al. (2017a) improved a mechanism for the extracellular production of Cladosporium cladosporioides gold nanoparticles. Dependent reductases and phenolic compounds were discovered to participate in the biological reduction of gold metal salts into nanoparticles. Gold nanoparticles have potent antioxidant and antibacterial properties. Some fungi can produce nanoparticles through external and intracellular production. Manjunath Hulikere and Joshi (2019) employed Cladosporium cladosporioides to synthesize nanosilver ions, which were subsequently assessed for their antibacterial and antioxidant properties. Alfryyan et al. (2022) investigated the biosynthesis of metallic nanoparticles using two distinct methods. They also explored the structural characteristics and catalytic capabilities of the nanoparticles produced through these processes, employing various technologies.
In conclusion, the enzyme–nanoparticle/nanomaterial interactions field has experienced significant advancements. Researchers are increasingly drawn to microbial nanoparticle production owing to its environmental friendliness, cost-effectiveness, and simplicity. Selecting suitable microorganisms is crucial for practical metal nanoparticle synthesis, with growth rate, metabolic activity, and replication mechanisms playing pivotal roles. Studies have shown that modifying recombinant strains of bacterial species can enhance metal nanoparticle production. However, the creation of recombinant strains might contribute to increased nanoparticle production costs.
Use of reducing agents
The green solvent approach involves utilizing environmentally friendly solvents such as distilled water and ethanol, replacing conventional organic solvents for economical nanoparticle synthesis. These green solvents offer benefits, including environmental preservation, renewability, and low toxicity, minimizing harm to ecosystems and human health (Tilahun Bekele et al. 2021). Using hazardous and expensive compounds as reducing and capping agents may lead to larger particle sizes, driven by increased energy requirements. Green solvents can serve as alternatives to traditional organic solvents in diverse applications.
Ionic liquids are efficient solvents/media for lignocellulosic biomass utilization (Yoo et al. 2017). During the last few decades, ionic liquids have been investigated as potential substitutes. Because of the unique properties of liquids, they can dissolve and/or convert cellulose to a range of chemicals. Scientists and chemical engineers are becoming increasingly interested in the possible applications of ionic liquids. While the full potential of these exceptional solvents remains to be fully harnessed, the predominant application of ionic liquids has shifted from extraction to synthesis or catalysis. Initially utilized to extract biopolymers, these ionic liquids have found expanded use in the extraction of secondary metabolites. Polysaccharides and lignin are the primary biopolymers of interest (Avirdi et al. 2022). Vanda et al. (2018) examined the manufacturing and stability of silver nanoparticles in common ionic liquids as green media. Meanwhile, Nikfarjam et al. (2021) proposed antibacterial ionic liquid derivatives as monomers and polymers and discussed the antibacterial efficacy of ionic liquids.
Presently, the availability of low eutectic solvents is somewhat limited. Like ionic liquids, deep eutectic solvents comprise a few solid components and can form eutectic mixtures through chemical interactions. These mixtures yield deep eutectic solvents with lower melting points than the individual components. The efficient interaction of enzymes with eutectic solvents makes the enzyme/eutectic solvent system a promising approach for future biotransformation processes (Hooshmand et al. 2020). Juneja and Pandey (2022) categorized deep eutectic solvents for intramolecular in-dimer synthesis, aiding polymeric resolution. Maia et al. (2021) emphasized the diverse roles of deep eutectic solvents in synthesizing metal covalent organic frameworks. Ghigo et al. (2022) employed diazonium salts as substitutes for aryl halides to establish a mild, simple, and effective Ullmann reaction in deep eutectic solvents, showcasing their potential as environmentally friendly and sustainable solvent media.
Considering the toxicity and environmental damage caused by conventional ionic liquids, the introduction of the concept of natural deep eutectic solvents was evidently promising as it offered environmentally friendly alternatives. These mixtures have a significantly lower melting point than their components and a significantly lower melting point than the ambient temperature (Vanda et al. 2018). Components of natural deep eutectic solvents are sourced from nature and are believed to possess physiological or biological effects in their natural contexts. Frequently, these components are naturally present in foods. Unlike organic solvents, the fundamental structural elements of natural deep eutectic solvents are primarily governed by intermolecular interactions among their constituents. Consequently, the organic matrix of deep eutectic solvents is susceptible to various conditions (Liu et al. 2018).
Magnetic nanoparticles are being researched more deeply because of their unique chemical and physical characteristics. Ferrite is one of the most studied magnetic materials due to several critical physical features, such as a low melting point, high specific heat, and low saturated magnetic moment at low temperatures. For instance, Nasrollahzadeh et al. (2017a) described the ecologically friendly synthesis of copper/reduced graphene oxide/ferroferric oxide nanocomposites for an innovative catalytic system for aldehyde cyanidation to nitriles. This nanocomposite catalytic system utilizes an aqueous extract of Euphorbia officinalis leaves both as a reducing agent and a stabilizer. Under optimal conditions, it yields various substituted aryl nitriles in moderate to good yields. In a related study, Kombaiah et al. (2018) presented a straightforward and an environmentally friendly method for producing nanoparticles using “local” microwave heating. They also employed Abelmoschus esculentus gel as a reducing agent to synthesize cobalt iron oxide.
Ferrites have a regular spinel structure, and the connection between their magnetic and conductivity properties has caught the curiosity of numerous investigators caused of a broad spectrum of potential applications. Bashir et al. (2020) synthesized nickel ferrite nanoparticles using a Persa americano seed extract as a reducing agent. Physical and electrochemical characteristics of nickel ferrite nanoparticles were also investigated. Patil and Kim (2018) employed juice from Saccharum officinarum, a high-carbohydrate source, as a combustion fuel. The aldehyde functional group in the Saccharum officinarum juice acts as a reduction agent in the combustion cycle, while the precursor’s nitrate is an oxidant. This method simplifies the production of zinc ferrite nanoparticles, offering low cost and scalability. Similarly, Matinise et al. (2018) utilized a natural plant extract from Moringa oleifera as both a reducing and stabilizing agent during the overshoot phase in synthesizing zinc ferrite nanocomposites.
Zinc nanoparticles have a robust catalytic activity as a degradant of a wide range of chemical reactions generated by direct wide band gap photocatalysts. As a result, in addition to being antibacterial, zinc oxide holds excellent promise in photocatalysis and the introduction of industrial pollutant pigments. Chen et al. (2019) used Scutellaria baicalensis root extract as a lowering agent, indicating that other metabolites can be employed as end-cap compounds in the one-pot synthesis of zinc nanoparticles. Pai et al. (2019) utilized a water extract of Pterocarpus santalinus. Phenolic compounds within the leaf extract facilitated the conversion of zinc acetate into zinc oxide nanoparticles, forming the underlying principle. In a similar vein, Rupa et al. (2018) used polyphenols, flavonoids, and anthocyanins in the fruit of Rubus coreanus, to transform zinc oxide nanoparticles. Employing a green approach, Golmohammadi et al. (2020) synthesized zinc oxide nanoparticles using jujube fruit extract as both a reducing and stabilizing agent. In a remarkable achievement, they achieved a 92% degradation efficiency of methylene blue within 5 h.
An effective strategy for eliminating dye toxicity is photocatalytic degradation activity. The high specific surface area of zinc oxide nanoparticles renders them suitable for dye degradation. Chakraborty et al. (2020) synthesized zinc oxide nanoparticles from Averrhoa carambola and investigated their photocatalytic activity in Congo red dye. Averrhoa carambola biomolecules such as reducing sugar, flavonoids, and proteins are also critical green stabilizers. Rambabu et al. (2021) described a method for producing zinc oxide nanoparticles from discarded jujube pulp. Dye degradation investigations have shown that zinc oxide nanoparticles have amphoteric and fast photocatalytic activity and can break down hazardous dyes in synthetic wastewater. Mirgane et al. (2021) created zinc oxide nanoparticles from waste pineapple peel, which contains a variety of phytochemical substances and can be utilized as an agent for capping and reducing.
In summary, green solvents must adhere to strict criteria of non-toxicity, biodegradability, recyclability, sustainability, availability, and affordability to qualify as environmentally friendly media. Utilizing green solvents allows for producing superior products while mitigating the environmental harm associated with conventional methods employing organic solvents. Researchers exploring innovative green solvents opt for high-quality natural resources derived from plants, animals, microorganisms, and marine organisms. The search for potential components should extend beyond an increase in animal-based research and encompass biological or physiological processes within living organisms that can inspire concepts or serve as templates for simulation.
Plant extract-mediated synthesis
Numerous phytochemical compounds present in plants, such as flavonoids, alkaloid compounds, tannins, saponins, and others, significantly influence the production of nanomaterials (Mohammadzadeh et al. 2022). Secondary metabolites, which plants generate for therapeutic purposes, encompass diverse biological activities and can serve as crucial markers in nanoparticle synthesis. Extracts function similarly to stabilizers and reducing agents (Jadoun et al. 2021). The mechanism of nanoparticle production using lignin as both a reducing agent and stabilizer notably depends on pH, with lignin concentration affecting the size and dispersion of silver nanoparticles (Iravani and Varma 2020). Zinc oxide nanoparticles derived from Ficus microcarpa leaf extracts exhibited high larvicidal efficacy against mosquitoes and demonstrated antibacterial solid properties (Ragavendran et al. 2023).
Plants serve as abundant, non-toxic, and harmless sources with significant economic benefits. Plant extracts such as phenols, saponins, terpenoids, and vitamins have medical potential while being environmentally safe (Shanavas et al. 2020; Aslam et al. 2021). These plant extracts can manufacture nanoparticles as reducing agents and stabilizers. Garcinia L. plants are high in phenolic metabolites, potent antioxidants, and free radical scavengers. Compared to other plants, they can effectively lower nanoparticles (Demenciano et al. 2020; Sarip et al. 2022). Garcinia hanburyi is combined with silver nitrate, gold chloride, copper nitrate, and other metal salt solutions at room temperature and stably interacts with groups with functions or electrostatic interaction of nanoparticles (Lee et al. 2019; Akintelu et al. 2021). Table 2 lists the preparation of metal nanoparticles using different parts of different plants.
Some biological components found in plant extracts have a vital role in reducing the number of metal ions and forming the top layer of nanoparticles of gold. For example, Abdoli et al. (2021) produced gold nanoparticles from cornflower leaves. Similarly, Balalakshmi et al. (2017) suggested a green synthesis approach for gold nanoparticles based on low-cost Sphaeranthus indicus leaf extracts, in which leaf extract components act as blocking and lowering agents during the gold nanoparticles process. Hamelian et al. (2018) produced gold nanoparticles with Thymus serpyllum extract in a green and environmentally friendly manner. Desai et al. (2018) described a simple, fast, and environmentally friendly method of manufacturing photoluminescent gold nanoparticles with catalytic and antioxidant activities utilizing kokum fruit extract. Naraginti and Li (2017) synthesized environmentally sustainable multi-functional gold and silver nanoparticles using an Actinidia lindl extract and subsequently evaluated their efficacy against Pseudomonas aeruginosa.
Gold nanoparticles have numerous applications, such as sensing probes, imaging, therapeutic agents and drug administration, diagnosis, cancer treatment, catalysis, and environmental applications. Gold nanoparticle manufacturing through traditional processes is expensive and may also harm the environment. Plant-based gold nanoparticle manufacturing is less costly and more environmentally friendly. Hosny and Fawzy (2021) used water extracts from Persicaria salicifolia leaves to create gold nanoparticles in an immediate, one-step, economical, and ecologically friendly biosynthesis. Zhao et al. (2021) created gold nanoparticles from Tribulus terrestris extract. Natural oxygen-containing phytochemistry promotes the conversion of green-reducing ions of trivalent gold ions to matching nanoparticles, which are then stabilized by encapsulation. This alteration inhibits the gold nanoparticles from aggregating. Gangapuram et al. (2018) created spherical gold nanoparticles from Anna squamosa L. fruit waste as a depressant and stabilizer, with an extensive list of possibilities for treating hazardous industrial effluent.
Silver nanoparticles exhibit remarkable antibacterial properties, and there is a growing trend of using plants as biological sources for their synthesis. Giri et al. (2022) focused on creating and characterizing silver nanoparticles from fully matured Eugenia roxburghii leaves while assessing their efficacy in reducing biofilm formation. Abdi et al. (2019) bio-synthesized silver nanoparticles from aqueous extracts of Rhizophora mucronata stems, roots, and leaves, exploring their antibacterial attributes. Shah et al. (2021) generated silver nanoparticles from an aqueous crude extract of Plantago asiatica and examined their antibacterial and antioxidant properties. Gopu et al. (2022) successfully synthesized silver nanoparticles from a solution of silver nitrate using Momordica charantia leaf extract, with the change in color indicating the formation of silver nanoparticles.
The production of silver nanoparticles is gradually transitioning toward a less harmful biological approach, which holds promise for environmental benefits. Using plant extracts in the biological synthesis of metal nanoparticles seems to be a viable alternative alongside chemical and physical methods. Dogiparthi et al. (2021) described a bioreduction method for producing silver nanoparticles using an aqueous leaf extract of Micrargeria wightii. Madivoli et al. (2020) employed a water extract of Lantana camara L. to produce silver nanoparticles, which were subsequently tested for antibacterial activity. Vakili et al. (2022) used the leaves of Biarum chaduchrum to make 100-nm spherical silver nanoparticles. Kumavat and Mishra (2021) investigated silver nanoparticles with antimicrobial and antimicrobial properties in vitro using Borago officinalis leaf extracts as environmentally friendly reducing agents. This research introduces a simple, efficient, and rapid procedure. Plant extracts incorporate biological components that serve as stabilizers and reducers in nanoparticle synthesis.
Platinum, a valuable silver-white precious metal with high density, has piqued the interest of many researchers in its nanoparticle form produced from plants. The green attributes, sustainability, and economic significance of palladium nanoparticles derived from plants also have garnered significant attention among academicians. Lebaschi et al. (2017) developed a unique approach for reducing aqueous palladium ions utilizing biologically active palladium@Btea nanoparticle catalysts. The polyols and carbonyls are reduction factors in the water-based extract and blocking/stabilizing agents. Nasrollahzadeh and Sajadi (2016a) detailed the eco-friendly synthesis of palladium nanoparticles using water-based extracts from Euphorbia officinalis leaves. They also highlighted the notable catalytic efficacy of these nanoparticles in both the Stille and Hiyama crossover bonding processes, achieved without additional reagents. Aygun et al. (2020) used Nigella sativa L. extract as a reducing agent to synthesize spherical platinum nanoparticles with sizes between 1 and 6 nm.
Copper nanoparticles are functional in catalysis, water purification, data storage, and other applications. However, the conventional synthesis methods of copper nanoparticles are associated with environmental pollution and toxicity concerns. Nagar and Devra (2018) utilized Azadirachta indica mesophyll soup to synthesize copper nanoparticles and investigated the effect of reaction parameters on nanoparticle conversion efficiency and morphology. Rajesh et al. (2018) used Syzygium aromaticum and Syzygium aromatic bud extracts to produce copper nanoparticles through a simple and environmentally friendly green technique. They studied their structural, morphological, optical, and antibacterial properties. Nasrollahzadeh et al. (2017b) synthesized copper nanoparticles under eco-friendly reaction conditions using Plantago depressa leaves extract as a natural solvent and biological medium. Sharma et al. (2019b) discussed the application of Tinospora cardifolia leaf extract in producing copper nanoparticles. The studies highlighted the potential of biological agents for the green synthesis of copper nanoparticles with reduced toxicity and improved properties, suitable for various applications in drugs, health care, and the environment.
Zinc oxide is an inorganic material that acts as a semiconductor, and various studies have shown that zinc oxide nanoparticles produced using green synthesis processes have good antibacterial properties. Khatami et al. (2020) examined the biosynthesis of rod-shaped zinc oxide nanoparticles using Lilium brownii var. viridulum extract and the anti-Leishmania spp. impact of zinc oxide nanoparticles. Doan Thi et al. (2020) suggested a green production of zinc oxide nanoparticles using orange peel extract that is efficient, environmentally beneficial, and simple. Thakur et al. (2020) used extracts from Jatropha curcas and Tinospora cordifolia leaves to create zinc oxide nanoparticles utilizing a green synthesis approach. Antibacterial efficacy against Staphylococcus aureus was most remarkable in zinc oxide nanoparticles derived from jatropha plants. Kombaiah et al. (2017) produced rectangular zinc oxide nanoparticles in the 10–90 nm range using Stevia rebaudiana (Bertoni) Hemsl extract and investigated antimicrobial solid activity against Leishmania spp., S. aureus, and Escherichia coli. These studies have demonstrated the potential of green synthesis processes for producing zinc oxide nanoparticles.
The application of potentially hazardous components in chemical synthesis can be avoided using plant-based nanoparticle synthesis. Plant-based biosynthesis is not only inexpensive, but it also has high biocompatibility and minimal environmental toxicity. Thakur et al. (2020) developed the technology for manufacturing barium ferrite nanoparticles from Acorus calamus rhizome extract. They examined the antifungal activity of various dosages of barium ferrite nanoparticles against pathogenic fungi affecting multiple plant species. Madhukara et al. (2019) produced zinc ferrite nanoparticles from Limonia acidissima using the green synthesis method, and the resulting zinc ferrite nanoparticles demonstrated excellent photodegradation of Evans blue and methylene blue when exposed to visible light. In the work of Kombaiah et al. (2017), Opuntia mill plant extracts were used as organic reagents to synthesize nanoparticles. In the study, two approaches were used to synthesize zinc iron oxide nanoparticles, namely the conventional and microwave methods, using plant extracts to synthesize nanoparticles.
Incorporating plant-based approaches for nanoparticle synthesis is not the only avenue. Transforming biomass waste into value-added nanoparticle products can contribute to the circular bioeconomy. Due to their ability to efficiently extract pollutants from solutions using a magnetic field, magnetic oxides have great potential for water pollution remediation applications (Abdel Maksoud et al. 2022). For the first time, Osman et al. (2022) carried out an in-depth investigation on the application of fruit residue extract as a reducing agent in the synthesis of magnetite carbon composite nanoparticles, in addition to the manufacture of magnetite carbon composite materials by pyrolysis of biomass waste and waste plastic to remove crystal violet dye from water. Osman et al. (2020) utilized brewer’s spent grain to manufacture carbon in two ways, employing a new approach of a large surface area of carbon and hydrophilic carbon nanotubes to recover biomass waste raw materials, realizing the circular economy concept. In addition, El-Nahas et al. (2020) proposed converting waste aluminum and silica gel into active zeolite materials for water hardness treatment using standard household microwave ovens. This approach requires fewer chemical supplies, templates, and multi-step programs and is 70% less expensive than readily accessible zeolites.
In conclusion, plant extracts boast a rich reservoir of bioactive compounds, rendering them a highly explored avenue for green synthesis methods, particularly in heavy metal accumulation and detoxification. The efficiency and eco-friendliness of biosynthesis have led to the successful production of diverse nanoparticles utilizing plant extracts. This approach has been extensively investigated for its potential across various domains. Moreover, plants house a variety of distinctive compounds that expedite synthesis and enhance synthesis rates. The realm of green nanomaterial synthesis from plants stands as an intriguing facet of nanotechnology, significantly contributing to environmental sustainability and the progression of nanotechnology.
Green hybrid nanoparticles
Due to its excellent light stability, oxidation resistance, and high electron mobility, zinc oxide is a critical inorganic semiconductor component. Silver-doped zinc oxide, synthesized through green methods, possesses advantageous properties like high thermal conductivity, setting it apart from other precious metals. Mousavi-Kouhi et al. (2021) utilized the green approach of Verbascum speciosum to synthesize silver–zinc oxide nanoparticles and evaluated their cytotoxicity. Azizi et al. (2016) used Zingiber officinale roscoe essential oil to develop an innovative green method for producing zinc oxide silver core–shell nanocomposites. The essential oil serves dual roles, acting as a reaction medium for zinc oxide synthesis and a reducer for silver ions. Several plants have been successfully utilized for producing green mixed nanoparticles, as highlighted in Table 3.
Due to their remarkable antibacterial and antifungal properties, silver–zinc oxide nanocomposites are extensively studied for their medical applications, such as dye degradation reagents, dye absorbers, wound healing, cancer chemotherapy, and drug delivery. Sohrabnezhad and Seifi (2016) synthesized silver–zinc oxide nanocomposites using Urtica fissae leaves extract, incorporating silver metal nanoparticles to enhance their photocatalytic efficiency. Rajaboopathi and Thambidurai (2018) described the chemical coprecipitation synthesis of silver–zinc oxide nanoparticles mediated by Sargassum pallidum and its extracts. These nanoparticles exhibit potential as catalysts for the photodegradation of industrial dye effluents. Swati et al. (2020) utilized Moringa oleifera Lam. seeds extract to fabricate well-aligned, uniform silver–zinc oxide nanostructures.
Nanoparticles generated by doping two or more metal elements exhibit more substantial reduction effects than undoped oxide nanoparticles. For the first time, Khan et al. (2018) developed undoped zinc oxide nanoparticles from Cistanche deserticola water extract, as well as manganese-doped zinc oxide nanoparticles from Clerodendrum infortunatum and Clerodendrum inerme water extracts. These findings suggest that Clerodendrum informationatum and Clerodendrum inerme aqueous extracts are fantastic agents for reducing the environmentally friendly synthesis of undoped and copper-doped zinc oxide nanoparticles with potent antibacterial, antioxidant, and antifungal potential. Nasrollahzadeh et al. (2018) developed a unique and ecologically conscious method of producing copper nanoparticles loaded with manganese dioxide nanoparticles. Centella asiatica leaves extract was employed as a natural reducing agent without adding a stabilizer or surfactant. Phenolic hydroxyl groups from the leaf extract are hypothesized to reduce copper ions in the medium, leading to the formation of copper nanoparticles that are subsequently anchored to the outermost layer of the manganese dioxide nanoparticles. Nasrollahzadeh et al. (2018) developed copper nanoparticles held by sodium borosilicate glass using Acalypha indica L. leaf extract as a reducing agent and stabilizer, and they also investigated the potential for catalyst reuse in the decrease reaction.
Hybrid nanoparticles with more than two metal components have better photocatalytic activity than single nanoparticles. Pakzad et al. (2019) initially directed their attention toward employing phenolic compounds derived from Euphorbia pekinensis Rupr. extract. These compounds were utilized to reduce and obstruct copper oxide and nickel@ferroferric oxide nanoparticles during their biosynthesis. Subsequent investigations have delved into the photocatalytic detoxification of color-related biosynthetic nanoparticles. In a study by Sayadi et al. (2022), zinc oxide-stannic oxide nanocomposite nanoparticles were synthesized using an extract from Viscum album L. leaves. These nanoparticles were then utilized to degrade Congo red, biphenyl-A, and tetracycline. Remarkably, even after being used four times, the nanoparticles retained their effectiveness, highlighting the durability and recyclability of the zinc oxide-stannic oxide nanocomposite nanoparticles produced through this method. Somanathan et al. (2019) used a Tinospora cardifolia extract to manufacture cerium-nickel@ferric oxide utilizing a microwave-aided combustion technique. When compared to pure cerium-nickel@ferric oxide, cerium-nickel@ferric oxide had higher photocatalytic activity.
Creating cost-effective and environmentally friendly nanoparticles for contaminant removal is achievable through the eco-friendly synthesis of iron-based bimetallic nanoparticles using various plant extracts, which helps overcome certain synthesis limitations. In the work of Pakzad et al. (2019), eco-synthesized ferrum/nickel nanoparticles were employed to remove triclosan and copper ions from aqueous solutions. In the study of Lin et al. (2021), green tea functioned as a reducing and capping agent in synthesizing a composite of reduced graphite oxide loaded with iron-nickel nanoparticles. This composite exhibited the capability to eliminate trivalent antimony ions from aqueous solutions. Gao et al. (2019) employed Ginkgo biloba linn extract as a green stabilizer to create ferrum/cobalt bimetallic nanoparticles to remove triclosan from aqueous solutions. Through carbon green, Gong et al. (2022) produced ferrum/nickel nanoparticles to remove 17 β-estradiol. The effectiveness of estradiol removal exceeded 98.3% in their study.
In summary, the comparison between single nanoparticles produced through green synthesis and mixed nanoparticles reveals that mixed nanoparticles exhibit superior performance across all aspects. The green synthesis approach can amalgamate the attributes of various nanoparticles, yielding hybrid nanomaterials that harness the benefits of individual nanoparticles, as shown in Fig. 3. In contrast to conventional techniques, the green synthesis method for effectively creating mixed nanoparticles addresses synthesis constraints, diminishes environmental repercussions stemming from the synthesis process, minimizes production expenses related to nanoparticles, and facilitates the sustainable advancement of nanoparticle manufacturing.
Mechanisms of green synthesis of nanoparticles using plant extracts, bacteria, and fungi. The foremost goal of researchers has always been to produce green nanoparticles through eco-friendly methods. The synthesis of nanoparticles mediated by microorganisms and plants is a critical step in nanotechnology. Enzymes and other chemical substances in microorganisms and plants can act as reducing and blocking agents in biosynthesis. These methods can produce stable and capped nanoparticles with high yield. Many characterization techniques can be used to study the properties of produced nanoparticles. Green nanoparticles can be used in biomedical, agriculture, environmental, food industry, cosmetics, and energy applications
In conclusion, the increasing demand for environmentally friendly science and nanostructures has driven the adoption of green synthesis methods for crafting nanomaterials. This approach curbs pollution and reduces costs while enhancing nanoparticle stability and performance. This section has provided insights into the mechanisms and principles of green nanoparticle synthesis, covering aspects such as nanoparticle biosynthesis, biological reduction, extraction-mediated synthesis, and mixed nanoparticles. Future endeavors in green nanoparticle synthesis should prioritize improving preparation efficiency, bolstering nanoparticle stability, and maximizing the utilization of eco-generated nanoparticles.
Applications
Over the past decades, there have been remarkable advancements in green nanomaterials, positioning them as top contenders across various domains, including biomedical, agriculture, environmental, food, sensors, electronics, and more, as shown in Fig. 4 and Table 4. Whether used to improve medical diagnosis and treatment, enhance agricultural production efficiency, reduce environmental pollution, improve food quality, develop efficient sensors, or promote innovation in electronic technology, green nanoparticles have shown strong application prospects. The exceptional capabilities of nanoparticles in these diverse sectors stem from their small size, distinctive morphology, extensive surface area, and distinct physical and chemical attributes. This versatility and potential for widespread application have made green nanomaterials an important research direction in the fields of science and engineering.
Applications of nanomaterials in various sectors. Nanomaterials demonstrate significant promise in biomedicine, featuring attributes like antimicrobial, anticancer, and antioxidant properties, alongside drug delivery functions. In agriculture, nanomaterials primarily contribute to nanofertilizers and nanopesticides. Notably, they play a substantial role in water and soil purification. Within the food industry, nanoparticles are widely employed in nanopackaging, nanonutraceuticals, and nanocarriers
Biomedical applications
Antibacterial effect
Undoubtedly, harmful bacteria in our surroundings pose a significant health risk, as they can easily infiltrate the human body. Additionally, the excessive use of antibiotics has led to drug resistance, complicating the treatment of various infections. Hence, ongoing research aims to uncover effective antibacterial agents for these challenging infections. Green nanoparticles have shown promising antibacterial activity against a wide range of gram-negative and gram-positive bacteria. However, the mechanisms behind growth inhibition and bactericidal effects remain unclear. Notably, the properties of nanoparticles, including their morphology, size, and surface area, among others, play a crucial role in determining how they damage bacterial cells. This could involve interactions between nanoparticles and cell walls/membranes or the penetration of nanoparticles into bacterial cells, leading to their destruction.
Anand et al. (2019) fabricated zinc oxide nanoparticles from Prunus dulcis via the disk diffusion approach. The scanning electron microscopy, i.e., SEM showed the nearly spherical shape of zinc oxide with a size of around 25 nm. Moreover, the antibacterial action of zinc oxide was studied using the agar diffusion technique. The concrete results elucidated the propitious antibacterial activity of zinc oxide against gram-positive where its inhibition zones on S. aureus was 18 mm, while the diameter of the inhibition zones of zinc oxide on E. coli and Salmonella paratyphi was in the range of 32–25 mm. On the contrary, zinc oxide did not exhibit antibacterial activity toward gram-negative bacteria, such as Proteus mirabilius and Klebsiella pneumoniae (Anand et al. 2019). In another study, Stan et al. (2016) reported the synthesis of zinc oxide via a chemical approach and a green method from three green resources: garlic, basil, and rosemary. The experimental results showed a higher antibacterial action of the green zinc oxide than the chemically prepared zinc oxide. Furthermore, the maximal zone diameters of the as-fabricated zinc oxide from garlic, basil, and rosemary against S. aureus were 22.0 mm, 19.3 mm, and 19.2 mm, respectively.
In one more attempt, Behravan et al. (2019) prepared silver nanoparticles from Berberis vulgaris and studied their antibacterial action on two bacterial species: S. aureus and E. coli. The excellent antibacterial effect of silver nanoparticles against both bacterial species was deduced. More importantly, the antibacterial mechanism of silver involves impairing the respiratory process of bacteria through potential interactions between silver and thiol groups. In addition, the high affinity of silver nanoparticles toward sulfur and phosphate-containing membrane cells facilitates the interaction between silver nanoparticles and the bacterial cell membrane. This plausible mechanism was consistent with Sathishkumar et al. (2018), suggesting that magnetite's antibacterial effect against S. aureus and E. coli occurred via the collapse of the cell membrane. Besides, deoxyribonucleic acid, i.e., DNA destroying and reactive oxygen species formation mechanisms, contributed to the damage of bacterial cells.
In conclusion, green nanoparticles have demonstrated impressive antibacterial effects against numerous bacterial species. The antibacterial activity of nanoparticles is greatly influenced by their morphology, size, and surface area, which play a significant role in causing damage to bacterial cells. Typically, the breakdown of bacterial cells occurs through membrane disruption and/or damage to the deoxyribonucleic acid, along with the formation of reactive oxygen species. However, further research is needed to understand the precise interaction pathways between nanoparticles and bacterial cells.
Anticancer effect
Globally, a staggering number of deaths occur annually due to cancer. This has prompted ongoing research to develop effective therapies for this malignant ailment (Jahangirian et al. 2017). Treatment approaches such as chemotherapy, surgery, and radiotherapy entail substantial patient risks. These include potential harm to healthy cells, insufficient drug delivery to tumors, and drug instability (Garg et al. 2021). Consequently, finding an innovative therapeutic approach to address these drawbacks is paramount. Green nanoparticles have demonstrated remarkable anticancer properties against diverse cancer cell types.
In this context, Devanesan et al. (2021) examined the anti-tumor activity of the green-synthesized silver nanoparticles from Carica papaya against the human liver cancer cell line, such as HepG2. Cytotoxicity of the green silver nanoparticles revealed that half maximal inhibitory concentration, namely IC50 of silver nanoparticles to treat human breast cancer cell line, i.e., MCF-7 was about 10 μg/mL during 24 h, while it increased to threefold during the next 24 h. Furthermore, the anti-proliferation action of silver was scrutinized against MCF-7, implying a diminution in the volume of the cell line. It demonstrated the capacity of silver nanoparticles to induce apoptosis in the HepG2, MCF-7, and human lung cancer cell lines such as A549, as evidenced by acridine orange and ethidium bromide staining after 24–72 h. Moreover, Li et al. (2021) prepared gold nanoparticles from Mentha longifolia to kill breast cancer cells. Scanning electron microscopy elucidated the spheroidal morphology of the green gold nanoparticles with a size of 30–45 nm. Such a particle size is a preference for anti-tumor applications since it is less than 50 nm (Hemmati et al. 2020). Thence, gold nanoparticles exhibited promising anti-breast cancer actions against MCF-7, human mammary gland/breast adenocarcinoma cell line, i.e., Hs 578Bst, human mammary gland/breast adenocarcinoma cell line such as UACC-313, and breast cancer cells namly Hs 319.T without toxicity to the normal cell. In addition, the bio-fabricated magnetite with an extract concentration of 10 wt% revealed higher anti-tumor activity. In addition, the IC50 of the colon cancer and normal cells were 99.8 μg/mL and 140.8 μg/mL, respectively (Yusefi et al. 2021). Shortly, green nanoparticles elucidated an auspicious anti-tumor action against several cancer cells without cytotoxicity. This new therapeutic way opens a new avenue to finding the finest treatment for this deadly disease. Nevertheless, most of the studies lack the in vivo analysis to confirm the viability of these green nanoparticles as anti-tumor drugs.
Antioxidant effect
Antioxidants, whether natural or synthetic, play a crucial role in preserving biomolecules like sugars, nucleic acids, and proteins from damage caused by free radicals. These antioxidants can be categorized into two main groups: natural compounds, which encompass exogenous sources like vitamins, carotenoids, polyphenols, and metal elements, as well as endogenous sources like enzymatic and nonenzymatic substances. Furthermore, there exist synthetic antioxidants such as phenolic compounds and nanooxidants (Bendary et al. 2013). Notably, many antioxidant mechanisms, such as the scavenging of reactive oxygen species, depend on the number and position of active groups. In addition, an antioxidant also acts via the inhibition of enzymes, hydrogen peroxide/hydroperoxide decomposition, and metal ions chelation mechanism (Flieger et al. 2021).
In one study by Hosny et al. (2022), platinum nanoparticles were synthesized using Atriplex halimus for potential biological applications. The high-resolution transmission electron microscopy images displayed the spherical structure of these bio-synthesized platinum nanoparticles, characterized by a small particle size ranging from 1 to 3 nm. Interestingly, the study observed a significant increase in the scavenging percentage of the 1,1-diphenyl-2-picrylhydrazyl radical, namely DPPH when exposed to these platinum nanoparticles. The DPPH scavenging percentage rose dramatically from an initial value of 13.8% to a remarkable 72% as the concentration of the platinum nanoparticles increased from 12.5% to 50 mg/mL. Furthermore, the scavenging activity of A. halimus against DPPH was measured at 48.35% when the concentration of A. halimus was elevated to 50 mg/mL. The positive control group using vitamin C demonstrated a lower scavenging percentage against DPPH compared to the platinum nanoparticles and A. halimus. The scavenging rate of vitamin C reached 47.8% at a concentration of 50 mg/mL.
In another study, Vitta et al. (2020) tested the antioxidant actions of Eucalyptus robusta and the derived bio-synthesized zerovalent iron from it against DPPH radicals. The scavenging capacity of zerovalent iron nanoparticles was observed to be higher than pure Eucalyptus robusta. While assessing the scavenging capabilities of the extract and bio-fabricated zerovalent iron, it is essential to note that not all previous studies have concurred with these findings. Some investigations have suggested that the scavenging capacity of zerovalent iron nanoparticles is either lower or occasionally on par with that of the unaltered extract (Rosli et al. 2018; Ibrahim et al. 2019). It was detected that lower concentrations of polyphenols and flavonoids in zerovalent iron nanoparticles than in the pure extract reflected the contribution of these compounds to the reduction process during the fabrication of zerovalent iron nanomaterial. More importantly, the presented polyphenols and flavonoids in zerovalent iron nanoparticles could act as stabilizing agents.
In summary, antioxidants derived from environmentally friendly sources have shown remarkable effectiveness in neutralizing free radicals such as 1,1-diphenyl-2-picrylhydrazyl radical, namely DPPH. Nanoparticles synthesized from natural sources can capture free radicals through diverse mechanisms, including enzyme inhibition, chelation of metal ions, and the direct scavenging of reactive oxygen species. Notably, in certain instances, the inherent antioxidant potency of the unprocessed extract might surpass that of the synthesized nanoparticles, while in other scenarios, the opposite could hold. The antioxidative efficiency of these eco-friendly nanoparticles hinges on the concentrations of phenolic compounds and flavonoids present within the extract.
Drug delivery
Advancements in drug delivery systems have become necessary to enhance the effectiveness of pharmaceutical compounds, including vaccines, proteins, enzymes, and drugs. Conventional drug delivery methods such as solutions, emulsions, and suspensions encounter various challenges, including high dosages, limited selectivity and availability, reduced stability, and the potential for fluctuations in plasma drug levels. As a result, extensive efforts have been dedicated to achieving targeted delivery that avoids clearance or rapid degradation. The evolution of drug delivery systems can be categorized into three generations. The first generation primarily focused on achieving sustained transdermal and oral administration release. The second generation emphasized the utilization of environmentally friendly nanomaterials. The third generation tackles the physicochemical and biological limitations of drug delivery systems (Kanwar et al. 2019). Interestingly, nanosubstances have exhibited promising drug delivery systems for treating varied diseases owing to their controlled drug release, high drug-loading capacity, bioavailability, targeted delivery, and conserving loaded drugs of moisture, physiological pH, and enzymes.
In this perception, Owoseni-Fagbenro et al. (2019) investigated utilizing the egg protein as a stabilizing agent for silver nanoparticles (protein-capped silver) for fabricating a drug vehicle to hesperidin. It was found that the hesperidin loading rate reached 83.3%, suggesting the protein-capped silver capability to deliver hesperidin to the targeted cells with a high concentration. Notably, the polydispersity index of free protein-capped silver was 0.3, which increased to 0.5 after the loading of hesperidin, implying successful drug loading. Furthermore, zeta potential measurements demonstrated that the net charge onto free protein-capped silver was − 18.5 mV. In comparison, the carried charge onto the surface of hesperidin-loaded protein-capped silver was − 20.2 mV. The observed augmentation in the net surface charge of protein-capped silver after loading with hesperidin can be attributed to negatively charged groups within the hesperidin molecules.
In another study, Fang et al. (2019) reported the preparation of gold nanoparticles from Juglans regia for using them as a release vehicle for zonisamide. The zonisamide release rate from zonisamide-gold nanoparticles was compared to the free zonisamide, revealing that zonisamide released gradually from the zonisamide-gold system, whereas the free zonisamide demonstrated an earlier release for ten days. Then, after 11 days, the total release percentages of zonisamide from the zonisamide-gold system and free zonisamide were 76.2% and 96%, respectively, inferring the viability of the as-prepared zonisamide-gold system as a release vehicle for zonisamide.
In this connection, Küp et al. (2020) explored the drug-release behavior of silver nanoparticles derived from Aesculus hippocastanum. The study initially examined the Fourier transform infrared, i.e., FTIR spectrum of resveratrol-loaded silver nanoparticles, confirming the binding of resveratrol to the silver nanoparticles. Furthermore, the zeta potential measurements indicated an increase in the negative charge carried by resveratrol upon binding with silver nanoparticles, transitioning from − 12 to − 34 mV. This shift in zeta potential reflected the successful conjugation between resveratrol and silver nanoparticles. The release profile of resveratrol was then assessed. Within the initial half-hour, the release rate reached 11.3% at pH 5.2 and 8% at pH 7.4. After 5 h, the release percentages increased to 45.6% at pH 5.2 and 32.3% at pH 7.4. This observation indicated that the release rate of resveratrol was notably quicker in an acidic environment, which is consistent with prior research (Kou et al. 2013). This accelerated release in an acidic medium aligns with the potential effectiveness of the acidic conditions found in cancer cells on the resveratrol release profile. In conclusion, using green nanomaterials has shown promising results regarding drug-loading efficiency and controlled release for various medications. However, the lack of in vivo studies remains a constraint, hampering their broader application in the field of pharmacology.
Agriculture applications
Nanofertilizers
Bio/nanofertilizers represent environmentally friendly substances capable of supplying essential nutrients to plants, fostering their growth, and increasing crop productivity. Bio-fertilizers, also known as bacterial fertilizers or microbial cultures, utilize live or dormant microorganisms to stimulate plant growth. These bio-fertilizers are categorized into beneficial nutrients, nitrogen fixation, phosphorus stabilization, phosphorus mobilization, and plant growth-promoting rhizobacteria (El-Ghamry et al. 2018). On the other hand, nanofertilizers can be divided into three categories: (i) nanoparticulates, including nanoparticles like titanium dioxide, silica, and carbon nanotubes, which enhance plant growth; (ii) micronutrients, including copper, zinc, iron, molybdenum, nickel, and manganese; and (iii) macronutrients combine phosphorus, calcium, nitrogen, and potassium. Notably, research has indicated that phosphatic nanofertilizers led to a remarkable 32% increase in soybean growth and a 20% boost in seed production compared to conventional fertilizers (Liu and Lal 2014). Furthermore, calcium phosphate nanomaterials decorated with potassium and nitrogen have the potential to reduce plant nitrogen requirements by approximately 40% more than conventional fertilizers.
Bio/nanofertilizers can foster plant growth via many mechanisms: (i) reduce hazardous impacts of abiotic and biotic stresses on the plants; (ii) increase the nutrient solubility; (iii) induce the phytohormone production in soils that foster plant nutrition; (iv) improve the growth of plants by nitrogen fixation; (v) enhance the fertility of soils; and (vi) sustain a suitable environment (El-Ghamry et al. 2018). In this context, de França Bettencourt et al. (2020) reported the bio-synthesized silver nanoparticles from onion as a nanofertilizer for brinjal and tomato. The green plants were sprayed with varied concentrations of silver nanoparticles ranging from 5 to 15 mL/L. The plant's vigor and weight were higher with raising the nanofertilizer concentration to 15 mL/L. This result may be ascribed to the abundance of proteins, phosphorous, carbohydrates, and potassium in onions that can improve the growth of plants (Gosavi et al. 2020). Additionally, de França Bettencourt et al. (2020) fabricated a bio-fertilizer incorporating bacteria-containing auxin complexes to promote the growth of maize plantlets. The results deduced the positive impact of the singlet oxides (iron oxide and manganese oxide) and the mixed oxides on boosting seed germination, fresh weight, and developing the roots of maize plantlets.
In summary, green nanomaterials offer a viable avenue for utilization as bio/nanofertilizers, facilitating plant growth, seed germination, and overall fresh weight enhancement. Nanofertilizers can not only help plants better cope with various pressures such as drought, diseases, and pests but also trigger a series of biochemical reactions in the plant body, such as enhancing the plant's immune capacity and stimulating the production of auxin in the plant. In addition, the use of nanofertilizers also helps to increase the solubility of nutrients in the soil, improving soil structure and fertility while further promoting healthy plant growth. These environmentally friendly fertilizers are produced through straightforward and rapid methods. The augmentation of plant growth can be attributed to a diverse range of mechanisms.
Pesticides
Plant diseases pose an urgent challenge, resulting in an annual cost of approximately 220 billion dollars to global agriculture (Wang and Nguyen 2018). These diseases encompass a variety of sources, including protozoa, insects, fungi, worms, parasites, bacteria, and viruses, all of which contribute to crop damage. In response, biological and chemical pesticides have advanced to address the complexities of modern agriculture (Kaur et al. 2022). Recent years have seen the emergence of novel technology in the agricultural sector: nanopesticides. These nanopesticides involve encapsulating pesticides to regulate their release, enhance stability, and improve selectivity. Nanopesticides offer a range of notable advantages, including (i) augmenting the solubility of otherwise insoluble active ingredients, (ii) refining the release dynamics of the pesticide, (iii) enhancing mobility due to their diminutive particle size, (iv) fortifying pesticide stability and deterring premature degradation, (v) extending effective functioning over time, and (vi) diminishing the presence of harmful components (Athanassiou et al. 2018). Remarkably, adopting nanopesticides exhibits substantial potential in combating plant diseases, boasting benefits such as heightened water solubility, controlled release mechanisms, improved mobility, prolonged effectiveness, and reduced inclusion of detrimental elements. This comprehensive array of advantages presents a viable and promising solution to the pressing agricultural challenges faced on a global scale.
In this context, El-Saadony et al. (2020) compared the insecticidal effects of copper nanoparticles derived from Pseudomonas fluorescens with those of chemically synthesized copper nanoparticles. The results revealed that as the concentration of bio-fabricated copper nanoparticles increased, the mortality percentage of Tribolium castaneum also rose, reaching 100% after five days when using 300 ppm of bio-copper. On the other hand, the chemically manufactured copper nanoparticles did not exhibit an apparent detrimental effect on Tribolium castaneum, potentially due to insufficient copper concentrations in the chemical formulation to effectively eliminate the pest. This observation aligns with the findings of Shaker et al. (2016), who reported a mortality rate of 95% for Spodoptera littoralis when using 1000 ppm of chemical copper oxide (Shaker et al. 2016). Moreover, the chemical copper nanoparticles tended to aggregate, highlighting the need to incorporate a stabilizing agent to mitigate this aggregation issue.
In another study, properties of bio-fabricated iron oxide sourced from Trigonella foenum-graecum (fenugreek) against Tuta absoluta. Employing a low concentration of 100 μg/mL of iron oxide, the mortality rate of T. absoluta reached 72% within 3 days. Similarly, Vivekanandhan et al. (2018) demonstrated the remarkable larvicidal efficacy of bio-fabricated silver nanoparticles against three mosquito species. Additionally, Benelli (2018) underscored the substantial toxicity of green nanoparticles to mosquito larvae without causing adverse effects on aquatic organisms. However, it is crucial to acknowledge that the potential adverse effects of nanomaterials on the environment remain incompletely understood. Thus, a comprehensive understanding of the environmental behavior of nanopesticides, both during and after their application, necessitates further assessments to discern their potential impacts on ecosystems.
Environmental remediation
Water remediation
Water pollution represents a profound and widespread challenge that threatens the overall health of the planet. In response, environmental experts have been dedicated to developing effective methods for water remediation, including coagulation, precipitation, catalysis, ozonation, and adsorption. Among these techniques, adsorption and catalysis stand out due to their commendable efficiency, straightforward processing, low toxicity, and potential for reusability. These attributes make them particularly attractive options for the purification of wastewater. Green nanomaterials have showcased impressive adsorption and catalytic capabilities, particularly in removing hazardous pollutants such as pharmaceutical residues, heavy metals, aromatic compounds, and organic dyes.
In this context, Jain et al. (2021) fabricated iron nanomaterial derived from Artocarpus heterophyllus to degrade Fuchsin's basic dye. X-ray diffraction analysis confirmed the successful synthesis of zerovalent iron, hematite, and iron oxyhydroxide. Employing an iron/hydrogen peroxide system, they achieved excellent Fenton-like degradation of Fuchsin basic, with a degradation rate of 87.5% within the first 20 min. In contrast, the degradation rate was merely 4.8% when using hydrogen peroxide alone, devoid of iron nanoparticles. Fuchsin basic degradation involved adsorption and Fenton-like degradation, as Eqs. (1–8) outlined.
(1) Adsorption
(2) Fenton-like process
Moreover, Prakash et al. (2021) delved into the degradation of acid blue-15 using magnetite synthesized through green methods in a sono-Fenton reaction. The study inferred that the activation of hydrogen peroxide to generate hydroxyl radicals could occur through multiple pathways: (i) the Fe2+/Fe3+ redox reaction, (ii) leached iron ions initiating a homogeneous Fenton process, (iii) the power of ultrasonic waves splitting hydrogen peroxide, and (iv) water splitting leading to the production of hydroxyl radicals. Subsequently, these generated hydroxyl radicals could effectively target acid blue-15, generating intermediates that react with hydroxyl radicals and ultimately transform into carbon dioxide and water.
In one attempt, Rahmayanti et al. (2022) explored the adsorption capabilities of magnetite nanoparticles derived from Jengkol toward methylene blue. The increase in Jengkol extract volume, ranging from 5 to 15 mL, led to a reduction in the particle size of the magnetite. This phenomenon is likely attributed to the significant role of Jengkol's phenolic content as a capping agent, preventing particle agglomeration. Analyzing the magnetism using a vibrating sample magnetometer revealed that pure magnetite exhibited higher magnetism than the magnetite derived from Jengkol. Specifically, the saturation magnetization values were 68.4, 25.6, and 12.9 emu/g for pure magnetite and Jengkol-derived magnetite using 5 mL and 15 mL of extract, respectively. Notably, according to the Langmuir model, the calculated adsorption capacities of methylene blue onto magnetite were 65.4 and 68.5 for Jengkol-derived magnetite using 5 mL and 15 mL extract, respectively. The adsorption mechanism for methylene blue onto Jengkol-derived magnetite primarily occurred through hydrogen bonding and electrostatic interactions.
In another attempt by Debnath et al. (2020), zirconium oxide was synthesized using Pseudomonas aeruginosa to adsorb tetracycline from wastewater. The efficacy of tetracycline removal by zirconium oxide increased as the pH was raised, reaching its peak (more than 98%) at pH 6. This phenomenon can be attributed to the versatile nature of tetracycline, encompassing cationic form (pH less than 3.3), anionic form (pH more than 7.7), and zwitterionic form (pH 3.3–7.7). This indicates the likelihood of electrostatic interactions between the zwitterionic tetracycline and the positively charged zirconium oxide (7.2 point of zero charge). Remarkably, zirconium oxide exhibited a distinct adsorption behavior compared to other green adsorbents of relevance. Its maximum adsorption efficiency reached an impressive 526.32 mg/g in a concise span of 15 min. Furthermore, zirconium oxide showcased excellent recyclability, with a removal efficiency of 81.6% even after undergoing five use cycles.
Soil remediation
The issue of severe soil contamination has worsened over time, resulting in increasing detrimental effects on both ecosystems and human health. Various methods have been employed to address soil pollution, including excavation/disposal and excavation/ex situ treatment. However, these approaches are time-consuming, costly, and highly toxic. As a result, in situ treatment techniques, such as introducing nanoparticles into the soil, have gained popularity. Specific green nanomaterials have demonstrated remarkable efficacy in remediating contaminants like dyes and heavy metals within the soil. This is attributed to their heightened mobility, exceptional reactivity, low toxicity, and substantial surface area (Wang et al. 2019). Generally, soil remediation is not the main factor in boosting the quality of soil; there are other factors, including (i) decline of the aggregation of the soil, (ii) enhance the organic material-containing soil, (iii) boost the nitrogen-phosphorus-potassium cycle, and (iv) promote the soil nutrition.
In one study, Soliemanzadeh and Fekri (2017) prepared a bentonite-green zerovalent iron nanocomposite for removing chromium from polluted soil. The Fourier transform infrared pattern showed the characteristic bands of the polyphenols-containing green tea, reflecting the dual function of green tea that acts as a reducing and stabilizing agent. The experimental results demonstrated the lower released chromium ions from the treated soil by bentonite-green zerovalent iron than the control one. In addition, a decline in the mobile fraction and an increase in residual and oxide-bound fractions were recorded. These findings inferred the preference for bentonite-green zerovalent iron nanocomposite to stabilize and adsorb chromium ions from pollutant soils. Furthermore, Noman et al. (2020) concluded that the biogenic copper nanomaterials boosted the length of wheat plants and their biomass by declining the cadmium influx and enhancing the nutrients delivered. Thus, copper could be exploited instead of remote techniques to dwindle the cadmium stress in wheat plants. Although the confirmed ability of green nanoparticles to decline the heavy metals stress in several plants, the molecular pathways of nanoparticles in plants during the alleviation of the metal stress need more investigations.
In another study, Zaki et al. (2021) reported the application of the bio-fabricated zinc oxide from Trichoderma harzianum as an antifungal agent against soil pathogens, including Macrophomina phaseolina, Rhizoctonia solani, and Fusarium sp. in different cotton cultivars. It was concluded that the antifungal action of zinc oxide changed according to the cotton cultivar since in Giza90, the plant survival, length, and weight were improved, inferring the high efficacy of zinc oxide 200 μg/mL in controlling the disease. In the Giza94 cultivar, although the plant survival was boosted compared to the controlled infested plant using 200 μg/mL of zinc oxide, the disease control was not preferable in terms of survival. Nevertheless, zinc oxide showed eminent treatments in the weight and length of the Giza94 cultivar. Moreover, Kalaba et al. (2021) used the bio-prepared zinc oxide from Streptomyces plicatus for controlling plant microbes. The experimental results elucidated that the excellent death rate of Meloidogyne incognita attained 96.7% within three days. Furthermore, zinc oxide promoted the seed germination of Vicia faba using a concentration in the range of 12.5–50 μg/mL. Green nanoparticles have demonstrated remarkable efficacy in soil remediation, particularly in removing heavy metals such as cadmium, nickel, arsenic, and chromium from contaminated soils. Moreover, these nanoparticles have antimicrobial solid effects against various plant pathogens, influencing plant weight, length, and overall survival.
Food applications
Ensuring the safety and freshness of food products is the primary objective of the food industry. Maintaining the freshness of fruits and vegetables for extended periods presents a critical challenge due to potential microbial contamination and inherent natural deterioration. Additionally, the quality of food products can deteriorate during storage and the supply chain (Martínez-Molina et al. 2022). Consequently, there is a pressing need to enhance the food packaging process to extend the shelf life of these products. Green nanomaterials have been extensively applied to the food sector, including anticaking agents, nanoadditives, and antimicrobial agents. They are precious in food packaging due to their potential to act as quality sensors, capable of monitoring and recording food quality (Ezhilarasi et al. 2013). However, a comprehensive assessment of nanomaterial toxicity is imperative to unlock its full potential in the food industry (Dikshit et al. 2021). Moreover, establishing regulations governing the production and application of nanomaterials in the food sector is crucial.
In one investigation, Gao et al. (2020) compared the preservative effects of green-fabricated zinc oxide and its commercial counterpart. The investigation evaluated weight loss, firmness, and rotting rates of strawberries treated with green zinc oxide/carboxymethyl cellulose, commercial zinc oxide/carboxymethyl cellulose, and carboxymethyl cellulose, compared to control strawberries. The outcomes indicated the beneficial impact of carboxymethyl cellulose in reducing weight loss in strawberries. Furthermore, after five days, the strawberries coated with commercial zinc oxide/carboxymethyl cellulose exhibited lower weight loss than those coated with green nanoparticles. Both green and commercial zinc oxide demonstrated the ability to inhibit mold growth and extend the shelf life of treated strawberries, attributed to their potent antifungal properties.
Additionally, the treated strawberries with green and commercial zinc oxide exhibited higher firmness than the control samples. Likewise, Kumar et al. (2019) investigated the packaging effectiveness of green zinc oxide-incorporated agar for preserving green grapes. Practical experiments revealed moldy spots on a significant portion of plastic-wrapped green grapes after a week, accompanied by a foul smell. Control green grapes exhibited mildew at the ends and yellow spots on the surface. In contrast, green grapes wrapped with 2% zinc oxide-incorporated agar remained fresh and visually appealing after two weeks. Interestingly, increasing the proportion of zinc oxide to 4% extended the shelf life of green grapes to 21 days. In summary, green nanoparticles have demonstrated a notable preservation capability across various food products. Their potent antifungal properties make them particularly suitable for packaging materials. However, comprehensive toxicity studies are required to harness their potential in the food industry.
Photovoltaics and energy storage
Carbon, a fundamental component of life and a pivotal element in coal, never ceases to amaze us with its remarkable versatility. Its latest manifestation as graphene has sparked renewed fascination. Graphene quantum dots, a captivating nanomaterial comprising one or a few graphene layers, boast exceptional and distinctive properties. These quantum dots find utility in diverse fields by merging carbon dots and graphene attributes. Graphene quantum dot-based materials could be used in sensing, bioimaging, and energy storage (Rathnasamy et al. 2017; Kumar et al. 2020b). A meticulous spotlight is directed toward prevalent applications, encompassing electrochemical and photoluminescence sensors, electrochemiluminescence sensors, humidity, and gas sensors, bioimaging, lithium-ion batteries, supercapacitors, and dye-sensitized solar cells. With a quest for innovative production pathways, researchers seek to unearth novel techniques for synthesizing graphene quantum dots, emphasizing their distinctive traits and properties.
Intriguingly, the synthesis of graphene quantum dots materializes through green synthesis routes, employing an array of carbon precursors such as fruit extracts, peels, food waste, algal blooms, bacteria, milk, cabbage, and even human urine (Li et al. 2011; Chen et al. 2018; Kumar et al. 2020b). A comparative analysis against conventional synthesis underlines the varying morphology and ultraviolet–visible spectroscopy absorbance achieved via green chemistry methods. A comparative analysis against conventional synthesis emphasizes the variable morphology and ultraviolet–visible spectroscopy absorbance achieved via green chemistry methods. For instance, Teymourinia et al. (2017) employed a green chemistry approach to synthesize graphene quantum dots using corn powder as a precursor. The synthesized graphene quantum dots were subjected to an ultraviolet light-induced rhodamine B degradation test. Compared to traditional titanium oxide materials, the graphene quantum dots/titanium oxide composite exhibited remarkable photocatalytic activity, achieving approximately 53% degradation of rhodamine B within 80 min.
Similarly, Chen et al. (2018) undertook a green and efficient hydrothermal approach, utilizing natural polymer starch as a graphene quantum dot production precursor. The outcome yielded graphene quantum dots, along with water and carbide precipitate. Notably, the diameter of the generated graphene quantum dots fell within the range of 2.3–3.5 nm. This green chemistry method facilitated carbonization and functionalization, harnessing biomass carbon sources and employing low reaction temperatures. The resultant quantum dots exhibited distinctive fluorescence characteristics contingent on the surface functionalities present. These examples underscore the potential of green synthesis approaches in producing graphene quantum dots with tailored properties, promising advancements in fields like photocatalysis and fluorescence modulation.
Many investigators have discussed the role of graphene quantum dots and the effect of their concentration as a sensitizer material in different types of solar cells to achieve better power conversion efficiency. Solar energy is paramount to replacing traditional non-renewable sources like fossil fuels. The core of a solar cell, a p–n junction, operates via the photovoltaic effect, where electrons and holes serve as charge carriers contributing to photocurrent (Chuhadiya et al. 2021). Among these advancements are organic and perovskite solar cells; dye-sensitized solar cells have emerged. Dye-sensitized solar cells encompass a dye-loaded photoanode, an electrolyte with a redox couple, and a catalytic counter electrode.
Teymourinia et al. (2018) have synthesized two types of graphene quantum dots at two different temperatures for 8 h and 48 h, respectively, through the green synthesis method using corn powder as a starting material. It has been investigated as a dye-sensitized solar cell that achieved 14.8% and 21.6% power conversion efficiency while using graphene quantum dots as down conversion material.
Additionally, Rathnasamy et al. (2017) employed a green synthesis technique, utilizing C. papaya leaf extract, to synthesize hexagonal wurtzite zinc oxide nanoparticles. Through a comprehensive characterization involving various analytical methods, they delved into the resulting sample’s structural phase purity, morphology, and size. The outcomes of these analyses unveiled that the synthesized zinc oxide nanoparticles exhibited a singularly pure hexagonal wurtzite structure, adopting a spherical form with an approximate particle size of 50 nm. Notably, the application potential of these synthesized zinc oxide nanoparticles extended to photocatalysis and photovoltaics. In the context of photocatalysis, these nanoparticles demonstrated the capability to entirely degrade methylene blue dye within 180 min under ultraviolet light irradiation.
Moreover, when integrated as a photoanode in dye-sensitized solar cells, they showcased an energy conversion efficiency of 1.6% alongside a current density of 8.1 mA cm−2. In essence, this study underscores the successful utilization of green synthesis techniques to fabricate zinc oxide nanoparticles, highlighting their multifaceted utility in both environmental remediation and energy-related applications. In conclusion, the synergy between green nanotechnology and energy cells, exemplified by photovoltaics, illuminates a path toward a greener and more energy-efficient future. By harnessing the potential of nanomaterials, sustainable synthesis methods, and innovative solar cell technologies, we can contribute to the global transition to renewable energy sources and mitigate the environmental challenges of conventional energy generation.
Advantages and limitations
A pioneering approach in green nanotechnology has introduced a novel paradigm that focuses on creating nanotechnologies with zero carbon emissions and minimal pollution. Notably, this process avoids using hazardous synthetic chemicals for reduction, setting it apart from other non-chemical approaches like bacterial, fungal, and algal nanoparticle synthesis (González-Ballesteros et al. 2017; Thipe et al. 2022). Beyond the inherent advantages of traditional green synthesis, these green nanotechnology techniques offer the additional benefits of speed and cost-effectiveness. Leveraging agricultural waste materials, they ensure environmental sustainability (Joseph et al. 2021; Mbatha et al. 2021; Palombarini et al. 2021; Kadkhoda et al. 2022; Kumar and Lim 2022; Yang et al. 2022).
The ambition of green nanotechnology is to eliminate all harmful substances from production processes, as noted by various researchers (Al-Yasiri et al. 2017; Gamal-Eldeen et al. 2017; Katti et al. 2018; Thipe et al. 2019; Khoobchandani et al. 2020; Sibuyi et al. 2021; Tangthong et al. 2021). An integral facet of green nanotechnology involves the use of plant-derived phytochemicals as agents for reducing and stabilizing metal ions into metallic nanoparticles (Thipe et al. 2022). Green chemistry is governed by 12 widely acknowledged principles that serve as a framework for creating or enhancing materials, procedures, products, and systems: (i) hazardous waste minimization, (ii) maximizing the end product through atom-level synthesis route economy, (iii) the synthetic pathways don’t contain any dangerous substances, (iv) creating safe and biocompatible materials, (v) application of environmentally friendly solvents like water, (vi) low energy inputs are used to provide high yields with little adverse effects on the environment and the economy, (vii) sustainability through the utilization of renewable feedstock or raw materials, (viii) minimize derivatives to prevent extra steps, (ix) using a catalyst instead of inefficient stoichiometric reactions, (x) design that produces harmless breakdown products, (xi) life cycle analysis with the purpose of preventing and reducing environmental pollution, and (xii) more efficient producing, storing, and transporting processes (DeVierno Kreuder et al. 2017, Thipe et al. 2022).
Recognizing the prevalent use of hazardous and ecologically detrimental compounds in many synthetic nanoparticle production processes is paramount. Conventional methods for synthesizing metallic nanoparticles involve risky chemical-reducing agents like hydrazine, sodium citrate, and sodium borohydride (Sengani et al. 2017; Mat Isa et al. 2022). Similarly, specific abrasive physical techniques, such as pyrolysis and attrition, employed for synthesizing various nanomaterials yield immediate and long-term environmental repercussions (Soares and Soares 2021). In comparison to chemical and physical approaches, green synthesis offers numerous advantages. It is characterized by its benign nature (Devi et al. 2019), environmentally friendly, cost-effectiveness (Kataria and Garg 2018), pollution-free (Alsammarraie et al. 2018), and more sustainable (Nasrollahzadeh and Sajadi 2016b). Furthermore, the realm of green nanotechnology demonstrates its potential in diverse applications, including air purification (Cao et al. 2021), site remediation (Kumar 2021), waste and byproduct reduction (Dhingra et al. 2010), and wastewater treatment (Cao et al. 2021). Consequently, there exists a critical need to devise nanoparticle production methodologies that minimize or eliminate the usage of hazardous chemicals, while concurrently implementing engineering solutions to mitigate or entirely eradicate the presence of imperceptible and perilous nanoparticulate contaminants within the overall production process.
Limitations
The utilization of green technology for synthesizing nanoparticles offers significant advantages characterized by cost-effectiveness and ease of application (Hano and Abbasi 2022). This production process avoids the use of harmful chemicals and solvents, enabling the precise fabrication of consistent nanoparticle sizes and shapes, and consequently reducing waste generation (Harish et al. 2023). Moreover, it operates at lower pressure and temperature conditions compared to traditional methodologies, thereby reducing the likelihood of unexpected incidents (Dikshit et al. 2021). Additionally, it facilitates the utilization of readily available sustainable raw materials for large-scale production (Soltys et al. 2021). Nevertheless, green synthesis is not without its shortcomings. Foremost among these is the consideration of human health risks associated with nanotechnology. Currently, research on the environmental toxicity and accumulation of nanoparticles is quite limited, and the small size of these particles may facilitate their entry into the human body, potentially leading to respiratory issues and severe illnesses (Pietroiusti et al. 2018). Furthermore, the application of green synthesis in industry remains relatively limited, posing challenges in controlling particle size and shape, as well as issues related to storage stability (Dikshit et al. 2021). A comprehensive toxicological assessment and genetic modifications are required to enhance the synthesis and application efficiency. Specific issues, such as the lack of universally applicable plants for biogenic synthesis and seasonal factors, also serve as constraints hindering the widespread application of green technology. These limitations are comprehensively evaluated, as shown in Fig. 5.
Potential limitations and challenges in the technology of green synthesis of nanoparticles. The synthesis of nanoscale metals through green methods faces challenges such as high energy consumption, lengthy reaction times, and reliance on specific plant extracts. Variability in particle sizes and forms further complicates the process, limiting precise control. The lack of understanding regarding biosynthesis mechanisms and the inability to address complex environmental pollutants with consistent efficiency pose significant hurdles. Storage stability and reusability of green-synthesized nanoparticles need careful consideration. Lastly, comprehensive toxicological assessments and optimization of genetically modified microorganisms are essential for expanding nanoparticle applications
Availability of raw material
Numerous plant components are available for the eco-friendly synthesis of nanoparticles, and many researchers have extensively explored utilizing locally accessible plant resources. These investigations present an opportunity for harnessing the potential of indigenous flora. Nevertheless, the global-scale production of environmentally synthesized nanoscale metals poses significant challenges. One challenge is the lack of a universal plant to synthesize green nanomaterials. For instance, palladium nanoparticles were synthesized using Lithodora hispidula Griseb. leaf, a plant species exclusively found in regions such as Cyrenaica, Cyprus, the southern Aegean Sea, and southern Turkey (Turunc et al. 2017). Sapium sebiferum and Euphorbia plants, two additional botanical components essential for palladium nanoparticle synthesis, are predominantly located in subtropical zones (Tahir et al. 2016; Turunc et al. 2017). In the case of silver nanoparticles, coconut, widespread in the Philippines, India, Malaysia, Sri Lanka, and southern China (Roopan et al. 2013), and Acacia, mainly distributed in Arabia, Africa, and mainland China (Ying et al. 2022), have been utilized.
Efforts have also been made to capitalize on native plants for the green production of other nanoparticles like copper nanoparticles, nanoscale zerovalent iron, iron oxide nanoparticles, and gold nanoparticles. Although peppermint originates from central and western Europe, fenugreek, employed in gold nanoparticle synthesis, is extensively dispersed across China and the eastern Mediterranean (Aromal and Philip 2012; Ying et al. 2022). Notably, there are regions in China where both Galaxaura elongata and A. calamus coexist, with A. calamus growing at an elevation of 2600 m along shorelines (Abdel-Raouf et al. 2017; Ganesan and Prabu 2019). In producing nanometer zerovalent iron, a unique form of Chinese tea known as Oolong tea is used (Huang et al. 2014b). While nanoscale zerovalent iron is primarily produced in Fujian Province, key production centers for nanosized iron oxide are Taiwan and Hainan (Balamurughan et al. 2014). Iron oxide nanoparticles can also be synthesized using alternative substances like psoralen, mainly sourced from India, Myanmar, and Sri Lanka (Nagajyothi et al. 2017b). Furthermore, Andean blackberry Rubus glucus Benth, abundant in Ecuador, Colombia, and the Andean region of central and South America, has successfully synthesized copper nanoparticles (Kumar et al. 2017). Given these considerations, it becomes imperative to assess the feasibility of utilizing local plant resources for large-scale production of nanoscale metals when making choices regarding synthetic materials.
Time constraints also limit the utilization of raw materials in practical production processes. As noted by Sana and Dogiparthi (2018), the constituents essential for crafting silver nanoparticles necessitate extraction from cotton leaves during the flowering season or from Sargassum fusiforme, whose growth cycle varies significantly depending on the geographical location (Ghaemi and Gholamipoor 2017). Similarly, Arabica coffee is employed in this context, yet the Arabica tree requires approximately seven years to reach full maturity and is typically cultivated at altitudes around 1500 m (Dhand et al. 2016). Peach blossoms necessitate meticulous harvesting during the flowering season, reducing availability. Trigonella trifoliata seeds, crucial for gold nanoparticle synthesis, are only obtainable during the fruiting season of the plant, spanning from July to September.
Moreover, the blooming and fruit-bearing period of White willow is limited to April and May, imposing a narrower timeframe for leaf collection. Pelargonium and Cymodocea serrulate share similar constraints (Jafarizad et al. 2015; Swain et al. 2016). Moreover, not all raw materials are suitable for environmentally friendly nanoscale metal synthesis due to the requirement for additional processing of certain secondary products, which elevates complexity and increases technology implementation costs. Therefore, it is imperative to determine the practicality, economic feasibility, and cost-effectiveness of these materials. Wang et al. (2017) directly integrated pure tea polyphenols for nanoscale zerovalent iron synthesis, but the extraction and purification processes proved economically impractical. Carboxymethyl cellulose presents a similar scenario, being used for palladium nanoparticle synthesis (Li et al. 2017). Although cellulose is an ecologically favorable raw material, carboxymethyl cellulose necessitates carboxymethylation, involving material sourced from other natural plants, such as Sago pulp. While sodium monochloroacetate, sodium hydroxide, and other reagents aid the process, their compatibility with green synthesis may be a concern (Ying et al. 2022).
Synthesis process
The main challenges in the synthesis process involve high energy consumption, long reaction times, and the use of additional chemical reagents. For example, Nasiri et al. (2018) used Ferula persica extracts at 600 °C for three hours to create silver nanoparticles, while Caroling et al. (2015) employed Guava fruit extract at 800 °C for copper nanoparticles. Copper oxide nanoparticles can be made more efficiently in two hours through ultrasonic stirring at 80 °C (Muthuvel et al. 2020). Consequently, specific green synthesis methods involve high temperatures and lengthy processes, consuming significant energy and potentially harming the environment. These procedures might not fully qualify as green synthesis despite using environmentally friendly materials. González-Ballesteros et al. (2017) synthesized gold nanoparticles at 24 °C using the brown alga Cystoseira baccata, which also required substantial energy. Certain plant extracts must also be stored at low temperatures, requiring further energy consumption. For instance, Azadirachta indica leaf extract should be stored at 4 °C (Ahmed et al. 2016), and dried grass extract should be kept below 4 °C (Khatami et al. 2018). This entails the use of energy-intensive equipment like freezers in these cold conditions. Producing nanoscale metals at room temperature is advisable to address this energy consumption and streamline the synthesis process.
Reaction time is crucial in manufacturing efficiency and cost, with a preference for quicker reactions. However, several studies have shown that longer reaction times are sometimes necessary. For example, a combination of mint leaf extract and iron nitrate must be placed in a rotary track shaker for 72 h at 30 °C and 200 rpm in the dark. Furthermore, in synthesizing iron chloride solution involving algae, agitation in an orbital shaker for 48 h at 24 °C in complete darkness is essential (Subramaniyam et al. 2015).
Similarly, the process of creating iron nanoparticles from a mixture of black tea and iron sulfate takes a full day, followed by 24 h of drying at 250 °C in an oven (Ali et al. 2016). On the other hand, producing copper oxide nanoparticles from coffee powder extract requires microwave radiation, involving three hours of boiling and four to five hours of drying in a hot air oven (Ahmed et al. 2016). In contrast, the sol–gel method for synthesizing nanoscale metal oxides is much simpler, necessitating continuous heating at 60 °C for an hour, followed by 30 min of stirring and centrifugation (Perveen et al. 2020). Additionally, resorting to lengthy extraction procedures is not ideal. For instance, extracting oil from mango peel involves a 12-h boiling process (Yirsaw et al. 2016). Cherry, mulberry, and oak leaf extracts must be pre-dried in an oven for 48 h at 50 °C, according to Poguberović et al. (2016).
Similarly, Sargassum acinarium and Padina pavonica must be washed with distilled water and freeze-dried at 20 °C for three days to obtain algal extract (El-Kassas et al. 2016). It is important to note that metal nanoparticles are susceptible to oxidation in the air. Even chemically inert metals can oxidize under mild conditions due to the impact of surface coordination, which is significantly influenced by the high surface-to-volume ratio and the disruption of three-dimensional symmetry (Ngom et al. 2016; Ismail et al. 2017). To mitigate this, some studies propose conducting synthesis under inert conditions to prevent oxidation. For example, Nasrollahzadeh and Sajadi (2016b) used Euphorbia granulate leaf extract in the centrifugal separation of palladium nanoparticles under an inert argon atmosphere. In another case, nitrogen was used to synthesize nanoscale zerovalent iron (Leili et al. 2018). However, this approach adds complexity and expense to the synthesis procedure.
Describing the synthesis process accurately through chemical reactions is a major obstacle in achieving green biosynthesis. Another significant limitation is the lack of comprehensive understanding regarding the biosynthesis mechanism. For instance, Fuku et al. (2016) observed that Punica granatum L. peel extract can be an end-capping agent for copper/copper oxide/zinc oxide nanostructures synthesis. Similarly, Sageretia thea has been identified as a chelating agent for iron oxide nanoparticle synthesis (Singh et al. 2015), and an extract of Zingiber officinale root has been used as a reducing and capping agent for the synthesis of silver nanoparticles (Kora and Rastogi 2016). Essentially, while the role of green extracts in synthesis can be established, the precise details of the reaction processes remain challenging to comprehend. To compare green and non-green syntheses, life cycle assessments have been conducted. Clearly, non-green synthesis methods demand substantial energy, with a significant portion attributed to electricity consumption. Furthermore, these methods exert more pressure on the environment, particularly regarding energy resources and greenhouse gas emissions (Bobba et al. 2016; Zhang et al. 2017).
Nanoparticles quality
The size and shape of nanoparticles generated by different extracts exhibit significant variability. Recent reports indicate a wide range of particle sizes, making it challenging to control size uniformity in production, rendering green technology less suitable for large-scale manufacturing. For instance, silver nanoparticles synthesized from Nigella arvensis leaves exhibited sizes ranging from 5 to 100 nm (Chahardoli et al. 2018), while nanoscale zerovalent iron nanoparticles produced from grape seeds displayed a size range of 63–381 nm (Gao et al. 2016). Scanning electron microscopy analysis revealed varying particle diameters for gold nanoparticles synthesized from Pistacia integerrimagall, aqueous Elaise guineensis, and Galaxaura elongata varied substantially, spanning 20–200 nm, 13–97 nm, and 2–100 nm, respectively (Ahmed et al. 2016; Abdel-Raouf et al. 2017; Islam et al. 2019). Similar challenges extend to other substances like green tea (Mystrioti et al. 2016), citron juice (Shende et al. 2015), Cassia alata flower (Sutradhar et al. 2014), and Rosa canina fruit (Veisi et al. 2016).
Moreover, some materials present structural imperfections. Leili et al. (2018) observed irregular cluster formation in nanoscale zerovalent iron synthesized from nettle and thyme leaf extracts, and KumaráBorah et al. (2015) noted irregular morphologies in palladium nanoparticles made from Colocasia esculenta leaf extract. Copper oxide nanoparticles derived from black soya beans exhibited spherical and hexagonal forms and diverse shapes (Nagajyothi et al. 2017b). Nanoscale zerovalent iron produced from blueberries showed high heterogeneity (Manquián-Cerda et al. 2017); however, non-crystalline nanoscale zerovalent iron was produced using Urtica dioica leaf extract or Eichhornia crassipes aqueous extract (Ebrahiminezhad et al. 2017; Wei et al. 2017). Conversely, smaller palladium nanoparticles from Camellia sinensis extract tended to aggregate into non-spherical shapes (Lebaschi et al. 2017). These variations limit the practical utility of the end product, restrict its applicability, and pose challenges for mass production.
Additionally, nanoparticles synthesized from green resources exhibit poor yield and conversion rates. For example, using extracts of Menthaspicata (spearmint, SM), Syzygium aromaticum (clove), and Camellia sinensis (green tea), minimal iron reduction was observed under all conditions tested, with less than 50% of iron converted into nanoparticles (Wang et al. 2014a). Similarly, Eucalyptus tereticornis, Melaleuca nesophila, and Rosemarinus officinalis leaf extract yielded iron-palladium nanoparticles with iron concentrations of 0.2%, 8.6%, and 0.5%, respectively (Arsiya et al. 2017). In contrast, physical methods for nanoparticle synthesis can produce highly pure materials with consistent particle sizes (Xu et al. 2020). This phenomenon stems from the low utilization and conversion rates of metal ions, resulting in a limited number of nanoparticles produced from a high metal ion concentration, which hinders economic feasibility.
Application constraints
Metal nanoparticles possess significant potential for contaminant removal due to their extensive surface area, strong adsorption capabilities, and high reducibility. However, not all metal nanoparticles produced through green synthesis effectively address environmental pollutants. For instance, nanoscale zerovalent iron created from Spinacia oleracea, as studied by Turakhia et al. (2018), exhibited removal efficiencies of only 73.8% for chemical oxygen demand and 60.3% for biochemical oxygen demand after 15 days. In another study, nanoscale zerovalent iron derived from mandarin, lemon, lime, and orange showed degradation efficiencies ranging from 12 to 37% when applied to chromium reduction (Machado et al. 2014). Similarly, nanoscale zerovalent iron produced from Eucalyptus leaf extract could remove 30.4% of total phosphorus from wastewater (Wang et al. 2014b). These insufficient removal rates raise concerns due to the ineffective utilization or wastage of iron.
Green-synthesized nanoscale metals also struggle to remove hazardous metal mixtures. Weng et al. (2016) employed iron nanoparticles from Eucalyptus leaf extract to eliminate mixed copper and chromium, resulting in 33.0% and 58.9% efficiencies, respectively. In contrast, when copper and chromium were treated separately, removal efficiencies improved to 45.2% and 74.2%, respectively. Furthermore, the combined presence of metal ions leads to decreased removal effectiveness. Since real-world wastewater typically contains combinations of heavy metals and contaminants, enhancing efficiency is crucial for using environmentally friendly synthesized nanoscale materials in such scenarios. The application of nanoscale materials produced through green synthesis in vitro cells remains relatively unexplored. Research by Nagajyothi et al. (2017b) revealed that copper oxide nanoparticles synthesized from black soya beans damaged the mitochondrial membrane of Hela cells. In a separate study utilizing the sulforhodamine assay, iron oxide nanoparticles produced from Psoralea corylifolia seeds aqueous extract exhibited dose-dependent suppression of human breast cancer cells. These nanoparticles also displayed the ability to inhibit renal tumor cell growth at low concentrations (Nagajyothi et al. 2017a). However, the outcomes of utilizing green nanoparticles in vivo remain uncertain as these experiments have primarily been conducted in vitro. Hence, further research is required to ascertain their impact on other cells within a living organism.
Anticipated areas of future research
Ideal raw materials
The year-round availability of plants and the geographical limitations pose significant drawbacks. A viable alternative material could be evergreen plants, such as Filicium decipiens, which could overcome limitations imposed by seasonal time constraints. Filicium decipiens is known for its unhindered growth regardless of soil conditions and is commonly used as a shade tree (Sharmila et al. 2017). Other plants, including Murraya koenigii, Stachys lavandulifolia, and Mediterranean cypress (Cupressus sempervirens), have also been harnessed for creating iron oxide nanoparticles (Mohanraj et al. 2014; Ebrahiminezhad et al. 2018; Shahriary et al. 2018). These plant resources, characterized by their consistent availability and absence of seasonal or temporal restrictions, hold considerable promise for practical green synthesis. Additionally, Urtica dioica, commonly known as stinging nettle or common nettle, is extensively cultivated in North Africa, Asia, America, and Europe and is frequently used for iron nanoparticle synthesis. Eucalyptus and grape leaves are similarly employed for copper nanoparticle synthesis (Edison et al. 2016; Rostami-Vartooni et al. 2016). Malva sylvestris, accessible across Europe, Asia, and America, is another readily available source for producing copper oxide nanoparticles (Kuppusamy et al. 2016).
Moreover, agricultural waste aligned with waste utilization for environmental preservation can be a suitable material. For instance, the water hyacinth, Eichhornia crassipes, an invasive weed species causing ecological concerns due to its rapid growth and fertility, can be harnessed for iron nanoparticle creation, thus reducing its environmental impact. Grape seeds, a common byproduct of the wine industry, have been employed for green synthesis (Gao et al. 2016). Furthermore, peels from mandarin, orange, lime, and lemon trees, along with pistachio shells, have been utilized to synthesize nanoscale zerovalent iron, providing a means of resource recycling with tangible benefits (Machado et al. 2014; Soliemanzadeh et al. 2016). Citron juice extraction is comparatively easier than obtaining plant extracts (Shende et al. 2015). Therefore, ensuring the practicality and simplicity of the material extraction process should be a crucial consideration for future research, aiming to facilitate the extraction process as much as possible. In essence, future research should primarily focus on materials that do not face constraints based on seasonal and geographical availability (Ebrahiminezhad et al. 2017). Researchers should prioritize materials that can be readily collected within specific seasons and regions while also considering the ease of the extraction procedure.
Reduce energy consumption
Researchers should explore alternative methods that eliminate the need for heating to address excessive energy consumption in certain synthesis processes. Promising advancements in energy-saving approaches demonstrate the feasibility of this goal. For instance, the synthesis of iron nanoparticles can be achieved without resorting to hazardous chemical reagents, high reaction energy, or elevated temperatures by harnessing leaf extracts from sources like Hippophae rhamnoides, Gardenia, and Henna (Naseem and Farrukh 2015; Nasrollahzadeh and Sajadi 2015). Several extraction techniques that demand minimal energy input are currently in use. These include reaction temperatures below 100 °C for producing silver nanoparticles from grape seed extract (Ping et al. 2018) or at 22 and 25 °C for generating gold nanoparticles from Genipa americana fruit extract (Kumar et al. 2016). In a similar vein, the synthesis of iron nanoparticles using nettle and thyme leaf extracts required only 80 °C (Leili et al. 2018). Methods characterized by comparably low energy requirements should be prioritized over procedures necessitating more than 600 °C temperatures.
Product optimization
Future studies should prioritize the synthesis of consistently small nanoparticles with a substantial surface area. Gloriosa superba L. extract, for instance, has shown promise in generating copper oxide nanoparticles with homogenous sizes ranging from 5 to 10 nm (Naika et al. 2015). Mint (Mentha spicata L.) leaf extract has demonstrated the ability to produce uniformly dispersed nanoscale zerovalent iron particles without aggregation. In contrast, Cupressus sempervirens leaf extract yielded particles approximately 1.5 nm in diameter (Ebrahiminezhad et al. 2018). Certain green materials, such as Sapindus mukorossi leaf extract, Gum olibanum, Filicium decipiens leaves, Hippophae rhamnoides Linn leaves, or carboxymethyl cellulose, have been successful in generating palladium nanoparticles with tiny particle sizes, ranging from 1.5 to 5.0 nm (Kora and Rastogi 2016; Li et al. 2017; Nagajyothi et al. 2017a; Sharmila et al. 2017; Devi et al. 2019). Ensuring nanoparticle stability is another critical aspect of their quality. Palladium nanoparticles synthesized with carboxymethyl cellulose remained stable for nearly a year (Li et al. 2017). In contrast, Nasrollahzadeh and Sajadi (2016b) found that palladium nanoparticles produced with leaf extract from Euphorbia granulata remained stable even after one week. Employing polyethylene glycol 6000 as a capping agent has proven effective in stabilizing metal colloids, and preventing agglomeration or sedimentation even after 15 days of storage (Caroling et al. 2015). Notably, freeze-dried nanoscale zerovalent iron created using grape seed extract maintained its activity under ambient conditions, making it suitable for practical applications (Gao et al. 2016).
The reusability of green materials is a crucial consideration. A notable advantage of black tea for palladium nanoparticle synthesis is that the tea can be easily extracted from the reaction mixture, recycled, and employed for an additional five runs without significant activity loss. Similarly, green-synthesized nanoparticles exhibit the potential for recycling. For instance, palladium nanoparticles created using Euphorbia granulata leaf extract demonstrated catalytic activity in four successive cycles (Nasrollahzadeh and Sajadi 2016b). Moreover, Rosa canina fruit extract could generate palladium nanoparticles employed for seven cycles without substantial activity loss (Veisi et al. 2016). Even after five cycles of use, copper nanoparticles supported on bentonite and derived from Thymus vulgaris L. leaf extract maintained efficient catalytic reduction of Congo red or methylene blue (Issaabadi et al. 2017).
Similarly, copper nanoparticles generated from Ginkgo biloba L. leaf extract displayed reusability for at least four cycles (Nasrollahzadeh and Sajadi 2015). Furthermore, functionalized iron nanoparticles synthesized using black tea were successfully regenerated seven times, retaining 80–88% removal capabilities for ametrine from water (Ali et al. 2016). Additionally, the catalytic activity for producing 2-aryl benzimidazoles remained relatively unaffected when Passiflora tripartita var. mollissima fruit extract-derived iron oxide nanoparticles were recycled four times, aided by easy separation through magnetization (Kumar et al. 2014). In summary, exploring and optimizing the reusability of green-synthesized nanoparticles can significantly contribute to their practical applicability and sustainability in various catalytic and environmental remediation processes.
Storage of the products
Careful consideration must be given to the storage of nanoscale metals. The cost associated with preserving and storing nanoscale metals could significantly decrease if they could be stored under ambient conditions. The stability of nanoscale metals during storage plays a crucial role in their cost-effectiveness. Notably, the stability of silver nanoparticles produced from A. calamus rhizome was observed for 24 h (Tahir et al. 2016); in contrast, silver nanoparticles derived from Carissa carandas fruits displayed their peak absorption for only the initial 4 h using ultraviolet–visible absorption spectroscopy, indicating compromised stability beyond that time frame (Anupama and Madhumitha 2017). In a similar vein, copper nanoparticles synthesized from Thymus vulgaris L. leaf extract and supported on bentonite were found to be stable for 20 days in an inert environment, as reported by Taghavi Fardood and Ramazani (2016). Copper nanoparticles produced using aqueous Guava extract remained stable at room temperature for 15 days, thanks to the use of polyethylene glycol 6000 as a stabilizing capping agent to prevent rapid oxidation (Caroling et al. 2015). The stability of the products can be influenced by various synthetic materials and techniques, prompting researchers to carefully select suitable materials and processing methods to ensure the synthesis of stable compounds. Moreover, treatments can be employed to enhance the stability of the product under ambient conditions. For instance, common nanoscale zerovalent iron is prone to oxidation and aggregation. Still, freeze-dried nanoscale zerovalent iron has been shown to retain its activity when stored in ambient air (Dhand et al. 2016).
Nevertheless, a comprehensive toxicological assessment of the impact of these nanoparticles on plants and animals is imperative to broaden their application across various fields. Furthermore, genetically modified microorganisms, beyond wild-type strains, could play a pivotal role in enhancing biosynthesis and stabilizing nanoparticles. These engineered microorganisms have the potential to produce higher levels of proteins, enzymes, and biomolecules, thereby contributing to improved nanoparticle synthesis. Additionally, enhancing the metal tolerance of genetically modified microbes offers a promising avenue for advancing the production and utilization of metal nanoparticles derived from green synthesis.
Conclusion
The green synthesis of nanoparticles has garnered increasing attention as a non-toxic, environmentally friendly, and cost-effective method. This revolutionary approach yields composite nanoparticles that amalgamate the strengths of diverse metals while minimizing chemical reagents and showcases the potential of microorganisms and plants in driving synthesis. These natural compounds, acting as capping and reducing agents, have unveiled applications ranging from medicine to environmental remediation, creating a transformative impact. Enhancing the sustainable development and utilization of green synthetic particles is paramount. As we strive for sustainable development and expanded applications of green synthetic particles, a thorough assessment ranging from biomedical applications to agriculture, environmental remediation, and food quality enhancement underscores the transformative influence of these particles in diverse domains. This review has illuminated a path toward a more sustainable and resilient future, showcasing the pivotal role of green-synthesized nanoparticles. Nonetheless, challenges persist, including the need to precisely control particle characteristics, unravel biosynthesis mechanisms, and ensure consistent pollutant removal efficiency. The diversity in particle sizes and forms and concerns about storage stability underscore the necessity of perfecting synthesis techniques. A holistic assessment of toxicity and optimizing genetically modified microorganisms are crucial steps to fully harness the potential of green-synthesized nanoparticles. To unlock the full potential of green-synthesized nanoparticles, a comprehensive toxicological evaluation and the optimization of genetically modified microorganisms warrant exploration. Despite these hurdles, the path toward sustainable and efficient nanoscale metal synthesis is an enticing route for future scientific inquiry and innovation across disciplines. As we traverse this path, the promise of greener, more responsible nanoparticle synthesis shines brighter than ever.
References
Abbasifar A, Shahrabadi F, ValizadehKaji B (2020) Effects of green synthesized zinc and copper nano-fertilizers on the morphological and biochemical attributes of basil plant. J Plant Nutr 43(8):1104–1118. https://doi.org/10.1080/01904167.2020.1724305
Abd El-Aziz A, Al-Othman MR (2019) Gold nanoparticles biosynthesis using zingiber officinale and their impact on the growth and chemical composition of lentil (Lens culinaris medic.). Pak J Bot 51(2):443–450. https://doi.org/10.30848/PJB2019-2(21)
Abd El-Monaem EM, Eltaweil AS, El-Subruiti GM, Mohy-Eldin MS, Omer AM (2023) Adsorption of nitrophenol onto a novel Fe3O4-κ-carrageenan/MIL-125 (Ti) composite: process optimization, isotherms, kinetics, and mechanism. Environ Sci Pollut Res 30(17):49301–49313. https://doi.org/10.1007/s11356-023-25678-2
Abdel Maksoud MIA, Fahim RA, Bedir AG, Osman AI, Abouelela MM, El-Sayyad GS, Elkodous MA, Mahmoud AS, Rabee MM, AaH A-M, Rooney DW (2022) Engineered magnetic oxides nanoparticles as efficient sorbents for wastewater remediation: a review. Environ Chem Lett 20(1):519–562. https://doi.org/10.1007/s10311-021-01351-3
Abdelghaffar F (2021) Biosorption of anionic dye using nanocomposite derived from chitosan and silver Nanoparticles synthesized via cellulosic banana peel bio-waste. Environ Technol Innov 24:101852. https://doi.org/10.1016/j.eti.2021.101852
Abdelmigid HM, Morsi MM, Hussien NA, Alyamani AA, Alhuthal NA, Albukhaty S (2022) Green synthesis of phosphorous-containing hydroxyapatite nanoparticles (nHAP) as a novel nano-fertilizer: preliminary assessment on pomegranate (Punica granatum L.). Nanomaterials 12(9):1527. https://doi.org/10.3390/nano12091527
Abdel-Rahman LH, Al-Farhan BS, Abou El-ezz D, Abd-El Sayed M, Zikry MM, Abu-Dief AM (2022) Green biogenic synthesis of silver nanoparticles using aqueous extract of Moringa oleifera: access to a powerful antimicrobial, anticancer, pesticidal and catalytic agents. J Inorg Organomet Polym Mater 32(4):1422–1435. https://doi.org/10.1007/s10904-021-02186-9
Abdel-Raouf N, Al-Enazi NM, Ibraheem IBM (2017) Green biosynthesis of gold nanoparticles using Galaxaura elongata and characterization of their antibacterial activity. Arab J Chem 10:S3029–S3039. https://doi.org/10.1016/j.arabjc.2013.11.044
Abdi V, Sourinejad I, Yousefzadi M, Ghasemi Z (2019) Biosynthesis of silver nanoparticles from the mangrove Rhizophora mucronata: its characterization and antibacterial potential. Iran J Sci Technol Trans A Sci 43(5):2163–2171. https://doi.org/10.1007/s40995-019-00739-9
Abdoli M, Arkan E, Shekarbeygi Z, Khaledian S (2021) Green synthesis of gold nanoparticles using Centaurea behen leaf aqueous extract and investigating their antioxidant and cytotoxic effects on acute leukemia cancer cell line (THP-1). Inorg Chem Commun 129:108649. https://doi.org/10.1016/j.inoche.2021.108649
Acharya P, Jayaprakasha G, Crosby KM, Jifon JL, Patil BS (2019) Green-synthesized nanoparticles enhanced seedling growth, yield, and quality of onion (Allium cepa L.). ACS Sustain Chem Eng 7(17):14580–14590. https://doi.org/10.1021/acssuschemeng.9b02180
Ahmed S, Ahmad M, Swami BL, Ikram S (2016) Green synthesis of silver nanoparticles using Azadirachta indica aqueous leaf extract. J Radiat Res Appl Sci 9(1):1–7. https://doi.org/10.1016/j.jrras.2015.06.006
Ahmed T, Noman M, Manzoor N, Shahid M, Hussaini KM, Rizwan M, Ali S, Maqsood A, Li B (2021) Green magnesium oxide nanoparticles-based modulation of cellular oxidative repair mechanisms to reduce arsenic uptake and translocation in rice (Oryza sativa L.) plants. Environ Pollut 288:117785. https://doi.org/10.1016/j.envpol.2021.117785
Aisida SO, Ugwu K, Akpa PA, Nwanya AC, Nwankwo U, Botha SS, Ejikeme PM, Ahmad I, Maaza M, Ezema FI (2019) Biosynthesis of silver nanoparticles using bitter leave (Veronica amygdalina) for antibacterial activities. Surf Interfaces 17:100359. https://doi.org/10.1016/j.surfin.2019.100359
Akbarian M, Mahjoub S, Elahi SM, Zabihi E, Tashakkorian H (2020) Green synthesis, formulation and biological evaluation of a novel ZnO nanocarrier loaded with paclitaxel as drug delivery system on MCF-7 cell line. Colloids Surf B 186:110686. https://doi.org/10.1016/j.colsurfb.2019.110686
Akintelu SA, Yao B, Folorunso AS (2021) Green synthesis, characterization, and antibacterial investigation of synthesized gold nanoparticles (AuNPs) from Garcinia kola pulp extract. Plasmonics 16:157–165. https://doi.org/10.1007/s11468-020-01274-9
Alam H, Khatoon N, Khan MA, Husain SA, Saravanan M, Sardar M (2020) Synthesis of selenium nanoparticles using probiotic bacteria Lactobacillus acidophilus and their enhanced antimicrobial activity against resistant bacteria. J Cluster Sci 31(5):1003–1011. https://doi.org/10.1007/s10876-019-01705-6
Alfryyan N, Kordy MGM, Abdel-Gabbar M, Soliman HA, Shaban M (2022) Characterization of the biosynthesized intracellular and extracellular plasmonic silver nanoparticles using Bacillus cereus and their catalytic reduction of methylene blue. Sci Rep 12(1):12495. https://doi.org/10.1038/s41598-022-16029-1
Ali I, Al-Othman ZA, Alwarthan A (2016) Green synthesis of functionalized iron nano particles and molecular liquid phase adsorption of ametryn from water. J Mol Liq 221:1168–1174. https://doi.org/10.1016/j.molliq.2016.06.089
Alsammarraie FK, Wang W, Zhou P, Mustapha A, Lin M (2018) Green synthesis of silver nanoparticles using turmeric extracts and investigation of their antibacterial activities. Colloids Surf B 171:398–405. https://doi.org/10.1016/j.colsurfb.2018.07.059
Al-Yasiri AY, Khoobchandani M, Cutler CS, Watkinson L, Carmack T, Smith CJ, Kuchuk M, Loyalka SK, Lugão AB, Katti KV (2017) Mangiferin functionalized radioactive gold nanoparticles (MGF-198 AuNPs) in prostate tumor therapy: green nanotechnology for production, in vivo tumor retention and evaluation of therapeutic efficacy. Dalton Trans 46(42):14561–14571. https://doi.org/10.1039/C7DT00383H
Ameen F, AlYahya S, Govarthanan M, Aljahdali N, Al-Enazi N, Alsamhary K, Alshehri WA, Alwakeel SS, Alharbi SA (2020) Soil bacteria Cupriavidus sp. mediates the extracellular synthesis of antibacterial silver nanoparticles. J Mol Struct 1202:127233. https://doi.org/10.1016/j.molstruc.2019.127233
Amin HH (2020) Biosynthesized silver nanoparticles using Ulva lactuca as a safe synthetic pesticide (in vitro). Open Agric. 5(1):291–299. https://doi.org/10.1515/opag-2020-0032
Anand GT, Renuka D, Ramesh R, Anandaraj L, Sundaram SJ, Ramalingam G, Magdalane CM, Bashir A, Maaza M, Kaviyarasu K (2019) Green synthesis of ZnO nanoparticle using Prunus dulcis (Almond Gum) for antimicrobial and supercapacitor applications. Surf Interfaces 17:100376. https://doi.org/10.1016/j.surfin.2019.100376
Anupama N, Madhumitha G (2017) Green synthesis and catalytic application of silver nanoparticles using Carissa carandas fruits. Inorg Nano-Met Chem 47(1):116–120. https://doi.org/10.1080/15533174.2016.1149731
Aromal SA, Philip D (2012) Green synthesis of gold nanoparticles using Trigonella foenum-graecum and its size-dependent catalytic activity. Spectrochim Acta Part A Mol Biomol Spectrosc 97:1–5. https://doi.org/10.1016/j.saa.2012.05.083
Arsiya F, Sayadi MH, Sobhani S (2017) Green synthesis of palladium nanoparticles using Chlorella vulgaris. Mater Lett 186:113–115. https://doi.org/10.1016/j.matlet.2016.09.101
Aslam M, Abdullah AZ, Rafatullah M (2021) Recent development in the green synthesis of titanium dioxide nanoparticles using plant-based biomolecules for environmental and antimicrobial applications. J Ind Eng Chem 98:1–16. https://doi.org/10.1016/j.jiec.2021.04.010
Athanassiou C, Kavallieratos N, Benelli G, Losic D, Usha Rani P, Desneux N (2018) Nanoparticles for pest control: current status and future perspectives. J Pest Sci 91:1–15. https://doi.org/10.1007/s10340-017-0898-0
Avirdi E, Hooshmand SE, Sepahvand H, Vishwanathan V, Bahadur I, Katata-Seru LM, Varma RS (2022) Ionic liquids-assisted greener preparation of silver nanoparticles. Curr Opin Green Sustain Chem 33:100581. https://doi.org/10.1016/j.cogsc.2021.100581
Aygun A, Gülbagca F, Ozer LY, Ustaoglu B, Altunoglu YC, Baloglu MC, Atalar MN, Alma MH, Sen F (2020) Biogenic platinum nanoparticles using black cumin seed and their potential usage as antimicrobial and anticancer agent. J Pharm Biomed Anal 179:112961. https://doi.org/10.1016/j.jpba.2019.112961
Azizi S, Mohamad R, Rahim RA, Moghaddam AB, Moniri M, Ariff A, Saad WZ, Namvab F (2016) ZnO–Ag core shell nanocomposite formed by green method using essential oil of wild ginger and their bactericidal and cytotoxic effects. Appl Surf Sci 384:517–524. https://doi.org/10.1016/j.apsusc.2016.05.052
Balalakshmi C, Gopinath K, Govindarajan M, Lokesh R, Arumugam A, Alharbi NS, Kadaikunnan S, Khaled JM, Benelli G (2017) Green synthesis of gold nanoparticles using a cheap Sphaeranthus indicus extract: impact on plant cells and the aquatic crustacean Artemia nauplii. J Photochem Photobiol B 173:598–605. https://doi.org/10.1016/j.jphotobiol.2017.06.040
Balamurughan MG, Mohanraj S, Kodhaiyolii S, Pugalenthi V (2014) Ocimum sanctum leaf extract mediated green synthesis of iron oxide nanoparticles: spectroscopic and microscopic studies. J Chem Pharm Sci 4:201–204
Bao H, Lu Z, Cui X, Qiao Y, Guo J, Anderson JM, Li CM (2010) Extracellular microbial synthesis of biocompatible CdTe quantum dots. Acta Biomater 6(9):3534–3541. https://doi.org/10.1016/j.actbio.2010.03.030
Barai AC, Paul K, Dey A, Manna S, Roy S, Bag BG, Mukhopadhyay C (2018) Green synthesis of Nerium oleander-conjugated gold nanoparticles and study of its in vitro anticancer activity on MCF-7 cell lines and catalytic activity. Nano Converg 5(1):1–9. https://doi.org/10.1186/s40580-018-0142-5
Baran A, Fırat Baran M, Keskin C, Hatipoğlu A, Yavuz Ö, İrtegün Kandemir S, Adican MT, Khalilov R, Mammadova A, Ahmadian E (2022) Investigation of antimicrobial and cytotoxic properties and specification of silver nanoparticles (AgNPs) derived from Cicer arietinum L. green leaf extract. Front Bioeng Biotechnol 10:263. https://doi.org/10.3389/fbioe.2022.855136
Barzinjy AA, Hamad SM, Aydın S, Ahmed MH, Hussain FH (2020) Green and eco-friendly synthesis of Nickel oxide nanoparticles and its photocatalytic activity for methyl orange degradation. J Mater Sci Mater Electron 31:11303–11316. https://doi.org/10.1007/s10854-020-03679-y
Bashir AKH, Matinise N, Sackey J, Kaviyarasu K, Madiba IG, Kodseti L, Ezema FI, Maaza M (2020) Investigation of electrochemical performance, optical and magnetic properties of NiFe2O4 nanoparticles prepared by a green chemistry method. Phys E 119:114002. https://doi.org/10.1016/j.physe.2020.114002
Behravan M, Panahi AH, Naghizadeh A, Ziaee M, Mahdavi R, Mirzapour A (2019) Facile green synthesis of silver nanoparticles using Berberis vulgaris leaf and root aqueous extract and its antibacterial activity. Int J Biol Macromol 124:148–154. https://doi.org/10.1016/j.ijbiomac.2018.11.101
Bendary E, Francis R, Ali H, Sarwat M, El Hady S (2013) Antioxidant and structure–activity relationships (SARs) of some phenolic and anilines compounds. Ann Agric Sci 58(2):173–181. https://doi.org/10.1016/j.aoas.2013.07.002
Benelli G (2018) Mode of action of nanoparticles against insects. Environ Sci Pollut Res 25(13):12329–12341. https://doi.org/10.1007/s11356-018-1850-4
Bhatia D, Mittal A, Malik DK (2021) Antimicrobial potential and in vitro cytotoxicity study of polyvinyl pyrollidone-stabilised silver nanoparticles synthesised from Lysinibacillus boronitolerans. IET Nanobiotechnol 15(4):427–440. https://doi.org/10.1049/nbt2.12054
Bilal M, Iqbal HMN, Adil SF, Shaik MR, Abdelgawad A, Hatshan MR, Khan M (2022) Surface-coated magnetic nanostructured materials for robust bio-catalysis and biomedical applications—a review. J Adv Res 38:157–177. https://doi.org/10.1016/j.jare.2021.09.013
Bilal M, Khaliq N, Ashraf M, Hussain N, Baqar Z, Zdarta J, Jesionowski T, Iqbal HMN (2023) Enzyme mimic nanomaterials as nanozymes with catalytic attributes. Colloids Surf B 221:112950. https://doi.org/10.1016/j.colsurfb.2022.112950
Bishnoi S, Kumar A, Selvaraj R (2018) Facile synthesis of magnetic iron oxide nanoparticles using inedible Cynometra ramiflora fruit extract waste and their photocatalytic degradation of methylene blue dye. Mater Res Bull 97:121–127. https://doi.org/10.1016/j.materresbull.2017.08.040
Biswal AK, Misra PK (2020) Biosynthesis and characterization of silver nanoparticles for prospective application in food packaging and biomedical fields. Mater Chem Phys 250:123014. https://doi.org/10.1016/j.matchemphys.2020.123014
Bobba S, Deorsola FA, Blengini GA, Fino D (2016) LCA of tungsten disulphide (WS2) nano-particles synthesis: state of art and from-cradle-to-gate LCA. J Clean Prod 139:1478–1484. https://doi.org/10.1016/j.jclepro.2016.07.091
Brady NG, O’Leary SL, Moormann GC, Singh MK, Watt J, Bachand GD (2023) Mycosynthesis of zinc oxide nanoparticles exhibits fungal species dependent morphological preference. Small 19(15):e2205799. https://doi.org/10.1002/smll.202205799
Cai W, Guo M, Weng X, Zhang W, Owens G, Chen Z (2020) Modified green synthesis of Fe3O4@SiO2 nanoparticles for pH responsive drug release. Mater Sci Eng C 112:110900. https://doi.org/10.1016/j.msec.2020.110900
Cao J-j, Huang Y, Zhang Q (2021) Ambient air purification by nanotechnologies: from theory to application. Catalysts 11(11):1276. https://doi.org/10.3390/catal11111276
Caroling G, Priyadharshini MN, Vinodhini E, Ranjitham AM, Shanthi P (2015) Biosynthesis of copper nanoparticles using aqueous guava extract-characterisation and study of antibacterial effects. Int J Pharm Biol Sci 5(2):25–43
Chahardoli A, Karimi N, Fattahi A (2018) Nigella arvensis leaf extract mediated green synthesis of silver nanoparticles: their characteristic properties and biological efficacy. Adv Powder Technol 29(1):202–210. https://doi.org/10.1016/j.apt.2017.11.003
Chakraborty S, Farida JJ, Simon R, Kasthuri S, Mary NL (2020) Averrhoe carrambola fruit extract assisted green synthesis of zno nanoparticles for the photodegradation of congo red dye. Surf Interfaces 19:100488. https://doi.org/10.1016/j.surfin.2020.100488
Chapman R, Stenzel MH (2019) All wrapped up: stabilization of enzymes within single enzyme nanoparticles. J Am Chem Soc 141(7):2754–2769. https://doi.org/10.1021/jacs.8b10338
Chen W, Li D, Tian L, Xiang W, Wang T, Hu W, Hu Y, Chen S, Chen J, Dai Z (2018) Synthesis of graphene quantum dots from natural polymer starch for cell imaging. Green Chem 20(19):4438–4442. https://doi.org/10.1039/C8GC02106F
Chen L, Batjikh I, Hurh J, Han Y, Huo Y, Ali H, Li JF, Rupa EJ, Ahn JC, Mathiyalagan R, Yang DC (2019) Green synthesis of zinc oxide nanoparticles from root extract of Scutellaria baicalensis and its photocatalytic degradation activity using methylene blue. Optik 184:324–329. https://doi.org/10.1016/j.ijleo.2019.03.051
Chen Z, Yao J, Ma B, Liu B, Kim J, Li H, Zhu X, Zhao C, Amde M (2022) A robust biocatalyst based on laccase immobilized superparamagnetic Fe3O4@SiO2–NH2 nanoparticles and its application for degradation of chlorophenols. Chemosphere 291:132727. https://doi.org/10.1016/j.chemosphere.2021.132727
Chuhadiya S, Himanshu SD, Patel SL, Dhaka MS (2021) Metal organic frameworks as hybrid porous materials for energy storage and conversion devices: a review. Coord Chem Rev 446:214115. https://doi.org/10.1016/j.ccr.2021.214115
Das M, Borah D, Patowary K, Borah M, Khataniar A, Bhusan Kakoti B (2019) Antimicrobial activity of silver nanoparticles synthesised by using microbial biosurfactant produced by a newly isolated Bacillus vallismortis MDU6 strain. IET Nanobiotechnol 13(9):967–973. https://doi.org/10.1049/iet-nbt.2019.0038
de França Bettencourt GM, Degenhardt J, Torres LAZ, de Andrade Tanobe VO, Soccol CR (2020) Green biosynthesis of single and bimetallic nanoparticles of iron and manganese using bacterial auxin complex to act as plant bio-fertilizer. Biocatal Agric Biotechnol 30:101822
Debnath B, Majumdar M, Bhowmik M, Bhowmik KL, Debnath A, Roy DN (2020) The effective adsorption of tetracycline onto zirconia nanoparticles synthesized by novel microbial green technology. J Environ Manag 261:110235. https://doi.org/10.1016/j.jenvman.2020.110235
Demenciano S, Silva M, Alexandrino C, Junior W, Bogo D (2020) Antiproliferative activity and antioxidant potential of extracts of Garcinia gardneriana. Molecules. https://doi.org/10.3390/molecules25143201
Desai MP, Sangaokar GM, Pawar KD (2018) Kokum fruit mediated biogenic gold nanoparticles with photoluminescent, photocatalytic and antioxidant activities. Process Biochem 70:188–197. https://doi.org/10.1016/j.procbio.2018.03.027
Devanesan S, AlSalhi MS (2021) Green synthesis of silver nanoparticles using the flower extract of Abelmoschus esculentus for cytotoxicity and antimicrobial studies. Int J Nanomed 16:3343. https://doi.org/10.2147/IJN.S307676
Devanesan S, Jayamala M, AlSalhi MS, Umamaheshwari S, Ranjitsingh AJA (2021) Antimicrobial and anticancer properties of Carica papaya leaves derived di-methyl flubendazole mediated silver nanoparticles. J Infect Public Health 14(5):577–587. https://doi.org/10.1016/j.jiph.2021.02.004
Devi HS, Boda MA, Shah MA, Parveen S, Wani AH (2019) Green synthesis of iron oxide nanoparticles using Platanus orientalis leaf extract for antifungal activity. Green Process Synth 8(1):38–45. https://doi.org/10.1515/gps-2017-0145
DeVierno Kreuder A, House-Knight T, Whitford J, Ponnusamy E, Miller P, Jesse N, Rodenborn R, Sayag S, Gebel M, Aped I (2017) A method for assessing greener alternatives between chemical products following the 12 principles of green chemistry. ACS Sustain Chem Eng 5(4):2927–2935. https://doi.org/10.1021/acssuschemeng.6b02399
Dhand V, Soumya L, Bharadwaj S, Chakra S, Bhatt D, Sreedhar B (2016) Green synthesis of silver nanoparticles using Coffea arabica seed extract and its antibacterial activity. Mater Sci Eng C 58:36–43. https://doi.org/10.1016/j.msec.2015.08.018
Dhingra R, Naidu S, Upreti G, Sawhney R (2010) Sustainable nanotechnology: through green methods and life-cycle thinking. Sustainability 2(10):3323–3338. https://doi.org/10.3390/su2103323
Dikshit PK, Kumar J, Das AK, Sadhu S, Sharma S, Singh S, Gupta PK, Kim BS (2021) Green synthesis of metallic nanoparticles: Applications and limitations. Catalysts 11(8):902. https://doi.org/10.3390/catal11080902
Ding C, Cheng W, Sun Y, Wang X (2015) RETRACTED: Effects of Bacillus subtilis on the reduction of U(VI) by nano-Fe0. Elsevier, Amsterdam
Doan Thi TU, Nguyen TT, Thi YD, Ta Thi KH, Phan BT, Pham KN (2020) Green synthesis of ZnO nanoparticles using orange fruit peel extract for antibacterial activities. RSC Adv 10(40):23899–23907. https://doi.org/10.1039/D0RA04926C
Dogiparthi LK, Sana SS, Shaik SZ, Kalvapalli MR, Kurupati G, Kumar GS, Gangadhar L (2021) Phytochemical mediated synthesis of silver nanoparticles and their antibacterial activity. SN Appl Sci 3(6):631. https://doi.org/10.1007/s42452-021-04641-1
Ebrahiminezhad A, Zare-Hoseinabadi A, Berenjian A, Ghasemi Y (2017) Green synthesis and characterization of zero-valent iron nanoparticles using stinging nettle (Urtica dioica) leaf extract. Green Process Synth 6(5):469–475. https://doi.org/10.1515/gps-2016-0133
Ebrahiminezhad A, Taghizadeh S, Ghasemi Y, Berenjian A (2018) Green synthesized nanoclusters of ultra-small zero valent iron nanoparticles as a novel dye removing material. Sci Total Environ 621:1527–1532. https://doi.org/10.1016/j.scitotenv.2017.10.076
Edison TNJI, Lee YR, Sethuraman MG (2016) Green synthesis of silver nanoparticles using Terminalia cuneata and its catalytic action in reduction of direct yellow-12 dye. Spectrochim Acta Part A Mol Biomol Spectrosc 161:122–129. https://doi.org/10.1016/j.saa.2016.02.044
El-Ansary MSM, Hamouda RA, Elshamy MM (2022) Using biosynthesized zinc oxide nanoparticles as a pesticide to alleviate the toxicity on banana infested with parasitic-nematode. Waste Biomass Valorization 13:405–415. https://doi.org/10.1007/s12649-021-01527-6
El-Ghamry A, Mosa AA, Alshaal T, El-Ramady H (2018) Nanofertilizers vs. biofertilizers: new insights. Environ Biodivers Soil Secur 2(2018):51–72
El-Kassas HY, Aly-Eldeen MA, Gharib SM (2016) Green synthesis of iron oxide (Fe3O4) nanoparticles using two selected brown seaweeds: characterization and application for lead bioremediation. Acta Oceanol Sin 35:89–98. https://doi.org/10.1007/s13131-016-0880-3
El-Nahas S, Osman AI, Arafat AS, AaH A-M, Salman HM (2020) Facile and affordable synthetic route of nano powder zeolite and its application in fast softening of water hardness. J Water Process Eng 33:101104. https://doi.org/10.1016/j.jwpe.2019.101104
El-Saadony MT, Abd El-Hack ME, Taha AE, Fouda MM, Ajarem JS, N. Maodaa S, Allam AA, Elshaer N, (2020) Ecofriendly synthesis and insecticidal application of copper nanoparticles against the storage pest Tribolium castaneum. Nanomaterials 10(3):587. https://doi.org/10.3390/nano10030587
Eltaweil AS, Abd El-Monaem EM, El-Subruiti GM, Ali BM, Abd El-Latif MM, Omer AM (2023) Graphene oxide incorporated cellulose acetate beads for efficient removal of methylene blue dye; isotherms, kinetic, mechanism and co-existing ions studies. J Porous Mater 30(2):607–618. https://doi.org/10.1007/s10934-022-01347-6
Es’haghi Z, Vafaeinezhad F, Hooshmand S, (2016) Green synthesis of magnetic iron nanoparticles coated by olive oil and verifying its efficiency in extraction of nickel from environmental samples via UV–vis spectrophotometry. Process Saf Environ Prot 102:403–409. https://doi.org/10.1016/j.psep.2016.04.011
Ezhilarasi P, Karthik P, Chhanwal N, Anandharamakrishnan C (2013) Nanoencapsulation techniques for food bioactive components: a review. Food Bioprocess Technol 6:628–647. https://doi.org/10.1007/s11947-012-0944-0
Fahimmunisha BA, Ishwarya R, AlSalhi MS, Devanesan S, Govindarajan M, Vaseeharan B (2020) Green fabrication, characterization and antibacterial potential of zinc oxide nanoparticles using Aloe socotrina leaf extract: a novel drug delivery approach. J Drug Deliv Sci Technol 55:101465. https://doi.org/10.1016/j.jddst.2019.101465
Fang C, Ma Z, Chen L, Li H, Jiang C, Zhang W (2019) Biosynthesis of gold nanoparticles, characterization and their loading with zonisamide as a novel drug delivery system for the treatment of acute spinal cord injury. J Photochem Photobiol B 190:72–75. https://doi.org/10.1016/j.jphotobiol.2018.11.011
Farmakes J, Schuster I, Overby A, Alhalhooly L, Lenertz M, Li Q, Ugrinov A, Choi Y, Pan Y, Yang Z (2020) Enzyme immobilization on graphite oxide (GO) surface via one-pot synthesis of GO/metal–organic framework composites for large-substrate biocatalysis. ACS Appl Mater Interfaces 12(20):23119–23126. https://doi.org/10.1021/acsami.0c04101
Flieger J, Flieger W, Baj J, Maciejewski R (2021) Antioxidants: classification, natural sources, activity/capacity measurements, and usefulness for the synthesis of nanoparticles. Materials 14(15):4135. https://doi.org/10.3390/ma14154135
Fuku X, Kaviyarasu K, Matinise N, Maaza M (2016) Punicalagin green functionalized Cu/Cu2O/ZnO/CuO nanocomposite for potential electrochemical transducer and catalyst. Nanoscale Res Lett 11:386. https://doi.org/10.1186/s11671-016-1581-8
Gamal-Eldeen AM, Moustafa D, El-Daly SM, Abo-Zeid MAM, Saleh S, Khoobchandani M, Katti K, Shukla R, Katti KV (2017) Gum Arabic-encapsulated gold nanoparticles for a non-invasive photothermal ablation of lung tumor in mice. Biomed Pharmacother 89:1045–1054. https://doi.org/10.1016/j.biopha.2017.03.006
Ganesan RM, Prabu HG (2019) Synthesis of gold nanoparticles using herbal Acorus calamus rhizome extract and coating on cotton fabric for antibacterial and UV blocking applications. Arab J Chem 12(8):2166–2174. https://doi.org/10.1016/j.arabjc.2014.12.017
Gangapuram BR, Bandi R, Alle M, Dadigala R, Kotu GM, Guttena V (2018) Microwave assisted rapid green synthesis of gold nanoparticles using Annona squamosa L peel extract for the efficient catalytic reduction of organic pollutants. J Mol Struct 1167:305–315. https://doi.org/10.1016/j.molstruc.2018.05.004
Gao J-F, Li H-Y, Pan K-L, Si C-Y (2016) Green synthesis of nanoscale zero-valent iron using a grape seed extract as a stabilizing agent and the application for quick decolorization of azo and anthraquinone dyes. RSC Adv 6(27):22526–22537. https://doi.org/10.1039/C5RA26668H
Gao J-F, Wu Z-L, Duan W-J, Zhang W-Z (2019) Simultaneous adsorption and degradation of triclosan by Ginkgo biloba L. stabilized Fe/Co bimetallic nanoparticles. Sci Total Environ 662:978–989. https://doi.org/10.1016/j.scitotenv.2019.01.194
Gao Y, Xu D, Ren D, Zeng K, Wu X (2020) Green synthesis of zinc oxide nanoparticles using Citrus sinensis peel extract and application to strawberry preservation: a comparison study. LWT Food Sci Technol 126:109297. https://doi.org/10.1016/j.lwt.2020.109297
Garg R, Rani P, Garg R, Eddy NO (2021) Study on potential applications and toxicity analysis of green synthesized nanoparticles. Turk J Chem 45(6):1690–1706. https://doi.org/10.3906/kim-2106-59
Ghaemi M, Gholamipoor S (2017) Controllable synthesis and characterization of silver nanoparticles using Sargassum angostifolium. Iran J Chem Chem Eng 36:1–10
Ghigo G, Bonomo M, Antenucci A, Damin A, Dughera S (2022) Ullmann homocoupling of arenediazonium salts in a deep eutectic solvent. Synth Mech Asp RSC Adv 12(41):26640–26647. https://doi.org/10.1039/D2RA05272E
Giri AK, Jena B, Biswal B, Pradhan AK, Arakha M, Acharya S, Acharya L (2022) Green synthesis and characterization of silver nanoparticles using Eugenia roxburghii DC. extract and activity against biofilm-producing bacteria. Sci Rep 12(1):8383. https://doi.org/10.1038/s41598-022-12484-y
Golmohammadi M, Honarmand M, Ghanbari S (2020) A green approach to synthesis of ZnO nanoparticles using jujube fruit extract and their application in photocatalytic degradation of organic dyes. Spectrochim Acta Part A Mol Biomol Spectrosc 229:117961. https://doi.org/10.1016/j.saa.2019.117961
Gong K, Lin Y, Wu P, Jin X, Owens G, Chen Z (2022) Removal mechanism of 17β-estradiol by carbonized green synthesis of Fe/Ni nanoparticles. Chemosphere 291:132777. https://doi.org/10.1016/j.chemosphere.2021.132777
González-Ballesteros N, Prado-López S, Rodríguez-González JB, Lastra M, Rodríguez-Argüelles MC (2017) Green synthesis of gold nanoparticles using brown algae Cystoseira baccata: its activity in colon cancer cells. Colloids Surf B 153:190–198. https://doi.org/10.1016/j.colsurfb.2017.02.020
Gopu C, Chirumamilla P, Kagithoju S, Taduri S (2022) Green synthesis of silver nanoparticles using Momordica cymbalaria aqueous leaf extracts and screening of their antimicrobial activity. Proc Natl Acad Sci India Sect B Biol Sci 92(4):771–782. https://doi.org/10.1007/s40011-022-01367-x
Gosavi VC, Daspute AA, Patil A, Gangurde A, Wagh SG, Sherkhane A, AnandraoDeshmukh V (2020) Synthesis of green nanobiofertilizer using silver nanoparticles of Allium cepa extract Short title: green nanofertilizer from Allium cepa. Int J Chem Stud 8(4):1690–1694. https://doi.org/10.22271/chemi.2020.v8.i4q.9854
Gu Y, Yuan L, Li M, Wang X, Rao D, Bai X, Shi K, Xu H, Hou S, Yao H (2022) Co-immobilized bienzyme of horseradish peroxidase and glucose oxidase on dopamine-modified cellulose–chitosan composite beads as a high-efficiency biocatalyst for degradation of acridine. RSC Adv 12(35):23006–23016. https://doi.org/10.1039/D2RA04091C
Gudikandula K, Charya Maringanti S (2016) Synthesis of silver nanoparticles by chemical and biological methods and their antimicrobial properties. J Exp Nanosci 11(9):714–721. https://doi.org/10.1080/17458080.2016.1139196
Gul A, Shaheen A, Ahmad I, Khattak B, Ahmad M, Ullah R, Bari A, Ali SS, Alobaid A, Asmari MM (2021) Green synthesis, characterization, enzyme inhibition, antimicrobial potential, and cytotoxic activity of plant mediated silver nanoparticle using Ricinus communis leaf and root extracts. Biomolecules 11(2):206. https://doi.org/10.3390/biom11020206
Gupta D, Boora A, Thakur A, Gupta TK (2023) Green and sustainable synthesis of nanomaterials: recent advancements and limitations. Environ Res 231:116316. https://doi.org/10.1016/j.envres.2023.116316
Hamelian M, Varmira K, Veisi H (2018) Green synthesis and characterizations of gold nanoparticles using Thyme and survey cytotoxic effect, antibacterial and antioxidant potential. J Photochem Photobiol B 184:71–79. https://doi.org/10.1016/j.jphotobiol.2018.05.016
Hano C, Abbasi BH (2022) Plant-based green synthesis of nanoparticles: production. Charact Appl Biomol 12(1):31. https://doi.org/10.3390/biom12010031
Harish V, Ansari MM, Tewari D, Yadav AB, Sharma N, Bawarig S, García-Betancourt M-L, Karatutlu A, Bechelany M, Barhoum A (2023) Cutting-edge advances in tailoring size, shape, and functionality of nanoparticles and nanostructures: a review. J Taiwan Inst Chem Eng 149:105010. https://doi.org/10.1016/j.jtice.2023.105010
Hemmati S, Joshani Z, Zangeneh A, Zangeneh MM (2020) Biosynthesis and chemical characterization of polydopamine-capped silver nanoparticles for the treatment of acute myeloid leukemia in comparison to doxorubicin in a leukemic mouse model. Appl Organomet Chem 34(2):e5277. https://doi.org/10.1002/aoc.5277
Hoag GE, Collins JB, Holcomb JL, Hoag JR, Nadagouda MN, Varma RS (2009) Degradation of bromothymol blue by ‘greener’ nano-scale zero-valent iron synthesized using tea polyphenols. J Mater Chem 19(45):8671–8677. https://doi.org/10.1039/B909148C
Hooshmand SE, Afshari R, Ramón DJ, Varma RS (2020) Deep eutectic solvents: cutting-edge applications in cross-coupling reactions. Green Chem 22(12):3668–3692. https://doi.org/10.1039/D0GC01494J
Hosny M, Fawzy M (2021) Instantaneous phytosynthesis of gold nanoparticles via Persicaria salicifolia leaf extract, and their medical applications. Adv Powder Technol 32(8):2891–2904. https://doi.org/10.1016/j.apt.2021.06.004
Hosny M, Fawzy M, El-Fakharany EM, Omer AM, Abd El-Monaem EM, Khalifa RE, Eltaweil AS (2022) Biogenic synthesis, characterization, antimicrobial, antioxidant, antidiabetic, and catalytic applications of platinum nanoparticles synthesized from Polygonum salicifolium leaves. J Environ Chem Eng 10(1):106806. https://doi.org/10.1016/j.jece.2021.106806
Huang L, Weng X, Chen Z, Megharaj M, Naidu R (2014a) Green synthesis of iron nanoparticles by various tea extracts: comparative study of the reactivity. Spectrochim Acta Part A Mol Biomol Spectrosc 130:295–301. https://doi.org/10.1016/j.saa.2014.04.037
Huang L, Weng X, Chen Z, Megharaj M, Naidu R (2014b) Synthesis of iron-based nanoparticles using oolong tea extract for the degradation of malachite green. Spectrochim Acta Part A Mol Biomol Spectrosc 117:801–804. https://doi.org/10.1016/j.saa.2013.09.054
Huong LM, Cong CQ, Dat NM, Hai ND, Nam NTH, An H, Tai LT, Do Dat T, Dat NT, Phong MT, Hieu NH (2023) Green synthesis of carbon-doped zinc oxide using Garcinia mangostana peel extract: characterization, photocatalytic degradation, and hydrogen peroxide production. J Clean Prod 392:136269. https://doi.org/10.1016/j.jclepro.2023.136269
Ibrahim FY, El-Khateeb AY, Mohamed AH (2019) Rhus and safflower extracts as potential novel food antioxidant, anticancer, and antimicrobial agents using nanotechnology. Foods 8(4):139. https://doi.org/10.3390/foods8040139
Iravani S (2011) Green synthesis of metal nanoparticles using plants. Green Chem 13(10):2638–2650. https://doi.org/10.1039/C1GC15386B
Iravani S, Varma RS (2020) Greener synthesis of lignin nanoparticles and their applications. Green Chem 22(3):612–636. https://doi.org/10.1039/C9GC02835H
Islam NU, Jalil K, Shahid M, Muhammad N, Rauf A (2019) Pistacia integerrima gall extract mediated green synthesis of gold nanoparticles and their biological activities. Arab J Chem 12(8):2310–2319. https://doi.org/10.1016/j.arabjc.2015.02.014
Ismail E, Khenfouch M, Dhlamini M, Dube S, Maaza M (2017) Green palladium and palladium oxide nanoparticles synthesized via Aspalathus linearis natural extract. J Alloy Compd 695:3632–3638. https://doi.org/10.1016/j.jallcom.2016.11.390
Issaabadi Z, Nasrollahzadeh M, Sajadi SM (2017) Green synthesis of the copper nanoparticles supported on bentonite and investigation of its catalytic activity. J Clean Prod 142:3584–3591. https://doi.org/10.1016/j.jclepro.2016.10.109
Jabir MS, Hussien AA, Sulaiman GM, Yaseen NY, Dewir YH, Alwahibi MS, Soliman DA, Rizwana H (2021) Green synthesis of silver nanoparticles from Eriobotrya japonica extract: a promising approach against cancer cells proliferation, inflammation, allergic disorders and phagocytosis induction. Artif Cells Nanomed Biotechnol 49(1):48–60. https://doi.org/10.1080/21691401.2020.1867152
Jadoun S, Arif R, Jangid NK, Meena RK (2021) Green synthesis of nanoparticles using plant extracts: a review. Environ Chem Lett 19(1):355–374. https://doi.org/10.1007/s10311-020-01074-x
Jafarizad A, Safaee K, Gharibian S, Omidi Y, Ekinci D (2015) Biosynthesis and in-vitro study of gold nanoparticles using Mentha and Pelargonium extracts. Procedia Mater Sci 11:224–230. https://doi.org/10.1016/j.mspro.2015.11.113
Jafir M, Ahmad JN, Arif MJ, Ali S, Ahmad SJN (2021) Characterization of Ocimum basilicum synthesized silver nanoparticles and its relative toxicity to some insecticides against tobacco cutworm, Spodoptera litura Feb. (Lepidoptera; Noctuidae). Ecotoxicol Environ Saf 218:112278. https://doi.org/10.1016/j.ecoenv.2021.112278
Jahangirian H, Lemraski EG, Webster TJ, Rafiee-Moghaddam R, Abdollahi Y (2017) A review of drug delivery systems based on nanotechnology and green chemistry: green nanomedicine. Int J Nanomed 12:2957. https://doi.org/10.2147/IJN.S127683
Jain R, Mendiratta S, Kumar L, Srivastava A (2021) Green synthesis of iron nanoparticles using Artocarpus heterophyllus peel extract and their application as a heterogeneous Fenton-like catalyst for the degradation of Fuchsin Basic dye. Curr Res Green Sustain Chem 4:100086. https://doi.org/10.1016/j.crgsc.2021.100086
Jena J, Pradhan N, Nayak RR, Dash BP, Sukla LB, Panda PK, Mishra BK (2014) Microalga Scenedesmus sp.: a potential low-cost green machine for silver nanoparticle synthesis. J Microbiol Biotechnol 24(4):522–533. https://doi.org/10.4014/jmb.1306.06014
Ji C, Nguyen LN, Hou J, Hai FI, Chen V (2017) Direct immobilization of laccase on titania nanoparticles from crude enzyme extracts of P. ostreatus culture for micro-pollutant degradation. Sep Purif Technol 178:215–223. https://doi.org/10.1016/j.seppur.2017.01.043
Jin Z, Dridi N, Palui G, Palomo V, Jokerst JV, Dawson PE, Amy Sang Q-X, Mattoussi H (2023) Evaluating the catalytic efficiency of the human membrane-type 1 matrix metalloproteinase (MMP-14) using AuNP–peptide conjugates. J Am Chem Soc 145(8):4570–4582. https://doi.org/10.1021/jacs.2c12032
Jose V, Raphel L, Aiswariya K, Mathew P (2021) Green synthesis of silver nanoparticles using Annona squamosa L. seed extract: characterization, photocatalytic and biological activity assay. Bioprocess Biosyst Eng 44:1819–1829. https://doi.org/10.1007/s00449-021-02562-2
Joseph C, Daniels A, Singh S, Singh M (2021) Histidine-tagged folate-targeted gold nanoparticles for enhanced transgene expression in breast cancer cells in vitro. Pharmaceutics 14(1):53. https://doi.org/10.3390/pharmaceutics14010053
Jovanov D, Vujić B, Vujić G (2018) Optimization of the monitoring of landfill gas and leachate in closed methanogenic landfills. J Environ Manag 216:32–40. https://doi.org/10.1016/j.jenvman.2017.08.039
Juneja S, Pandey S (2022) Classifying deep eutectic solvents for polymer solvation via intramolecular dimer formation. Phys Chem Chem Phys 24(36):21655–21665. https://doi.org/10.1039/D2CP03114K
Kadam J, Dhawal P, Barve S, Kakodkar S (2020) Green synthesis of silver nanoparticles using cauliflower waste and their multifaceted applications in photocatalytic degradation of methylene blue dye and Hg2+ biosensing. SN Appl Sci 2:1–16. https://doi.org/10.1007/s42452-020-2543-4
Kadkhoda J, Aghanejad A, Safari B, Barar J, Rasta SH, Davaran S (2022) Aptamer-conjugated gold nanoparticles for targeted paclitaxel delivery and photothermal therapy in breast cancer. J Drug Deliv Sci Technol 67:102954. https://doi.org/10.1016/j.jddst.2021.102954
Kalaba MH, Moghannem SA, El-Hawary AS, Radwan AA, Sharaf MH, Shaban AS (2021) Green synthesized ZnO nanoparticles mediated by Streptomyces plicatus: characterizations, antimicrobial and nematicidal activities and cytogenetic effects. Plants 10(9):1760. https://doi.org/10.3390/plants10091760
Kanwar R, Rathee J, Salunke DB, Mehta SK (2019) Green nanotechnology-driven drug delivery assemblies. ACS Omega 4(5):8804–8815. https://doi.org/10.1021/acsomega.9b00304
Karim N, Liu S, Rashwan AK, Xie J, Mo J, Osman AI, Rooney DW, Chen W (2023) Green synthesis of nanolipo-fibersomes using Nutriose® FB 06 for delphinidin-3-O-sambubioside delivery: characterization, physicochemical properties, and application. Int J Biol Macromol 247:125839. https://doi.org/10.1016/j.ijbiomac.2023.125839
Kataria N, Garg VK (2018) Green synthesis of Fe3O4 nanoparticles loaded sawdust carbon for cadmium(II) removal from water: regeneration and mechanism. Chemosphere 208:818–828. https://doi.org/10.1016/j.chemosphere.2018.06.022
Katti KV, Khoobchandani M, Thipe VC, Al-Yasiri AY, Katti KK, Loyalka SK, Sakr TM, Lugao AB (2018) Prostate tumor therapy advances in nuclear medicine: green nanotechnology toward the design of tumor specific radioactive gold nanoparticles. J Radioanal Nucl Chem 318:1737–1747. https://doi.org/10.1007/s10967-018-6320-4
Kaur A, Gupta U (2009) A review on applications of nanoparticles for the preconcentration of environmental pollutants. J Mater Chem 19(44):8279–8289. https://doi.org/10.1039/B901933B
Kaur A, Bhatt D, Raja L (2022) Applications of nanotechnology in agriculture, with a focus on insect pest management. NanoWorld J 8(S1):S76–S82. https://doi.org/10.17756/nwj.2022-s1-015
Khan SA, Noreen F, Kanwal S, Iqbal A, Hussain G (2018) Green synthesis of ZnO and Cu-doped ZnO nanoparticles from leaf extracts of Abutilon indicum, Clerodendrum infortunatum, Clerodendrum inerme and investigation of their biological and photocatalytic activities. Mater Sci Eng C 82:46–59. https://doi.org/10.1016/j.msec.2017.08.071
Khanna P, Kaur A, Goyal D (2019) Algae-based metallic nanoparticles: synthesis, characterization and applications. J Microbiol Methods 163:105656. https://doi.org/10.1016/j.mimet.2019.105656
Khatami M, Sharifi I, Nobre MAL, Zafarnia N, Aflatoonian MR (2018) Waste-grass-mediated green synthesis of silver nanoparticles and evaluation of their anticancer, antifungal and antibacterial activity. Green Chem Lett Rev 11(2):125–134. https://doi.org/10.1080/17518253.2018.1444797
Khatami M, Khatami S, Mosazade F, Raisi M, Haghighat M, Sabaghan M, Yaghoubi S, Sarani M, Bamorovat M, Malekian L (2020) Greener synthesis of rod shaped zinc oxide nanoparticles using lilium ledebourii tuber and evaluation of their leishmanicidal activity. Iran J Biotechnol 18(1):e2196. https://doi.org/10.30498/IJB.2020.119481.2196
Khoobchandani M, Katti KK, Karikachery AR, Thipe VC, Srisrimal D, Dhurvas Mohandoss DK, Darshakumar RD, Joshi CM, Katti KV (2020) New approaches in breast cancer therapy through green nanotechnology and nano-ayurvedic medicine–pre-clinical and pilot human clinical investigations. Int J Nanomed. https://doi.org/10.2147/IJN.S219042
Kombaiah K, Vijaya JJ, Kennedy LJ, Bououdina M, Al-Lohedan HA, Ramalingam RJ (2017) Studies on Opuntia dilenii haw mediated multifunctional ZnFe2O4 nanoparticles: optical, magnetic and catalytic applications. Mater Chem Phys 194:153–164. https://doi.org/10.1016/j.matchemphys.2017.03.020
Kombaiah K, Vijaya JJ, Kennedy LJ, Bououdina M, Ramalingam RJ, Al-Lohedan HA (2018) Okra extract-assisted green synthesis of CoFe2O4 nanoparticles and their optical, magnetic, and antimicrobial properties. Mater Chem Phys 204:410–419. https://doi.org/10.1016/j.matchemphys.2017.10.077
Kora AJ, Rastogi L (2016) Catalytic degradation of anthropogenic dye pollutants using palladium nanoparticles synthesized by gum olibanum, a glucuronoarabinogalactan biopolymer. Ind Crops Prod 81:1–10. https://doi.org/10.1016/j.indcrop.2015.11.055
Kou L, Sun J, Zhai Y, He Z (2013) The endocytosis and intracellular fate of nanomedicines: implication for rational design. Asian J Pharm Sci 8(1):1–10. https://doi.org/10.1016/j.ajps.2013.07.001
Kowsalya E, MosaChristas K, Balashanmugam P, Rani JC (2019) Biocompatible silver nanoparticles/poly(vinyl alcohol) electrospun nanofibers for potential antimicrobial food packaging applications. Food Packag Shelf Life 21:100379. https://doi.org/10.1016/j.fpsl.2019.100379
Kulkarni D, Sherkar R, Shirsathe C, Sonwane R, Varpe N, Shelke S, More MP, Pardeshi SR, Dhaneshwar G, Junnuthula V, Dyawanapelly S (2023) Biofabrication of nanoparticles: sources, synthesis, and biomedical applications. Front Bioeng Biotechnol 11:1159193. https://doi.org/10.3389/fbioe.2023.1159193
Kumar V (2021) Rhizomicrobiome dynamics in bioremediation. CRC Press, Taylor & Francis Group, Boca Raton
Kumar PPP, Lim D-K (2022) Gold-polymer nanocomposites for future therapeutic and tissue engineering applications. Pharmaceutics 14(1):70. https://doi.org/10.3390/pharmaceutics14010070
Kumar B, Smita K, Cumbal L, Debut A (2014) Biogenic synthesis of iron oxide nanoparticles for 2-arylbenzimidazole fabrication. J Saudi Chem Soc 18(4):364–369. https://doi.org/10.1016/j.jscs.2014.01.003
Kumar V, Bano D, Mohan S, Singh DK, Hasan SH (2016) Sunlight-induced green synthesis of silver nanoparticles using aqueous leaf extract of Polyalthia longifolia and its antioxidant activity. Mater Lett 181:371–377. https://doi.org/10.1016/j.matlet.2016.05.097
Kumar S, Boro JC, Ray D, Mukherjee A, Dutta J (2019) Bionanocomposite films of agar incorporated with ZnO nanoparticles as an active packaging material for shelf life extension of green grape. Heliyon 5(6):e01867. https://doi.org/10.1016/j.heliyon.2019.e01867
Kumar S, Mudai A, Roy B, Basumatary IB, Mukherjee A, Dutta J (2020a) Biodegradable hybrid nanocomposite of chitosan/gelatin and green synthesized zinc oxide nanoparticles for food packaging. Foods 9(9):1143. https://doi.org/10.3390/foods9091143
Kumar YR, Deshmukh K, Sadasivuni KK, Pasha SKK (2020b) Graphene quantum dot based materials for sensing, bio-imaging and energy storage applications: a review. RSC Adv 10(40):23861–23898. https://doi.org/10.1039/d0ra03938a
KumaráBorah R, JyotiáSaikia H, KumaráDas V, JyotiáThakur A (2015) Biosynthesis of poly(ethylene glycol)-supported palladium nanoparticles using Colocasia esculenta leaf extract and their catalytic activity for Suzuki–Miyaura cross-coupling reactions. RSC Adv 5(89):72453–72457. https://doi.org/10.1039/C5RA12657F
Kumavat SR, Mishra S (2021) Green synthesis of silver nanoparticles using Borago officinalis leaves extract and screening its antimicrobial and antifungal activity. Int Nano Lett 11(4):355–370. https://doi.org/10.1007/s40089-021-00345-x
Küp FÖ, Çoşkunçay S, Duman F (2020) Biosynthesis of silver nanoparticles using leaf extract of Aesculus hippocastanum (horse chestnut): evaluation of their antibacterial, antioxidant and drug release system activities. Mater Sci Eng C 107:110207. https://doi.org/10.1016/j.msec.2019.110207
Kuppusamy P, Yusoff MM, Maniam GP, Govindan N (2016) Biosynthesis of metallic nanoparticles using plant derivatives and their new avenues in pharmacological applications—an updated report. Saudi Pharm J 24(4):473–484. https://doi.org/10.1016/j.jsps.2014.11.013
Lebaschi S, Hekmati M, Veisi H (2017) Green synthesis of palladium nanoparticles mediated by black tea leaves (Camellia sinensis) extract: catalytic activity in the reduction of 4-nitrophenol and Suzuki–Miyaura coupling reaction under ligand-free conditions. J Colloid Interface Sci 485:223–231. https://doi.org/10.1016/j.jcis.2016.09.027
Lee M, Wells E, Wong YK, Koenig J, Adrian L, Richnow HH, Manefield M (2015) Relative contributions of Dehalobacter and zerovalent iron in the degradation of chlorinated methanes. Environ Sci Technol 49(7):4481–4489. https://doi.org/10.1021/es5052364
Lee KX, Shameli K, Mohamad SE, Yew YP, Mohamed Isa ED, Yap H-Y, Lim WL, Teow S-Y (2019) Bio-mediated synthesis and characterisation of silver nanocarrier, and its potent anticancer action. Nanomaterials 9(10):1423. https://doi.org/10.3390/nano9101423
Leili M, Fazlzadeh M, Bhatnagar A (2018) Green synthesis of nano-zero-valent iron from Nettle and Thyme leaf extracts and their application for the removal of cephalexin antibiotic from aqueous solutions. Environ Technol 39(9):1158–1172. https://doi.org/10.1080/09593330.2017.1323956
Li Y, Hu Y, Zhao Y, Shi G, Deng L, Hou Y, Qu L (2011) An electrochemical avenue to green-luminescent graphene quantum dots as potential electron-acceptors for photovoltaics. Adv Mater 23(6):776–780. https://doi.org/10.1002/adma.201003819
Li G, He D, Qian Y, Guan B, Gao S, Cui Y, Yokoyama K, Wang L (2012) Fungus-mediated green synthesis of silver nanoparticles using Aspergillus terreus. Int J Mol Sci 13(1):466-476. https://doi.org/10.3390/ijms13010466
Li G, Li Y, Wang Z, Liu H (2017) Green synthesis of palladium nanoparticles with carboxymethyl cellulose for degradation of azo-dyes. Mater Chem Phys 187:133–140. https://doi.org/10.1016/j.matchemphys.2016.11.057
Li S, Al-Misned FA, El-Serehy HA, Yang L (2021) Green synthesis of gold nanoparticles using aqueous extract of Mentha longifolia leaf and investigation of its anti-human breast carcinoma properties in the in vitro condition. Arab J Chem 14(2):102931. https://doi.org/10.1016/j.arabjc.2020.102931
Li X, Mao X, Xie W, Liu B, Chen F (2022) Intracellular biosynthesis of gold nanoparticles for monitoring microalgal biomass via surface-enhanced Raman spectroscopy. ACS Sustain Chem Eng 10(15):4872–4880. https://doi.org/10.1021/acssuschemeng.1c07432
Lin Z, Weng X, Khan NI, Owens G, Chen Z (2021) Removal mechanism of Sb(III) by a hybrid rGO-Fe/Ni composite prepared by green synthesis via a one-step method. Sci Total Environ 788:147844. https://doi.org/10.1016/j.scitotenv.2021.147844
Liu R, Lal R (2014) Synthetic apatite nanoparticles as a phosphorus fertilizer for soybean (Glycine max). Sci Rep 4(1):5686. https://doi.org/10.1038/srep05686
Liu Q, Wang H, Shi X, Wang Z-G, Ding B (2017) Self-assembled DNA/peptide-based nanoparticle exhibiting synergistic enzymatic activity. ACS Nano 11(7):7251–7258. https://doi.org/10.1021/acsnano.7b03195
Liu Y, Friesen JB, McAlpine JB, Lankin DC, Chen S-N, Pauli GF (2018) Natural deep eutectic solvents: properties, applications, and perspectives. J Nat Prod 81(3):679–690. https://doi.org/10.1021/acs.jnatprod.7b00945
Luangpipat T, Beattie IR, Chisti Y, Haverkamp RG (2011) Gold nanoparticles produced in a microalga. J Nanopart Res 13(12):6439–6445. https://doi.org/10.1007/s11051-011-0397-9
Machado S, Pinto S, Grosso J, Nouws H, Albergaria JT, Delerue-Matos C (2013a) Green production of zero-valent iron nanoparticles using tree leaf extracts. Sci Total Environ 445:1–8. https://doi.org/10.1016/j.scitotenv.2012.12.033
Machado S, Stawiński W, Slonina P, Pinto AR, Grosso JP, Nouws HPA, Albergaria JT, Delerue-Matos C (2013b) Application of green zero-valent iron nanoparticles to the remediation of soils contaminated with ibuprofen. Sci Total Environ 461–462:323–329. https://doi.org/10.1016/j.scitotenv.2013.05.016
Machado S, Grosso J, Nouws H, Albergaria JT, Delerue-Matos C (2014) Utilization of food industry wastes for the production of zero-valent iron nanoparticles. Sci Total Environ 496:233–240. https://doi.org/10.1016/j.scitotenv.2014.07.058
Machado S, Pacheco J, Nouws H, Albergaria JT, Delerue-Matos C (2015) Characterization of green zero-valent iron nanoparticles produced with tree leaf extracts. Sci Total Environ 533:76–81. https://doi.org/10.1016/j.scitotenv.2015.06.091
Madhukara Naik M, Bhojya Naik HS, Nagaraju G, Vinuth M, Raja Naika H, Vinu K (2019) Green synthesis of zinc ferrite nanoparticles in Limonia acidissima juice: characterization and their application as photocatalytic and antibacterial activities. Microchem J 146:1227–1235. https://doi.org/10.1016/j.microc.2019.02.059
Madivoli ES, Kareru PG, Gachanja AN, Mugo SM, Makhanu DS, Wanakai SI, Gavamukulya Y (2020) Facile synthesis of silver nanoparticles using Lantana trifolia aqueous extracts and their antibacterial activity. J Inorg Organomet Polym Mater 30(8):2842–2850. https://doi.org/10.1007/s10904-019-01432-5
Maia RA, Louis B, Baudron SA (2021) Deep eutectic solvents for the preparation and post-synthetic modification of metal- and covalent organic frameworks. CrystEngComm 23(29):5016–5032. https://doi.org/10.1039/D1CE00714A
Majeed S, Danish M, Mohamad Ibrahim MN, Sekeri SH, Ansari MT, Nanda A, Ahmad G (2021) Bacteria mediated synthesis of iron oxide nanoparticles and their antibacterial, antioxidant, cytocompatibility properties. J Cluster Sci 32(4):1083–1094. https://doi.org/10.1007/s10876-020-01876-7
Manjunath HM, Joshi CG, Raju NG (2017a) Biofabrication of gold nanoparticles using marine endophytic fungus—Penicillium citrinum. IET Nanobiotechnol 11(1):40–44. https://doi.org/10.1049/iet-nbt.2016.0065
Manjunath Hulikere M, Joshi CG (2019) Characterization, antioxidant and antimicrobial activity of silver nanoparticles synthesized using marine endophytic fungus—Cladosporium cladosporioides. Process Biochem 82:199–204. https://doi.org/10.1016/j.procbio.2019.04.011
Manquián-Cerda K, Cruces E, Rubio MA, Reyes C, Arancibia-Miranda N (2017) Preparation of nanoscale iron (oxide, oxyhydroxides and zero-valent) particles derived from blueberries: Reactivity, characterization and removal mechanism of arsenate. Ecotoxicol Environ Saf 145:69–77. https://doi.org/10.1016/j.ecoenv.2017.07.004
Mariadoss AVA, Saravanakumar K, Sathiyaseelan A, Venkatachalam K, Wang M-H (2020) Folic acid functionalized starch encapsulated green synthesized copper oxide nanoparticles for targeted drug delivery in breast cancer therapy. Int J Biol Macromol 164:2073–2084. https://doi.org/10.1016/j.ijbiomac.2020.08.036
Martínez-Molina EC, Freile-Pelegrin Y, Ovando-Chacón SL, Gutiérrez-Miceli FA, Ruiz-Cabrera MÁ, Grajales-Lagunes A, Luján-Hidalgo MC, Abud-Archila M (2022) Development and characterization of alginate-based edible film from Sargassum fluitans incorporated with silver nanoparticles obtained by green synthesis. J Food Meas Charact 16:126–136. https://doi.org/10.1007/s11694-021-01156-6
Mat Isa SZ, Zainon R, Tamal M (2022) State of the art in gold nanoparticle synthesisation via pulsed laser ablation in liquid and its characterisation for molecular imaging: a review. Materials 15(3):875. https://doi.org/10.3390/ma15030875
Matinise N, Kaviyarasu K, Mongwaketsi N, Khamlich S, Kotsedi L, Mayedwa N, Maaza M (2018) Green synthesis of novel zinc iron oxide (ZnFe2O4) nanocomposite via Moringa oleifera natural extract for electrochemical applications. Appl Surf Sci 446:66–73. https://doi.org/10.1016/j.apsusc.2018.02.187
Mbatha LS, Maiyo F, Daniels A, Singh M (2021) Dendrimer-coated gold nanoparticles for efficient folate-targeted mRNA delivery in vitro. Pharmaceutics 13(6):900. https://doi.org/10.3390/pharmaceutics13060900
Mh M, Joshi CG, Danagoudar A, Poyya J, Kudva AK, Bl D (2017) Biogenic synthesis of gold nanoparticles by marine endophytic fungus-Cladosporium cladosporioides isolated from seaweed and evaluation of their antioxidant and antimicrobial properties. Process Biochem 63:137–144. https://doi.org/10.1016/j.procbio.2017.09.008
Mirgane NA, Shivankar VS, Kotwal SB, Wadhawa GC, Sonawale MC (2021) Waste pericarp of Ananas comosus in green synthesis zinc oxide nanoparticles and their application in waste water treatment. Mater Today Proc 37:886–889. https://doi.org/10.1016/j.matpr.2020.06.045
Mohamed AA, Abu-Elghait M, Ahmed NE, Salem SS (2021) Eco-friendly mycogenic synthesis of ZnO and CuO nanoparticles for in vitro antibacterial, antibiofilm, and antifungal applications. Biol Trace Elem Res 199(7):2788–2799. https://doi.org/10.1007/s12011-020-02369-4
Mohammadzadeh V, Barani M, Amiri MS, Taghavizadeh Yazdi ME, Hassanisaadi M, Rahdar A, Varma RS (2022) Applications of plant-based nanoparticles in nanomedicine: a review. Sustain Chem Pharm 25:100606. https://doi.org/10.1016/j.scp.2022.100606
Mohan SV, Katakojwala R (2021) The circular chemistry conceptual framework: a way forward to sustainability in industry 4.0. Curr Opin Green Sustain Chem 28:100434. https://doi.org/10.1016/j.cogsc.2020.100434
Mohanraj S, Kodhaiyolii S, Rengasamy M, Pugalenthi V (2014) Green synthesized iron oxide nanoparticles effect on fermentative hydrogen production by Clostridium acetobutylicum. Appl Biochem Biotechnol 173:318–331. https://doi.org/10.1007/s12010-014-0843-0
Molnar Z, Bodai V, Szakacs G, Erdelyi B, Fogarassy Z, Safran G, Varga T, Konya Z, Toth-Szeles E, Szucs R, Lagzi I (2018) Green synthesis of gold nanoparticles by thermophilic filamentous fungi. Sci Rep 8(1):3943. https://doi.org/10.1038/s41598-018-22112-3
Mondal P, Anweshan A, Purkait MK (2020) Green synthesis and environmental application of iron-based nanomaterials and nanocomposite: a review. Chemosphere 259:127509 https://doi.org/10.1016/j.chemosphere.2020.127509
Monga Y, Kumar P, Sharma RK, Filip J, Varma RS, Zbořil R, Gawande MB (2020) Sustainable synthesis of nanoscale zerovalent iron particles for environmental remediation. Chemsuschem 13(13):3288–3305. https://doi.org/10.1002/cssc.202000290
Morales-Lozoya V, Espinoza-Gómez H, Flores-López LZ, Sotelo-Barrera EL, Núñez-Rivera A, Cadena-Nava RD, Alonso-Nuñez G, Rivero IA (2021) Study of the effect of the different parts of Morinda citrifolia L. (noni) on the green synthesis of silver nanoparticles and their antibacterial activity. Appl Surf Sci 537:147855. https://doi.org/10.1016/j.apsusc.2020.147855
Mousavi-Kouhi SM, Beyk-Khormizi A, Amiri MS, Mashreghi M, Taghavizadeh Yazdi ME (2021) Silver–zinc oxide nanocomposite: from synthesis to antimicrobial and anticancer properties. Ceram Int 47(15):21490–21497. https://doi.org/10.1016/j.ceramint.2021.04.160
Muthuvel A, Jothibas M, Manoharan C (2020) Biological synthesis and characterization of silver nanoparticles with Capparis zeylanica L. leaf extract for potent antimicrobial and anti proliferation efficiency. Nanotechnol Environ Eng 5:1–19. https://doi.org/10.1016/j.mset.2020.02.008
Mystrioti C, Xanthopoulou TD, Tsakiridis P, Papassiopi N, Xenidis A (2016) Comparative evaluation of five plant extracts and juices for nanoiron synthesis and application for hexavalent chromium reduction. Sci Total Environ 539:105–113. https://doi.org/10.1016/j.scitotenv.2015.08.091
Nagajyothi PC, Muthuraman P, Sreekanth TVM, Kim DH, Shim J (2017a) Green synthesis: in-vitro anticancer activity of copper oxide nanoparticles against human cervical carcinoma cells. Arab J Chem 10(2):215–225. https://doi.org/10.1016/j.arabjc.2016.01.011
Nagajyothi PC, Pandurangan M, Kim DH, Sreekanth TVM, Shim J (2017b) Green synthesis of iron oxide nanoparticles and their catalytic and in vitro anticancer activities. J Cluster Sci 28:245–257. https://doi.org/10.1007/s10876-016-1082-z
Nagar N, Devra V (2018) Green synthesis and characterization of copper nanoparticles using Azadirachta indica leaves. Mater Chem Phys 213:44–51. https://doi.org/10.1016/j.matchemphys.2018.04.007
Naika HR, Lingaraju K, Manjunath K, Kumar D, Nagaraju G, Suresh D, Nagabhushana H (2015) Green synthesis of CuO nanoparticles using Gloriosa superba L. extract and their antibacterial activity. J Taibah Univ Sci 9(1):7–12. https://doi.org/10.1016/j.jtusci.2014.04.006
Naraginti S, Li Y (2017) Preliminary investigation of catalytic, antioxidant, anticancer and bactericidal activity of green synthesized silver and gold nanoparticles using Actinidia deliciosa. J Photochem Photobiol B 170:225–234. https://doi.org/10.1016/j.jphotobiol.2017.03.023
Narasaiah BP, Mandal BK (2020) Remediation of azo-dyes based toxicity by agro-waste cotton boll peels mediated palladium nanoparticles. J Saudi Chem Soc 24(2):267–281. https://doi.org/10.1016/j.jscs.2019.11.003
Naseem T, Farrukh MA (2015) Antibacterial activity of green synthesis of iron nanoparticles using Lawsonia inermis and Gardenia jasminoides leaves extract. J Chem 2015:912342. https://doi.org/10.1155/2015/912342
Nasiri J, Rahimi M, Hamezadeh Z, Motamedi E, Naghavi MR (2018) Fulfillment of green chemistry for synthesis of silver nanoparticles using root and leaf extracts of Ferula persica: solid-state route vs. solution-phase method. J Clean Prod 192:514–530. https://doi.org/10.1016/j.jclepro.2018.04.218
Nasrollahzadeh M, Sajadi SM (2015) Green synthesis of copper nanoparticles using Ginkgo biloba L. leaf extract and their catalytic activity for the Huisgen [3 + 2] cycloaddition of azides and alkynes at room temperature. J Colloid Interface Sci 457:141–147. https://doi.org/10.1016/j.jcis.2015.07.004
Nasrollahzadeh M, Sajadi SM (2016a) Green synthesis of Pd nanoparticles mediated by Euphorbia thymifolia L. leaf extract: catalytic activity for cyanation of aryl iodides under ligand-free conditions. J Colloid Interface Sci 469:191–195. https://doi.org/10.1016/j.jcis.2016.02.024
Nasrollahzadeh M, Sajadi SM (2016b) Pd nanoparticles synthesized in situ with the use of Euphorbia granulate leaf extract: catalytic properties of the resulting particles. J Colloid Interface Sci 462:243–251. https://doi.org/10.1016/j.jcis.2015.09.065
Nasrollahzadeh M, Atarod M, Sajadi SM (2017a) Biosynthesis, characterization and catalytic activity of Cu/RGO/Fe3O4 for direct cyanation of aldehydes with K4[Fe(CN)6]. J Colloid Interface Sci 486:153–162. https://doi.org/10.1016/j.jcis.2016.09.053
Nasrollahzadeh M, Momeni SS, Sajadi SM (2017b) Green synthesis of copper nanoparticles using Plantago asiatica leaf extract and their application for the cyanation of aldehydes using K4Fe(CN)6. J Colloid Interface Sci 506:471–477. https://doi.org/10.1016/j.jcis.2017.07.072
Nasrollahzadeh M, Sajjadi M, Dasmeh HR, Sajadi SM (2018) Green synthesis of the Cu/sodium borosilicate nanocomposite and investigation of its catalytic activity. J Alloys Compd 763:1024–1034. https://doi.org/10.1016/j.jallcom.2018.05.012
Ngom BD, Mpahane T, Manikandan E, Maaza M (2016) ZnO nano-discs by lyophilization process: size effects on their intrinsic luminescence. J Alloys Compd 656:758–763. https://doi.org/10.1016/j.jallcom.2015.09.230
Nikfarjam N, Ghomi M, Agarwal T, Hassanpour M, Sharifi E, Khorsandi D, Ali Khan M, Rossi F, Rossetti A, Nazarzadeh Zare E, Rabiee N, Afshar D, Vosough M, Kumar Maiti T, Mattoli V, Lichtfouse E, Tay FR, Makvandi P (2021) Antimicrobial ionic liquid-based materials for biomedical applications. Adv Funct Mater 31(42):2104148. https://doi.org/10.1002/adfm.202104148
Nilavukkarasi M, Vijayakumar S, Kumar SP (2020) Biological synthesis and characterization of silver nanoparticles with Capparis zeylanica L. leaf extract for potent antimicrobial and anti proliferation efficiency. Mater Sci Energy Technol 3:371–376. https://doi.org/10.1016/j.mset.2020.02.008
Noman M, Ahmed T, Hussain S, Niazi MBK, Shahid M, Song F (2020) Biogenic copper nanoparticles synthesized by using a copper-resistant strain Shigella flexneri SNT22 reduced the translocation of cadmium from soil to wheat plants. J Hazard Mater 398:123175. https://doi.org/10.1016/j.jhazmat.2020.123175
Omran BA (2020) Biosynthesized nanomaterials via processing of different plant parts (phytonanotechnology) and biovalorization of agro-industrial wastes to nano-sized valuable products. Nanobiotechnol A Multidiscip Field Sci 2020:145-184. https://doi.org/10.1007/978-3-030-46071-6_5
Omran BA, Whitehead KA, Baek K-H (2021) One-pot bioinspired synthesis of fluorescent metal chalcogenide and carbon quantum dots: applications and potential biotoxicity. Colloids Surf B 200:111578. https://doi.org/10.1016/j.colsurfb.2021.111578
Orooji Y, Mortazavi-Derazkola S, Ghoreishi SM, Amiri M, Salavati-Niasari M (2020) Mesopourous Fe3O4@SiO2-hydroxyapatite nanocomposite: green sonochemical synthesis using strawberry fruit extract as a capping agent, characterization and their application in sulfasalazine delivery and cytotoxicity. J Hazard Mater 400:123140. https://doi.org/10.1016/j.jhazmat.2020.123140
Osman AI, O’Connor E, McSpadden G, Abu-Dahrieh JK, Farrell C, AaH A-M, Harrison J, Rooney DW (2020) Upcycling brewer’s spent grain waste into activated carbon and carbon nanotubes for energy and other applications via two-stage activation. J Chem Technol Biotechnol 95(1):183–195. https://doi.org/10.1002/jctb.6220
Osman AI, Elgarahy AM, Mehta N, AaH A-M, Al-Fatesh AS, Rooney DW (2022) Facile synthesis and life cycle assessment of highly active magnetic sorbent composite derived from mixed plastic and biomass waste for water remediation. ACS Sustain Chem Eng 10(37):12433–12447. https://doi.org/10.1021/acssuschemeng.2c04095
Owaid MN, Ibraheem IJ (2017) Mycosynthesis of nanoparticles using edible and medicinal mushrooms. Eur J Nanomed 9(1):5–23. https://doi.org/10.1515/ejnm-2016-0016
Owoseni-Fagbenro KA, Saifullah S, Imran M, Perveen S, Rao K, Fasina TM, Olasupo IA, Adams LA, Ali I, Shah MR (2019) Egg proteins stabilized green silver nanoparticles as delivery system for hesperidin enhanced bactericidal potential against resistant S. aureus. J Drug Deliv Sci Technol 50:347–354. https://doi.org/10.1016/j.jddst.2019.02.002
Pai S, Sridevi H, Varadavenkatesan T, Vinayagam R, Selvaraj R (2019) Photocatalytic zinc oxide nanoparticles synthesis using Peltophorum pterocarpum leaf extract and their characterization. Optik 185:248–255. https://doi.org/10.1016/j.ijleo.2019.03.101
Pakzad K, Alinezhad H, Nasrollahzadeh M (2019) Green synthesis of Ni@Fe3O4 and CuO nanoparticles using Euphorbia maculata extract as photocatalysts for the degradation of organic pollutants under UV-irradiation. Ceram Int 45(14):17173–17182. https://doi.org/10.1016/j.ceramint.2019.05.272
Palombarini F, Masciarelli S, Incocciati A, Liccardo F, Di Fabio E, Iazzetti A, Fabrizi G, Fazi F, Macone A, Bonamore A (2021) Self-assembling ferritin-dendrimer nanoparticles for targeted delivery of nucleic acids to myeloid leukemia cells. J Nanobiotechnol 19(1):1–12. https://doi.org/10.1186/s12951-021-00921-5
Pan Y, Li Q, Liu W, Armstrong Z, MacRae A, Feng L, McNeff C, Zhao P, Li H, Yang Z (2023) Unveiling the orientation and dynamics of enzymes in unstructured artificial compartments of metal–organic frameworks (MOFs). Nanoscale 15(6):2573–2577. https://doi.org/10.1039/D2NR06659A
Patil MP, Kim G-D (2018) Marine microorganisms for synthesis of metallic nanoparticles and their biomedical applications. Colloids Surf B 172:487–495. https://doi.org/10.1016/j.colsurfb.2018.09.007
Patil MP, Kang M-J, Niyonizigiye I, Singh A, Kim J-O, Seo YB, Kim G-D (2019) Extracellular synthesis of gold nanoparticles using the marine bacterium Paracoccus haeundaensis BC74171T and evaluation of their antioxidant activity and antiproliferative effect on normal and cancer cell lines. Colloids Surf B 183:110455. https://doi.org/10.1016/j.colsurfb.2019.110455
Perveen R, Shujaat S, Qureshi Z, Nawaz S, Khan MI, Iqbal M (2020) Green versus sol-gel synthesis of ZnO nanoparticles and antimicrobial activity evaluation against panel of pathogens. J Mater Res Technol 9(4):7817–7827. https://doi.org/10.1016/j.jmrt.2020.05.004
Pietroiusti A, Stockmann-Juvala H, Lucaroni F, Savolainen K (2018) Nanomaterial exposure, toxicity, and impact on human health. Wires Nanomed Nanobiotechnol 10(5):e1513. https://doi.org/10.1002/wnan.1513
Ping Y, Zhang J, Xing T, Chen G, Tao R, Choo K-H (2018) Green synthesis of silver nanoparticles using grape seed extract and their application for reductive catalysis of Direct Orange 26. J Ind Eng Chem 58:74–79. https://doi.org/10.1016/j.jiec.2017.09.009
Poguberović SS, Krčmar DM, Maletić SP, Kónya Z, Pilipović DDT, Kerkez DV, Rončević SD (2016) Removal of As(III) and Cr(VI) from aqueous solutions using “green” zero-valent iron nanoparticles produced by oak, mulberry and cherry leaf extracts. Ecol Eng 90:42–49. https://doi.org/10.1016/j.ecoleng.2016.01.083
Pooja D, Panyaram S, Kulhari H, Reddy B, Rachamalla SS, Sistla R (2015) Natural polysaccharide functionalized gold nanoparticles as biocompatible drug delivery carrier. Int J Biol Macromol 80:48–56. https://doi.org/10.1016/j.ijbiomac.2015.06.022
Prakash LV, Gopinath A, Gandhimathi R, Velmathi S, Ramesh S, Nidheesh P (2021) Ultrasound aided heterogeneous Fenton degradation of Acid Blue 15 over green synthesized magnetite nanoparticles. Sep Purif Technol 266:118230. https://doi.org/10.1016/j.seppur.2020.118230
Qayyum S, Oves M, Khan AU (2017) Obliteration of bacterial growth and biofilm through ROS generation by facilely synthesized green silver nanoparticles. PLoS ONE 12(8):e0181363. https://doi.org/10.1371/journal.pone.0181363
Ragavendran C, Kamaraj C, Jothimani K, Priyadharsan A, Kumar DA, Natarajan D, Malafaia G (2023) Eco-friendly approach for ZnO nanoparticles synthesis and evaluation of its possible antimicrobial, larvicidal and photocatalytic applications. Sustain Mater Technol 36:e00597. https://doi.org/10.1016/j.susmat.2023.e00597
Rahmayanti M, Syakina AN, Fatimah I, Sulistyaningsih T (2022) Green synthesis of magnetite nanoparticles using peel extract of jengkol (Archidendron pauciflorum) for methylene blue adsorption from aqueous media. Chem Phys Lett 803:139834. https://doi.org/10.1016/j.cplett.2022.139834
Rajaboopathi S, Thambidurai S (2018) Enhanced photocatalytic activity of Ag-ZnO nanoparticles synthesized by using Padina gymnospora seaweed extract. J Mol Liq 262:148–160. https://doi.org/10.1016/j.molliq.2018.04.073
Rajesh KM, Ajitha B, Reddy YAK, Suneetha Y, Reddy PS (2018) Assisted green synthesis of copper nanoparticles using Syzygium aromaticum bud extract: physical, optical and antimicrobial properties. Optik 154:593–600. https://doi.org/10.1016/j.ijleo.2017.10.074
Rajeshkumar S, Sivapriya D (2020) Fungus-mediated nanoparticles: characterization and biomedical advances. Nanoparticles in medicine 2020:185–199. https://doi.org/10.1007/978-981-13-8954-2_7
Rajeshkumar S, Kumar SV, Ramaiah A, Agarwal H, Lakshmi T, Roopan SM (2018) Biosynthesis of zinc oxide nanoparticles using Mangifera indica leaves and evaluation of their antioxidant and cytotoxic properties in lung cancer (A549) cells. Enzyme Microb Technol 117:91–95. https://doi.org/10.1016/j.enzmictec.2018.06.009
Rajeshkumar S, Menon S, Kumar SV, Tambuwala MM, Bakshi HA, Mehta M, Satija S, Gupta G, Chellappan DK, Thangavelu L (2019) Antibacterial and antioxidant potential of biosynthesized copper nanoparticles mediated through Cissus arnotiana plant extract. J Photochem Photobiol B 197:111531. https://doi.org/10.1016/j.jphotobiol.2019.111531
Rambabu K, Bharath G, Banat F, Show PL (2021) Green synthesis of zinc oxide nanoparticles using Phoenix dactylifera waste as bioreductant for effective dye degradation and antibacterial performance in wastewater treatment. J Hazard Mater 402:123560. https://doi.org/10.1016/j.jhazmat.2020.123560
Ramkumar G, Asokan R, Ramya S, Gayathri G (2021) Characterization of Trigonella foenum-graecum derived iron nanoparticles and its potential pesticidal activity against Tuta absoluta (Lepidoptera). J Cluster Sci 32:1185–1190. https://doi.org/10.1007/s10876-020-01867-8
Rashwan AK, Karim N, Xu Y, Hanafy NAN, Li B, Mehanni A-HE, Taha EM, Chen W (2022a) An updated and comprehensive review on the potential health effects of curcumin-encapsulated micro/nanoparticles. Crit Rev Food Sci Nutr 63(29):9731-9751. https://doi.org/10.1080/10408398.2022.2070906
Rashwan AK, Younes HA, Karim N, Taha EM, Mozafari MR, Tawfeuk HZ, Chen W (2022b) Health properties of bioactive food compounds-loaded micro and nano-encapsulation systems: a review. SVU-Int J Agric Sci 4(2):178–203. https://doi.org/10.21608/svuijas.2022.134530.1208
Rashwan AK, Bai H, Osman AI, Eltohamy KM, Chen Z, Younis HA, Al-Fatesh A, Rooney DW, Yap P-S (2023a) Recycling food and agriculture by-products to mitigate climate change: a review. Environ Chem Lett 21(6): 3351-3375. https://doi.org/10.1007/s10311-023-01639-6
Rashwan AK, Karim N, Xu Y, Xie J, Cui H, Mozafari MR, Chen W (2023b) Potential micro-/nano-encapsulation systems for improving stability and bioavailability of anthocyanins: an updated review. Crit Rev Food Sci Nutr 63(19):3362–3385. https://doi.org/10.1080/10408398.2021.1987858
Rather MY, Sundarapandian S (2022) Facile green synthesis of copper oxide nanoparticles and their rhodamine-b dye adsorption property. J Cluster Sci 2022:1–9. https://doi.org/10.1007/s10876-021-02025-4
Rathnasamy R, Thangasamy P, Thangamuthu R, Sampath S, Alagan V (2017) Green synthesis of ZnO nanoparticles using Carica papaya leaf extracts for photocatalytic and photovoltaic applications. J Mater Sci Mater Electron 28:10374–10381. https://doi.org/10.1007/s10854-017-6807-8
Roopan SM, Madhumitha G, Rahuman AA, Kamaraj C, Bharathi A, Surendra TV (2013) Low-cost and eco-friendly phyto-synthesis of silver nanoparticles using Cocos nucifera coir extract and its larvicidal activity. Ind Crops Prod 43:631–635. https://doi.org/10.1016/j.indcrop.2012.08.013
Rose GK, Soni R, Rishi P, Soni SK (2019) Optimization of the biological synthesis of silver nanoparticles using Penicillium oxalicum GRS-1 and their antimicrobial effects against common food-borne pathogens. Green Process Synth 8(1):144–156. https://doi.org/10.1515/gps-2018-0042
Rosli I, Zulhaimi H, Ibrahim S, Gopinath S, Kasim K, Akmal H, Nuradibah M, Sam T (2018) Phytosynthesis of iron nanoparticle from Averrhoa Bilimbi Linn. IOP Publishing 318(1):012012. https://doi.org/10.1088/1757-899X/318/1/012012
Rostami-Vartooni A, Nasrollahzadeh M, Alizadeh M (2016) Green synthesis of seashell supported silver nanoparticles using Bunium persicum seeds extract: application of the particles for catalytic reduction of organic dyes. J Colloid Interface Sci 470:268–275. https://doi.org/10.1016/j.jcis.2016.02.060
Rostamizadeh E, Iranbakhsh A, Majd A, Arbabian S, Mehregan I (2020) Green synthesis of Fe2O3 nanoparticles using fruit extract of Cornus mas L. and its growth-promoting roles in Barley. J Nanostruct Chem 10:125–130. https://doi.org/10.1007/s40097-020-00335-z
Rupa EJ, Anandapadmanaban G, Mathiyalagan R, Yang D-C (2018) Synthesis of zinc oxide nanoparticles from immature fruits of Rubus coreanus and its catalytic activity for degradation of industrial dye. Optik 172:1179–1186. https://doi.org/10.1016/j.ijleo.2018.07.115
Sabouri Z, Akbari A, Hosseini HA, Khatami M, Darroudi M (2021a) Green-based bio-synthesis of nickel oxide nanoparticles in Arabic gum and examination of their cytotoxicity, photocatalytic and antibacterial effects. Green Chem Lett Rev 14(2):404–414. https://doi.org/10.1080/17518253.2021.1923824
Sabouri Z, Rangrazi A, Amiri MS, Khatami M, Darroudi M (2021b) Green synthesis of nickel oxide nanoparticles using Salvia hispanica L. (chia) seeds extract and studies of their photocatalytic activity and cytotoxicity effects. Bioprocess Biosyst Eng 44:2407–2415. https://doi.org/10.1007/s00449-021-02613-8
Sagar N, Pareek S, Sharma S, Yahia E, Lobo M (2018) Fruit and vegetable waste: bioactive compounds, their extraction, and possible utilization. Compr Rev Food Sci Food Saf 17:512–531. https://doi.org/10.1111/1541-4337.12330
Saif S, Tahir A, Chen Y (2016) Green synthesis of iron nanoparticles and their environmental applications and implications. Nanomaterials 6(11):209. https://doi.org/10.3390/nano6110209
Salmani MH, Abedi M, Mozaffari SA, Mahvi AH, Sheibani A, Jalili M (2021) Simultaneous reduction and adsorption of arsenite anions by green synthesis of iron nanoparticles using pomegranate peel extract. J Environ Health Sci Eng 19:603–612. https://doi.org/10.1007/s40201-021-00631-y
Sana SS, Dogiparthi LK (2018) Green synthesis of silver nanoparticles using Givotia moluccana leaf extract and evaluation of their antimicrobial activity. Mater Lett 226:47–51. https://doi.org/10.1016/j.matlet.2018.05.009
Saravanakumar K, Chelliah R, MubarakAli D, Oh D-H, Kathiresan K, Wang M-H (2019) Unveiling the potentials of biocompatible silver nanoparticles on human lung carcinoma A549 cells and Helicobacter pylori. Sci Rep 9(1):1–8. https://doi.org/10.1038/s41598-019-42112-1
Saravanan A, Kumar PS, Karishma S, Vo D-VN, Jeevanantham S, Yaashikaa PR, George CS (2021) A review on biosynthesis of metal nanoparticles and its environmental applications. Chemosphere 264:128580. https://doi.org/10.1016/j.chemosphere.2020.128580
Sarip NA, Aminudin NI, Danial WH (2022) Green synthesis of metal nanoparticles using Garcinia extracts: a review. Environ Chem Lett 2022:1-25. https://doi.org/10.1007/s10311-021-01319-3
Sathishkumar G, Logeshwaran V, Sarathbabu S, Jha PK, Jeyaraj M, Rajkuberan C, Senthilkumar N, Sivaramakrishnan S (2018) Green synthesis of magnetic Fe3O4 nanoparticles using Couroupita guianensis Aubl. fruit extract for their antibacterial and cytotoxicity activities. Artif Cells Nanomed Biotechnol 46(3):589–598. https://doi.org/10.1080/21691401.2017.1332635
Sayadi MH, Ghollasimood S, Ahmadpour N, Homaeigohar S (2022) Biosynthesis of the ZnO/SnO2 nanoparticles and characterization of their photocatalytic potential for removal of organic water pollutants. J Photochem Photobiol A 425:113662. https://doi.org/10.1016/j.jphotochem.2021.113662
Sengani M, Grumezescu AM, Rajeswari VD (2017) Recent trends and methodologies in gold nanoparticle synthesis—a prospective review on drug delivery aspect. OpenNano 2:37–46. https://doi.org/10.1016/j.onano.2017.07.001
Shah MZ, Guan Z-H, Din AU, Ali A, Rehman AU, Jan K, Faisal S, Saud S, Adnan M, Wahid F, Alamri S, Siddiqui MH, Ali S, Nasim W, Hammad HM, Fahad S (2021) Synthesis of silver nanoparticles using Plantago lanceolata extract and assessing their antibacterial and antioxidant activities. Sci Rep 11(1):20754. https://doi.org/10.1038/s41598-021-00296-5
Shahriary M, Veisi H, Hekmati M, Hemmati S (2018) In situ green synthesis of Ag nanoparticles on herbal tea extract (Stachys lavandulifolia)-modified magnetic iron oxide nanoparticles as antibacterial agent and their 4-nitrophenol catalytic reduction activity. Mater Sci Eng C 90:57–66. https://doi.org/10.1016/j.msec.2018.04.044
Shaik AM, David Raju M, Rama Sekhara Reddy D (2020) Green synthesis of zinc oxide nanoparticles using aqueous root extract of Sphagneticola trilobata Lin and investigate its role in toxic metal removal, sowing germination and fostering of plant growth. Inorg Nano-Met Chem 50(7):569–579. https://doi.org/10.1080/24701556.2020.1722694
Shaker A, Zaki A, Abdel-Rahim E, Khedr M (2016) Novel CuO nanoparticles for pest management and pesticides photodegradation. Adv Environ Biol 10(12):274–283
Shanavas S, Priyadharsan A, Karthikeyan S, Dharmaboopathi K, Ragavan I, Vidya C, Acevedo R, Anbarasana PM (2020) Green synthesis of titanium dioxide nanoparticles using Phyllanthus niruri leaf extract and study on its structural, optical and morphological properties. Mater Today Proc 26:3531–3534. https://doi.org/10.1016/j.matpr.2019.06.715
Sharma A, Kumar S, Tripathi P (2019a) A facile and rapid method for green synthesis of Achyranthes aspera stem extract-mediated silver nano-composites with cidal potential against Aedes aegypti L. Saudi J Biol Sci 26(4):698–708. https://doi.org/10.1016/j.sjbs.2017.11.001
Sharma P, Pant S, Dave V, Tak K, Sadhu V, Reddy KR (2019b) Green synthesis and characterization of copper nanoparticles by Tinospora cardifolia to produce nature-friendly copper nano-coated fabric and their antimicrobial evaluation. J Microbiol Methods 160:107–116. https://doi.org/10.1016/j.mimet.2019.03.007
Sharmila G, Fathima MF, Haries S, Geetha S, Kumar NM, Muthukumaran C (2017) Green synthesis, characterization and antibacterial efficacy of palladium nanoparticles synthesized using Filicium decipiens leaf extract. J Mol Struct 1138:35–40. https://doi.org/10.1016/j.molstruc.2017.02.097
Shende S, Ingle AP, Gade A, Rai M (2015) Green synthesis of copper nanoparticles by Citrus medica Linn. (Idilimbu) juice and its antimicrobial activity. World J Microbiol Biotechnol 31:865–873. https://doi.org/10.1007/s11274-015-1840-3
Shirzadi-Ahodashti M, Mortazavi-Derazkola S, Ebrahimzadeh MA (2020) Biosynthesis of noble metal nanoparticles using Crataegus monogyna leaf extract (CML@X-NPs, X= Ag, Au): antibacterial and cytotoxic activities against breast and gastric cancer cell lines. Surf Interfaces 21:100697. https://doi.org/10.1016/j.surfin.2020.100697
Shukla G, Gaurav SS, Singh A (2020) Synthesis of mycogenic zinc oxide nanoparticles and preliminary determination of its efficacy as a larvicide against white grubs (Holotrichia sp.). Int Nano Lett 10:131–139. https://doi.org/10.1007/s40089-020-00302-0
Sibuyi NRS, Thipe VC, Panjtan-Amiri K, Meyer M, Katti KV (2021) Green synthesis of gold nanoparticles using Acai berry and Elderberry extracts and investigation of their effect on prostate and pancreatic cancer cells. Nanobiomedicine 8:1849543521995310. https://doi.org/10.1177/1849543521995310
Singh J, Kumar S, Rathi B, Bhrara K, Chhikara BS (2015) Therapeutic analysis of Terminalia arjuna plant extracts in combinations with different metal nanoparticles. J Mater NanoSci 2(1):1–7. Available at https://pubs.thesciencein.org/journal/index.php/jmns/article/view/2394.jmns.2015.2.1.1/187
Skiba MI, Vorobyova VI (2019) Synthesis of silver nanoparticles using orange peel extract prepared by plasmochemical extraction method and degradation of methylene blue under solar irradiation. Adv Mater Sci Eng 2019:1–8. https://doi.org/10.1155/2019/8306015
Soares EV, Soares HMVM (2021) Harmful effects of metal (loid) oxide nanoparticles. Appl Microbiol Biotechnol 105:1379–1394. https://doi.org/10.1007/s00253-021-11124-1
Sohrabnezhad S, Seifi A (2016) The green synthesis of Ag/ZnO in montmorillonite with enhanced photocatalytic activity. Appl Surf Sci 386:33–40. https://doi.org/10.1016/j.apsusc.2016.05.102
Soliemanzadeh A, Fekri M (2017) The application of green tea extract to prepare bentonite-supported nanoscale zero-valent iron and its performance on removal of Cr(VI): effect of relative parameters and soil experiments. Microporous Mesoporous Mater 239:60–69. https://doi.org/10.1016/j.micromeso.2016.09.050
Soliemanzadeh A, Fekri M, Bakhtiary S, Mehrizi MH (2016) Biosynthesis of iron nanoparticles and their application in removing phosphorus from aqueous solutions. Chem Ecol 32(3):286–300. https://doi.org/10.1080/02757540.2016.1139091
Soltys L, Olkhovyy O, Tatarchuk T, Naushad M (2021) Green synthesis of metal and metal oxide nanoparticles: principles of green chemistry and raw materials. Magnetochemistry 7(11):145. https://doi.org/10.3390/magnetochemistry7110145
Somanathan T, Abilarasu A, Jermy BR, Ravinayagam V, Suresh D (2019) Microwave assisted green synthesis Ce0.2Ni0.8Fe2O4 nanoflakes using calotropis gigantean plant extract and its photocatalytic activity. Ceram Int 45(14):18091–18098. https://doi.org/10.1016/j.ceramint.2019.06.031
Soto KM, Quezada-Cervantes CT, Hernández-Iturriaga M, Luna-Bárcenas G, Vazquez-Duhalt R, Mendoza S (2019) Fruit peels waste for the green synthesis of silver nanoparticles with antimicrobial activity against foodborne pathogens. LWT Food Sci Technol 103:293–300. https://doi.org/10.1016/j.lwt.2019.01.023
Srihasam S, Thyagarajan K, Korivi M, Lebaka VR, Mallem SPR (2020) Phytogenic generation of NiO nanoparticles using Stevia leaf extract and evaluation of their in-vitro antioxidant and antimicrobial properties. Biomolecules 10(1):89. https://doi.org/10.3390/biom10010089
Sriramulu M, Shukla D, Sumathi S (2018) Aegle marmelos leaves extract mediated synthesis of zinc ferrite: antibacterial activity and drug delivery. Mater Res Express 5(11):115404. https://doi.org/10.1088/2053-1591/aadd88
Stan M, Popa A, Toloman D, Silipas T-D, Vodnar DC (2016) Antibacterial and antioxidant activities of ZnO nanoparticles synthesized using extracts of Allium sativum, Rosmarinus officinalis and Ocimum basilicum. Acta Metall Sin (engl Lett) 29:228–236. https://doi.org/10.1007/s40195-016-0380-7
Subramaniyam V, Subashchandrabose SR, Thavamani P, Megharaj M, Chen Z, Naidu R (2015) Chlorococcum sp. MM11—a novel phyco-nanofactory for the synthesis of iron nanoparticles. J Appl Phycol 27:1861–1869. https://doi.org/10.1007/s10811-014-0492-2
Sun H, Wu H, Teng Q, Liu Y, Wang H, Wang Z-G (2022) Enzyme-mimicking materials from designed self-assembly of lysine-rich peptides and G-quadruplex DNA/Hemin DNAzyme: charge effect of the key residues on the catalytic functions. Biomacromol 23(8):3469–3476. https://doi.org/10.1021/acs.biomac.2c00620
Sundeep D, Vijaya Kumar T, Rao PS, Ravikumar R, Gopala Krishna A (2017) Green synthesis and characterization of Ag nanoparticles from Mangifera indica leaves for dental restoration and antibacterial applications. Prog Biomater 6:57–66. https://doi.org/10.1007/s40204-017-0067-9
Suresh AK, Pelletier DA, Wang W, Broich ML, Moon J-W, Gu B, Allison DP, Joy DC, Phelps TJ, Doktycz MJ (2011) Biofabrication of discrete spherical gold nanoparticles using the metal-reducing bacterium Shewanella oneidensis. Acta Biomater 7(5):2148–2152. https://doi.org/10.1016/j.actbio.2011.01.023
Sutradhar P, Saha M, Maiti D (2014) Microwave synthesis of copper oxide nanoparticles using tea leaf and coffee powder extracts and its antibacterial activity. J Nanostruct Chem 4:1–6. https://doi.org/10.1007/s40097-014-0086-1
Swain S, Barik SK, Behera T, Nayak SK, Sahoo SK, Mishra SS, Swain P (2016) Green synthesis of gold nanoparticles using root and leaf extracts of Vetiveria zizanioides and Cannabis sativa and its antifungal activities. BioNanoScience 6:205–213. https://doi.org/10.1007/s12668-016-0208-y
Swati VR, Chauhan A, Shandilya M, Li X, Kumar R, Kulshrestha S (2020) Antimicrobial potential of ag-doped ZnO nanostructure synthesized by the green method using Moringa oleifera extract. J Environ Chem Eng 8(3):103730. https://doi.org/10.1016/j.jece.2020.103730
Taghavi Fardood S, Ramazani A (2016) Green synthesis and characterization of copper oxide nanoparticles using coffee powder extract. J Nanostruct 6(2):167–171. https://doi.org/10.7508/JNS.2016.02.009
Tahir K, Nazir S, Li B, Ahmad A, Nasir T, Khan AU, Shah SAA, Khan ZUH, Yasin G, Hameed MU (2016) Sapium sebiferum leaf extract mediated synthesis of palladium nanoparticles and in vitro investigation of their bacterial and photocatalytic activities. J Photochem Photobiol B 164:164–173. https://doi.org/10.1016/j.jphotobiol.2016.09.030
Tangthong T, Piroonpan T, Thipe VC, Khoobchandani M, Katti K, Katti KV, Pasanphan W (2021) Water-soluble chitosan conjugated DOTA-Bombesin peptide capped gold nanoparticles as a targeted therapeutic agent for prostate cancer. Nanotechnol Sci Appl 2021:69-89 https://doi.org/10.2147/NSA.S301942
Tavker N, Yadav VK, Yadav KK, Cabral-Pinto MM, Alam J, Shukla AK, Ali FAA, Alhoshan M (2021) Removal of cadmium and chromium by mixture of silver nanoparticles and nano-fibrillated cellulose isolated from waste peels of citrus sinensis. Polymers 13(2):234. https://doi.org/10.3390/polym13020234
Teymourinia H, Salavati-Niasari M, Amiri O, Safardoust-Hojaghan H (2017) Synthesis of graphene quantum dots from corn powder and their application in reduce charge recombination and increase free charge carriers. J Mol Liq 242:447–455. https://doi.org/10.1016/j.molliq.2017.07.052
Teymourinia H, Salavati-Niasari M, Amiri O, Farangi M (2018) Facile synthesis of graphene quantum dots from corn powder and their application as down conversion effect in quantum dot-dye-sensitized solar cell. J Mol Liq 251:267–272. https://doi.org/10.1016/j.molliq.2017.12.059
Thakur A, Sharma N, Bhatti M, Sharma M, Trukhanov AV, Trukhanov SV, Panina LV, Astapovich KA, Thakur P (2020) Synthesis of barium ferrite nanoparticles using rhizome extract of Acorus calamus: characterization and its efficacy against different plant phytopathogenic fungi. Nano-Struct Nano-Objects 24:100599. https://doi.org/10.1016/j.nanoso.2020.100599
Thipe VC, Panjtan Amiri K, Bloebaum P, Raphael Karikachery A, Khoobchandani M, Katti KK, Jurisson SS, Katti KV (2019) Development of resveratrol-conjugated gold nanoparticles: interrelationship of increased resveratrol corona on anti-tumor efficacy against breast, pancreatic and prostate cancers. Int J Nanomed 2019:4413-4428. https://doi.org/10.2147/IJN.S204443
Thipe VC, Karikachery AR, Çakılkaya P, Farooq U, Genedy HH, Kaeokhamloed N, Phan D-H, Rezwan R, Tezcan G, Roger E (2022) Green nanotechnology—an innovative pathway towards biocompatible and medically relevant gold nanoparticles. J Drug Deliv Sci Technol 70:103256. https://doi.org/10.1016/j.jddst.2022.103256
Tilahun Bekele E, Gonfa BA, Sabir FK (2021) Use of different natural products to control growth of titanium oxide nanoparticles in green solvent emulsion, characterization, and their photocatalytic application. Bioinorg Chem Appl 2021:6626313. https://doi.org/10.1155/2021/6626313
Turakhia B, Turakhia P, Shah S (2018) Green synthesis of zero valent iron nanoparticles from Spinacia oleracea (spinach) and its application in waste water treatment. J Adv Res Appl Sci 5(1):46–51 Available at www.researchgate.net/profile/Bhavika-Turakhia/publication/325966452_Green_Synthesis_of_Zero_Valent_Iron_Nanoparticles_from_Spinacia_oleracea_spinach_and_Its_Application_in_waste_water_treatment/links/5b347e7e4585150d23dbf62d/Green-Synthesis-of-Zero-Valent-Iron-Nanoparticles-from-Spinacia-oleracea-spinach-and-Its-Application-in-waste-water-treatment.pdf
Turunc E, Binzet R, Gumus I, Binzet G, Arslan H (2017) Green synthesis of silver and palladium nanoparticles using Lithodora hispidula (Sm.) Griseb. (Boraginaceae) and application to the electrocatalytic reduction of hydrogen peroxide. Mater Chem Phys 202:310–319. https://doi.org/10.1016/j.matchemphys.2017.09.032
Tyagi P, Kawamura K, Fu P, Bikkina S, Kanaya Y, Wang Z (2016) Impact of biomass burning on soil microorganisms and plant metabolites: a view from molecular distributions of atmospheric hydroxy fatty acids over Mount Tai. J Geophys Res Biogeosci 121(10):2684–2699. https://doi.org/10.1002/2016JG003324
Uddin S, Safdar LB, Iqbal J, Yaseen T, Laila S, Anwar S, Abbasi BA, Saif MS, Quraishi UM (2021) Green synthesis of nickel oxide nanoparticles using leaf extract of Berberis balochistanica: characterization, and diverse biological applications. Microsc Res Tech 84(9):2004–2016. https://doi.org/10.1002/jemt.23756
Vakili S, Shabaninejad Z, Ameri M, Ebrahiminezhad A, Shojazadeh A, Safarpour H, Khalaf N, Noorozi S (2022) Green synthesis, characterization and antibacterial activity of silver nanoparticles by Biarum chaduchrum leaf extract. Appl Phys A 128(3):241. https://doi.org/10.1007/s00339-022-05384-5
Vanda H, Dai Y, Wilson EG, Verpoorte R, Choi YH (2018) Green solvents from ionic liquids and deep eutectic solvents to natural deep eutectic solvents. C R Chim 21(6):628–638. https://doi.org/10.1016/j.crci.2018.04.002
Veisi H, Rashtiani A, Barjasteh V (2016) Biosynthesis of palladium nanoparticles using Rosa canina fruit extract and their use as a heterogeneous and recyclable catalyst for Suzuki–Miyaura coupling reactions in water. Appl Organomet Chem 30(4):231–235. https://doi.org/10.1002/aoc.3421
Verma VC, Kharwar RN, Gange AC (2009) Biosynthesis of antimicrobial silver nanoparticles by the endophytic fungus Aspergillus clavatus. Nanomedicine 5(1):33–40. https://doi.org/10.2217/nnm.09.77
Vickers NJ (2017) Animal communication: When i’m calling you, will you answer too? Curr Biol 27(14):R713–R715. https://doi.org/10.1016/j.cub.2017.05.064
Vitta Y, Figueroa M, Calderon M, Ciangherotti C (2020) Synthesis of iron nanoparticles from aqueous extract of Eucalyptus robusta Sm and evaluation of antioxidant and antimicrobial activity. Mater Sci Energy Technol 3:97–103. https://doi.org/10.1016/j.mset.2019.10.014
Vivekanandhan P, Deepa S, Kweka EJ, Shivakumar MS (2018) Toxicity of Fusarium oxysporum-VKFO-01 derived silver nanoparticles as potential inseciticide against three mosquito vector species (Diptera: Culicidae). J Cluster Sci 29:1139–1149. https://doi.org/10.1007/s10876-018-1423-1
Vranish JN, Ancona MG, Oh E, Susumu K, Medintz IL (2017) Enhancing coupled enzymatic activity by conjugating one enzyme to a nanoparticle. Nanoscale 9(16):5172–5187. https://doi.org/10.1039/C7NR00200A
Wang S-L, Nguyen AD (2018) Effects of Zn/B nanofertilizer on biophysical characteristics and growth of coffee seedlings in a greenhouse. Res Chem Intermed 44:4889–4901. https://doi.org/10.1007/s11164-018-3342-z
Wang T, Jin X, Chen Z, Megharaj M, Naidu R (2014a) Green synthesis of Fe nanoparticles using Eucalyptus leaf extracts for treatment of eutrophic wastewater. Sci Total Environ 466:210–213. https://doi.org/10.1016/j.scitotenv.2013.07.022
Wang T, Lin J, Chen Z, Megharaj M, Naidu R (2014b) Green synthesized iron nanoparticles by green tea and Eucalyptus leaves extracts used for removal of nitrate in aqueous solution. J Clean Prod 83:413–419. https://doi.org/10.1016/j.jclepro.2014.07.006
Wang S, Chen S, Wang Y, Low A, Lu Q, Qiu R (2016) Integration of organohalide-respiring bacteria and nanoscale zero-valent iron (Bio-nZVI-RD): a perfect marriage for the remediation of organohalide pollutants? Biotechnol Adv 34(8):1384–1395. https://doi.org/10.1016/j.biotechadv.2016.10.004
Wang X, Wang A, Ma J, Fu M (2017) Facile green synthesis of functional nanoscale zero-valent iron and studies of its activity toward ultrasound-enhanced decolorization of cationic dyes. Chemosphere 166:80–88. https://doi.org/10.1016/j.chemosphere.2016.09.056
Wang Y, Xia R, Hu H, Peng T (2018) Biosynthesis, characterization and cytotoxicity of gold nanoparticles and their loading with N-acetylcarnosine for cataract treatment. J Photochem Photobiol B 187:180–183. https://doi.org/10.1016/j.jphotobiol.2018.08.014
Wang Y, O’connor D, Shen Z, Lo IM, Tsang DC, Pehkonen S, Pu S, Hou D (2019) Green synthesis of nanoparticles for the remediation of contaminated waters and soils: constituents, synthesizing methods, and influencing factors. J Clean Prod 226:540–549. https://doi.org/10.1016/j.jclepro.2019.04.128
Wang D, Xue B, Wang L, Zhang Y, Liu L, Zhou Y (2021) Fungus-mediated green synthesis of nano-silver using Aspergillus sydowii and its antifungal/antiproliferative activities. Sci Rep 11(1):10356. https://doi.org/10.1038/s41598-021-89854-5
Wei Y, Fang Z, Zheng L, Tsang EP (2017) Biosynthesized iron nanoparticles in aqueous extracts of Eichhornia crassipes and its mechanism in the hexavalent chromium removal. Appl Surf Sci 399:322–329. https://doi.org/10.1016/j.apsusc.2016.12.090
Weng X, Jin X, Lin J, Naidu R, Chen Z (2016) Removal of mixed contaminants Cr(VI) and Cu(II) by green synthesized iron based nanoparticles. Ecol Eng 97:32–39. https://doi.org/10.1016/j.ecoleng.2016.08.003
Wu Y, Lian J-J, Yan Y, Qi H (2017) Mechanism and applications of bio-mineralization induced by Sporosarcina pasteurii and related microorganisms. China Biotechnol 37(8):96–103. https://doi.org/10.13523/j.cb.20170814
Wu F, Zhu J, Li G, Wang J, Veeraraghavan VP, Krishna Mohan S, Zhang Q (2019) Biologically synthesized green gold nanoparticles from Siberian ginseng induce growth-inhibitory effect on melanoma cells (B16). Artif Cells Nanomed Biotechnol 47(1):3297–3305. https://doi.org/10.1080/21691401.2019.1647224
Wu J, Lin M, Weng X, Owens G, Chen Z (2021) Pre-adsorption and Fenton-like oxidation of mitoxantrone using hybrid green synthesized rGO/Fe nanoparticles. Chem Eng J 408:127273. https://doi.org/10.1016/j.cej.2020.127273
Xia Y, Xiao Z, Dou X, Huang H, Lu X, Yan R, Gan Y, Zhu W, Tu J, Zhang W, Tao X (2013) Green and facile fabrication of hollow porous MnO/C microspheres from microalgaes for lithium-ion batteries. ACS Nano 7(8):7083–7092. https://doi.org/10.1021/nn4023894
Xie Y, Dong H, Zeng G, Tang L, Jiang Z, Zhang C, Deng J, Zhang L, Zhang Y (2017) The interactions between nanoscale zero-valent iron and microbes in the subsurface environment: a review. J Hazard Mater 321:390–407. https://doi.org/10.1016/j.jhazmat.2016.09.028
Xu L, Yi-Yi W, Huang J, Chun-Yuan C, Zhen-Xing W, Xie H (2020) Silver nanoparticles: synthesis, medical applications and biosafety. Theranostics 10(20):8996. https://doi.org/10.7150/thno.45413
Xu Y, Rashwan AK, Osman AI, Abd El-Monaem EM, Elgarahy AM, Eltaweil AS, Omar M, Li Y, Mehanni A-HE, Chen W, Rooney DW (2023) Synthesis and potential applications of cyclodextrin-based metal–organic frameworks: a review. Environ Chem Lett 21(1):447–477. https://doi.org/10.1007/s10311-022-01509-7
Yang Z, Wang D, Zhang C, Liu H, Hao M, Kan S, Liu D, Liu W (2022) The applications of gold nanoparticles in the diagnosis and treatment of gastrointestinal cancer. Front Oncol 11:5855. https://doi.org/10.3389/fonc.2021.819329
Ying S, Guan Z, Ofoegbu PC, Clubb P, Rico C, He F, Hong J (2022) Green synthesis of nanoparticles: current developments and limitations. Environ Technol Innov 26:102336. https://doi.org/10.1016/j.eti.2022.102336
Yirsaw BD, Megharaj M, Chen Z, Naidu R (2016) Reduction of hexavalent chromium by green synthesized nano zero valent iron and process optimization using response surface methodology. Environ Technol Innov 5:136–147. https://doi.org/10.1016/j.eti.2016.01.005
Yoo CG, Pu Y, Ragauskas AJ (2017) Ionic liquids: promising green solvents for lignocellulosic biomass utilization. Curr Opin Green Sustain Chem 5:5–11. https://doi.org/10.1016/j.cogsc.2017.03.003
Yuan L, Chai J, Wang S, Li T, Yan X, Wang J, Yin H (2023) Biomimetic Laccase-Cu2O@MOF for synergetic degradation and colorimetric detection of phenolic compounds in wastewater. Environ Technol Innov 30:103085. https://doi.org/10.1016/j.eti.2023.103085
Yusefi M, Shameli K, Yee OS, Teow S-Y, Hedayatnasab Z, Jahangirian H, Webster TJ, Kuča K (2021) Green synthesis of Fe3O4 nanoparticles stabilized by a Garcinia mangostana fruit peel extract for hyperthermia and anticancer activities. Int J Nanomed 16:2515. https://doi.org/10.2147/IJN.S284134
Zaki SA, Ouf SA, Albarakaty FM, Habeb MM, Aly AA, Abd-Elsalam KA (2021) Trichoderma harzianum-mediated ZnO nanoparticles: a green tool for controlling soil-borne pathogens in cotton. J Fungi 7(11):952. https://doi.org/10.3390/jof7110952
Zare M, Namratha K, Alghamdi S, Mohammad YHE, Hezam A, Zare M, Drmosh QA, Byrappa K, Chandrashekar BN, Ramakrishna S (2019) Novel green biomimetic approach for synthesis of ZnO-Ag nanocomposite; antimicrobial activity against food-borne pathogen, biocompatibility and solar photocatalysis. Sci Rep 9(1):8303. https://doi.org/10.1038/s41598-019-44309-w
Zhang H, Hortal M, Dobon A, Jorda-Beneyto M, Bermudez JM (2017) Selection of nanomaterial-based active agents for packaging application: using life cycle assessment (LCA) as a tool. Packag Technol Sci 30(9):575–586. https://doi.org/10.1002/pts.2238
Zhang HL, Zhou H, Bai JD, Li Y, Yang J, Ma Q, Qu YY (2019) Biosynthesis of selenium nanoparticles mediated by fungus Mariannaea sp. HJ and their characterization. Colloids Surf A Physicochem Eng Asp 571:9–16. https://doi.org/10.1016/j.colsurfa.2019.02.070
Zhang X, Qiu D, Chen J, Zhang Y, Wang J, Chen D, Liu Y, Cheng M, Monchaud D, Mergny J-L, Ju H, Zhou J (2023) Chimeric biocatalyst combining peptidic and nucleic acid components overcomes the performance and limitations of the native horseradish peroxidase. J Am Chem Soc 145(8):4517–4526. https://doi.org/10.1021/jacs.2c11318
Zhao P, El-kott A, Ahmed AE, Khames A, Zein MA (2021) Green synthesis of gold nanoparticles (Au NPs) using Tribulus terrestris extract: Investigation of its catalytic activity in the oxidation of sulfides to sulfoxides and study of its anti-acute leukemia activity. Inorg Chem Commun 131:108781. https://doi.org/10.1016/j.inoche.2021.108781
Zinatloo-Ajabshir S, Morassaei MS, Amiri O, Salavati-Niasari M (2020) Green synthesis of dysprosium stannate nanoparticles using Ficus carica extract as photocatalyst for the degradation of organic pollutants under visible irradiation. Ceram Int 46(5):6095–6107. https://doi.org/10.1016/j.ceramint.2019.11.072
Acknowledgements
Dr. Ahmed I. Osman wishes to acknowledge the support of The Bryden Centre project (Project ID VA5048), which was awarded by The European Union’s INTERREG VA Program, managed by the Special EU Programs Body (SEUPB), with match funding provided by the Department for the Economy in Northern Ireland and the Department of Business, Enterprise, and Innovation in the Republic of Ireland.
Author information
Authors and Affiliations
Contributions
AIO contributed to conceptualization, writing, review and editing, and data editing. YZ contributed to data gathering, writing, and review. MF contributed to conceptualization, writing, review and editing, and data editing. AKR contributed to figure designing and editing, and review. ASE contributed to data gathering, writing, and review. EMA contributed to data gathering, writing and review. IMAM contributed to data gathering and writing and review. MAB contributed to writing and review. IH contributed to review and editing and supervision. DWR contributed to review and editing and supervision. PSY contributed to review and editing and supervision.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Disclaimer The views and opinions expressed in this review do not necessarily reflect those of the European Commission or the Special EU Programs Body (SEUPB).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Osman, A.I., Zhang, Y., Farghali, M. et al. Synthesis of green nanoparticles for energy, biomedical, environmental, agricultural, and food applications: A review. Environ Chem Lett 22, 841–887 (2024). https://doi.org/10.1007/s10311-023-01682-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10311-023-01682-3