Abstract
Essential Tremor (ET) is a prevalent neurological disease characterized by an 8–10 Hz action tremor. Molecular mechanisms of ET remain poorly understood. Clinical data suggest the importance of the cerebellum in disease pathophysiology, and pathological studies indicate Purkinje Cells (PCs) incur damage. Our recent cerebellar cortex and PC-specific transcriptome studies identified alterations in calcium (Ca2+) signaling pathways that included ryanodine receptor type 1 (RyR1) in ET. RyR1 is an intracellular Ca2+ release channel located on the Endoplasmic Reticulum (ER), and in cerebellum is predominantly expressed in PCs. Under stress conditions, RyR1 undergoes several post-translational modifications (protein kinase A [PKA] phosphorylation, oxidation, nitrosylation), coupled with depletion of the channel-stabilizing binding partner calstabin1, which collectively characterize a “leaky channel” biochemical signature. In this study, we found markedly increased PKA phosphorylation at the RyR1-S2844 site, increased RyR1 oxidation and nitrosylation, and calstabin1 depletion from the RyR1 complex in postmortem ET cerebellum. Decreased calstabin1-RyR1-binding affinity correlated with loss of PCs and climbing fiber-PC synapses in ET. This ‘leaky’ RyR1 signature was not seen in control or Parkinson’s disease cerebellum. Microsomes from postmortem cerebellum demonstrated excessive ER Ca2+ leak in ET vs. controls, attenuated by channel stabilization. We further studied the role of RyR1 in tremor using a mouse model harboring a RyR1 point mutation that mimics constitutive site-specific PKA phosphorylation (RyR1-S2844D). RyR1-S2844D homozygous mice develop a 10 Hz action tremor and robust abnormal oscillatory activity in cerebellar physiological recordings. Intra-cerebellar microinfusion of RyR1 agonist or antagonist, respectively, increased or decreased tremor amplitude in RyR1-S2844D mice, supporting a direct role of cerebellar RyR1 leakiness for tremor generation. Treating RyR1-S2844D mice with a novel RyR1 channel-stabilizing compound, Rycal, effectively dampened cerebellar oscillatory activity, suppressed tremor, and normalized cerebellar RyR1-calstabin1 binding. These data collectively support that stress-associated ER Ca2+ leak via RyR1 may contribute to tremor pathophysiology.









Similar content being viewed by others
Data availability
Basic, anonymized demographic, clinical and tissue data are available as Supplemental Online material.
References
Abu-Omar N, Das J, Szeto V, Feng ZP (2018) Neuronal ryanodine receptors in development and aging. Mol Neurobiol 55(2):1183–1192. https://doi.org/10.1007/s12035-016-0375-4
Almeida Lopes ACB, Peixe TS, Mesas AE, Paoliello MMB (2016) Lead exposure and oxidative stress: a systematic review. Rev Environ Contam Toxicol 236:193–238. https://doi.org/10.1007/978-3-319-20013-2_3
Andersson DC, Betzenhauser MJ, Reiken S, Meli AC, Umanskaya A, Xie W et al (2011) Ryanodine receptor oxidation causes intracellular calcium leak and muscle weakness in aging. Cell Metab 14(2):196–207. https://doi.org/10.1016/j.cmet.2011.05.014
Babij R, Lee M, Cortes E, Vonsattel JP, Faust PL, Louis ED (2013) Purkinje cell axonal anatomy: quantifying morphometric changes in essential tremor versus control brains. Brain 136(Pt 10):3051–3061. https://doi.org/10.1093/brain/awt238
Bellinger AM, Reiken S, Carlson C, Mongillo M, Liu X, Rothman L et al (2009) Hypernitrosylated ryanodine receptor calcium release channels are leaky in dystrophic muscle. Nat Med 15(3):325–330. https://doi.org/10.1038/nm.1916
Benito-Leon J, Labiano-Fontcuberta A (2016) Linking essential tremor to the cerebellum: clinical evidence. Cerebellum 15(3):253–262. https://doi.org/10.1007/s12311-015-0741-1
Bezprozvanny I, Watras J, Ehrlich BE (1991) Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature 351:751–754. https://doi.org/10.1038/351751a0
Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K (2006) Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol 112(4):389–404. https://doi.org/10.1007/s00401-006-0127-z
Braak H, Braak E (1997) Diagnostic criteria for neuropathologic assessment of Alzheimer’s disease. Neurobiol Aging 18:S85–S88. https://doi.org/10.1016/s0197-4580(97)00062-6
Cerasa A, Quattrone A (2016) Linking essential tremor to the cerebellum-neuroimaging evidence. Cerebellum 15(3):263–275. https://doi.org/10.1007/s12311-015-0739-8
Choe M, Cortes E, Vonsattel JP, Kuo SH, Faust PL, Louis ED (2016) Purkinje cell loss in essential tremor: random sampling quantification and nearest neighbor analysis. Mov Disord 31(3):393–401. https://doi.org/10.1002/mds.26490
Clodfelter GV, Porter NM, Landfield PW, Thibault O (2002) Sustained Ca 2+ −induced Ca 2+ −release underlies the postglutamate lethal Ca2+ plateau in older cultured hippocampal neurons. Eur J Pharmacol 447:189–200. https://doi.org/10.1016/s0014-2999(02)01843-5
De Zeeuw CI (2021) Bidirectional learning in upbound and downbound microzones of the cerebellum. Nat Rev Neurosci 22(2):92–110. https://doi.org/10.1038/s41583-020-00392-x
Dogu O, Louis ED, Tamer LT, Unal O, Yilmaz A, Kaleagasi H (2007) elevated blood lead concentrations in essential tremor: a case-control study in Mersin, Turkey. Environ Health Perspect 115(11):1564–1568. https://doi.org/10.1289/ehp.10352
Dridi H, Liu X, Yuan Q, Reiken S, Yehia M, Sittenfeld L et al (2020) Role of defective calcium regulation in cardiorespiratory dysfunction in Huntington’s disease. JCI Insight. https://doi.org/10.1172/jci.insight.140614
Duenas AM, Goold R, Giunti P (2006) Molecular pathogenesis of spinocerebellar ataxias. Brain 129(Pt 6):1357–1370. https://doi.org/10.1093/brain/awl081
Egorova PA, Bezprozvanny IB (2022) Electrophysiological studies support utility of positive modulators of SK channels for the treatment of spinocerebellar ataxia type 2. Cerebellum 21(5):742–749. https://doi.org/10.1007/s12311-021-01349-1
Erickson-Davis CR, Faust PL, Vonsattel J-PG, Gupta S, Honig LS, Louis ED (2010) ‘“Hairy Baskets”’ associated with degenerative Purkinje cell changes in essential tremor. Neuropathol Exp Neurol 69(3):262–271. https://doi.org/10.1097/NEN.0b013e3181d1ad04
Fierro L, Llano I (1996) High endogenous calcium buffering in Purkinje cells from rat cerebellar slices. J Physiol 496(3):617–625. https://doi.org/10.1113/jphysiol.1996.sp021713
Fierro L, DiPolo R, Llano I (1998) Intracellular calcium clearance in Purkinje cell somata from rat cerebellar slices. J Physiol 510:499–512. https://doi.org/10.1111/j.1469-7793.1998.499bk.x
Filip P, Lungu OV, Manto MU, Bares M (2016) Linking essential tremor to the cerebellum: physiological evidence. Cerebellum 15(6):774–780. https://doi.org/10.1007/s12311-015-0740-2
Giannini G, Conti A, Mammarella S, Scrobogna M, Sorrentino V (1995) The ryanodine receptor: calcium channel genes are widely and differentially expressed in murine brain and peripheral tissues. JCB 128(5):893–904. https://doi.org/10.1083/jcb.128.5.893
Gomez LC, Kawaguchi S-Y, Collin T, Jalil A, Del Pilar M, Gomez EN et al (2020) Influence of spatially segregated IP 3-producing pathways on spike generation and transmitter release in Purkinje cell axons. Proc Natl Acad Sci 117(20):11097–11108. https://doi.org/10.1073/pnas.2000148117
Haines DE, Dietrichs E (2012) The cerebellum—structure and connections. Handb Clin Neurol 103:3–36. https://doi.org/10.1016/B978-0-444-51892-7.00001-2
Hall T, Miller A, Corsellis J (1975) Variations in the human Purkinje cell population according to age and sex. Neuropathol Exp Neurol 1(3):267–292. https://doi.org/10.1111/j.1365-2990.1975.tb00652.x
Haubenberger D, Abbruzzese G, Bain PG, Bajaj N, Benito-León J, Bhatia KP et al (2016) Transducer-based evaluation of tremor. Mov Disord 31(9):1327–1336
Huang M, Verbeek DS (2019) Why do so many genetic insults lead to Purkinje Cell degeneration and spinocerebellar ataxia? Neurosci Lett 688:49–57. https://doi.org/10.1016/j.neulet.2018.02.004
Jayaraman T, Brillantes AM, Timerman AP, Fleischer S, Erdjument-Bromage H, Tempst P et al (1992) FK506 binding protein associated with the calcium release channel (ryanodine receptor). J Biol Chem 267(14):9474–9477. https://doi.org/10.1016/S0021-9258(19)50114-4
Jimenez-Jimenez FJ, Alonso-Navarro H, Garcia-Martin E, Alvarez I, Pastor P, Agundez JAG (2021) Genomic markers for essential tremor. Pharmaceuticals (Basel). https://doi.org/10.3390/ph14060516
Axelrad JE, Louis ED, Honig LS, Flores I, Ross W, Pahwa R et al (2008) Reduced Purkinje cell number in essential tremor: a postmortem study. Arch Neurol 65(1):101–107. https://doi.org/10.1001/archneurol.2007.8
Kakizawa S, Yamazawa T, Chen Y, Ito A, Murayama T, Oyamada H et al (2012) Nitric oxide-induced calcium release via ryanodine receptors regulates neuronal function. EMBO J 31(2):417–428. https://doi.org/10.1038/emboj.2011.386
Kakizawa S, Yamazawa T, Iino M (2013) Nitric oxide-induced calcium release: activation of type 1 ryanodine receptor by endogenous nitric oxide. Channels (Austin) 7(1):1–5. https://doi.org/10.4161/chan.22555
Khodakhah K, Armstrong CM (1997) Inositol trisphosphate and ryanodine receptors share a common functional Ca2+ pool in cerebellar Purkinje neurons. Biophys J 73(6):3349–3357. https://doi.org/10.1016/S0006-3495(97)78359-0
Kuo SH, Erickson-Davis C, Gillman A, Faust PL, Vonsattel JP, Louis ED (2011) Increased number of heterotopic Purkinje cells in essential tremor. JNNP 82(9):1038–1040. https://doi.org/10.1136/jnnp.2010.213330
Kushnir A, Todd JJ, Witherspoon JW, Yuan Q, Reiken S, Lin H et al (2020) Intracellular calcium leak as a therapeutic target for RYR1-related myopathies. Acta Neuropathol 136(6):1089–1104. https://doi.org/10.1007/s00401-020-02150-w
Kushnir A, Wajsberg B, Marks AR (2018) Ryanodine receptor dysfunction in human disorders. BBA-Mol Cell Res 1865(11 Pt B):1687–1697. https://doi.org/10.1016/j.bbamcr.2018.07.011
Lacampagne A, Liu X, Reiken S, Bussiere R, Meli AC, Lauritzen I et al (2017) Post-translational remodeling of ryanodine receptor induces calcium leak leading to Alzheimer’s disease-like pathologies and cognitive deficits. Acta Neuropathol 134(5):749–767. https://doi.org/10.1007/s00401-017-1733-7
Lawal TA, Todd JJ, Witherspoon JW, Bönnemann CG, Dowling JJ, Hamilton SL et al (2020) Ryanodine receptor 1-related disorders: an historical perspective and proposal for a unified nomenclature. Skelet Muscle. https://doi.org/10.1186/s13395-020-00243-4
Lee D, Gan S, Faust P, Louis E, Kuo S (2018) Climbing fiber-Purkinje cell synaptic pathology across essential tremor subtypes. Parkinsonism Relat Disord 51:24–29. https://doi.org/10.1016/j.parkreldis.2018.02.032
Lee PJ, Kerridge CA, Chatterjee D, Koeppen AH, Faust PL, Louis ED (2018) A quantitative study of empty baskets in essential tremor and other motor neurodegenerative diseases. J Neuropathol Exp Neurol. https://doi.org/10.1093/jnen/nly114
Lenka A, Jankovic J (2021) Tremor syndromes: an updated review. Front Neurol. https://doi.org/10.3389/fneur.2021.684835
Lin C-Y, Louis ED, Faust PL, Koeppen AH, Vonsattel J-PG, Kuo S-H (2014) Abnormal climbing fibre-Purkinje cell synaptic connections in the essential tremor cerebellum. Brain 137:3149–3159. https://doi.org/10.1093/brain/awu281
Liu J, Tang TS, Tu H, Nelson O, Herndon E, Huynh DP et al (2009) Deranged calcium signaling and neurodegeneration in spinocerebellar ataxia type 2. J Neurosci 29(29):9148–9162. https://doi.org/10.1523/JNEUROSCI.0660-09.2009
Liu X, Betzenhauser MJ, Reiken S, Meli AC, Xie W, Chen B-X et al (2012) Role of leaky neuronal ryanodine receptors in stress- induced cognitive dysfunction. Cell 150(5):1055–1067. https://doi.org/10.1016/j.cell.2012.06.052
Louis ED (2013) The primary type of tremor in essential tremor is kinetic rather than postural: cross-sectional observation of tremor phenomenology in 369 cases. Eur J Neurol 20(4):725–727. https://doi.org/10.1111/j.1468-1331.2012.03855.x
Louis ED (2016) Linking essential tremor to the cerebellum: neuropathological evidence. Cerebellum 15(3):235–242. https://doi.org/10.1007/s12311-015-0692-6
Louis ED (2019) The roles of age and aging in essential tremor: an epidemiological perspective. Neuroepidemiology 52(1–2):111–118. https://doi.org/10.1159/000492831
Louis ED, Factor-Litvak P, Gerbin M, Slavkovich V, Graziano JH, Jiang W et al (2011) Blood harmane, blood lead, and severity of hand tremor: evidence of additive effects. Neurotoxicology 32:227–232. https://doi.org/10.1016/j.neuro.2010.12.002
Louis ED, Faust PL (2020) Essential tremor: the most common form of cerebellar degeneration? Cerebellum Ataxias 7(12):1–10. https://doi.org/10.1186/s40673-020-00121-1
Louis ED, Faust PL (2020) Essential tremor pathology: neurodegeneration and reorganization of neuronal connections. Nat Rev Neurol 16(2):69–83. https://doi.org/10.1038/s41582-019-0302-1
Louis ED, Faust PL, Vonsattel JP, Honig LS, Rajput A, Rajput A et al (2009) Torpedoes in Parkinson’s disease, Alzheimer’s disease, essential tremor, and control brains. Mov Disord 24(11):1600–1605. https://doi.org/10.1002/mds.22567
Louis ED, Faust PL, Vonsattel JP, Honig LS, Rajput A, Robinson CA et al (2007) Neuropathological changes in essential tremor: 33 cases compared with 21 controls. Brain 130(Pt 12):3297–3307. https://doi.org/10.1093/brain/awm266
Louis ED, Jurewicz EC, Applegate L, Factor-Litvak P, Parides M, Andrews L et al (2003) Association between essential tremor and blood lead concentration. Environ Health Perspect 111(14):1707–1711. https://doi.org/10.1289/ehp.6404
Louis ED, Kerridge CA, Chatterjee D, Martuscello RT, Diaz DT, Koeppen AH et al (2019) Contextualizing the pathology in the essential tremor cerebellar cortex: a patholog-omics approach. Acta Neuropathol 138(5):859–876. https://doi.org/10.1007/s00401-019-02043-7
Louis ED, Kuo SH, Tate WJ, Kelly GC, Gutierrez J, Cortes EP et al (2018) Heterotopic Purkinje Cells: a Comparative Postmortem Study of Essential Tremor and Spinocerebellar Ataxias 1, 2, 3, and 6. Cerebellum 17(2):104–110. https://doi.org/10.1007/s12311-017-0876-3
Louis ED, Lee M, Babij R, Ma K, Cortes E, Vonsattel JP et al (2014) Reduced Purkinje cell dendritic arborization and loss of dendritic spines in essential tremor. Brain 137(Pt 12):3142–3148. https://doi.org/10.1093/brain/awu314
Louis ED, Martuscello RT, Gionco JT, Hartstone WG, Musacchio JB, Portenti M et al (2023) Histopathology of the cerebellar cortex in essential tremor and other neurodegenerative motor disorders: comparative analysis of 320 brains. Acta Neuropathol 145(3):265–283. https://doi.org/10.1007/s00401-022-02535-z
Louis ED, McCreary M (2021) How common is essential tremor? Update on the worldwide prevalence of essential tremor. TOHM. https://doi.org/10.5334/tohm.632
Louis ED, Ottman R (2014) How many people in the USA have essential tremor? Deriving a population estimate based on epidemiological data. TOHM. https://doi.org/10.7916/D8TT4P4B
Louis ED, Yi H, Erickson-Davis C, Vonsattel JP, Faust PL (2009) Structural study of Purkinje cell axonal torpedoes in essential tremor. Neurosci Lett 450(3):287–291. https://doi.org/10.1016/j.neulet.2008.11.043
Marks AR (1997) Intracellular calcium-release channels: regulators of cell life and death. Am J Physiol 2727(2):H597-605. https://doi.org/10.1152/ajpheart.1997.272.2.H597
Martuscello RT, Kerridge CA, Chatterjee D, Hartstone WG, Kuo SH, Sims PA et al (2020) Gene expression analysis of the cerebellar cortex in essential tremor. Neurosci Lett 721:134540. https://doi.org/10.1016/j.neulet.2019.134540
Martuscello RT, Sivprakasam K, Hartstone WG, Kuo S-H, Konopka G, Louis ED et al (2022) Gene expression analysis of laser captured Purkinje cells in the essential tremor cerebellum. Cerebellum. https://doi.org/10.1007/s12311-022-01483-4
Marx S, Reiken S, Hisamatsu Y, Gaburjakova M, Gaburjakova J, Yang Y et al (2001) Phosphorylation-dependent regulation of ryanodine receptors: a novel role for leucine/isoleucine zippers. J Cell Biol 153(4):699–708. https://doi.org/10.1083/jcb.153.4.699
Marx S, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N et al (2000) PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell 101(4):365–376. https://doi.org/10.1016/s0092-8674(00)80847-8
Mirra SS (1997) The CERAD neuropathology protocol and consensus recommendations for the postmortem diagnosis of Alzheimer’s disease: a commentary. Neurobiol Aging 18:S91–S94. https://doi.org/10.1016/s0197-4580(97)00058-4
Montine TJ, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Dickson DW et al (2011) National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta Neuropathol 123(1):1–11. https://doi.org/10.1007/s00401-011-0910-3
Mouton J, Marty I, Villaz M, Feltz A, Maulet Y (2001) Molecular interaction of dihydropyridine receptors with type-1 ryanodine receptors in rat brain. Biochem J 354:597–603. https://doi.org/10.1042/0264-6021:3540597
Odgerel Z, Hernandez N, Park J, Ottman R, Louis E, Clark L (2019) Whole genome sequencing and rare variant analysis in essential tremor families. PLoS ONE 14(8):e0220512. https://doi.org/10.1371/journal.pone.0220512
Otsu Y, Marcaggi P, Feltz A, Isope P, Kollo M, Nusser Z et al (2014) Activity-dependent gating of calcium spikes by A-type K+ channels controls climbing fiber signaling in Purkinje cell dendrites. Neuron 84(1):137–151. https://doi.org/10.1016/j.neuron.2014.08.035
Pan MK, Li YS, Wong SB, Ni CL, Wang YM, Liu WC et al (2020) Cerebellar oscillations driven by synaptic pruning deficits of cerebellar climbing fibers contribute to tremor pathophysiology. Sci Transl Med 12(526):eaay1769. https://doi.org/10.1126/scitranslmed.aay1769
Pchitskaya E, Popugaeva E, Bezprozvanny I (2018) Calcium signaling and molecular mechanisms underlying neurodegenerative diseases. Cell Calcium 70:87–94. https://doi.org/10.1016/j.ceca.2017.06.008
Prestori F, Moccia F, D’Angelo E (2019) Disrupted calcium signaling in animal models of human spinocerebellar ataxia (SCA). Int J Mol Sci. https://doi.org/10.3390/ijms21010216
Reiken S, Lacampagne A, Zhou H, Kherani A, Lehnart SE, Ward C et al (2003) PKA phosphorylation activates the calcium release channel (ryanodine receptor) in skeletal muscle: defective regulation in heart failure. J Cell Biol 160(7):919–928. https://doi.org/10.1083/jcb.200211012
Sammi SR, Agim ZS, Cannon JR (2018) Harmane-induced selective dopaminergic neurotoxicity in Caenorhabditis elegans. Toxicol Sci 161(2):335–348. https://doi.org/10.1093/toxsci/kfx223
Santulli G, Marks AR (2015) Essential roles of intracellular calcium release channels in muscle, brain, metabolism, and aging. Curr Mol Pharmacol 8(2):206–222. https://doi.org/10.2174/1874467208666150507105105
Thibault O, Gant JC, Landfield PW (2007) Expansion of the calcium hypothesis of brain aging and Alzheimer’s disease: minding the store. Aging Cell 6(3):307–317. https://doi.org/10.1111/j.1474-9726.2007.00295.x
Uhlén M, Fagerberg L, Hallström BM, Lindskog C, Oksvold P, Mardinoglu A et al (2015) Tissue-based map of the human proteome. Science. https://doi.org/10.1126/science.1260419
Vial F, Kassavetis P, Merchant S, Haubenberger D, Hallett M (2019) How to do an electrophysiological study of tremor. Clin Neurophysiol Pract 4:134–142. https://doi.org/10.1016/j.cnp.2019.06.002
Wilkins HM, Kirchhof D, Manning E, Joseph JW, Linseman DA (2013) Mitochondrial glutathione transport is a key determinant of neuronal susceptibility to oxidative and nitrosative stress. J Biol Chem 288(7):5091–5101. https://doi.org/10.1074/jbc.M112.405738
Womack MD, Walker JW, Khodakhah K (2000) Impaired calcium release in cerebellar Purkinje neurons maintained in culture. J Gen Physiol. https://doi.org/10.1085/jgp.115.3.339
Yu M, Ma K, Faust PL, Honig LS, Cortes E, Vonsattel JP et al (2012) Increased number of Purkinje cell dendritic swellings in essential tremor. Eur J Neurol 19(4):625–630. https://doi.org/10.1111/j.1468-1331.2011.03598.x
Zalk R, Lehnart SE, Marks AR (2007) Modulation of the ryanodine receptor and intracellular calcium. Annu Rev Biochem 76:367–85. https://www.annualreviews.org/doi/https://doi.org/10.1146/annurev.biochem.76.053105.094237
Acknowledgements
Human brain tissue was derived from the New York Brain Bank at Columbia University and the NIH NeuroBioBank (University of Miami, Miami FL; University of Maryland, Baltimore MD).
We would like to thank all the patients and families that contributed to brain donation and the staff at the ETCBR at the New York Brain Bank and the NIH NeuroBioBank. We thank Dr. Marco C. Miotti for creating the illustration of the RyR1 macromolecular complex of kinases, phosphatases, and accessory scaffold proteins.
Funding
Funding for this project was provided from the National Institutes of Health (NIH)/NINDS (R01 NS124854 [SHK and PLF], R01 NS118179 [SHK], R01 NS117745 [PLF and EDL], RF1 NS114570 [ARM], R01 NS104423 [SHK], R01 NS088257 [EDL], R01 NS086736 [EDL]; from the NIH/NHLBI (R25 HL156002 [ARM], R01 HL145473 [ARM], R01 HL140934 [ARM], R01 HL142903 [ARM], T32 HL120826 [ARM]) and from the NIH/NIDDK (R01 DK118240 [ARM]).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by RTM, M-LC, SR, LRS, DSR, C-LN, C-CL, M-KP, ARM, S-HK, and PLF. The first draft of the manuscript was written by RTM and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
ARM and Columbia University own shares in ARMGO Pharma, Inc. a biotech company developing RyR targeted therapeutics. All other authors declare no competing interests or conflicts.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
401_2023_2602_MOESM1_ESM.tif
Supplemental Figure 1: RyR1 macromolecular complex of kinases, phosphatases, and accessory scaffold proteins. Cryo-EM structures of RyR1 (grey, PDB: 7M6A) with subunit calstabin-1 bound (yellow) and endoplasmic reticulum (ER) membrane (black), PP1/Spinophilin complex (blue/green PDB: 3EGG), PDE4 (purple, PDB:5K1I), mAKAP (cyan, AlphaFold), and PKA (orange, PDB:6MM5). Domain-domain interaction information among proteins is still missing. Spatial placement of proteins is just schematic. mAKAP = muscle A-kinase anchoring protein; PDE4 = phosphodiesterase-4; PKA = protein kinase A; PP1 = protein phosphatase 1; RyR1 = ryanodine receptor type 1 (TIF 25619 KB)
401_2023_2602_MOESM2_ESM.tif
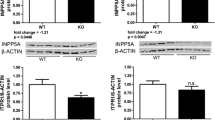
Supplemental Figure 2: Additional Western blot correlations for RyR1 macromolecular scaffold proteins. Quantification of immunoblot from Figure 1a for a. Spinophillin/RyR1, b. PDE4/RyR1 and c. PKA/RyR1 showing no significant difference between ET and control (n = 8 for each), although the decrease in spinophillin protein is trending towards significance (p = 0.063) (TIF 5730 KB)
401_2023_2602_MOESM3_ESM.xlsx
Supplemental Table 1: Clinical demographic and pathological metrics for all patient samples. Clinical and pathological information for each patient cerebellum that was utilized in the biochemical analysis of the ‘leaky’ RyR channel complex via immunoprecipitation and for correlation with calstabin1-RyR1 binding assay. Purkinje cell (PC)/mm and torpedo/mm quantifications were analyzed from a LH&E (luxol fast blue-hematoxylin & eosin) stained slide of cerebellum. LH&E is a general histologic tissue stain that identifies cell bodies, nuclei and myelin sheaths. VGlut2 immunostain is used to quantify density of climbing fiber (CF) synapses on PC dendrites and % CFs in outer 20% of the cerebellar molecular layer (ML). Samples are indicated as being used in RyR1, RyR2 or both Western blot assays, and RyR1 binding assay (XLSX 15 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Martuscello, R.T., Chen, ML., Reiken, S. et al. Defective cerebellar ryanodine receptor type 1 and endoplasmic reticulum calcium ‘leak’ in tremor pathophysiology. Acta Neuropathol 146, 301–318 (2023). https://doi.org/10.1007/s00401-023-02602-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-023-02602-z