Abstract
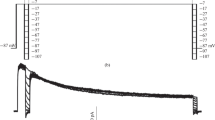
The volume-sensitive chloride current (IClVol) exhibit a time-dependent decay presumably due to channel inactivation. In this work, we studied the effects of chloride ions (Cl−) and H+ ions on IClVol decay recorded in HEK-293 and HL-60 cells using the whole-cell patch clamp technique. Under control conditions ([Cl−]e = [Cl−]i = 140 mM and pHi = pHe = 7.3), IClVol in HEK cells shows a large decay at positive voltages but in HL-60 cells IClVol remained constant independently of time. In HEK-293 cells, simultaneously raising the [Cl−]e and [Cl−]i from 25 to 140 mM (with pHe = pHi = 7.3) increased the fraction of inactivated channels (FIC). This effect was reproduced by elevating [Cl−]i while keeping the [Cl−]e constant. Furthermore, a decrease in pHe from 7.3 to 5.5 accelerated current decay and increased FIC when [Cl−] was 140 mM but not 25 mM. In HL-60 cells, a slight IClVol decay was seen when the pHe was reduced from 7.3 to 5.5. Our data show that inactivation of IClVol can be controlled by changing either the Cl− or H+ concentration or both. Based on our results and previously published data, we have built a model that explains VRAC inactivation. In the model the H+ binding site is located outside the electrical field near the extracellular entry whilst the Cl− binding site is intracellular. The model depicts inactivation as a pore constriction that happens by simultaneous binding of H+ and Cl− ions to the channel followed by a voltage-dependent conformational change that ultimately causes inactivation.








Similar content being viewed by others
References
Almac J, Tian Y, Aldehni F et al (2009) TMEM16 proteins produce volume-regulated chloride currents that are reduced in mice lacking TMEM16A. J Biol Chem 284:28571–28578
Arreola J, Begenisich T, Nehrke K et al (2002) Secretion and cell volume regulation by salivary acinar cells from mice lacking expression of the Clcn3 Cl- channel gene. J Physiol 545:207–216
Arreola J, Hallows KR, Knauf PA (1995) Volume-activated chloride channels in HL-60 cells: potent inhibition by an oxonol dye. Am J Physiol 269:C1063–C1072
Arreola J, Melvin JE, Begenisich T (1995) Volume-activated chloride channels in rat parotid acinar cells. J Physiol 484:677–687
Arreola J, Park K, Melvin JE et al (1996) Three distinct chloride channels control anion movements in rat parotid acinar cells. J Physiol 490:351–362
Coca-Prados M, Sánchez-Torres J, Peterson-Yantorno K et al (1996) Association of ClC-3 channel with Cl- transport by human nonpigmented ciliary epithelial cells. J Membr Biol 150:197–208
Duan D, Winter C, Cowley S et al (1997) Molecular identification of a volume-regulated chloride channel. Nature 390:417–421
Duan D, Zhong J, Hermoso M et al (2001) Functional inhibition of native volume-sensitive outwardly rectifying anion channels in muscle cells and Xenopus oocytes by anti-ClC-3 antibody. J Physiol 531:437–444
Fürst J, Gschwentner M, Ritter M et al (2002) Molecular and functional aspects of anionic channels activated during regulatory volume decrease in mammalian cells. Pflugers Arch 444:1–25
Hanrahan JW, Tabcharini JA (1990) Inhibition of an outwardly rectifying anion channel by HEPES and related buffers. J Membr Biol 116:65–77
Hille B (2001) Ion channels of excitable membranes. Sinauer Associates, Inc., USA
Lewis RS, Ross PE, Cahalan MD (1993) Chloride channels activated by osmotic stress in T lymphocytes. J Gen Physiol 101:801–26
Meyer K, Korbmacher C (1996) Cell swelling activates ATP-dependent voltage-gated chloride channels in M-1 mouse cortical collecting duct cells. J Gen Physiol 108:177–193
Miller C (2006) ClC chloride channels viewed through a transporter lens. Nature 440:484–489
Nilius B, Eggermont J, Voets T et al (1997) Properties of volume-regulated anion channels in mammalian cells. Prog Biophys Mol Biol 68:69–119
Nilius B, Prenen J, Droogmans G (1998) Modulation of volume-regulated anion channels by extra- and intracellular pH. Pflugers Arch 436:742–748
Niemeyer MI, Cid LP, Yusef YR et al (2009) Voltage-dependent and -independent titration of specific residues accounts for complex gating of a ClC chloride channel by extracellular protons. J Physiol 587:1387–1400
Okada Y (2006) Cell volume-sensitive chloride channels: phenotypic properties and molecular identity. Contrib Nephrol 152:9–24
Okada Y, Sato K, Numata T (2009) Pathophysiology and puzzles of the volume-sensitive outwardly rectifying anion channel. J Physiol 587:2141–2149
Perez-Cornejo P, Arreola J, Law FY et al (2004) Volume-sensitive chloride channels do not mediate activation-induced chloride efflux in human neutrophils. J Immunol 172:6988–6993
Poletto Chaves LA, Varanda WA (2008) Volume-activated chloride channels in mice Leydig cells. Pflugers Arch 457:493–504
Rossow CF, Duan D, Hatton WJ et al (2006) Functional role of amino terminus in ClC-3 chloride channel regulation by phosphorylation and cell volume. Acta Physiol (Oxf) 187:5–19
Sabirov RZ, Prenen J, Droogmans G et al (2000) Extra- and Intracellular Proton-Binding Sites of Volume-Regulated Anion Channels. J Membr Biol 177:13–22
Stobrawa SM, Breiderhoff T, Takamori S et al (2001) Disruption of ClC-3, a chloride channel expressed on synaptic vesicles, leads to a loss of the hippocampus. Neuron 29:185–196
Stoddard JS, Steinbach JH, Simchowitz L (1993) Whole cell Cl- currents in human neutrophils induced by cell swelling. Am J Physiol 265:C156–C165
Voets T, Droogmans G, Nilius B (1997) Modulation of Voltage-dependent Properties of a Swelling-activated Cl Current. J Gen Physiol 110:313–325
Acknowledgements
This work was supported by grants 79897, 59889, and 45895 (Consejo Nacional de Ciencia y Tecnologia, Mexico) and PO1-HL18208 (National Institutes of Health, USA). TRS and JADSC received a scholarship from Consejo Nacional de Ciencia y Tecnologia, Mexico.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Fig. 1
Hypothetical V m-dependence of volume-sensitive chloride channels. a Simple barrier model representing the energy landscape along the VRAC pore. This energy profile plus the kinetic model shown in Fig. 7 were used to qualitatively explain the V m (a) and external Cl−-dependence (b) of inactivation. The energy profiles depict the landscape along the pore that the permeant anions (with symmetrical [Cl−]i = [Cl−]e = 140 mM) experiment at −100, 0, and +100 mV. At each voltage, the pore occupancy changes and thus the probability that the pore is empty (P U) is greater at positive voltages. Moreover, P U changes as a function of the Cl− gradient across the membrane. b P U becomes larger as the Cl− gradient decreases. P U was calculated using the V m-dependent rate constants \( {\alpha_{\rm{V}}} = {\left[ {{\hbox{C}}{{\hbox{l}}^{-} }} \right]_{\rm{o}}} \times {k_1} + {\left[ {{\hbox{C}}{{\hbox{l}}^{-} }} \right]_{\rm{i}}} \times {k_{ - {2}}}\;{\hbox{and}}\;{\beta_{\rm{V}}} = {k_{ - {1} + }} \times {k_{ - {2}}} \) recorded in Table 2 (PPT 153 kb)
Rights and permissions
About this article
Cite this article
Hernández-Carballo, C.Y., De Santiago-Castillo, J.A., Rosales-Saavedra, T. et al. Control of volume-sensitive chloride channel inactivation by the coupled action of intracellular chloride and extracellular protons. Pflugers Arch - Eur J Physiol 460, 633–644 (2010). https://doi.org/10.1007/s00424-010-0842-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-010-0842-0