Abstract
Main conclusion
HRW increased the content of starch and sucrose via regulating a series of sucrose and starch synthesis genes, which induced the formation of bulblets and adventitious roots of Lilium davidii var. unicolor.
Abstract
Hydrogen gas (H2), as a signaling molecule, has been reported to be involved in plant growth and development. Here, the effect of hydrogen-rich water (HRW) on the formation of bulblets and adventitious roots in the scale cuttings of Lilium davidii var. unicolor and its mechanisms at the molecular levels were investigated. The results revealed that compared with distilled water treatment (Con), the number of bulblets and adventitious roots were significantly promoted by different concentrations of HRW treatment. Treatment with 100% HRW obtained the most positive effects. RNA sequencing (RNA-seq) analysis found that compared with Con, a total of 1702 differentially expressed genes (DEGs, upregulated 552 DEGs, downregulated 1150 DEGs) were obtained under HRW treatment. The sucrose and starch metabolism, cysteine and methionine metabolism and phenylalanine metabolism were significantly enriched in the analysis of the Kyoto encyclopedia of genes and genomes (KEGG). In addition, the genes involved in carbohydrate metabolism were significantly upregulated or downregulated (upregulated 22 DEGs, downregulated 15 DEGs), indicating that starch and sucrose metabolism held a central position. The expressions of 12 DEGs were identified as coding for key enzymes in metabolism of carbohydrates was validated by qPCR during bulblet formation progress. RNA-seq analysis and expression profiles indicated that the unigene levels such as glgc, Susy, otsA and glgP, BMY and TPS were well correlated with sucrose and starch metabolism during HRW-induced bulblet formation. The change of key enzyme content in starch and sucrose metabolism pathway was explored during bulblet formation in Lilium under HRW treatment. Meanwhile, compared with Con, 100% HRW treatment increased the levels of sucrose and starch, and decreased the trehalose content, which were agreed with the expression pattern of DEGs related to the biosynthesis pathway of sucrose, starch and trehalose. Therefore, this study suggested that HRW could promote the accumulation of sucrose and starch contents in mother scales, and decreased the trehalose content, this might provide more energy for bulblet formation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hydrogen (H2), a flammable, colorless and odorless gas, is the least dense gas in nature and known as a multifunctional signaling molecule. In different cell and disease model, H2 might have cytoprotective effects and act as a potential therapeutic medical gas by selectively decreasing hydroxyl radical (Zheng et al. 2011). H2 has been identified as a broad-spectrum anti-stress molecule to response the abiotic stresses in plants (Chen et al. 2014). Studies have mainly paid attention to its scavenging of ROS under abiotic stress, such as heat stress (Chen et al. 2017), salt stress (Xu et al. 2013), mercury (Hg) stress (Cui et al. 2014), cadmium (Cd) stress (Cui et al. 2013), aluminum (Al) stress (Zhao et al. 2017), and UV irradiation (Su et al. 2014), which attributed these alleviative effects to anti-oxidant ability of H2. Previous study found that H2 could decrease the respiration rate and delay the softening of the kiwi fruit by decreasing ethylene biosynthesis (Hu et al. 2018). Meanwhile, our previous results indicated that the vase life of cut lily (Lilium spp.) and cut rose (Rosa hybrid L.) flowers was improved under hydrogen-rich water (HRW) treatments by keeping water balance and membrane stability (Ren et al. 2017; Wang et al. 2020a). In addition, H2 also played an effective role in the development of adventitious root in cucumber and the germination in black barley (Guan et al. 2019; Zhu et al. 2016). However, the effect of H2 on the bulblet generation in lily and the deep mechanism of H2 in adventitious roots formation need to be further studied.
Lilium davidii is one of the lilies with sweet tasting. However, its reproductive coefficient is low. Scale cutting acts as an efficient method for rapid propagation in L. davidii and can provide healthy bulblets (Wu et al. 2016). Since bulbs are the production organs of lily plants, current studies on the bulblet formation of lilies mainly focus on its carbohydrate metabolism (Li et al. 2014). In the generation of bulblets in lily, sucrose plays an important role in increasing the number of bulblets. Higher sucrose concentrations enhanced the average fresh mass and diameter of bulblet (Keller 1993). Li et al. (2014) also found that the formation of bulblets was accelerated by upregulating SuSy and INV genes which is related to sucrose and starch metabolism. During bulblet initiation, the expression of Susy was significantly upregulated in mother scales, and sucrose was transported to bulblets for starch synthesis (Xu et al. 2020). The starch in mother scales of L. could be degraded into soluble sugars and contribute to the bulblet formation (Li et al. 2014).
TCP transcription factors, which could activate or inhibit cell proliferation, were identified as master integrator in tulip (Moreno-Pachon et al. 2016). Some studies showed that TCP transcription factors might be regulated by sucrose, cytokinin and strigolactone in several species (Manassero et al. 2013; Moreno-Pachon et al. 2017). A recent study suggested that TCP and its target miR319 were regulated by HUA ENHANCER 1 (HEN1) and played a crucial role in the shoot organogenesis of mutants and overexpression plants (Yang et al. 2020). However, the effects of TCP on bulblet generation in Lilium remain unclear.
The objective of the present study was to investigate the effect of exogenous H2 on the formation of bulblets and adventitious roots in L. davidii. In addition, transcriptomic comparative analysis was performed via RNA-seq to identify candidate genes associated with starch and glucose metabolism during the formation of bulblets and adventitious roots induced by H2. The result may provide new insights into the formation of bulblets in Lilium, which would be useful for further study on carbohydrate mechanism at the molecular level.
Materials and methods
Preparation of HRW
Purified H2 gas (99.99%, v/v) generated from a hydrogen gas generator (QL-300, Saikesaisi Hydrogen Energy Co., Ltd, Shandong, China) was bubbled into 1000 mL distilled water solution at a rate of 330 mL min−1 for 60 min. Then, the corresponding hydrogen-rich water (HRW) was rapidly diluted to the different saturations required (1, 10, 50 and 100%, [v/v]) (Wang et al. 2019a). The dissolved hydrogen portable meter was used to analyze the H2 concentration in freshly prepared HRW, and it was maintained at an effective concentration level at 25 °C for at least 12 h (Figs. S1 and S2).
Plant material and growth conditions
The fresh bulbs used in this study were gained from planters in the Xiguoyuan of Qilihe District, Lanzhou, Gansu province, China. Selected identical bulbs (diameter = 4.0 ± 0.2 cm) of Lilium davidii var. unicolor without obvious disease spot. Then, healthy bulb surfaces were sterilized by immersing in 500 times carbendazim solution for 30 min and washed with distilled water three times. After surface sterilization, cutting experiment was carried out with intermediate scales (3–5 layers). The scales were immersed in 1000 mL HRW of different concentrations for 3 h, and distilled water was used as the control (Con). Scales were embedded concave upward into pre-sterilized substrate comprising perlite and vermiculite (2:1, V/V). The growth conditions were first maintained in dark at 60% relative humidity, 23/18 ℃ day/night temperature for 14 days, and then cultured with a 14 h photoperiod (photosynthetically active radiation = 200 μmol m−2 s−1). The 1.5-cm-long segments of the mother scales base, which were treated with different concentration of HRW for 10 and 40 days, were stored at − 80 °C for total RNA extraction and RNA-seq analysis.
Measurement of morphological indexes
After 40 days of HRW treatment, the number of bulblets and roots was counted. The number of bulblet diameter above 1 mm was counted. Similarly, the number of root length above 1 mm was counted. The length of bulblet diameter and adventitious roots per mother scale was observed and recorded, and corresponding photographs were taken.
Total RNA isolation
Total RNA was isolated from more than 3 mother scales using TaKaRa MiniBEST plant RNA extraction kit (Takara Bio Inc, Kusatsu, Shiga, Japan) as described by the manufacturer’s protocol. RNA concentration and integrity were measured using NanoDrop spectrophotometer and Agilent 2100 Bioanalyzer. RNA samples were sent to Novogene Co.Ltd (Beijing, China) for cDNA library construction and sequencing.
Iso-Seq library construction, sequencing and de novo transcriptome assembly
The Iso-Seq library was constructed using the Clontech SMARTer PCR cDNA Synthesis Kit and the Blue Pippin Size Selection System protocol, according to the Iso form Sequencing protocol (Iso-Seq), as described by Pacific Biosciences (PN100-092-800-03). Oligo magnetic adsorption was used to enrich Poly (A) mRNA. cDNA fragments > 4 bp in size were selected for PCR amplification. Finally, sequencing was performed on PacBio Sequel platform. The SMRTlink 7.0 software was used to process the sequence data. The clean data were generated by CD-HIT (-c0.95-T6-G0aL0.00-aS0.99) to remove redundancy. Final transcripts were obtained and then used for all subsequent analyses.
Unigene functional annotation
Unigene functions were annotated based on seven public databases, including NCBI non-redundant protein sequences (NR), NCBI non-redundant nucleotide sequences (NT), Protein family (Pfam, http://pfam.sanger.ac.uk/), Clusters of Orthologous Groups of proteins (KOG/COG, http://pfam.sanger.ac.uk/), a manually annotated and reviewed protein sequence database (Swiss-Prot, http://www.ebi.ac.uk/uniprot/), KEGG Ortholog database (KO, http://www.genome.jp/kegg/), and Gene Ontology (GO, http://www.geneontology.org/). These databases were performed with a search e value of ‘1e−10’.
Analysis of the functional enrichment of differentially expressed genes (DEGs)
DEGs between the different treatments were identified using DESeqR package (1.10.1). Genes with an adjusted P value < 0.05 and |log2 fold change| ≥ 1 were defined as differentially expressed.
The sequence of unigenes was enriched to the KEGG database. Statistical enrichment of differentially expressed genes (DEGs) in KEGG pathway was detected using KOBAS software. GO enrichment was analyzed by the GOseq R package (corrected P value less than 0.05). The DEGs mainly enriched in biological process (BP), molecular function (MF) and cellular component (CC).
Quantitative real-time PCR verification
To verify the expression patterns of the genes in RNA-seq analysis, 9 DEGs were selected from carbohydrate metabolic pathways and TCP transcription factors for qRT-PCR verification. Among these genes, 7 DEGs are involved in starch and sucrose metabolism and 2 DEGs are related to TCP transcription factors. SYBR® Green Premix Pro Taq HS qPCR Kit was used to quantitative real-time RT-PCR by Light Cycler® 96 Real-Time PCR System (Roche). LoT1 was selected as a reference gene. The \(2^{{ - \Delta \Delta C_{T} }}\) approach was used to calculate the relative expression of selected genes (Vandesompele et al. 2002). All the genes were each performed in three biological replicates. Primers are listed in Supplementary Table S4.
Measurements of the content of starch and sucrose
After 20 days of HRW treatment, the content of starch and sucrose was measured. Starch content was determined using iodine colorimetry, while sucrose was measured by traditional anthrone colorimetry (Miao et al. 2016; Xu et al. 2020). Samples were ground in 2 mL distilled water, and approximately 0.5 g of powder was incubated with 3.2 mL 60% of perchloric acid for 10 min. Then, the extraction solution was centrifuged at 5000×r for 5 min. After filtration, the starch content of the extraction was determined using iodine colorimetry. Samples that weighted approximately 1.0 g were ground in liquid nitrogen, and the power was incubated with 100 mL of 80% ethanol at 100 °C for 30 min to measure the sucrose content. Then, the extraction solution was centrifuged and filtered. The content of sucrose was determined by hydrolysis and resorcinol reaction. Using 30% HCl and 1% C6H6O2 extracted the sucrose. The content of sucrose was determined and calculated at the absorbance of 480 nm.
Measurements of the content of trehalose
After 20 days of HRW treatment, the level of trehalose was determined using trehalose content detection kit (Beijing Solarbio Science & Technology Co., Ltd, China) according to the manufacturer’s protocol. The trehalose content in mother scales was determined in three independent replicate experiments.
Measurements of the content of adenosine diphosphoglucose pyrophosphorylase (AGPase), sucrose synthase (Susy) and sucrose-phosphate synthase (SPS)
After 20 days of HRW treatment, the content of AGPase, Susy and SPS enzymes was measured from mother scales separately according to the instruction of AGPase ELISA Kit, Susy ELISA Kit and SPS ELISA Kit (Andy Gene Biotechnology Co. Ltd., Beijing, China). About 1 g of mother scales was ground with 9 mL PBS (0.01 M, pH 7.4). To obtain the supernatant, the sample was centrifuged at 12,000g for 15 min at 4 ℃, and the supernatant was collected for measuring the content of AGPase, Susy and SPS enzymes. To measure the AGPase, Susy and SPS content in the mother scales, the purified AGPase, Susy and SPS antibodies were used to coat microtiter plate wells. After adding AGPase, Susy and SPS to wells, the antibodies of AGPase, Susy and SPS could combine with the corresponding enzymes to form the antibody–antigen–enzyme–antibody complex, and then the wells were washed for 5 times. TMB substrates became blue color after adding TMB substrate solution. The colors were changed to yellow and reactions were terminated after adding the sulphuric acid solutions, and then samples were measured by spectrophotometry at 450 nm. The content of AGPase, Susy and SPS which has activity in the mother scales were calculated by comparing the OD value of standard curve. All treatment experiments were carried out with three biological replicates.
Statistical analysis
All data were determined in three independent biological replicates for each experiment. The value of data was expressed as mean ± standard error (SE), and the data were analyzed using one-way analysis of variance (ANOVA) of SPSS 22.0 software (SPSS, Inc., Chicago, IL, USA). The different treatment was separated by Duncan’s multiple range test at P < 0.01 and P < 0.05.
Results
Effect of HRW on the number of adventitious roots and bulblets of scale cuttings in Lilium davidii var. unicolor
To examine the effect of H2 on the formation of bulblets and roots, the number of bulblets and adventitious roots in various concentrations of HRW treatments was measured. As shown in Fig. 1, compared with the control (Con), 1, 10, 50%, and 100% HRW treatments significantly increased the number of adventitious roots and bulblets. Compared with Con, 1, 10, 50 and 100% HRW treatments increased the number of roots by 23.57, 73.61, 47.13 and 285%, respectively. Compared with Con, the bulblet number in 1, 10, 50 and 100% HRW-treated mother scales increased by 7.18, 21.46, 46.52 and 53.59%, respectively. Under various HRW concentrations, 100% HRW obtained the maximum number of bulblets and adventitious roots (Fig. 1). Treatment with 100% HRW showed the most positive effect on the formation of bulblets and roots, so 100% HRW concentration was selected for all following experiments.
Effects of various concentrations of HRW treatments on the formation of bulblets and adventitious roots. The mother scales were cut in plug and treated by different concentrations of HRW (1, 10, 50, and 100%). Using distilled water as control (Con). Photos were taken after 40 days of HRW treatments (a). The mother scales were treated with HRW or distilled water every day for 40 days. After 40 days of HRW treatment, the number of bulblets and adventitious roots were measured (b). Different letters mean significant difference (P < 0.05). Means and SE values were calculated from at least three independent experiments
Transcriptome sequencing, de novo assembly, and functional annotation of unigenes in Lilium davidii var. unicolor
To better understand the role of H2 in promoting bulblets and adventitious roots formation, a RNA-seq was determined. RNA-seq obtained raw data from 53.0 to 79.2 million reads (Supplementary Table S1). After filtering, more than 51.92 million clean reads and 51.52G clean bases were produced and used for de novo assembly in total. All transcripts were assembled into 14,140 non-redundant unigenes with an average length of 1785 bp (N50 = 2270 bp). The quality scores (Q20 and Q30) of all samples were higher than 97 and 92%. Each sample included GC content more than 50% (Supplementary Table S1).
Under HRW treatment, the 14,140 unigenes were annotated based on BLASTx (E value = 10–10) to identify candidate genes and their potential functions in the bulblet initiation process. In total, 13,408 (94.82%) unigenes were mapped to least one public database, as following annotation distribution: 12,867 (NR; 91.00%), 11,521 (Swissprot; 81.48), 12,578 (KEGG; 88.95%), 8,097 (KOG; 57.26%), 9,969 (GO; 70.50%), 9806 (NT; 69.35%), 9969 (Pfam; 70.50%) (Fig. 2a). Among 14,140 unigenes, 5740 unigenes (40.59% of the total genes) were annotated in all databases. There were 8097 unigenes that could be classified into 25 KOG categories (Fig. 2b). The clusters were focus on general function, protein modification and turnover, signal transduction. In addition, the 510 unigenes of carbohydrate transport and metabolism occupied the central position (i.e., 6.30% of annotated of KOG).
KEGG and GO enrichment analysis of DEGs
To further study the metabolism pathway that occurs in bulblet formation after HRW treatment, KEGG and GO enrichment was used for analysis of the DEGs. As shown in Fig. 3, in HRW vs. Con, a total of 1702 DEGs were obtained. The number of downregulated DEGs (1150) was more than that of upregulated DEGs (552), and these genes were significantly affected by HRW treatment, which might be associated with several metabolic pathways.
The top 20 enriched KEGG pathways were used to predict the biochemical pathways of bulblet formation induced by HRW. The top 20 KEGG pathways from the comparisons were shown as follow: linoleic acid metabolism; starch and sucrose metabolism; phenylpropanoid biosynthesis; phenylalanine metabolism; isoquinoline alkaloid biosynthesis; carbon fixation in photosynthetic organisms; cysteine and methionine metabolism; alpha-Linolenic acid metabolism; stilbenoid, diarylheptanoid and gingerol biosynthesis; flavonoid biosynthesis; tyrosine metabolism; plant hormone signal transduction; plant-pathogen interaction; amino sugar and nucleotide sugar metabolism; ubiquinone and other terpenoid-quinone biosynthesis; tropane, piperidine and pyridine alkaloid biosynthesis; glycolysis/gluconeogenesis; DNA replication; carotenoid biosynthesis and fatty acid elongation (Fig. 4a). Among these pathways, the starch and sucrose metabolism, cysteine and methionine metabolism and phenylalanine metabolism were obviously enriched in the analysis of the KEGG pathway. Under HRW treatment, there were 37 (upregulated 22 DEGs, downregulated 15 DEGs), 27 (upregulated 11 DEGs, downregulated 16 DEGs) and 26 (upregulated 17 DEGs, downregulated 9 DEGs) DEGs during bulblet formation were involved in starch and sucrose metabolism (ko00500), cysteine and methionine metabolism (ko00270) and phenylalanine metabolism (ko00360) (Fig. 4a).
The GO database was used to categorize DEGs, as shown in Fig. 4b. In the GO enrichment analysis, the classification of DEGs was as follows: biological process (BP), cellular component (CC) and molecular function (MF). During the biological processes, 292 and 192 DEGs treated by HRW were mainly concentrated in single-organism metabolic process (GO: 0044710) and carbohydrate metabolic process (GO: 0005975). In the cellular component, the DEGs were mainly involved in virion part (GO: 0044423, 69 DEGs) and virion (GO: 0019012, 69 DEGs). During the molecular function, 695 DEGs and 349 DEGs were mainly enriched in catalytic activity (GO: 0003824) and transferase activity (GO: 0016740) (Fig. 4b). Thus, H2 might increase the enzyme activity of catalyticase and transferase, and promote the metabolism of starch and sucrose.
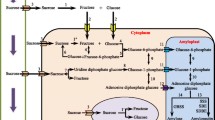
Expression of genes involved in metabolic pathways of sucrose and starch metabolic pathways under HRW treatment
In Con vs. HRW, 37 DEGs were identified, which contributed to sucrose and starch metabolic pathways. Among 37 DEGs, 31 unigenes were annotated to the specific sucrose and starch metabolic pathways from transcriptomic analysis (Fig. 5, Supplementary Table S2). Of these, 21 DEGs were upregulated, and 10 DEGs were downregulated under HRW treatment compared to Con, respectively. The unigene expression patterns are shown in the heat map in Fig. 6. At 10 DAT, sucrose could be degraded into trehalose and maltose by trehalose 6-phosphate synthase (ostA), trehalose 6-phosphate phosphatase (otsB), and beta-amylase (BMY) (Fig. 5), leading the decrease in sucrose content during bulblet initiation. However, compare with Con, HRW treatment significantly downregulated all of DEGs encoding ostA (3 DEGs), otsB (1 DEGs), and BYM (1 DEGs) (Fig. 5; Table 1). Meanwhile, sucrose could be synthesized by Susy which was encoded by 9 DGEs. Compared with Con, 7 genes encoding Susy were significantly upregulated, but other 2 genes were obviously downregulated, such as Susy (Lv-5835/f2p0/2787) and Susy (Lv-6141/f5p0/2804) (Fig. 5, Supplementary Table S2).
Overview of major carbohydrate metabolism. Pathways of sucrose and starch metabolism associated with bulblet formation under HRW treatment (a). The heatmap of the expression of DEGs related to sucrose and starch metabolism under HRW treatment at 10 DAT (b). Rectangle in red and blue represent upregulation and downregulation, respectively. Each column and row represented a sample and a DEG
Alpha-d-glucose 1-phosphate (α-UDP-glucose-1P), as an intermediate substance, was involved in the metabolism of starch and sucrose. α-UDP-glucose-1P could be converted into starch and glycogen via the catalysis of AGPase and GBE1. Then, the starch was converted into dextrin under the regulation of BMY (Lv-14655/f2p0/1768). In addition, starch and glycogen could be degraded into α-UDP-glucose-1P, contributing to a reduction in starch content. In this study, compared with Con, except for glgP (Lv-4329/f8p0/3070), the expressions of two genes (glgP Lv-3169/f3p0/3388 and glgP Lv-3276/f2p0/3343) encoding glycogen phosphorylase was upregulated in HRW treatment (Fig. 5; Table 1). Some starch and glycogen in mother scales could be inverted into sucrose during bulblet initiation in HRW treatment. α-UDP-glucose-1P could also be metabolized via another pathway that was catalyzed by phosphoglucomutase and glucose-6-phosphate isomerase (Fig. 5a; Table 1). However, in HRW treatment, genes encoding these enzymes, including pgm (Lv-10771/f9p0/2180) and GPI (Lv-10253/f3p0/2243), were significantly upregulated (Fig. 5b; Table 1). In addition, the synthesis and metabolism of cellobiose and D-glucose were mediated by β-glucosidase. RNA-seq data showed that bglX (Lv-9667/f2p0/2285) and bglX (Lv-11238/f19p0/2102) were kept at a higher expression in the Con, but they showed a significant downregulation expression in HRW treatment (Fig. 5b).
Identification and expression of the genes of transcription factor TCP under HRW treatment
TCP transcription factors have been identified as master integrators in the complex network of hormones and sugar signals that control the axillary bud outgrowth of bulbs in tulips (Moreno-Pachon et al. 2017). To investigate further the role of H2 in bulblet initiation, RNA-seq was used to assess the expression of encoding TCP transcription factors genes in mother scales. In this study, 7 unique transcripts which encoding members of TCP transcription factor families were identified including 5 DEGs, which showed different expression levels under HRW treatment (Supplementary Table S3). As showed in Fig. 6, 4 DEGs (Lv-26277/f3p0/772, Lv-9757/f2p0/2278, Lv-18038/f5p0/1428 and Lv-13348/f2p0/1896) were upregulated, and 1 DEG (Lv-11430/f31p0/2067) was downregulated. These data indicated that HRW treatment might influence the expression of TCP transcription factor families to regulate the bulblets’ formation.
Quantitative analysis of predominant genes involved in starch and sucrose metabolism
To verify the expression of DEGs, 10 carbohydrate metabolism-related genes and 2 TCP transcription factors were selected. As shown in Fig. 7, the expression level of Susy (Lv-11396/f10p0/2107) and Susy (Lv-6345/f6p0/2708) under HRW treatment increased at 10 DAT and showed about onefold of increase. However, compared with Con, Susy (Lv-5835/f2p0/2787) was significantly downregulated (about 3-fold), and the genes (otsA (Lv-3190/f5p0/3376), and otsB (Lv-5637/f3p0/2848)) were involved in trehalose accumulation were clearly downregulated by HRW treatment. At 10 DAT, expression profiles and RNA-seq analyses indicated that the mRNA levels of glgc (Lv-11836/f6p0/2065), glgP (Lv-3276/f2p0/3343) and GBE1 (Lv-5071/f12p0/2936), which encode key enzymes in starch synthesis pathway, showed > onefold of increase at 10 DAT under HRW treatment. The expression of glgc (Lv-10878/f2p0/2172) was increased at 10 DAT but no significant increase in HRW-induced. However, the expression of BMY (Lv-14655/f2p0/1768) was decreased in HRW treatment at 10 DAT which correlated with starch degradation (Fig. 7). These results indicate that the level of starch and sucrose could be accumulated with HRW treatment. Meanwhile, at 10 DAT, according to gene expression profiles, treatment with HRW significantly decreased the trehalose accumulation. Compare with Con, the expression of Lv-18038/f5p0/1428 and Lv-26277/f3p0/772, encoding TCP transcription factor genes were upregulated (> 2 fold) in the HRW treatment at 10 DAT (Fig. 7). The results of qRT-PCR were consistent with the expression levels of the transcript sequence.
Changes in starch and sucrose contents of mother scales in Lilium davidii var. unicolor under HRW treatment
To explain the stimulatory roles of H2 in the generation of bulblets and adventitious roots, the content of starch and sucrose in mother scales were determined in this study (Fig. 8). The starch content in mother scales deeply decreased from 0 to 15 days, and then it slightly increased from 15 to 20 days. In mother scales, compared with Con, HRW treatment significantly suppressed the decrease of starch content from 0 to 15 days. For example, on day 10, the decrease of starch content in Con was 39.93%, but it was 8.20% in HRW treatment (Fig. 8a). Compared with Con, 100% HRW treatment increased the content of starch by 52.83 and 7.65% at 10 and 15 days after treatment (DAT), respectively. However, on day 20, there was no significant difference in starch content between Con and HRW treatments. Thus, HRW treatment could strongly affect the change of starch content in mother scales at 10 DAT.
Changes of starch (a) and sucrose contents (b) during HRW-induced roots and bulblet formation. The mother scales were treated with HRW or distilled water (Con) every day for 20 days. After 20 days of HRW treatment, the starch and sucrose contents were measured. Asterisk (*) and double asterisk (**) indicate 5 and 1% significance level, respectively. Values represent the mean ± SE of three replicates. Each experiment was repeated three times independently
The results in Fig. 8b showed that HRW also could influence the levels of sucrose in mother scales. The sucrose content in mother scales sharply decreased within 10 days, and it lightly declined from 10 to 20 days. Compare with Con, at 10 and 20 DAT, HRW treatment extremely significantly suppressed the decrease in content of sucrose. For example, at 10 DAT, the decrease of sucrose content in Con was 69.62%, but HRW treatment showed a decrease of 61.43% in sucrose content. At 15 DAT, there was significant difference in sucrose content between Con and HRW treatments. Compared with Con, 100% HRW treatment obviously increased the content of sucrose by 26.95, 14.39 and 24.82% at 10 DAT, 15 DAT and 20 DAT, respectively (Fig. 8b). After HRW treatment, compared with Con, the content of sucrose and starch increased significantly at 10 DAT. Therefore, the 10 days might be the critical period of starch and sucrose metabolism during the formation of bulblets.
The content of starch and sucrose synthesis enzymes during bulblet formation in Lilium davidii var. unicolor under HRW treatment
To explore the changes of starch and sucrose levels under HRW treatment, the content of three starch and sucrose metabolism-related enzymes was measured. Compared with Con, AGPase content responded rapidly to HRW treatment. First, the AGPase content declined sharply within 15 days (Fig. 9a), and then increased gradually from 15 to 20 DAT. During the formation of bulblets and adventitious roots, compared with Con, 100% HRW treatment increased the AGPase content by 21.18 and 2.97% at 10 DAT and 15 DAT, respectively, and the AGPase content was significantly higher in HRW treatment than in Con at 10 DAT (Fig. 9a). However, on days 15 and 20, there was no significant difference in AGPase content between Con and HRW treatments. Similarly, the change of AGPase content was consistent with the starch content in HRW treatments during bulblet formation (Figs. 8a, 9a).
Changes in the content of AGPase (a), SS (b) and SPS enzymes (c) during HRW-induced root and bulblet formation. Using distilled water as control (Con). The mother scales were treated with HRW or distilled water every day for 20 days. After 20 days of HRW treatment, the enzyme contents of AGPase, SS and SPS were measured. Asterisk (*) and double asterisk (**) indicate 5 and 1% significance level, respectively. The bar represents the value of standard error. Means and SE values were calculated from at least three independent experiments
During bulblet formation, treatment with HRW significantly increased the content of Susy as compared to Con (Fig. 9b). At 10, 15 and 20 DAT, compared with Con, HRW treatment increased the Susy content in mother scales by 21.16, 24.84, and 36.37%, respectively. Among 9 genes encoding Susy enzyme, 7 genes were significantly upregulated, which was consistent with the changes in Susy content (Figs. 5b, 9b). The content of Susy in mother scales was shown to exceed considerably the content of SPS. Within 16 days, compared with Con, the content of SPS was inhibited under HRW treatment, which was decreased by 24.84% (Fig. 9c). Figure 9c shows that the content of SPS in HRW treatment was higher than Con from 15 to 20 DAT.
The trehalose level change of mother scales in Lilium davidii var. unicolor under HRW treatment
To further explore the mechanism of bulblet formation in HRW treatment, the trehalose level in mother scales was measured within 20 DAT (Fig. 10). As shown in Fig. 10, compared with Con, the trehalose content dramatically decreased at first and then increased gradually from 0 to 20 DAT. Compared with Con, HRW treatment dramatically decreased the content of trehalose by 16.51 and 13.01% at 10 DAT, 15 DAT, respectively (Fig. 10). At 10 and 15 DAT, treatment with HRW significantly decreased the trehalose accumulation. Similar to RNA-seq analysis, the genes involved in trehalose accumulation were clearly downregulated by HRW treatment, such as otsA (Lv-5254/f2p0/2951) and otsB (Lv-5637/f3p0/2848), which might lead to the decrease in trehalose level (Figs. 5b, 10). However, at 20 DAT, there was no significant difference in trehalose content between Con and HRW treatments.
Changes of the trehalose level during HRW-induced root and bulblet formation. The mother scales were treated with HRW or distilled water every day for 20 days. After 20 days of HRW treatment, the change of trehalose was measured. Asterisk (*) and double asterisk (**) indicate 5 and 1% significance level, respectively. The bar represents the value of standard error. Means and SE values were calculated from at least three independent experiments
Discussion
H2 plays an important role in plant growth and development
Hydrogen, known as a potential anti-oxidant, could effectively neutralize ·OH in living cells (Zheng et al. 2011). In general, cell membranes are considered to prevent some water-soluble anti-oxidants from entering cells and organelles. However, previous studies have shown that H2 could easily penetrate cellular and intracellular membranes and diffuse into the cytosol, mitochondria and nuclei because it is electronically neutral and much smaller than the oxygen molecule (Zheng et al. 2011). Hydrogen could selectively inactivate ·OH via forming water (Ohsawa et al. 2007). The authors also revealed that H2 could keep cellular morphology by preventing DNA oxidation, preserving mitochondrial membrane potential, and ATP synthesis. In addition, H2 also could increase the activity of anti-oxidant enzyme in response to abiotic stress in plants (Xie et al. 2012). Moreover, Xie et al. (2014) suggested that H2 treatment upregulated the GORK transcript levels. For Cd2+ transport, H2 treatment could significantly repress the expression of two main transporters (BcIRT1 and BcZIP2) during Cd uptake (Wu et al. 2019). These results indicated that H2 may play an important role in plant response to abiotic stresses. Previous study has shown that adventitious root formation could be induced by exogenous HRW treatment (Zhu et al. 2017). However, whether there are some other novel pathways involved in adventitious root formation remains to be investigated. In this study, the starch and sucrose metabolism and the content of key enzyme during bulblet formation in Lilium davidii var. unicolor were investigated for this process.
HRW promoted the bulblet formation in Lilium davidii var. unicolor
H2 is a gas signaling molecule that could promote the generation of adventitious roots and the germination of seed. Compared with HRW-free control sample, 50 and 100% HRW promoted the formation of adventitious root, and the length of adventitious root also was promoted (Lin et al. 2014). They also found the HRW promoted the development of adventitious root in a dose-dependent manner. In cucumber, H2 could upregulated the expressions of cell cycle-related genes and target genes related to adventitious rooting during adventitious root formation (Zhu et al. 2016). In our previous study, Zhu and Liao (2017) showed that exogenous H2 could promote the development of adventitious roots in marigold. HRW treatment was also shown to significantly promote the growth of mung bean seedlings because it could promote the elongation of hypocotyl and root cell by increasing GA and IAA contents (Wu et al. 2020). The results were also confirmed in experiments of cucumber and radish seedlings (Zhang et al. 2018). However, few detailed studies have explored the genetic and molecular mechanism about whether H2 could regulate the generation of bulblets in L. davidii var. unicolor. Treatments with 100% HRW treatment significantly increased the number of bulblets and adventitious roots in (Fig. 1). Thus, this is the first report to show that H2 could induce bulblet and adventitious root formation in L. davidii var. unicolor.
HRW increased the sucrose and starch contents during bulblet formation
HRW treatment promoted the adventitious root formation via complex process that usually involves a series of biochemical reactions. Bulblet formation is also a complex biological process that is regulated by both external environment and genetic material. In previous studies of L. davidii var. unicolor (Li et al. 2014), Sagittaria sagittifolia (Gao et al. 2018), and Lycoris radiata (Xu et al. 2020), starch and sucrose have been found to be vital to bulblet morphogenesis and emergence. The bulblet outgrowth and initiation process might need the continuous support of soluble sugar which from the mother scales (Xu et al. 2020). In this study, according to KEGG analysis, starch and sucrose metabolism held a central position, indicating that carbohydrate metabolism was the major pathway of Lilium in HRW treatment. Thus, the metabolism pathway of starch and sucrose was selected to further explore. During bulblet formation, the change of sucrose and starch contents in the mother scales showed a trend of first decrease and then increase and reach a nadir at 10 DAT (Fig. 8). Xu et al. (2020) also found that sucrose and starch contents first decreased and then increased during bulblet initiation in Lycoris radiata. HRW treatment significantly suppressed the decreases of sucrose and starch (Fig. 8), accompanied by regulation of the 37 genes (22 genes were upregulated and 15 gene were downregulated), which associated with sucrose and starch synthesis (Fig. 5). Among these genes, 31 unigenes were annotated to the specific sucrose and starch metabolic pathways via KEGG enrichment.
The sugars in bulblet of Lycoris radiata were largely derived from carbohydrate metabolism in the mother scales (Xu et al. 2020). Sucrose was supposed as the direct substrate of starch synthesis during bulblet formation (Zhang et al. 2013). Barbier et al. (2015) suggested that sucrose was the first trigger to control endogenous hormone signaling and promoted the bud formation. Sucrose synthesis and hydrolysis were catalyzed by SPS and Susy (Li et al. 2014). Previous studies revealed that Susy primarily catalyzed sucrose hydrolysis rather than the synthesis of sucrose (Li et al. 2014). However, in this study, the content of Susy was higher than SPS activity in mother scales (Fig. 9B, C), and treatment with HRW led to sucrose accumulation (Fig. 8B). Meanwhile, RNA-seq analysis showed that the genes encoding SPS were not significantly upregulated or downregulated after HRW treatment, even though SPS content had a change during bulblet formation. At 10 DAT, the number of upregulated genes encoding Susy (7 DEGs) were obvious more than downregulated genes (2 DEGs), accompanied by the increase of sucrose content, indicating that under HRW treatment the sucrose synthesis ability was higher than the sucrose degradation ability. This suggests that Susy might be involved in sucrose synthesis rather than sucrose hydrolysis, and the content of Susy was higher than the content of SPS at 10 DAT under 100% HRW treatment. In previous studies on sucrose metabolism pathway, about 60% pathways as shown in Fig. 5a have been reported in Lycoris radiata and Sagittaria sagittifolia (Xu et al. 2020; Gao et al. 2018). Another new pathway associated with sucrose hydrolysis was suggested in this study (Fig. 5a). UDP-glucose, which can also be synthesized from sucrose degradation catalyzed by Susy (Li et al. 2014), considered as the substrate of Trehalose-6P synthesis by trehalose-phosphate synthase (TPS) (Yadav et al. 2014). Trehalose-6P, as the intermediate of trehalose biosynthesis, plays a central role in signal metabolic pathway in plants (Yadav et al. 2014; Paul et al. 2008). They also found that the substrates of TPS and UDP-glucose could be increased with sucrose induction in Arabidopsis thaliana. Therefore, Yadav et al. (2014) proposed a hypothesized pathway that sucrose could activate TPS and promote the increase of Trehalose-6P level. In this experiment, according to RNA-seq enrichment analysis and verification, it is suggested that that another novel sucrose hydrolysis pathway might exist in the process of bulblet formation (Fig. 5a). As Fig. 5a shows that sucrose might convert into Trehalose-6P by Susy and TPS, and then Trehalose-6P convert into trehalose by trehalose 6-phosphate phosphatase (TPP). Does this novel pathway exist in carbohydrate metabolism pathway? More work should be done to solve the above problems. In this study, compared to Con, and the expressions of the genes involved in sucrose degradation were clearly downregulated by HRW treatment, such as otsA (Lv-5254/f2p0/2951) and otsB (Lv-5637/f3p0/2848), leading to a significant decrease in trehalose level at 10 DAT. Therefore, more sucrose from the mother scales was used for the starch synthesis in the bulblets treated with HRW. The starch synthesis of bulblet in Sagittaria sagittifolia needed the metabolism of sucrose and starch from the mother scales (Gao et al. 2018). Similar results were also reported in bulblet formation in Lycoris radiata (Xu et al. 2020). Their research showed that many transcripts related to sucrose degradation were significantly changed, and the hexose sugars of sucrose degradation were used for biosynthetic reactions of starch.
The development of underground organs, such as bulbous enlargement and bulblet formation, is based on the synthesis and accumulation of starch (Seng et al. 2017). In the bulblets of Tulipa edulis, the starch bodies are the main storage form and it could be synthesized from bulb by several enzymes, such as AGPase, Susy, and GBSS (Xu et al. 2020). In addition, the activity of essential enzymes during root formation was changed and energy was produced to support the process (Rosental et al. 2014). Xu et al. (2014) also found AGPase activity was strongly increased during bulblet initiation. In the starch synthesis pathway, the expression of the genes encoding AGPase and GBE1 were significantly upregulated during bulblet formation. However, in this study, the content of AGPase showed sharp decrease and then increase within 20 DAT. During bulblet formation, change in starch content was similar in AGPase content, showing a rapid decrease and then a subsequent increase (Figs. 8a, 9a). Meanwhile, compared to Con, the gene expressions of ADP-glucose and starch synthesis also were upregulated in HRW treatment (Fig. 5). Therefore, HRW treatment inhibited starch degradation and resulted in starch content accumulation. RNA-seq data showed that the gene expressions encoding the starch synthesis enzymes (i.e., AGPase and GBE1) in HRW treatment were significantly upregulated. The β-amylase enzyme degraded starch to dextrin, and then dextrin was converted to maltose and glucose (Wang et al. 2014). However, the expression of BMY (Lv-14655/f2p0/1768) encoding beta-amylase in HRW treatment was obviously downregulated (Fig. 5b). It is suggested that H2 could suppress the degradation of starch, so more starch in mother scales might be used to promote bulblet formation. Gene expression that related to sucrose and starch metabolism in HRW treatment, such as Susy (Lv-5835/f2p0/2787), otsA (Lv-4864/f4p0/2970) and glgc (Lv-10878/f2p0/2172), were confirmed by RT-qPCR analyses (Fig. 7), and the results confirmed the expression of the DEGs. Taken together, H2 positively promoted bulblet and adventitious root formation by mediating the metabolic pathways of sucrose and starch.
TCP transcription factors regulated the bulblet formation
The formation and development of bulblet is a complex process regulated by a series of transcription factors (Gao et al. 2018; Moreno-Pachon et al. 2016). The teosinte branched1/cycloidea/proliferating cell factor1 (TCP) is a special transcription factor family involved in activation or inhibition cell proliferation and related to branching, leaf development, and germination (He et al. 2020). TCP transcription factors have been reported in many plant species, such as 24 TCP in Arabidopsis (Li 2015), 39 TCP in Brassica rapa L. ssp. Pekinensis (Liu et al. 2018), 31 TCP in Solanum tuberosum (Wang et al. 2019b). During the tuber swollen stage of Brassica juncea var. tumida, the expression of 6 BjTCP genes were significantly upregulated (He et al. 2020). The root hair, density, and stem trichome were increased when the GbTCP5 gene in transgenic Arabidopsis was overexpressed (Wang et al. 2020b). In Arabidopsis, the leaf shape would be controlled by plant hormone-activated TCP domain protein 1 (Wang et al. 2020b). MicroRNA 319 (miR319) could regulate the expression of TCP genes and control leaf development (Bresso et al. 2018). In this study, seven TCP transcription factors were identified through RNA-seq, and five unique transcripts encoding TCP protein were showed different expression patterns under HRW treatment (Fig. 6). Among these genes, the expression levels of TCP (Lv-26277/f3p0/772, Lv-9757/f2p0/2278, Lv-18038/f5p0/1428 and Lv-13348/f2p0/1896) showed an increasing tendency under HRW treatment, which might indicate that bulblet and adventitious root formations were accelerated. These upregulated genes might be involved in the activation of cell regeneration, and the TCP (Lv-11430/f31p0/2067) might as negative regulation factor in the formation of bulblets.
Conclusion
In conclusion, exogenous H2 significantly promoted the bulblet and adventitious root formations, and the metabolism of sucrose and starch might play an important role in bulblet initiation. When bulblets gradually generated, the sucrose and starch contents in mother scales were rapidly reduced, which were regulated by several genes. When mother scales were treated with HRW, compared with Con, the starch and sucrose contents increased, which was primarily regulated by genes encoding AGPase and Susy, the crucial enzymes associated with sucrose and starch metabolism. There was no significant upregulation or downregulation of the genes encoding SPS under 10 DAT, but the expressions of the genes encoding Susy changed significantly. Furthermore, the content of Susy was increased under HRW treatment, although Susy was generally considered to be related to sucrose hydrolysis. These results suggested that 100% HRW treatment could promote sucrose and starch synthesis via regulating a series of genes, supporting the substance accumulation of bulblets. Interestingly, we also hypothesized that the decrease in trehalose content of mother scales might be regulated by sucrose, which needs to be explored in future studies. The study also revealed that 5 TCP transcription factors might be involved in the H2-induced bulblet formation in L. davidii. However, further studies should be conducted to explore the specific mechanism of H2-mediated regulation of bulblet formation at molecular levels.
Author contribution statement
The authors WBL and CLW contributed to the study conception and design. Material preparation, data collection and analysis were performed by XMH, NNQ, CXL, DJH, YHL and NW. The first draft of the manuscript was written by XMH. All the authors read and approved the manuscript.
Data availability
All data generated or analyzed during this study are included in this manuscript. The relevant RNA-seq and annotation information for this article can be found in the Supplementary Table S1, Supplementary Table S2, Supplementary Table S3 and Supplementary Table S4. The transcripts sequences were listed in the Supplementary Materials.
Abbreviations
- HRW:
-
Hydrogen-rich water
- Con:
-
Control
- GO:
-
Gene ontology
- KEGG:
-
Kyoto encyclopedia of genes and genomes
- DEGs:
-
Differentially expressed genes
- RNA-seq:
-
RNA sequencing
- qRT-PCR:
-
Quantitative real-time PCR
References
Barbier F, Péron T, Lecerf M, Perez-Garcia MD, Barrière Q, Rolčík J, Boutet-Mercey S, Citerne S, Lemoine R, Porcheron B, Roman H, Leduc N, Le Gourrierec J, Bertheloot J, Sakr S (2015) Sucrose is an early modulator of the key hormonal mechanisms controlling bud outgrowth in Rosa hybrida. J Exp Bot 66:2569–2582. https://doi.org/10.1093/jxb/erv047
Bresso EG, Chorostecki U, Rodriguez RE, Palatnik JF, Schommer C (2018) Spatial control of gene expression by miR319-regulated TCP transcription factors in leaf development. Plant Physiol 176:1694–1708. https://doi.org/10.1104/pp.17.00823
Chen M, Cui W, Zhu K, Xie Y, Zhang C, Shen W (2014) Hydrogen-rich water alleviates aluminum-induced inhibition of root elongation in alfalfa via decreasing nitric oxide production. J Hazard Mater 267:40–47. https://doi.org/10.1016/j.jhazmat.2013.12.029
Chen Q, Zhao X, Lei D, Hu S, Shen Z, Shen W, Xu X (2017) Hydrogen-rich water pretreatment alters photosynthetic gas exchange, chlorophyll fluorescence, and antioxidant activities in heat-stressed cucumber leaves. Plant Growth Regul 83:1–14. https://doi.org/10.1007/s10725-017-0284-1
Cui W, Gao C, Fang P, Lin G, Shen W (2013) Alleviation of cadmium toxicity in Medicago sativa by hydrogen-rich water. J Hazard Mater 260:715–724. https://doi.org/10.1016/j.jhazmat.2013.06.032
Cui W, Fang P, Zhu K, Mao Y, Gao C, Xie Y, Wang J, Shen W (2014) Hydrogen-rich water confers plant tolerance to mercury toxicity in alfalfa seedlings. Ecotoxicol Environ Saf 105:103–111. https://doi.org/10.1016/j.ecoenv.2014.04.009
Gao M, Zhang S, Luo C, He X, Wei S, Jiang W, He F, Lin Z, Yan M, Dong W (2018) Transcriptome analysis of starch and sucrose metabolism across bulb development in Sagittaria sagittifolia. Gene 649:99–112. https://doi.org/10.1016/j.gene.2018.01.075
Guan Q, Ding XW, Jiang R, Ouyang PL, Gui J, Feng L, Yang L, Song LH (2019) Effects of hydrogen-rich water on the nutrient composition and antioxidative characteristics of sprouted black barley. Food Chem 299:125095. https://doi.org/10.1016/j.foodchem.2019.125095
He J, He X, Chang P, Jiang H, Gong D, Sun Q (2020) Brassica juncea genome-wide identification and characterization of TCP family genes in var. tumida. PeerJ 8:e9130. https://doi.org/10.7717/peerj.9130
Hu H, Zhao S, Li P, Shen W (2018) Hydrogen gas prolongs the shelf life of kiwifruit by decreasing ethylene biosynthesis. Postharvest Biol Technol 135:123–130. https://doi.org/10.1016/j.postharvbio.2017.09.008
Keller ERJ (1993) Sucrose, cytokinin, and ethylene influence formation of in vitro bulblets in onion and leek. Genet Resour Crop Evol 40:113–120. https://doi.org/10.1007/BF00052642
Li S (2015) The Arabidopsis thaliana TCP transcription factors: a broadening horizon beyond development. Plant Signal Behav 10:e1044192. https://doi.org/10.1080/15592324.2015.1044192
Li X, Wang C, Cheng J, Zhang J, da Silva JA, Liu X, Duan X, Li T, Sun H (2014) Transcriptome analysis of carbohydrate metabolism during bulblet formation and development in Lilium davidii var. unicolor. BMC Plant Biol 14:358. https://doi.org/10.1186/s12870-014-0358-4
Lin Y, Zhang W, Qi F, Cui W, Xie Y, Shen W (2014) Hydrogen-rich water regulates cucumber adventitious root development in a heme oxygenase-1/carbon monoxide-dependent manner. J Plant Physiol 171:1–8. https://doi.org/10.1016/j.jplph.2013.08.009
Liu Y, Guan X, Liu S, Yang M, Ren J, Guo M, Huang Z, Zhang Y (2018) Genome-wide identification and analysis of TCP transcription factors involved in the formation of leafy head in Chinese cabbage. Int J Mol Sci 19(3):847. https://doi.org/10.3390/ijms19030847
Manassero NG, Viola IL, Welchen E, Gonzalez DH (2013) TCP transcription factors: architectures of plant form. Biomol Concepts 4:111–127. https://doi.org/10.1515/bmc-2012-0051
Miao Y, Zhu Z, Guo Q, Yang X, Liu L, Sun Y, Wang C (2016) Dynamic changes in carbohydrate metabolism and endogenous hormones during Tulipa edulis stolon development into a new bulb. J Plant Biol 59:121–132. https://doi.org/10.1007/s12374-016-0456-y
Moreno-Pachon NM, Leeggangers HA, Nijveen H, Severing E, Hilhorst H, Immink RG (2016) Elucidating and mining the Tulipa and Lilium transcriptomes. Plant Mol Biol 92(3):249–261. https://doi.org/10.1007/s11103-016-0508-1
Moreno-Pachon NM, Mutimawurugo MC, Heynen E, Sergeeva L, Benders A, Blilou I, Hilhorst HWM, Immink RGH (2017) Role of Tulipa gesneriana TEOSINTE BRANCHED1 (TgTB1) in the control of axillary bud outgrowth in bulbs. Plant Reprod 31(2):145–157. https://doi.org/10.1007/s00497-017-0316-z
Ohsawa I, Ishikawa M, Takahashi K, Watanabe M, Nishimaki K, Yamagata K, Katsura K, Katayama Y, Asoh S, Ohta S (2007) Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med 13(6):688–694. https://doi.org/10.1038/nm1577
Paul MJ, Primavesi LF, Jhurreea D, Zhang Y (2008) Trehalose metabolism and signaling. Annu Rev Plant Biol 59(1):417–441. https://doi.org/10.1146/annurev.arplant.59.032607.092945
Ren PJ, Jin X, Liao WB, Wang M, Niu LJ, Li XT, Zhu YC (2017) Effect of hydrogen-rich water on vase life and quality in cut lily and rose flowers. Hortic Environ Biotechnol 58:576–584. https://doi.org/10.1007/s13580-017-0043-2
Rosental L, Nonogaki H, Fait A (2014) Activation and regulation of primary metabolism during seed germination. Seed Sci Res 24(1):1–15. http://journals.cambridge.org/abstract_S0960258513000391
Seng S, Wu J, Liang J, Zhang F, Yang Q, He J, Yi M (2017) Silencing GhAGPL1 reduces the quality and quantity of corms and cormels in gladiolus. J Am Soc Hortic Sci 142:119–125. https://journals.ashs.org/jashs/view/journals/jashs/142/2/article-p119.xml
Su N, Wu Q, Liu Y, Cai J, Shen W, Xia K, Cui J (2014) Hydrogen-rich water reestablishes ROS homeostasis but exerts differential effects on anthocyanin synthesis in two varieties of radish sprouts under UV-A irradiation. J Agric Food Chem 62:6454–6462. https://doi.org/10.1021/jf5019593
Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3(7):RESEARCH0034. https://doi.org/10.1186/gb-2002-3-7-research0034
Wang J, Yang Y, Liu X, Huang J, Wang Q, Gu J, Lu Y (2014) Transcriptome profiling of the cold response and signaling pathways in Lilium lancifolium. BMC Genomics 15:203. https://doi.org/10.1186/1471-2164-15-203
Wang B, Bian B, Wang C, Li C, Fang H, Zhang J, Huang D, Huo J, Liao W (2019a) Hydrogen gas promotes the adventitious rooting in cucumber under cadmium stress. PLoS ONE 14:e0212639. https://doi.org/10.1371/journal.pone.0212639
Wang Y, Zhang N, Li T, Yang J, Zhu X, Fang C, Li S, Si H (2019b) Genome-wide identification and expression analysis of StTCP transcription factors of potato (Solanum tuberosum L.). Comput Biol Chem 78:53–63. https://doi.org/10.1016/j.compbiolchem.2018.11.009
Wang C, Fang H, Gong T, Zhang J, Niu L, Huang D, Huo J, Liao W (2020a) Hydrogen gas alleviates postharvest senescence of cut rose “Movie star” by antagonizing ethylene. Plant Mol Biol 102:271–285. https://doi.org/10.1007/s11103-019-00946-3
Wang Y, Yu Y, Wang J, Chen Q, Ni Z (2020b) Heterologous overexpression of the GbTCP5 gene increased root hair length, root hair and stem trichome density, and lignin content in transgenic Arabidopsis. Gene 758:144954. https://doi.org/10.1016/j.gene.2020.144954
Wu Y, Xia YP, Zhang JP, Du F, Zhang L, Ma YD, Zhou H (2016) Low humic acids promote in vitro lily bulblet enlargement by enhancing roots growth and carbohydrate metabolism. J Zhejiang Univ Sci B 17(11):892–904. https://doi.org/10.1631/jzus.B1600231
Wu X, Zhu Z, Chen J, Huang Y, Liu Z, Zou J, Chen Y, Su N, Cui J (2019) Transcriptome analysis revealed pivotal transporters involved in the reduction of cadmium accumulation in pak choi (Brassica chinensis L.) by exogenous hydrogen-rich water. Chemosphere 216:684–697. https://doi.org/10.1016/j.chemosphere.2018.10.152
Wu Q, Su N, Huang X, Ling X, Yu M, Cui J, Shabala S (2020) Hydrogen-rich water promotes elongation of hypocotyls and roots in plants through mediating the level of endogenous gibberellin and auxin. Funct Plant Biol 47(9):771–778. https://doi.org/10.1071/FP19107
Xie Y, Mao Y, Lai D, Zhang W, Shen W (2012) H2 enhances Arabidopsis salt tolerance by manipulating ZAT10/12-mediated antioxidant defence and controlling sodium exclusion. PLoS ONE 7(11):e49800. https://doi.org/10.1371/journal.pone.0049800
Xie Y, Mao Y, Zhang W, Lai D, Wang Q, Shen W (2014) Reactive oxygen species-dependent nitric oxide production contributes to hydrogen-promoted stomatal closure in Arabidopsis. Plant Physiol 165(2):759–773. https://doi.org/10.1104/pp.114.237925
Xu S, Zhu S, Jiang Y, Wang N, Wang R, Shen W, Xu X (2013) Hydrogen-rich water alleviates salt stress in rice during seed germination. Plant Soil 370:47–57. https://doi.org/10.1007/s11104-013-1614-3
Xu J, Li Q, Yang L, Li X, Wang Z, Zhang Y (2020) Changes in carbohydrate metabolism and endogenous hormone regulation during bulblet initiation and development in Lycoris radiata. BMC Plant Biol 20(1):180. https://doi.org/10.1186/s12870-020-02394-4
Yadav UP, Ivakov A, Feil R, Duan GY, Walther D, Giavalisco P, Piques M, Carillo P, Hubberten HM, Stitt M, Lunn JE (2014) The sucrose–trehalose 6-phosphate (Tre6P) nexus: specificity and mechanisms of sucrose signalling by Tre6P. J Exp Bot 65(4):1051–1068. https://doi.org/10.1093/jxb/ert457
Yang W, Choi MH, Noh B, Noh YS (2020) De novo shoot regeneration controlled by HEN1 and TCP3/4 in Arabidopsis. Plant Cell Physiol 61:1600–1613. https://doi.org/10.1093/pcp/pcaa083
Zhang W, Song LN, Jaime A, Silva D, Sun HM et al (2013) Effects of temperature, plant growth regulators and substrates and changes in carbohydrate content during bulblet formation by twin scale propagation in Hippeastrum vittatum “Red lion.” Sci Hortic-Amsterdam 160:230–237. https://doi.org/10.1016/j.scienta.2013.06.001
Zhang X, Su N, Jia L, Tian J, Li H, Huang L, Shen Z, Cui J (2018) Transcriptome analysis of radish sprouts hypocotyls reveals the regulatory role of hydrogen-rich water in anthocyanin biosynthesis under UV-A. BMC Plant Biol 18(1):227. https://doi.org/10.1186/s12870-018-1449-4
Zhao X, Chen Q, Wang Y, Shen Z, Shen W, Xu X (2017) Hydrogen-rich water induces aluminum tolerance in maize seedlings by enhancing antioxidant capacities and nutrient homeostasis. Ecotoxicol Environ Saf 144:369–379. https://doi.org/10.1016/j.ecoenv.2017.06.045
Zheng XF, Sun XJ, Xia ZF (2011) Hydrogen resuscitation, a new cytoprotective approach. Clin Exp Pharmacol Physiol 38:155–163. https://doi.org/10.1111/j.1440-1681.2011.05479.x
Zhu Y, Liao W (2017) The metabolic constituent and rooting-related enzymes responses of marigold explants to hydrogen gas during adventitious root development. Theor Exp Plant Phys 29:77–85. https://doi.org/10.1007/s40626-017-0085-y
Zhu Y, Liao W, Niu L, Wang M, Ma Z (2016) Nitric oxide is involved in hydrogen gas-induced cell cycle activation during adventitious root formation in cucumber. BMC Plant Biol 16:1–13. https://doi.org/10.1186/s12870-016-0834-0
Acknowledgements
This work was supported by the National Key Research and Development Program (2018YFD1000800); the National Natural Science Foundation of China (Nos. 32072559, 31860568, 31560563 and 31160398); the Key Research and Development Program of Gansu Province, China (No. 21YF5WA096); the Research Fund of Higher Education of Gansu, China (No. 2018C-14 and 2019B-082); the Natural Science Foundation of Gansu Province, China (Nos. 1606RJZA073, 1606RJZA077 and 1606RJYA252).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Anastasios Melis.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hou, X., Qi, N., Wang, C. et al. Hydrogen-rich water promotes the formation of bulblets in Lilium davidii var. unicolor through regulating sucrose and starch metabolism. Planta 254, 106 (2021). https://doi.org/10.1007/s00425-021-03762-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00425-021-03762-6