Abstract
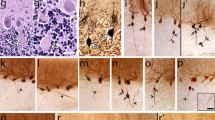
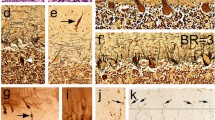
In recent years, numerous morphologic changes have been identified in the essential tremor (ET) cerebellar cortex, distinguishing ET from control brains. These findings have not been fully contextualized within a broader degenerative disease spectrum, thus limiting their interpretability. Building off our prior study and now doubling the sample size, we conducted comparative analyses in a postmortem series of 320 brains on the severity and patterning of cerebellar cortex degenerative changes in ET (n = 100), other neurodegenerative disorders of the cerebellum [spinocerebellar ataxias (SCAs, n = 47, including 13 SCA3 and 34 SCA1, 2, 6, 7, 8, 14); Friedreich’s ataxia (FA, n = 13); multiple system atrophy (MSA), n = 29], and other disorders that may involve the cerebellum [Parkinson’s disease (PD), n = 62; dystonia, n = 19] versus controls (n = 50). We generated data on 37 quantitative morphologic metrics, grouped into 8 broad categories: Purkinje cell (PC) loss, heterotopic PCs, PC dendritic changes, PC axonal changes (torpedoes), PC axonal changes (other than torpedoes), PC axonal changes (torpedo-associated), basket cell axonal hypertrophy, and climbing fiber-PC synaptic changes. Principal component analysis of z scored raw data across all diagnoses (11,651 data items) revealed that diagnostic groups were not uniform with respect to pathology. Dystonia and PD each differed from controls in only 4/37 and 5/37 metrics, respectively, whereas ET differed in 21, FA in 10, SCA3 in 10, MSA in 21, and SCA1/2/6/7/8/14 in 27. Pathological changes were generally on the milder end of the degenerative spectrum in ET, FA and SCA3, and on the more severe end of that spectrum in SCA1/2/6/7/8/14. Comparative analyses across morphologic categories demonstrated differences in relative expression, defining distinctive patterns of changes in these groups. In summary, we present a robust and reproducible method that identifies somewhat distinctive signatures of degenerative changes in the cerebellar cortex that mark each of these disorders.





Similar content being viewed by others
Data availability
Basic, anonymized demographic, clinical and tissue data are available as Supplemental Online material.
References
Babij R, Lee M, Cortes E, Vonsattel JP, Faust PL, Louis ED (2013) Purkinje cell axonal anatomy: quantifying morphometric changes in essential tremor versus control brains. Brain 136:3051–3061. https://doi.org/10.1093/brain/awt238
Beliveau E, Tremblay C, Aubry-Lafontaine E, Paris-Robidas S, Delay C, Robinson C et al (2015) Accumulation of amyloid-beta in the cerebellar cortex of essential tremor patients. Neurobiol Dis 82:397–408. https://doi.org/10.1016/j.nbd.2015.07.016
Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K (2006) Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol 112:389–404. https://doi.org/10.1007/s00401-006-0127-z
Braak H, Braak E (1997) Diagnostic criteria for neuropathologic assessment of Alzheimer’s disease. Neurobiol Aging 18:S85-88
Choe M, Cortes E, Vonsattel JG, Kuo SH, Faust PL, Louis ED (2016) Purkinje cell loss in essential tremor: random sampling quantification and nearest neighbor analysis. Mov Disord 31:393–401. https://doi.org/10.1002/mds.26490
Delay C, Tremblay C, Brochu E, Paris-Robidas S, Emond V, Rajput AH et al (2014) Increased LINGO1 in the cerebellum of essential tremor patients. Mov Disord 29:1637–1647. https://doi.org/10.1002/mds.25819
Erickson-Davis CR, Faust PL, Vonsattel JP, Gupta S, Honig LS, Louis ED (2010) “Hairy baskets” associated with degenerative Purkinje cell changes in essential tremor. J Neuropathol Exp Neurol 69:262–271. https://doi.org/10.1097/NEN.0b013e3181d1ad04
Fujishima K, Kurisu J, Yamada M, Kengaku M (2020) βIII spectrin controls the planarity of Purkinje cell dendrites by modulating perpendicular axon-dendrite interactions. Development 147:dev194530. https://doi.org/10.1242/dev.194530
Harasymiw JW, Bean P (2001) Identification of heavy drinkers by using the early detection of alcohol consumption score. Alcohol Clin Exp Res 25:228–235. https://doi.org/10.1111/j.1530-0277.2001.tb02203.x
Hirano A, Llena JF, French JH, Ghatak NR (1977) Fine structure of the cerebellar cortex in Menkes Kinky-hair disease. X-chromosome-linked copper malabsorption. Arch Neurol 34:52–56. https://doi.org/10.1001/archneur.1977.00500130072014
Ito H, Kawakami H, Wate R, Matsumoto S, Imai T, Hirano A et al (2006) Clinicopathologic investigation of a family with expanded SCA8 CTA/CTG repeats. Neurology 67:1479–1481. https://doi.org/10.1212/01.wnl.0000240256.13633.7b
Iwabuchi K, Koyano S, Yagishita S (2022) Simple and clear differentiation of spinocerebellar degenerations: overview of macroscopic and low-power view findings. Neuropathology 42:379–393. https://doi.org/10.1111/neup.12823
Koeppen AH (2018) The neuropathology of the adult cerebellum. Handb Clin Neurol 154:129–149. https://doi.org/10.1016/B978-0-444-63956-1.00008-4
Koeppen AH (1991) The Purkinje cell and its afferents in human hereditary ataxia. J Neuropathol Exp Neurol 50:505–514. https://doi.org/10.1097/00005072-199107000-00010
Koeppen AH, Davis AN, Morral JA (2011) The cerebellar component of Friedreich’s ataxia. Acta Neuropathol 122:323–330. https://doi.org/10.1007/s00401-011-0844-9
Koeppen AH, Mazurkiewicz JE (2013) Friedreich ataxia: neuropathology revised. J Neuropathol Exp Neurol 72:78–90. https://doi.org/10.1097/NEN.0b013e31827e5762
Kuo SH, Lin CY, Wang J, Sims PA, Pan MK, Liou JY et al (2017) Climbing fiber-Purkinje cell synaptic pathology in tremor and cerebellar degenerative diseases. Acta Neuropathol 133:121–138. https://doi.org/10.1007/s00401-016-1626-1
Kuo SH, Tang G, Louis ED, Ma K, Babji R, Balatbat M et al (2013) Lingo-1 expression is increased in essential tremor cerebellum and is present in the basket cell pinceau. Acta Neuropathol 125:879–889. https://doi.org/10.1007/s00401-013-1108-7
Lee PJ, Kerridge CA, Chatterjee D, Koeppen AH, Faust PL, Louis ED (2019) A quantitative study of empty baskets in essential tremor and other motor neurodegenerative diseases. J Neuropathol Exp Neurol 78:113–122. https://doi.org/10.1093/jnen/nly114
Lin CY, Louis ED, Faust PL, Koeppen AH, Vonsattel JP, Kuo SH (2014) Abnormal climbing fibre-Purkinje cell synaptic connections in the essential tremor cerebellum. Brain 137:3149–3159. https://doi.org/10.1093/brain/awu281
Louis ED, Babij R, Cortes E, Vonsattel JP, Faust PL (2013) The inferior olivary nucleus: a postmortem study of essential tremor cases versus controls. Mov Disord 28:779–786. https://doi.org/10.1002/mds.25400
Louis ED, Babij R, Lee M, Cortes E, Vonsattel JP (2013) Quantification of cerebellar hemispheric purkinje cell linear density: 32 ET cases versus 16 controls. Mov Disord 28:1854–1859. https://doi.org/10.1002/mds.25629
Louis ED, Faust PL (2020) Essential tremor pathology: neurodegeneration and reorganization of neuronal connections. Nat Rev Neurol 16:69–83. https://doi.org/10.1038/s41582-019-0302-1
Louis ED, Faust PL, Vonsattel JP, Honig LS, Rajput A, Pahwa R et al (2009) Torpedoes in Parkinson’s disease, Alzheimer’s disease, essential tremor, and control brains. Mov Disord 24:1600–1605. https://doi.org/10.1002/mds.22567
Louis ED, Ford B, Bismuth B (1998) Reliability between two observers using a protocol for diagnosing essential tremor. Mov Disord 13:287–293. https://doi.org/10.1002/mds.870130215
Louis ED, Kerridge CA, Chatterjee D, Martuscello RT, Diaz DT, Koeppen AH et al (2019) Contextualizing the pathology in the essential tremor cerebellar cortex: a patholog-omics approach. Acta Neuropathol 138:859–876. https://doi.org/10.1007/s00401-019-02043-7
Louis ED, Kuo SH, Tate WJ, Kelly GC, Gutierrez J, Cortes EP et al (2018) Heterotopic Purkinje cells: a comparative postmortem study of essential tremor and spinocerebellar ataxias 1, 2, 3, and 6. Cerebellum 17:104–110. https://doi.org/10.1007/s12311-017-0876-3
Louis ED, Kuo SH, Vonsattel JP, Faust PL (2014) Torpedo formation and Purkinje cell loss: modeling their relationship in cerebellar disease. Cerebellum 13:433–439. https://doi.org/10.1007/s12311-014-0556-5
Louis ED, Lee M, Babij R, Ma K, Cortes E, Vonsattel JP et al (2014) Reduced Purkinje cell dendritic arborization and loss of dendritic spines in essential tremor. Brain 137:3142–3148. https://doi.org/10.1093/brain/awu314
Louis ED, McCreary M (2021) How common is essential tremor? Update on the worldwide prevalence of essential tremor. Tremor Other Hyperkinet Mov (NY) 11:28. https://doi.org/10.5334/tohm.632
Louis ED, Ottman R (2014) How many people in the USA have essential tremor? Deriving a population estimate based on epidemiological data. Tremor Other Hyperkinet Mov (NY) 4:259. https://doi.org/10.7916/D8TT4P4B
Louis ED, Ottman R, Ford B, Pullman S, Martinez M, Fahn S et al (1997) The Washington heights-inwood genetic study of essential tremor: methodologic issues in essential-tremor research. Neuroepidemiology 16:124–133. https://doi.org/10.1159/000109681
Louis ED, Vonsattel JP, Honig LS, Ross GW, Lyons KE, Pahwa R (2006) Neuropathologic findings in essential tremor. Neurology 66:1756–1759. https://doi.org/10.1212/01.wnl.0000218162.80315.b9
Louis ED, Wendt KJ, Albert SM, Pullman SL, Yu Q, Andrews H (1999) Validity of a performance-based test of function in essential tremor. Arch Neurol 56:841–846. https://doi.org/10.1001/archneur.56.7.841
Louis ED, Zheng W, Mao X, Shungu DC (2007) Blood harmane is correlated with cerebellar metabolism in essential tremor: a pilot study. Neurology 69:515–520. https://doi.org/10.1212/01.wnl.0000266663.27398.9f
Mavroudis I, Kazis D, Petridis F, Chatzikonstantinou S, Karantali E, Njau SN et al (2022) Morphological and morphometric changes in the Purkinje cells of patients with essential tremor. Exp Ther Med 23:167. https://doi.org/10.3892/etm.2021.11090
McKay BE, Turner RW (2005) Physiological and morphological development of the rat cerebellar Purkinje cell. J Physiol 567:829–850. https://doi.org/10.1113/jphysiol.2005.089383
Mirra SS (1997) The CERAD neuropathology protocol and consensus recommendations for the postmortem diagnosis of Alzheimer’s disease: a commentary. Neurobiol Aging 18:S91-94. https://doi.org/10.1016/S0197-4580(97)00058-4
Paris-Robidas S, Brochu E, Sintes M, Emond V, Bousquet M, Vandal M et al (2012) Defective dentate nucleus GABA receptors in essential tremor. Brain 135:105–116. https://doi.org/10.1093/brain/awr301
Poewe W, Stankovic I, Halliday G, Meissner WG, Wenning GK, Pellecchia MT et al (2022) Multiple system atrophy. Nat Rev Dis Primers 8:56. https://doi.org/10.1038/s41572-022-00382-6
Sakai K, Ishida C, Morinaga A, Takahashi K, Yamada M (2015) Case study: somatic sprouts and halo-like amorphous materials of the purkinje cells in Huntington’s disease. Cerebellum 14:707–710. https://doi.org/10.1007/s12311-015-0678-4
Seidel K, Siswanto S, Brunt ER, den Dunnen W, Korf HW, Rub U (2012) Brain pathology of spinocerebellar ataxias. Acta Neuropathol 124:1–21. https://doi.org/10.1007/s00401-012-1000-x
Tanabe K, Kani S, Shimizu T, Bae YK, Abe T, Hibi M (2010) Atypical protein kinase C regulates primary dendrite specification of cerebellar Purkinje cells by localizing Golgi apparatus. J Neurosci 30:16983–16992. https://doi.org/10.1523/JNEUROSCI.3352-10.2010
Yang Q, Hashizume Y, Yoshida M, Wang Y, Goto Y, Mitsuma N et al (2000) Morphological Purkinje cell changes in spinocerebellar ataxia type 6. Acta Neuropathol 100:371–376. https://doi.org/10.1007/s004010000201
Yoshida K, Asakawa M, Suzuki-Kouyama E, Tabata K, Shintaku M, Ikeda S et al (2014) Distinctive features of degenerating Purkinje cells in spinocerebellar ataxia type 31. Neuropathology 34:261–267. https://doi.org/10.1111/neup.12090
Yu M, Ma K, Faust PL, Honig LS, Cortes E, Vonsattel JP et al (2012) Increased number of Purkinje cell dendritic swellings in essential tremor. Eur J Neurol 19:625–630. https://doi.org/10.1111/j.1468-1331.2011.03598.x
Acknowledgements
Brain tissue was derived from: New York Brain Bank at Columbia University; Dr. Arnulf H. Koeppen, Veterans Affairs Medical Center, Albany, New York, USA; the National Institutes of Health NeuroBioBank (University of Maryland, Baltimore, MD, University of Miami, Miami, FL, and Harvard Brain Tissue Resource Center, McLean Hospital, Belmont, MA); Dr. Laura Ranum, Center for NeuroGenetics Ataxia Brain Bank at the University of Florida, Gainesville FL; Dr. Todd Goldie, University of Florida Neuromedicine Human Brain and Tissue Bank, Gainesville FL; Dr. C. Dirk Keene, University of Washington, Seattle WA; and The Sheffield Biorepository at the University of Sheffield, Sheffield UK. We would like to thank all the patients and families that contributed to brain donation and the National Ataxia Foundation for providing funding to investigators for banking of brains from individuals with ataxia.
Funding
This work was supported by NINDS R01 NS088257 and NINDS R01 NS117745 (Drs. Louis and Faust, PIs) and R01 NS086736 (Dr. Louis, PI), which provide funding for the Essential Tremor Centralized Brain Repository.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by EDL, RTM, JTG, WGH, JBM, MP, MM, S-HK, J-PGV, and PLF. The first draft of the manuscript was written by EDL and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
None of the authors report any competing or financial interests.
Ethical approval
All study subjects signed informed consent forms approved by the respective university or institutional ethics boards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Louis, E.D., Martuscello, R.T., Gionco, J.T. et al. Histopathology of the cerebellar cortex in essential tremor and other neurodegenerative motor disorders: comparative analysis of 320 brains. Acta Neuropathol 145, 265–283 (2023). https://doi.org/10.1007/s00401-022-02535-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-022-02535-z