Abstract
Phytoremediation is gaining momentum among bioremediation strategies for addressing high levels of metal(loid)s and organic pollutants in the environment, which threaten plants, wildlife, and human safety due to their cytotoxic, mutagenic, and carcinogenic effects. An impediment to this bioremediation method is the limitation in the innate abilities of phytoremediation species to efficiently cope with pollutant-mediated stress, which often restricts growth, development, and efficient pollutant removal. Phlorotannins, a class of polyphenols derived from marine brown algae, possess a number of bioactivities that may be beneficial for boosting phytoremediation efficiency. This review provides a concise overview of phlorotannins, their chemical nature and structural classes, and the few (indicating a paucity of research data) bioactivities of phlorotannins that have been reported in plants. In addition, included are synopses on different phytoremediation strategies and highlights of major future research perspectives on harnessing phlorotannin bioactivities to ameliorate growth, development, and stress tolerance in phytoremediation species for the benefit of phytoremediation efforts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Heavy metal(loid)s and a diverse class of organic pollutants from natural sources and anthropogenic activities constitute a contamination problem in soils, surface water, and ground water. Their recalcitrant nature and presence in the environment threaten plants, wildlife, and human safety due to their cytotoxic, mutagenic, and carcinogenic effects (Olguín and Sánchez-Galván, 2011; Allamin et al. 2020). These afore, and the quest to fulfill stringent environmental laws and lower the cost of remediation, have spurred the development of several plant-dependent contaminant containment and removal strategies that rely on using different collections of species for phytoremediation.
A limitation of phytoremediation is that very few plant species possess the natural abilities to tolerate and survive in the highly toxic and growth-limiting environments that characterize polluted sites. These have further necessitated the search and development of biotechnologies that can assist phytoremediation species in coping with pollutant-induced limitations of growth and development while enhancing their phytoremediation efficiencies. Organic and inorganic soil amendments (Shrestha et al. 2019; Gul et al. 2020; Kafle et al. 2022) and plant growth promoting bacteria (Ullah et al. 2015; Kong and Glick 2017; Mesa-Marín et al. 2020) have been employed to improve phytoremediation efficiencies. In addition, plant biostimulants which help plants counteract various environmental stress, including stresses resulting from exposure to toxic pollutants, have been employed to augment plant growth and ameliorate phytoremediation performance [See recent review by Bartucca et al. (2022)].
Marine brown algae produce a class of polyphenols called phlorotannins which possess a broad category of bioactivities ranging from potent antioxidant and reducing power to metal chelation capacities and augmentation of stress tolerance in plants. An emerging body of research demonstrates that phlorotannins, when supplied as biostimulants, enhanced plant growth and development (Aremu et al. 2015; Rengasamy et al. 2015), altered primary metabolism and promoted the accumulation of secondary metabolites (Aremu et al. 2015; Kulkarni et al. 2019), and boosted tolerance to osmotic and salinity stress (Masondo et al. 2018). However, research reports on the potential(s) of phlorotannins in enhancing phytoremediation performance are not readily available. In this review, we present a concise overview of phlorotannins, their chemical nature and different structural classes, and the few (indicating a paucity of research data) phlorotannin bioactivities that have been reported in plants. We then provide synopses of different phytoremediation strategies that have been developed so far. Finally, we highlight major research areas that must be addressed if the bioactivities of phlorotannins are to be exploited to facilitate growth, development, and stress tolerance in species to enhance phytoremediation efficiencies and performance of phytoremediation species.
Phlorotannins
Phlorotannins are a distinct class of polyphenols that are synthesized by marine brown algae [Phaeophyta; essentially Fucales and Laminariales (Meslet-Cladière et al. 2013)], and are structurally and functionally analogous to condensed tannins that occur in terrestrial plants. They are accumulated within the vegetative cells of the outer cortical layer of the thalli, irrespective of the species, stage of growth, tissue or organ (Shibata et al. 2004) and have been seen to represent up to 25% of dry algal biomass (Meslet-Cladière et al. 2013). Phlorotannins occur in soluble forms in cellular compartments, such as specialized secretion vesicles called physodes, and as components of extracellular exudates (Meslet-Cladière et al. 2013; Imbs and Zvyagintseva 2018) or insoluble forms in complexation with alginic acid in cell walls (Imbs and Zvyagintseva 2018).
Phlorotannins are polymerization products of phloroglucinol (1,3,5-trihydroxybenzene) residues (Fig. 1) with diverse permutations and combinations of aryl-aryl and/or diaryl ether linkages (Shibata et al. 2004; Meslet-Cladière et al. 2013), thus yielding a wide range of highly hydrophilic compounds with molecular sizes ranging between 126 Da and 650 kDa (Li et al. 2011). The monomeric unit is synthesized in the acetate–malonate (polyketide) pathway, which is catalyzed by a type III polyketide synthase and cyclase complex (Achkar et al. 2005; Abe and Morita 2010) in a series of sequential reactions including; (i) the conversion of acetyl-coenzyme A to malonyl-coenzyme A by the addition of CO2; (ii) condensation of three malonyl-coenzyme A molecules to yield a polyketide intermediate; and (iii) subsequent cyclization and tautomerization of the resulting polyketide chain to form phloroglucinol (Achkar et al. 2005; Meslet-Cladière et al. 2013).
Structurally, phlorotannins are highly diverse due to the occurrence of structural and conformational isomers, which arise from the linkage of monomers at different positions of the phloroglucinol ring as well as variations in the number of additional hydroxyl groups. Six different classes of phlorotannins are delineated based on the type(s) of inter-monomeric linkages as described below.
Fucols
The phloroglucinol residues are linked by C—C phenyl linkage in the meta position as seen in trifucol, tetrafucol A, tetrafucol B, and heptafucol (Fig. 2). Fucols have been widely isolated from species of Cystoseira (Sánchez-Camargo et al. 2016) and Fucus (Cérantola et al. 2006; Birkemeyer et al. 2020), as well as some in Fucaceae genera such as Pelvetia (Birkemeyer et al. 2020).
Phlorethols
Monomeric phloroglucinol units are linked via ether bonds as seen in heptaphlorethol, triphlorethol A, and Tetraphlorethol B (Fig. 3). Phlorethol-class of phlorotannins was found to be the predominant phlorotannin in Cystoseira osmundacea, Egregia mensienzii, and Pterigophora californica (Múzquiz de la Garza et al. 2019) and have also been isolated from Sargassum species (Li et al. 2017).
Fucophloroethols
Each phloroglucinol unit may be linked to the next via an ether and/or phenyl bond, e.g., 947-B, fucodiphlorethol G, and fucotriphlorethol A (Fig. 4). This class of phlorotannins is common in species of Fucaceae, such as Ascophyllum, Fucus, Pelvetia, Pelvetiopsis, and Silvetia (Catarino et al. 2017), and Laminariaceae, e.g., Eisenia (Choi et al. 2015).
Eckols
This class of phlorotannins bear one or more 1,4-dibenzodioxin element(s) formed by two phloroglucinol units, e.g., 8,8’-bieckol, dibenzo[1,4] dioxin-2,4,7,9-tetraol, dieckol, and eckol (Fig. 5). Eckol-type phlorotannins have been found mainly in Ecklonia, Eisenia, and Sargassum species (Rengasamy et al. 2013; Li et al. 2017; Murata et al. 2020; Sugiura et al. 2021).
Fuhalols
The phloroglucinol units are interconnected by ortho-/para-arranged ether linkages with an extra hydroxyl group on one unit, making that unit vicinally trihydroxylated, e.g., bifuhalol and tetrafuhalol A (Fig. 6). The additional hydroxyl group differentiates fuhalols from phlorethols. Some fuhalols possess more than one extra hydroxyl group and are termed hydroxyfuhalols, e.g., hydroxytrifuhalol B, hydroxypentafuhalol A, and hydroxyheptafuhalol B. Fuhalols are the dominant categories of phlorotannins in Sargassum and Sargassaceae species, such as Halidrys siliquosa, Cystoseira baccata, and Bifurcaria bifurcata [See Li et al. (2017) and references therein].
Carmalols
These are basically phlorethols with a dibenzodioxin moiety at positions 3 and 7 in their structures as seen in diphlorethohydroxycarmalol and triphlorethohydroxycarmalol (Fig. 7) from Carpophyllum maschalocarpum (Sargassaceae) (Li and Glombitza 1991) and Ishige okamurae (Ishigeaceae) (Kim et al. 2020).
Phlorotannin Bioactivities and their Implications for Abiotic Stress Mitigation in Plants
Phlorotannins exhibit a broad range of bioactivities which include antioxidant and radioprotective actions (Wang et al. 2012; Imbs and Zvyagintseva 2018; Bogolitsyn et al. 2019), enzyme inhibitory effects (Li et al. 2011; Rengasamy et al. 2015), antimicrobial and antiviral activities (Tang et al. 2020; Lomartire and Gonçalves 2022), as well as anti-inflammation, anticancer, and anti-allergic actions (Catarino et al. 2021; Sugiura et al. 2021). Due to the enormous research attention that phlorotannins have attracted in these fields, their bioactivities have been the subject of cosmeceutical, medical, and pharmaceutical research. In contrast, the bioactivities of specific phlorotannins in plants and the attenuation of abiotic and biotic stresses have not been extensively investigated. The following sections concisely highlight major reports on phlorotannin-bioactivities and their potentials or implications in the mitigation of abiotic stresses in plants.
Antioxidant Activities
A major bioactivity associated with phlorotannins is their strong antioxidant activities against free radical-mediated oxidative damage. For instance, 974-B, a heterocyclic fucophlorethol-type phlorotannin derived from Eisenia bicyclis, exhibited significant inhibitory effects against DPPH, ONOO−, and total reactive oxygen species (ROS), with scavenging potencies indicated by IC50 values of 0.86, 1.80, 6.45 µM, respectively (Choi et al. 2015). Similarly, eckol and dibenzo[1,4]dioxine-2,4,7,9-tetraol, extracted from Ecklonia maxima, exhibited strong antioxidant activities on DPPH free radicals with EC50 values of 0.008 and 0.012 µM, respectively (Rengasamy et al. 2013). The antioxidant properties of phlorotannins are attributed to their ability to scavenge radicals and oxygen-containing compounds, strong reducing power (Budhiyanti et al. 2011; Li et al. 2011; Wang et al. 2012), as well as their metal-chelating properties (Ragan et al. 1979). Phlorotannins are polyphenols that are electron-rich compounds. Polyphenolics are generally known to readily partake in efficient electron-donation reactions to produce phenoxyl radical species as intermediates in the presence of oxidizing agents. The resulting phenoxyl radicals maybe stabilized by resonance delocalization of the unpaired electrons to the ortho and para positions of the ring or by hydrogen bonding with an adjacent hydroxyl group (Parshad et al. 2016).
Antioxidants feature among the first strategies deployed by plants in the mitigation of oxidative stress. While the bioactivities of different specific phlorotannins in the mitigation of oxidative stress have been extensively investigated in animal systems (Catarino et al. 2017; Shrestha et al. 2021), not much has been done on in vivo evaluation of the attenuating effects of phlorotannins on abiotic stresses in plants. Despite the paucity of data on this subject, the potential of phlorotannins to mitigate oxidative stress in plants can be inferred from observations with many seaweed-derived biostimulants which have been seen to augment plant tolerance to abiotic stresses, e.g., drought and salinity (Goñi et al. 2018; Deolu-Ajayi et al. 2022). Among other bioactive agents in these seaweed-derived extracts, polyphenols (including phlorotannins) have been suggested as one of the bioactive compounds that mediate these oxidative stress mitigation properties (Bogolitsyn et al. 2019). In agreement with the afore, phloroglucinol, the monomeric units of phlorotannins, improved seedling growth in Ceratotheca triloba under polyethylene glycol-induced osmotic and NaCl-induced salinity stress (Masondo et al. 2018).
Metal-Binding Capacities
Metal-mediated initiation and propagation of excessive ROS production is characteristic of mitochondria in the presence of excess free (redox-active) metal cations such as Fe2+ and Cu+ (Keunen et al. 2011). For instance, cations of transition metals catalyze the Fenton reaction, a single-electron reduction of H2O2 molecules to produce hydroxyl radicals, and the Haber–Weiss reaction, i.e., the formation of a hydroxyl radical from H2O2 and superoxide anion, (Richards et al. 2015) thus increasing the production of hydroxyl radicals when such cations are in excess. These metals impose a mitochondrial oxidative challenge, a result of metal-induced electron transport chain over-reduction and dysfunction at the level of the cytochrome pathway, which is typified by increased ROS generation and alteration of antioxidative defenses [See review by Keunen et al. (2011)]. Phlorotannins, through their metal-binding activities (Ragan et al. 1979), can sequester free metal cations and allay metal-induced oxidative damage. Although this metal chelating capacity has been well characterized (Toth and Pavia 2000; Connan and Stengel 2011), an assessment of how specific or different classes of phlorotannins may influence tolerance to heavy metal(loid)-induced stress in plants is yet to be carried out.
Stimulation of Vegetative Growth, Plant Metabolism, and Stress Responses
Many seaweed extracts have been assessed and successfully employed as biostimulants to boost plant growth and overall productivity, e.g., Kelpak® (Digruber et al. 2018; Gupta et al. 2021) and extracts from Laminaria species and Ascophyllum nodosum (Ertani et al. 2018). Despites these observations, studies that evaluate how specific classes and/or individual phlorotannins influence vegetative growth, biomass production, and abiotic stress tolerance in candidate phytoremediation species are scarce. However, assessments of the growth stimulatory effects of phloroglucinol and eckol, isolated from Ecklonia maxima in our laboratory, demonstrated that these phlorotannins are effective at augmenting vegetative growth and boosting biomass production when supplied as exogenous treatments (Aremu et al. 2015; Rengasamy et al. 2015; Kulkarni et al. 2019). Eckol was particularly efficient at stimulating root development (Rengasamy et al. 2015) in an auxin-mimicking manner. With the above, it will be interesting to see if phlorotannins can elicit these effects in phytoremediation species that are subjected to the limiting ambience that characterise polluted sites.
Similarly, eckol was noted to have altered primary and secondary metabolic processes in treated Spinacia oleracea (Kulkarni et al. 2019). These included enhanced cytokinin, photosynthetic pigments, free phenolic acids, and protein contents, in addition to the induction of phenylalanine ammonia-lyase activity. Phenylalanine ammonia-lyase is a key enzyme of the phenylpropanoid pathway that catalyzes the first step in the synthesis of a variety of polyphenyl compounds, and it is a primary inducible defense response in plants against several biotic and abiotic stresses (Rasool et al. 2021). These observations hint at the ability of eckol to augment stress tolerance responses via the induction of plant enzymatic defense mechanisms. Further analyses are, therefore, required to demonstrate if eckol and other classes of phlorotannins can stimulate primary and secondary metabolism in phytoremediation species to boost their remediation efficiencies.
Phytoremediation: A Concise Overview
Phytoremediation is a plant-based bioremediation and environmental restoration technology that involves in situ application of plants, and associated soil microbiota, to remove or reduce the bioavailability or toxic effects of contaminants in the environment (Jacob et al. 2018; Yan et al. 2020). The development of different phytoremediation strategies as a clean-up approach for environmental (soil and wetland) pollutants was necessitated by the difficulties and impracticalities encountered with traditional remediation techniques such as excavation and disposal of contaminated soils, vitrification, soil flushing and washing, solidification/stabilization, and thermal desorption (Khalid et al. 2017; Liu et al. 2018). These traditional remediation methods are energy intensive or/and expensive and are thus limited to small-scale applications (Khalid et al. 2017; Liu et al. 2018). Other benefits that fostered the gravitation towards phytoremediation as the go-to-technique for contaminant removal include (i) feasibility and applicability on a very large scale and in terms of a wide range of toxic heavy metals; (ii) inherently environment- and eco-friendly; (iii) non-invasive and non-destructive technologies which leave the soil intact and biologically productive; (iv) it is an autotrophic system that requires little or no energy input; and (v) improvements in soil fertility via the release of various organic matter (Sabir et al. 2015; Thakur et al. 2016; Jacob et al. 2018; Yan et al. 2020).
The reclamation of contaminated/derelict soils and wetlands using phytoremediation strategies relies on a plethora of plant and edaphic factors. Soil/edaphic factors are critical determinants of phytoremediation efficiencies due to their significance in plant growth and development. These include soil physicochemical properties—pH, redox potential, electrical conductivity, cation exchange capacity, water-holding capacity, organic matter contents (Sheoran et al. 2016); concentrations and bioavailability of the contaminant(s); and the composition and activity of soil/rhizosphere microbiome (Philippot et al. 2013; Eisenhauer et al. 2017; Canarini et al. 2019).
Plant-specific factors are also critical for phytoremediation success. Some major species-dependent factors are discussed below.
Plant Species
Due to high diversity in morphophysiological traits, plants vary in their phytoremediation capabilities (Mitton et al. 2014; Bian et al. 2018; Guidi Nissim et al. 2018). These variations in phytoremediation capacities arose from differences in one or a combination of plant characteristics, some of which are discussed below. For instance, notable differences in bioconcentration factors were reported for tomato, sunflower, soybean, and alfalfa in the remediation of an organochlorine insecticide, p,p′-DDT [2,2-bis(chlorophenyl)-1,1,1-trichloroethane] (Mitton et al. 2014).
Pollutant Uptake, Transport, and Translocation Efficiencies
The uptake and transportation of metal(loid)s and organic pollutants from soils into root cells/tissues occur either by passive diffusion through the cell membrane (apoplastic pathway) or via active transport against concentration and/or electrochemical potential gradients mediated by carriers, channels, or transporters (symplastic pathway). For example, As and Se uptake is mediated via phosphate and sulfur transporters, respectively (Li et al. 2015; Pilon-Smits 2017). Similarly, organic pollutants such as polycyclic aromatic hydrocarbons are transported via apoplastic and symplastic pathways (Zhan et al. 2018). The significance of these traits in candidate phytoremediation species is evident in the fact that a species’ bioconcentration factor, i.e., the ratio of pollutant concentration in tissues to the concentration in the environment (Yadav et al. 2022), is a critical parameter in the evaluation of the pollutant uptake capacity of macrophytes. Translocation of absorbed metal(loid)s from the root to the shoot, which is requisite for sequestration, biotransformation, or volatilization, occurs predominantly via symplastic movement through the xylem and phloem [See Kvesitadze et al. (2015) and references therein]. Membrane transport proteins such as ATP-binding cassette (ABC), P-type ATPases, natural resistance-associated macrophage proteins (Nramps), and ZIP (ZRT, IRT-like proteins) families of proteins mediate the translocation of metal(loid)s [see reviews by Thakur et al. (2016) and (Yan et al. 2020)]. Therefore, genetic and epigenetic factors that foster the induction, expression, and activities of these transporters enhance contaminant uptake and sequestration and, in effect, the phytoremediation efficiencies of such species.
High Biomass Production Capacities
The ability to produce significantly high biomass, especially under stress conditions that characterize polluted sites, translate into better phytoremediation efficiencies in remediation plants (Sheoran et al. 2016; Guidi Nissim et al. 2018; Kafle et al. 2022). Several independent research groups have demonstrated that the application of different fertilizers to boost biomass production in phytoremediation species translated into higher phytoextraction efficiencies [See review by Sheoran et al. (2016)]. These observations of positive correlations between high biomass and increased efficiencies of plants in the phytoremediation of heavy metals (Hamlin and Barker 2006; Sheoran et al. 2016) spurred the development of phytoremediation strategies that are assisted by plant growth promoting bacteria (Ullah et al. 2015; Kong and Glick 2017; Mesa-Marín et al. 2020) as well as the use of organic and inorganic soil amendments, e.g., compost, biochar, and others, which boost vegetative growth in plants (Shrestha et al. 2019; Gul et al. 2020; Kafle et al. 2022).
The Efficiency of Tolerance and Detoxification Mechanisms
Resistance to metal(loid)s and organic contaminants in plants is achieved by either avoidance and/or tolerance strategies (Toth and Pavia 2000; Yan et al. 2020). Avoidance strategies employ exclusion mechanisms that limit pollutant uptake and accumulation to restrict their movement into plant tissues through root cells. These can be achieved via the alteration of membrane permeability, extracellular degradation of organopollutants, active efflux of metal(loid)s, and production of extracellular or cell wall-associated metal(loid) chelating compounds (Thakur et al. 2016). Tolerance strategies include several internal detoxification mechanisms, such as the binding of metal(loid)s by ligands and compartmentalization within cells or in vivo enzymatic degradation of absorbed organopollutants.
Plants immediately detoxify accumulated metal(loid)s through oxidation, reduction, chelation, or a combination of these processes (Duan et al. 2005; Bleeker et al. 2006; Pilon-Smits and Quinn 2010; Li et al. 2015; Pilon-Smits 2017). After oxidation to the appropriate oxidation state, complexation of metal(loid) ions occurs either spontaneously or enzymatically to organic ligands such as amino acids, organic acids, metallothioneins, phytochelatins, and cell wall proteins/pectins/phenolics (Tahara et al. 2013; Yan et al. 2020). These chelates are then compartmentalized in vacuoles or apoplast or specialized cell types, e.g., epidermal cells, mesophyll cells, and trichomes (Javed et al. 2019). For instance, tolerance to high Al levels was mediated via Al chelation by organic acids (e.g., oxalate, malate, and citrate) in Al-resistant buckwheat and Hydrangea in contrast to the complexation of Al with phenolics in maize, followed by compartmentalization in vacuoles (Yan et al. 2022). Also, in Eucalyptus camaldulensis, Al tolerance is mediated via oenothein B, a gallate-derived polyphenol, to sequester and precipitate Al in the vacuoles (Tahara et al. 2013). Ligands like glutathione, phytochelatins, and metallothioneins proficiently detoxify As due to the strong affinity of As3+ for peptides with sulfhydryl (—SH) groups (Roy et al. 2021).
On the other hand, the detoxification of organic pollutants is primarily mediated through enzymatic transformations. For instance, oxidases (e.g., cytochrome P450 monooxygenases, peroxidases, and laccase), dehalogenases, and esterases (e.g., phosphatases, carboxylesterases, and arylesterases) metabolize a diverse class of phytotoxic exogenous compounds including pesticides and organic environmental pollutants via functionalization while transferases (e.g., glutathione S-transferase, N-glucosyltransferase, O-glucosyl-transferase) detoxify pollutants via conjugation (Wolfe and Hoehamer 2003; Kvesitadze et al. 2015). Therefore, the higher the quantity of specific enzymes produced and the more diverse the enzyme classes a species possesses, the more efficient it is in degrading organic contaminants in its environment. Although plants possess these innate abilities to detoxify organic pollutants, complete detoxification of organic pollutants (i.e., total mineralization or degradation to cell standard metabolites) may not always be possible due to insufficient enzyme capacity, extreme resistance of pollutants to biotransformation (e.g., polycyclic aromatic hydrocarbons, and polyhalogenated biphenyls), or their high concentration in the environment (Kvesitadze et al. 2015). These have necessitated the development of transgenic plants, which carry genes of some enzymes from other organisms or the overexpression of the genes that encode some important enzymes for enhanced phytoremediation efficiencies.
A critical effect of heavy contaminant levels in the environment and the bioaccumulation of these contaminants by root tissues is the induction of ROS and reactive nitrogen species (RNS) production and accumulation of ROS up to deleterious levels. For instance, heavy metal(loid) contamination induces excessive ROS production (Georgiadou et al. 2018; Berni et al. 2019; Singh et al. 2020), thereby upsetting cell and tissue redox balance in plants. Similarly, oxidative stress in plants exposed to organic pollutants has been attributed to excessive ROS and RNS production (Kreslavski et al. 2014; Ahammed et al. 2015). As a result, plant antioxidant and redox balancing/regulatory systems feature prominently in enhancing plant tolerance to environmental contamination and ameliorating the phytoremediation efficiencies of such plants. Several studies have demonstrated that the deleterious effects of heavy metal(loid)s and organic pollutants can be mitigated via the activation of innate antioxidant systems, regulation of ROS levels, and promotion of redox homeostasis (Ahammed et al. 2015, 2017; Singh et al. 2020; Małecka et al. 2021; Yan et al. 2022).
Quantity and Composition of Root Exudates
Root exudates, and other rhizodeposits, play crucial roles in the ability of plants to remove both organic and inorganic contaminants from contaminated soils. This is primarily due to the significant influences that root exudates exert in shaping rhizosphere ecology. Although the composition of root exudates varies between species, significant components of root exudates include ions (especially H+), saccharides (Koroney et al. 2016; Galloway et al. 2020), and organic acids, such as amino, citric, malic, malonic, and oxalic acids (Lesuffleur et al. 2007; Canarini et al. 2019). Others include hormones, e.g., auxins, salicylic acid, and strigolactones (Zhalnina et al. 2018; Omoarelojie et al. 2019), peptide ligands (Ma et al. 2016), secondary metabolites, e.g., phenolics, flavonoids, and terpenoids (Zhalnina et al. 2018; Huang et al. 2019), phytosiderophores (Chen et al. 2017), and extracellular enzymes, e.g., hydrolases, oxidoreductases, and phosphatases (Gianfreda 2015). Due to the diverse bioactivities of root exudate components, they influence the rhizosphere in diverse ways to augment the phytoremediation efficiencies of plants. These, among many others, include:
Modulation of Soil pH
Protons and organic acids in root exudates facilitate rhizosphere acidification, thereby fostering metal bioavailability and sequestration via the solubilization of insoluble metal complexes (Ma et al. 2016).
Nutrient Sources
Saccharide, carboxylate, and amino acid components of root exudates serve as carbon/energy and nitrogen sources for rhizospheric microbiomes. Thus, plant roots (via rhizodeposition and root exudation) create nutrient-rich environments, which support microbial activity in the rhizosphere and, in turn, foster rhizodegradation and microbiota-mediated immobilization of contaminants (Lu et al. 2017; Zhalnina et al. 2018).
Modulation of Pollutant Bioavailability
The availability for plant uptake, especially metal(loid)s, can be tuned by root exudates through solubilization, precipitation, and chelation. Low molecular weight organic acids may solubilize and mobilize otherwise insoluble metals, e.g., Fe and Zn, thus making them available for uptake (Chen et al. 2017; Jiang et al. 2022). These organic acids also facilitate the precipitation and desorption of some heavy metals, e.g., Al, As, Cd, Cu, and Pb, from soils, thus rendering them immobile and unavailable (Agnello et al. 2014; Chen et al. 2017). Metal(loid)s mobility and bioavailability can be altered by root exudate components through chelation—complexation with organic acids, phenolics, peptide ligands (such as phytochelatins and metallothioneins) (Tahara et al. 2013; Yan et al. 2020), as well as the sequestration of metal-siderophore complexes in root apoplasts or soils (Ma et al. 2016).
Molecular Signalling
Some components of root exudate function as molecular cues that signal plant–soil microbiota interactions. For example, flavonoids, triterpenes, and strigolactones present in root exudates elicit microbial growth and modulate microbial activity in the rhizosphere (Philippot et al. 2013; Huang et al. 2019; Omoarelojie et al. 2019).
Enzymes—released into the rhizosphere via root exudation, play a crucial role in the remediation of biodegradable organic and inorganic contaminants. Enzymes mediate the oxidation, reduction, functional group conversions, or polymerization that transform toxic metals and organic contaminants into non-toxic forms [See review by Jacob et al. (2018)]. Important enzyme classes that occur in root exudates and with significant roles in phytoremediation include oxidoreductases, such as dehydrogenases, oxygenases, laccases, and peroxidases (Wolfe and Hoehamer 2003; Kvesitadze et al. 2015; Jacob et al. 2018); and hydrolytic enzymes such as cellulases, dehalogenases, esterases, lipases, nitrilases, and proteases (Wolfe and Hoehamer 2003; Tu et al. 2011; Kvesitadze et al. 2015; Jacob et al. 2018; Zhao et al. 2018).
Phytoremediation Strategies
A variety of phytoremediation approaches have been developed to reclaim soils that are contaminated with different classes of pollutants. Soil pollutants are diverse in their chemical nature, and these range from polycyclic aromatic hydrocarbons (PAHs), petroleum, organophosphorus and carbamate insecticides, herbicides, fungicides, polychlorinated/polybrominated biphenyls and dibenzofurans to biowastes (sewage and animal wastes) and heavy metals (Khalid et al. 2017; Duarte et al. 2018; Alengebawy et al. 2021; Raklami et al. 2022). Figure 8 depicts a graphical summary of different phytoremediation strategies.
Phytodegradation or Phytotransformation
This phytoremediation strategy involves plant-mediated elimination of organic and biodegradable soil contaminants via metabolic processes within plant tissues or the activities of extracellular enzymes released into the soil (Schwitzguébel 2017). These processes make the most of the diversity of plant metabolic pathways and enzymes to chemically transform, thereby partially or totally degrading, xenobiotics to detoxify the soil. The end products of degradation, which are non-toxic, may be assimilated or released into the environment. For instance, species such as alfalfa (Medicago sativa), Brassica campestris, white clover (Trifolium repens), and some aquatic species have been shown to degrade two polycyclic aromatic hydrocarbons, phenanthrene and pyrene (Wei and Pan 2010; He and Chi 2016). Also, phytodegradation of polychlorinated biphenyls has been reportedly observed in Medicago sativa and some cucurbit species (Tu et al. 2011; Wyrwicka et al. 2014). Some important specific enzyme classes that feature in phytodegradation processes include: dehalogenases—which mediate the dehalogenation of a wide variety of halogenated pollutants (Zhao et al. 2018); laccases—which catalyze the transformation of phenolics such as azo dyes and bisphenol A into less toxic compounds (Sridharan et al. 2021; Xing et al. 2022); nitrilases—degrade cyanide and nitrile group-containing compounds (Park et al. 2017); nitroreductases—catalyze the reduction of aromatic nitro groups (Wolfe and Hoehamer 2003; Hannink et al. 2007); oxidoreductases (Hazarika et al. 2022); phosphatases—hydrolyze ester and anhydride linkages of organophosphate compounds (Wolfe and Hoehamer 2003).
Phytoextraction
This is also called phytoaccumulation, phytoabsorption, or phytosequestration and it is a phytoremediation strategy that employs in situ cultivation of plant species that take up the target contaminant(s) from the soil, translocate, and concentrate them in aerial tissues thereby depleting the soil concentration of the contaminant(s). The contaminant-laden biomass is then harvested and removed for treatments such as composting, incineration, pyrolysis, gasification, and phytomining (Corzo Remigio et al. 2020; Singh et al. 2022). Phytoaccumulation is mainly employed for the remediation of soils that are polluted with heavy metals (e.g., Ag, Cd, Cr, Cu, Hg, Ni, Pb, and Zn) and metalloids (e.g., As, Se, Sb, and Te) (Ogra et al. 2015; Reeves et al. 2018). A group of plant species, known as hyperaccumulators, preferentially accumulate metal(loid)s in their shoots over a wide range of concentrations in the growth medium without showing any noticeable adverse effects or symptoms that depict toxicity (Corzo Remigio et al. 2020). Hyperaccumulators are able to accumulate metal(loid)s up to hundreds or thousands of times greater than normal when grown in soils with high levels of metal contaminants (Suman et al. 2018). Some species which have been established as hyper accumulators include Sedum alfredii (Tian et al. 2017), Pteris vittata (Kohda et al. 2021), Haumaniastrum robertii (Bastien et al. 2017), Berkheya coddii (Hipfinger et al. 2022), and Astragalus bisulcatus (Pilon-Smits 2017). In addition to effective metal(loid) uptake and accumulation, metallotolerance [when grown in soils with toxic metal(loid)s levels], fast growth, and high biomass production are essential traits in candidate species for phytoextraction efforts. For instance, non-accumulator species such as Salix sp., Populus sp., and Zea mays have been assessed for the phytoextraction of Cd and Zn with significant success (Ghori et al. 2016). These have been attributed to rapid growth, high biomass production, and Cd tolerance in Z. mays (Rizwan et al. 2017) while a high biomass production in relatively short time, a high water uptake and transpiration, and deep root system accounted for the phytoextraction efficiencies of Salix and Populus sp. (Suman et al. 2018).
Phytofiltration
This involves the in situ use of plants to absorb, concentrate, and/or precipitate contaminants, usually heavy metals, from aqueous medium (Olguín and Sánchez-Galván, 2011; Favas et al. 2016). Hydroponically grown plants are transplanted into metal-laden water, and the heavy metals are absorbed or adsorbed through the roots (rhizofiltration) or other submerged parts of the plants and concentrated in their biomass (Olguín and Sánchez-Galván, 2011; Jacob et al. 2018). Phytofiltration is also aided by root exudates and root-mediated alterations in the rhizosphere, which fosters the precipitation [or chelation with ligands, such as phytochelatins, metallothioneins, metal-binding proteins (Olguín and Sánchez-Galván, 2012; Favas et al. 2016)] of heavy metals from solution and adsorption of heavy metal precipitates and/or chelates onto root surfaces (Javed et al. 2019). This phytoremediation strategy is particularly suited for wastewater treatment, polluted water bodies, and wetlands. Candidate species for phytofiltration are required to possess dense root systems with high biomass production capacities; high absorption and adsorption surface areas; ability to grow efficiently in hydroponic/aquatic systems; high metal accumulation and/or adsorption capacities; a high root-to-shoot translocation factor; as well as significant metallotolerance levels (Olguín and Sánchez-Galván, 2011; Kaur 2020). Some aquatic and wetland species which have been employed and documented for their phytofiltration applications include species of Eichhornia, Salvinia, Pistia, Lemna, Phragmites, Scirpus, Monosoleum and Potamogeton (Rezania et al. 2016; Javed et al. 2019; Sut-Lohmann et al. 2020).
Phytostabilization or Phytoimmobilization
This strategy employs tolerant plant species for in situ immobilization of soil pollutants, mainly heavy metal(loid)s, thereby reducing their bioavailability and mobility in the contaminated soil and ecosystem. This is achieved via root absorption (and sequestration within root tissues) or adsorption (onto root apoplasts), exudate complexation/precipitation, rhizospheric reduction of metal valence, and soil stabilization (Liu et al. 2018; Yan et al. 2020). Phytostabilization is simply a plant-mediated containment approach as it does not diminish the soil concentration of the pollutant but mainly contains them and prevents off-site movement, leaching, and dispersal of the contaminant (Khalid et al. 2017). Candidate species for phytostabilization must be tolerant to high metal(loid) concentrations; fast growing with high biomass production capacities; minimal root-to-shoot translocation of metal(loid)s (i.e., metal excluder); and produce dense/extensive root systems since the root is pivotal in phytostabilization (Galal et al. 2017; Burges et al. 2018). Since root exudates and microorganisms facilitate phytostabilization via metal precipitation, chelation, and immobilization (Favas et al. 2016; Ma et al. 2016; Yan et al. 2020), root exudate composition and the ability to stimulate microbial activities are also essential factors in the selection of species for phytostabilization efforts. Phytostabilization is particularly suitable for soils contaminated by a mixture of different metal(loid)s (e.g., Al, As, Cd, Cr, Cu, Ni, Pb, Sb, and Zn). Some species that have been employed for phytostabilization include Agrostis sp. (Burges et al. 2018), Festuca rubra L. (Radziemska et al. 2017), Lolium perenne (Burges et al. 2018), Salix sp. (Sylvain et al. 2016; Burges et al. 2018), and Vossia cuspidate (Galal et al. 2017).
Phytovolatilization
This involves root uptake, transport to aerial parts, and transformation of toxic metal(loid)s into non-toxic, gaseous forms, which are then released into the atmosphere via the leaves. Metal(loid)s, such as Se, Hg, and As, are assimilated into volatile organic compounds and released via transpiration. For instance, arsenate (As5+), the prevalent form of arsenic in oxic soils/environments, is absorbed through the phosphate uptake pathway via active transport by phosphate transporters (Zhao et al. 2009; Li et al. 2015). Passive uptake of arsenite (As3+), the prevalent form in reduced environments such as flooded paddies, occurs via the plasma membrane by aquaporins/aquaglyceroporins (Ma et al. 2008). The absorbed As5+ is detoxified by enzymatic or non-enzymatic reduction to As3+ in processes involving As5+ reductase activity (Duan et al. 2005; Bleeker et al. 2006) or glutathione (Delnomdedieu et al. 1994; Bleeker et al. 2006), respectively. Due to the high affinity of As3+ for sulphhydryl groups of peptides, As3+ is sequestered by complexation with GSH and phytochelatins (Zhao et al. 2009). The volatilization of As3+ is believed to be achieved via the actions of As3+-S-adenosyl methionine methyltransferase homologs, which mediates the methylation of As3+ to form the volatile trimethylarsine (Roy et al. 2021). Phytovolatilization of Se, as observed in some species of the Brassicaceae family, occurs through the uptake and assimilation of inorganic Se into organic selenoamino acids, i.e., selenocysteine and selenomethionine, via sulfur transporters and assimilation pathways (Li et al. 2008; Pilon-Smits and Quinn 2010). Selenocysteine and selenomethionine are methylated via the catalytic actions of methyltransferases (Pilon-Smits 2017) and further converted into dimethyl selenide or dimethyl diselenide, which are volatile and released from the leaves into the atmosphere (Gupta and Gupta 2016). Organic contaminants may also be removed from polluted soils and ground water through phytovolatilization. For example, common groundwater contaminants, trichloroethylene and tetrachloroethylene, have been removed using traditional phytoremediation plants such as Salix and Populus species [See review by Limmer and Burken (2016)].
Phytostimulation
This is also known as rhizodegradation, and it is a phytoremediation strategy that uses plant-mediated stimulation of microbial activities in the rhizosphere to promote the degradation of organic pollutants and decrease or increase the bioavailability of toxic metal(loid)s to foster exclusion from plant tissue or uptake from the soil, respectively. Plants, through rhizodeposition and root exudation of assimilates, create nutrient-rich environments around their roots, thus attracting and fostering microbial growth, colonization, and activity (Meier et al. 2020). For instance, Zhalnina et al. (2018) demonstrated that a combination of traits associated with plant exudation and microbial substrate uptake interacted to yield the patterns of microbial community assembly observed in the rhizosphere of Avena barbata. Similarly, root exudates were noted to have enhanced the abundance of metal-reducing bacteria Geobacter and Anaeromyxobacter in paddy soils (Jiang et al. 2022). It is clear that the diverse classes of compounds in root exudates act individually and/or synergistically to shape rhizospheric microbiota, which, in turn, contribute to the degradation and remediation of contaminated soils.
Soil microbes contribute to phytoremediation in a number of ways. In the microbe-assisted phytoremediation of metal(loid)s, rhizospheric microbes can foster metal(loid) mobilization and bioavailability. This may be achieved through: (i) soil pH modification—rhizosphere acidification and protonation by influencing the quantity of root exudates (Chen et al. 2014), proton export (Ma et al. 2016), and/or organic acid export into the rhizosphere (Rajkumar et al. 2012); and (ii) metal(loid) chelation—microbes produce, or induce root cells to secrete, organic chelators, such as metallophores, metallothioneins, phytochelatins, and siderophores, which bind metal(loid)s and facilitate uptake into root tissues (Bolchi et al. 2011; Deicke et al. 2013; Yuan et al. 2014). On the other hand, rhizospheric microbes can facilitate metal(loid) immobilisation and decrease bioavailability by one or more of the following mechanisms: (i) alkalinization—through the release of (OH−); (ii) complexation and biosorption via glomalin and/or microbe-derived extracellular polymeric substances (Rajkumar et al. 2012; Ma et al. 2016; Costa et al. 2018; Riaz et al. 2021); and (iii) via precipitation and immobilisation—through enzyme-catalysed microbial reduction or oxidation of metal(loid)s (Chatterjee et al. 2009; Rajkumar et al. 2012; Oves et al. 2013; Jiang et al. 2022).
Microorganisms possess a plethora of biochemical pathways with diverse classes of enzymes that are able to degrade different classes of organic pollutants. Plants use their root exudates to facilitate microbe-mediated degradation of organic pollutants. For instance, in demonstrating the role of root exudates in the degradation of pyrene, Lu et al. (2017) showed that artificial root exudate components altered soil microbial community and significantly enhanced Mycobacterium activity which was dominant in the degradation of pyrene. In addition, the detoxification of polychlorinated biphenyls has been demonstrated in several dehalogenation microbes, e.g., some species of Thermithiobacillus, Pseudomonas, Burkholderia, Comamonas, Rhodococcus, Phenylobacterium, and Sphingomonas [See review by Jing et al. (2018)]. Further demonstrating a plant-induced stimulation of soil microbiota for contaminant degradation, Allamin et al. (2020) showed that Cajanus cajan enhanced the diversity of soil microbial community and fostered the rhizodegradation of petroleum oil sludge in the contaminated soil.
Do Phlorotannins Hold Benefits for Phytoremediation?
The concept of employing phlorotannins to enhance phytoremediation efficiencies via the incorporation of phlorotannin treatment regimes into phytoremediation strategies is a subject that has received little to no research attention. Here we suggest a number of candidate physiological and developmental aspects of phytoremediation species where phlorotannins may feature to boost phytoremediation efficiencies of such species. Figure 9 is a graphical highlight of potential research areas/questions that must be addressed to evaluate the potential benefits of incorporating phlorotannin treatment regimes into phytoremediation strategies, as discussed below.
As mentioned above, phlorotannins promote extensive root growth and development, thereby fostering an increase in total root surface area and biomass. This enables the plant to reach more areas and increase the total soil volume explored within the rhizosphere for nutrient and water uptake. An advantage of this phlorotannin bioactivity for phytoremediation efforts is the potential to enhance the total volume of polluted soil/medium explored and pollutant uptake by roots of phytoremediation species, thereby boosting pollutant mobilization, uptake, and removal, which ultimately promote phytoremediation efficiency.
Another direct implication of enhanced root growth and development is an increase in root exudation and rhizodeposition (Meier et al. 2020; Virk et al. 2022). Root exudation of primary and secondary metabolites, the deposition of polymerized sugars (e.g., mucilage), root border cells, and dead root cap cells influence the composition and functioning of soil biota (Philippot et al. 2013; Eisenhauer et al. 2017). Through root exudates and other rhizodeposits, plants furnish soil microorganisms in the rhizosphere with an easily accessible and important energy source as well as modify the physicochemical properties (i.e., pH, oxygen pressure, and water status) of the soil (Gargallo-Garriga et al. 2018; Jiang et al. 2022). In the light of phlorotannin-mediated augmentation of root growth and development, research into the question of whether phlorotannins can directly or indirectly enhance root exudation and rhizodeposition to augment the phytoremediation capacities of plant species becomes pertinent.
Root exudates contain secondary metabolites that function as chemical signals, antimicrobial compounds, and nematicides, thereby playing significant roles in the establishment of symbiosis or in warding off pathogens and pests in the rhizosphere (Faure et al. 2009; Philippot et al. 2013; Gargallo-Garriga et al. 2018). Soil microbes have been shown to increase [phytoextraction (Guarino et al. 2018)] or decrease metal availability [phytostabilization (Lebrun et al. 2021)] in soils. Also, metal translocation from soil into root tissues (bioaccumulation) or from the roots to shoot tissues (translocation) is significantly influenced by soil microbiota (Singh et al. 2019; Xu et al. 2019; Zhan et al. 2019). These observations further demonstrate that root exudation and rhizodeposition are important in phytoremediation because of their significant influence on soil microbiota. Reports of how phlorotannins may influence root exudation and rhizodeposition are, to our knowledge, not readily available. However, it is safe to infer from available reports of phlorotannin bioactivities in enhancing root growth and development that phlorotannins may indirectly enhance the volume of exudates and rhizodeposits that are released into the rhizosphere and thereby stimulate soil microbial growth and activities. The ideas that phlorotannins may influence root exudation and rhizodeposition, the mechanisms that underpin such bioactivities if found to exist, are research subjects that must be addressed to provide a scientific basis for the development of feasible phlorotannin-assisted phytoremediation strategies.
Metal(loid) avoidance and tolerance are critical traits for the success of any species in the remediation of heavy metal(loid)-polluted sites. It is therefore important to determine if, and how phlorotannins influence heavy metal chelation capacities of candidate species for phytoremediation. Are phlorotannins able to induce or augment the biosynthesis of low molecular weight organic acids, phytosiderophores, phytochelatins, and metallothioneins in phytoremediation species? All of these play important roles in metal(loid) chelation and plant tolerance to high metal(loid) pollution in soils (Philippot et al. 2013). Eckol has been seen to enhance photosynthetic activities and primary metabolism (Kulkarni et al. 2019). Can this translate into increased availability of photosynthate for release into the rhizosphere as carboxylates in root exudates to enhance phytostabilization and rhizodegradation? Phytochelatins, peptides with repeating γ-GluCys dipeptide units, are synthesized from glutathione in an enzymatic reaction catalyzed by phytochelatin synthase. At the same time, metallothioneins, low molecular weight, cysteine-rich, metal-binding proteins, are products of mRNA translation (Cobbett and Goldsbrough 2002). Can phlorotannins exert modulatory effects on the expression and biosynthesis of these chelators to promote tolerance to heavy metal levels in candidate phytoremediation plants? These are critical research questions that must be addressed to determine if there are avenues to be explored in the incorporation of phlorotannins as biostimulants in phytoremediation strategies.
In addition, phenolics are known to bind and mobilize metals, e.g., Fe, from the soil (Chen et al. 2017). As discussed above, heavy metal(loid) mobilization from soil/water and uptake by root cells are pivotal processes in the clean-up of metal(loid) contamination through phytoextraction, phytovolatilization, and phytofiltration. It has been noted that eckol enhanced phenylalanine ammonia-lyase activities (Kulkarni et al. 2019), an enzyme of the phenylpropanoid pathway which produces precursors for the biosynthesis of phenolic compounds and a diverse class of secondary metabolites (Rasool et al. 2021). This observation raises the question of whether eckol, and other classes of phlorotannins, can enhance phenolic metabolic production to boost metal binding, mobilization, and uptake from the soil in phytoremediation species.
As discussed above, the deleterious and toxic effects that different classes of environmental pollutants exert on plants necessitates the possession of efficient antioxidant and redox homeostasis regulatory systems in phytoremediation species. These are critical for contaminant avoidance and/or tolerance, phytoremediation species survival under pollutant-induced toxicity and stresses, and, ultimately, optimum decontamination of polluted sites. A robust and efficient antioxidant capacity is one of the significant beneficial bioactivities of phlorotannins (Li et al. 2011; Rengasamy et al. 2013; Choi et al. 2015; Bogolitsyn et al. 2019). Thus, phlorotannins hold significant promises for boosting phytoremediation species decontamination mechanisms through phlorotannin-mediated augmentation of plant antioxidant capacities and redox homeostasis maintenance. As with most other phlorotannin bioactivities, the ability of phlorotannins to improve plant tolerance to oxidative and abiotic stresses and the underlying biochemical and molecular mechanisms are yet to be exhaustively researched. Hence, it is crucial to assess/explore these as a starting point to providing foundational knowledge for applying phlorotannins to augment phytoremediation species tolerance and survival.
The uptake of pollutants from the soil (or water body during phytofiltration), their transportation within root tissues, and translocation from tissues of the root to shoot tissues have been earlier highlighted as critical physiological processes with significant implications for phytoremediation success. If the bioactivities of phlorotannins are to be explored to enhance the phytoremediation efficiencies of species, it is also important to evaluate and assess how phlorotannins influence these processes. Can phlorotannins enhance root uptake of metal(loid)s and organopollutants? The mobilization and uptake of pollutants from the soil/water medium into root cells/tissues is mediated via either apoplastic, symplastic, or both pathways (Zhan et al. 2018). Can phlorotannins exert ameliorative influence(s) on these pollutant acquisition and transport strategies? Metal(loid)s are taken into cells via membrane-localized channels/transport proteins (Li et al. 2015; Gupta and Gupta 2016; Pilon-Smits 2017). Also, can phlorotannins alter and boost the genetic expression and activities of these channels? Are phlorotannins able to alter the expression or boost the activities of metal transporters, such as the P-type ATPases, ATP-binding cassette (ABC) superfamily, natural resistance-associated macrophage proteins (Nramps), and ZIP family of transporters [see review by Thakur et al. (2016)], that mediate the transport of a wide range of cations across the cell membranes?
Efficient root-to-shoot transport of metal(loid)s is crucial for phytoextraction and phytofiltration species. Hence, it is essential to determine if phlorotannins are able to enhance the inter-tissue and aerial movement of absorbed metal(loid)s. If found to do so, what are the biochemical and molecular mechanisms that underlie such bioactivities? Other vital traits highlighted above are the efficient exclusion of contaminants from cells/tissues and active efflux mechanisms of the plasma membrane to prevent the accumulation of metal(loid) ions in the cytosol as part of plants’ avoidance strategies. These are crucial for phytoremediation efficiency in phytostabilization species. Are phlorotannins able to augment phytoremediation species’ contaminant avoidance strategies via the alteration of innate underlying biochemical and molecular mechanisms?
Conclusions
Due to their diverse bioactivities, phlorotannins hold immense value for enhancing innate detoxification and survival mechanisms in phytoremediation species as well as contaminant removal capacities thus ameliorating their phytoremediation efficiencies. Since pollutant removal efficiency is highly correlated to species growth rate, tolerance to supra-optimal metal(loid) or pollutant levels, and high-level adaptivity to different environments, it is crucial to garner research data on how phlorotannins may influence each of these processes. Specific morphological and physiological areas of plant development where phlorotannins may act to achieve the above include vegetative growth and biomass accumulation, extensive root development for rhizodeposition, microbiota stimulation, and pollutant mobilization/uptake, detoxification mechanisms, redox homeostasis and antioxidative stress prevention, and metal(loid) accumulation and transport, etc. Further research will also be required to delineate how the different classes of phlorotannins influence each of the afore mentioned areas of plant development and metabolic adaptations as well as how phlorotannins that elicit sufficient bioactivities may be further incorporated into phytoremediation strategies.
Data Availability
Data sharing is not applicable to this article as no new data were created or analyzed.
References
Abe I, Morita H (2010) Structure and function of the chalcone synthase superfamily of plant type III polyketide synthases. Nat Prod Rep 27:809–838. https://doi.org/10.1039/B909988N
Achkar J, Xian M, Zhao H, Frost JW (2005) Biosynthesis of phloroglucinol. J Am Chem Soc 127:5332–5333. https://doi.org/10.1021/ja042340g
Agnello AC, Huguenot D, Van Hullebusch ED, Esposito G (2014) Enhanced phytoremediation: a review of low molecular weight organic acids and surfactants used as amendments. Crit Rev Environ Sci Technol 44:2531–2576. https://doi.org/10.1080/10643389.2013.829764
Ahammed GJ, He B-B, Qian X-J, Zhou Y-H, Shi K, Zhou J, Yu J-Q, Xia X-J (2017) 24-Epibrassinolide alleviates organic pollutants-retarded root elongation by promoting redox homeostasis and secondary metabolism in Cucumis sativus L. Environ Pollut 229:922–931. https://doi.org/10.1016/j.envpol.2017.07.076
Ahammed GJ, Li X, Xia X-J, Shi K, Zhou Y-H, Yu J-Q (2015) Enhanced photosynthetic capacity and antioxidant potential mediate brassinosteriod-induced phenanthrene stress tolerance in tomato. Environ Pollut 201:58–66. https://doi.org/10.1016/j.envpol.2015.02.024
Alengebawy A, Abdelkhalek ST, Qureshi SR, Wang MQ (2021) Heavy metals and pesticides toxicity in agricultural soil and plants: ecological risks and human health implications. Toxics 9:42. https://doi.org/10.3390/toxics9030042
Allamin IA, Halmi MIE, Yasid NA, Ahmad SA, Abdullah SRS, Shukor Y (2020) Rhizodegradation of petroleum oily sludge-contaminated soil using Cajanus cajan increases the diversity of soil microbial community. Sci Rep 10:4094. https://doi.org/10.1038/s41598-020-60668-1
Aremu AO, Masondo NA, Rengasamy KRR, Amoo SO, Gruz J, Bíba O, Šubrtová M, Pěnčík A, Novák O, Doležal K, van Staden J (2015) Physiological role of phenolic biostimulants isolated from brown seaweed Ecklonia maxima on plant growth and development. Planta 241:1313–1324. https://doi.org/10.1007/s00425-015-2256-x
Bartucca ML, Cerri M, Del Buono D, Forni C (2022) Use of biostimulants as a new approach for the improvement of phytoremediation performance-a review. Plants (basel, Switzerland) 11:1946. https://doi.org/10.3390/plants11151946
Berni R, Luyckx M, Xu X, Legay S, Sergeant K, Hausman J-F, Lutts S, Cai G, Guerriero G (2019) Reactive oxygen species and heavy metal stress in plants: impact on the cell wall and secondary metabolism. Environ Exp Bot 161:98–106. https://doi.org/10.1016/j.envexpbot.2018.10.017
Bian F, Zhong Z, Wu S, Zhang X, Yang C, Xiong X (2018) Comparison of heavy metal phytoremediation in monoculture and intercropping systems of Phyllostachys praecox and Sedum plumbizincicola in polluted soil. Int J Phytorem 20:490–498. https://doi.org/10.1080/15226514.2017.1374339
Birkemeyer C, Lemesheva V, Billig S, Tarakhovskaya E (2020) Composition of intracellular and cell wall-bound phlorotannin fractions in fucoid algae indicates specific functions of these metabolites dependent on the chemical structure. Metabolites 10:369. https://doi.org/10.3390/metabo10090369
Bleeker PM, Hakvoort HWJ, Bliek M, Souer E, Schat H (2006) Enhanced arsenate reduction by a CDC25-like tyrosine phosphatase explains increased phytochelatin accumulation in arsenate-tolerant Holcus lanatus. Plant J 45:917–929. https://doi.org/10.1111/j.1365-313X.2005.02651.x
Bogolitsyn K, Druzhinina A, Kaplitsin P, Ovchinnikov D, Parshina A, Kuznetsova M (2019) Relationship between radical scavenging activity and polymolecular properties of brown algae polyphenols. Chem Pap 73:2377–2385. https://doi.org/10.1007/s11696-019-00760-7
Bolchi A, Ruotolo R, Marchini G, Vurro E, di Toppi LS, Kohler A, Tisserant E, Martin F, Ottonello S (2011) Genome-wide inventory of metal homeostasis-related gene products including a functional phytochelatin synthase in the hypogeous mycorrhizal fungus Tuber melanosporum. Fungal Genet Biol 48:573–584. https://doi.org/10.1016/j.fgb.2010.11.003
Budhiyanti SA, Raharjo S, Marseno DW, Lelana IYB (2011) Free radical scavenging, metal chelating and singlet oxygen quenching activity of fractionated brown seaweed Sargassum hystrix extract. J Biol Sci 11:288–298. https://doi.org/10.3923/jbs.2011.288.298
Burges A, Alkorta I, Epelde L, Garbisu C (2018) From phytoremediation of soil contaminants to phytomanagement of ecosystem services in metal contaminated sites. Int J Phytorem 20:384–397. https://doi.org/10.1080/15226514.2017.1365340
Canarini A, Kaiser C, Merchant A, Richter A, Wanek W (2019) Root exudation of primary metabolites: mechanisms and their roles in plant responses to environmental stimuli. Front Plant Sci 10:00157. https://doi.org/10.3389/fpls.2019.00157
Catarino MD, Fernandes I, Oliveira H, Carrascal M, Ferreira R, Silva AMS, Cruz MT, Mateus N, Cardoso SM (2021) Antitumor activity of Fucus vesiculosus-derived phlorotannins through activation of apoptotic signals in gastric and colorectal tumor cell lines. Int J Mol Sci 22:7604. https://doi.org/10.3390/ijms22147604
Catarino MD, Silva AMS, Cardoso SM (2017) Fucaceae: a source of bioactive phlorotannins. Int J Mol Sci 18:1327. https://doi.org/10.3390/ijms18061327
Cérantola S, Breton F, Gall EA, Deslandes E (2006) Co-occurrence and antioxidant activities of fucol and fucophlorethol classes of polymeric phenols in Fucus spiralis. Bot Mar 49:347–351. https://doi.org/10.1515/BOT.2006.042
Chatterjee S, Sau GB, Mukherjee SK (2009) Plant growth promotion by a hexavalent chromium reducing bacterial strain, Cellulosimicrobium cellulans KUCr3. World J Microbiol Biotechnol 25:1829–1836. https://doi.org/10.1007/s11274-009-0084-5
Chen B, Zhang Y, Rafiq MT, Khan KY, Pan F, Yang X, Feng Y (2014) Improvement of cadmium uptake and accumulation in Sedum alfredii by endophytic bacteria Sphingomonas SaMR12: effects on plant growth and root exudates. Chemosphere 117:367–373. https://doi.org/10.1016/j.chemosphere.2014.07.078
Chen Y-T, Wang Y, Yeh K-C (2017) Role of root exudates in metal acquisition and tolerance. Curr Opin Plant Biol 39:66–72. https://doi.org/10.1016/j.pbi.2017.06.004
Choi JS, Haulader S, Karki S, Jung HJ, Kim HR, Jung HA (2015) Acetyl- and butyryl-cholinesterase inhibitory activities of the edible brown alga Eisenia bicyclis. Arch Pharmacal Res 38:1477–1487. https://doi.org/10.1007/s12272-014-0515-1
Cobbett C, Goldsbrough P (2002) Phytochelatins and metallothioneins: roles in heavy metal detoxification and homeostasis. Annu Rev Plant Biol 53:159–182. https://doi.org/10.1146/annurev.arplant.53.100301.135154
Connan S, Stengel DB (2011) Impacts of ambient salinity and copper on brown algae: 2. Interactive effects on phenolic pool and assessment of metal binding capacity of phlorotannin. Aquat Toxicol 104:1–13. https://doi.org/10.1016/j.aquatox.2011.03.016
Corzo Remigio A, Chaney RL, Baker AJM, Edraki M, Erskine PD, Echevarria G, van der Ent A (2020) Phytoextraction of high value elements and contaminants from mining and mineral wastes: opportunities and limitations. Plant Soil 449:11–37. https://doi.org/10.1007/s11104-020-04487-3
Costa OYA, Raaijmakers JM, Kuramae EE (2018) Microbial extracellular polymeric substances: ecological function and impact on soil aggregation. Front Microbiol 9:1636. https://doi.org/10.3389/fmicb.2018.01636
Deicke M, Bellenger J-P, Wichard T (2013) Direct quantification of bacterial molybdenum and iron metallophores with ultra-high-performance liquid chromatography coupled to time-of-flight mass spectrometry. J Chromatogr A 1298:50–60. https://doi.org/10.1016/j.chroma.2013.05.008
Delnomdedieu M, Basti MM, Otvos JD, Thomas DJ (1994) Reduction and binding of arsenate and dimethylarsinate by glutathione: a magnetic resonance study. Chem Biol Interact 90:139–155. https://doi.org/10.1016/0009-2797(94)90099-X
Deolu-Ajayi AO, van der Meer IM, van der Werf A, Karlova R (2022) The power of seaweeds as plant biostimulants to boost crop production under abiotic stress. Plant, Cell Environ 45:2537–2553. https://doi.org/10.1111/pce.14391
Digruber T, Sass L, Cseri A, Paul K, Nagy AV, Remenyik J, Molnár I, Vass I, Toldi O, Gyuricza C, Dudits D (2018) Stimulation of energy willow biomass with triacontanol and seaweed extract. Ind Crops Prod 120:104–112. https://doi.org/10.1016/j.indcrop.2018.04.047
Duan GL, Zhu YG, Tong YP, Cai C, Kneer R (2005) Characterization of arsenate reductase in the extract of roots and fronds of Chinese brake fern, an arsenic hyperaccumulator. Plant Physiol 138:461–469. https://doi.org/10.1104/pp.104.057422
Duarte RMBO, Matos JTV, Senesi N (2018) Organic Pollutants in Soils. In: Duarte AC, Cachada A, Rocha-Santos T (eds) Soil Pollution. Academic Press, Cambridge, pp 103–126
Eisenhauer N, Lanoue A, Strecker T, Scheu S, Steinauer K, Thakur MP, Mommer L (2017) Root biomass and exudates link plant diversity with soil bacterial and fungal biomass. Sci Rep 7:44641. https://doi.org/10.1038/srep44641
Ertani A, Francioso O, Tinti A, Schiavon M, Pizzeghello D, Nardi S (2018a) Evaluation of seaweed extracts from Laminaria and Ascophyllum nodosum spp. as biostimulants in Zea mays L. using a combination of chemical, biochemical and morphological approaches. Front Plant Sci 9:428. https://doi.org/10.3389/fpls.2018.00428
Faure D, Vereecke D, Leveau JHJ (2009) Molecular communication in the rhizosphere. Plant Soil 321:279–303. https://doi.org/10.1007/s11104-008-9839-2
Favas PJC, Pratas J, Paul MS, Sarkar SK, Prasad MNV (2016) Phytofiltration of metal(loid)-contaminated water: The potential of native aquatic plants. In: Ansari AA, Gill SS, Gill R, Lanza GR, Newman L (eds) Phytoremediation: Management of Environmental Contaminants, vol 3. Springer International Publishing, Cham, pp 305–343
Galal TM, Gharib FA, Ghazi SM, Mansour KH (2017) Phytostabilization of heavy metals by the emergent macrophyte Vossia cuspidata (Roxb.) Griff.: A phytoremediation approach. Int J Phytorem 19:992–999. https://doi.org/10.1080/15226514.2017.1303816
Galloway AF, Akhtar J, Marcus SE, Fletcher N, Field K, Knox P (2020) Cereal root exudates contain highly structurally complex polysaccharides with soil-binding properties. Plant J 103:1666–1678. https://doi.org/10.1111/tpj.14852
Gargallo-Garriga A, Preece C, Sardans J, Oravec M, Urban O, Peñuelas J (2018) Root exudate metabolomes change under drought and show limited capacity for recovery. Sci Rep 8:12696. https://doi.org/10.1038/s41598-018-30150-0
Georgiadou EC, Kowalska E, Patla K, Kulbat K, Smolińska B, Leszczyńska J, Fotopoulos V (2018b) Influence of heavy metals (Ni, Cu, and Zn) on nitro-oxidative stress responses, proteome regulation and allergen production in basil (Ocimum basilicum L.) plants. Front Plant Sci 9:862. https://doi.org/10.3389/fpls.2018.00862
Ghori Z, Iftikhar H, Bhatti MF, Sharma I, Kazi AG, Ahmad P (2016) Phytoextraction: The use of plants to remove heavy metals from soil. In: Ahmad P (ed) Plant Metal Interaction: Emerging remediation techniques. Elsevier, Amsterdam, pp 385–409
Gianfreda L (2015) Enzymes of importance to rhizosphere processes. J Soil Sci Plant Nutr 15:283–306. https://doi.org/10.4067/S0718-95162015005000022
Goñi O, Quille P, O’Connell S (2018) Ascophyllum nodosum extract biostimulants and their role in enhancing tolerance to drought stress in tomato plants. Plant Physiol Biochem 126:63–73. https://doi.org/10.1016/j.plaphy.2018.02.024
Guarino C, Paura B, Sciarrillo R (2018) Enhancing phytoextraction of HMs at real scale, by combining Salicaceae trees with microbial consortia. Front Environ Sci. https://doi.org/10.3389/fenvs.2018.00137
Guidi Nissim W, Palm E, Mancuso S, Azzarello E (2018) Trace element phytoextraction from contaminated soil: a case study under Mediterranean climate. Environ Sci Pollut Res 25:9114–9131. https://doi.org/10.1007/s11356-018-1197-x
Gul I, Manzoor M, Kallerhoff J, Arshad M (2020) Enhanced phytoremediation of lead by soil applied organic and inorganic amendments: Pb phytoavailability, accumulation and metal recovery. Chemosphere 258:127405. https://doi.org/10.1016/j.chemosphere.2020.127405
Gupta M, Gupta S (2016) An overview of selenium uptake, metabolism, and toxicity in plants. Front Plant Sci 7:2074. https://doi.org/10.3389/fpls.2016.02074
Gupta S, Stirk WA, Plačková L, Kulkarni MG, Doležal K, van Staden J (2021) Interactive effects of plant growth-promoting rhizobacteria and a seaweed extract on the growth and physiology of Allium cepa L. (onion). J Plant Physiol 262:153437. https://doi.org/10.1016/j.jplph.2021.153437
Hamlin RL, Barker AV (2006) Influence of ammonium and nitrate nutrition on plant growth and zinc accumulation by indian mustard. J Plant Nutr 29:1523–1541. https://doi.org/10.1080/01904160600837709
Hannink NK, Subramanian M, Rosser SJ, Basran A, Murray JAH, Shanks JV, Bruce NC (2007) Enhanced transformation of tnt by tobacco plants expressing a bacterial nitroreductase. Int J Phytorem 9:385–401. https://doi.org/10.1080/15226510701603916
Hazarika A, Saikia S, Devi B, Yadav M, Yadav HS (2022) 6 - Oxidoreductase metalloenzymes as green catalyst for phytoremediation of environmental pollutants. In: Kumar V, Shah MP, Shahi SK (eds) Phytoremediation Technology for the Removal of Heavy Metals and Other Contaminants from Soil and Water. Elsevier, Amsterdam, pp 141–172
He Y, Chi J (2016) Phytoremediation of sediments polluted with phenanthrene and pyrene by four submerged aquatic plants. J Soils Sediments 16:309–317. https://doi.org/10.1007/s11368-015-1221-4
Hipfinger C, Laux M, Puschenreiter M (2022) Comparison of four nickel hyperaccumulator species in the temperate climate zone of Central Europe. J Geochem Explora 234:106933. https://doi.org/10.1016/j.gexplo.2021.106933
Huang AC, Jiang T, Liu Y-X, Bai Y-C, Reed J, Qu B, Goossens A, Nützmann H-W, Bai Y, Osbourn A (2019) A specialized metabolic network selectively modulates Arabidopsis root microbiota. Science 364:eaau6389. https://doi.org/10.1126/science.aau6389
Imbs TI, Zvyagintseva TN (2018) Phlorotannins are polyphenolic metabolites of brown algae. Russ J Mar Biol 44:263–273. https://doi.org/10.1134/S106307401804003X
Jacob JM, Karthik C, Saratale RG, Kumar SS, Prabakar D, Kadirvelu K, Pugazhendhi A (2018) Biological approaches to tackle heavy metal pollution: a survey of literature. J Environ Manage 217:56–70. https://doi.org/10.1016/j.jenvman.2018.03.077
Javed MT, Tanwir K, Akram MS, Shahid M, Niazi NK, Lindberg S (2019) Phytoremediation of cadmium-polluted water/sediment by aquatic macrophytes: Role of plant-induced pH changes. In: Hasanuzzaman M, Prasad MNV, Fujita M (eds) Cadmium Toxicity and Tolerance in Plants. Academic Press, London, pp 495–529
Jiang O, Li L, Duan G, Gustave W, Zhai W, Zou L, An X, Tang X, Xu J (2022) Root exudates increased arsenic mobility and altered microbial community in paddy soils. J Environ Sci 127:410–420. https://doi.org/10.1016/j.jes.2022.05.036
Jing R, Fusi S, Kjellerup BV (2018) Remediation of polychlorinated biphenyls (PCBs) in contaminated soils and sediment: State of knowledge and perspectives. Front Environ Sci 6:79. https://doi.org/10.3389/fenvs.2018.00079
Kafle A, Timilsina A, Gautam A, Adhikari K, Bhattarai A, Aryal N (2022) Phytoremediation: Mechanisms, plant selection and enhancement by natural and synthetic agents. Environmen Adv 8:100203. https://doi.org/10.1016/j.envadv.2022.100203
Kaur L (2020) Role of phytoremediation strategies in removal of heavy metals. In: Kumar M, Snow DD, Honda R (eds) Emerging Issues in the Water Environment during Anthropocene: A South East Asian Perspective. Springer, Singapore, pp 223–259
Keunen E, Remans T, Bohler S, Vangronsveld J, Cuypers A (2011) Metal-induced oxidative stress and plant mitochondria. Int J Mol Sci 12:6894–6918. https://doi.org/10.3390/ijms12106894
Khalid S, Shahid M, Niazi NK, Murtaza B, Bibi I, Dumat C (2017) A comparison of technologies for remediation of heavy metal contaminated soils. J Geochem Explor 182:247–268. https://doi.org/10.1016/j.gexplo.2016.11.021
Kim S-Y, Ahn G, Kim H-S, Je J-G, Kim K-N, Jeon Y-J (2020) Diphlorethohydroxycarmalol (DPHC) isolated from the brown alga Ishige okamurae acts on inflammatory myopathy as an inhibitory agent of TNF-α. Mar Drugs 18:529. https://doi.org/10.3390/md18110529
Kohda YH-T, Qian Z, Chien M-F, Miyauchi K, Endo G, Suzui N, Yin Y-G, Kawachi N, Ikeda H, Watabe H, Kikunaga H, Kitajima N, Inoue C (2021) New evidence of arsenic translocation and accumulation in Pteris vittata from real-time imaging using positron-emitting 74As tracer. Sci Rep 11:12149. https://doi.org/10.1038/s41598-021-91374-1
Kong, Z., Glick, B.R. 2017. The role of plant growth-promoting bacteria in metal phytoremediation. In R.K. Poole (Ed.), Advances in Microbial Physiology (Vol. 71, pp. 97–132): Academic Press.
Koroney AS, Plasson C, Pawlak B, Sidikou R, Driouich A, Menu-Bouaouiche L, Vicré-Gibouin M (2016) Root exudate of Solanum tuberosum is enriched in galactose-containing molecules and impacts the growth of Pectobacterium atrosepticum. Ann Bot 118:797–808. https://doi.org/10.1093/aob/mcw128
Kreslavski VD, Lankin AV, Vasilyeva GK, Luybimov VY, Semenova GN, Schmitt F-J, Friedrich T, Allakhverdiev SI (2014) Effects of polyaromatic hydrocarbons on photosystem II activity in pea leaves. Plant Physiol Biochem 81:135–142. https://doi.org/10.1016/j.plaphy.2014.02.020
Kulkarni MG, Rengasamy KRR, Pendota SC, Gruz J, Plačková L, Novák O, Doležal K, van Staden J (2019) Bioactive molecules derived from smoke and seaweed Ecklonia maxima showing phytohormone-like activity in Spinacia oleracea L. New Biotechnol 48:83–89. https://doi.org/10.1016/j.nbt.2018.08.004
Kvesitadze G, Khatisashvili G, Sadunishvili T, Kvesitadze E (2015) Plants for remediation: Uptake, translocation and transformation of organic pollutants. In: Öztürk M, Ashraf M, Aksoy A, Ahmad MSA, Hakeem KR (eds) Plants, Pollutants and Remediation. Springer, Dordrecht, pp 241–308
Lange B, van Der Ent A, Baker AJ, Echevarria G, Mahy G, Malaisse F, Meerts P, Pourret O, Verbruggen N, Faucon MP (2017) Copper and cobalt accumulation in plants: a critical assessment of the current state of knowledge. New Phytol 213:537–551. https://doi.org/10.1111/nph.14175
Lebrun M, Michel C, Joulian C, Morabito D, Bourgerie S (2021) Rehabilitation of mine soils by phytostabilization: does soil inoculation with microbial consortia stimulate Agrostis growth and metal(loid) immobilization? Sci Total Environ 791:148400. https://doi.org/10.1016/j.scitotenv.2021.148400
Lesuffleur F, Paynel F, Bataillé M-P, Le Deunff E, Cliquet J-B (2007) Root amino acid exudation: measurement of high efflux rates of glycine and serine from six different plant species. Plant Soil 294:235–246. https://doi.org/10.1007/s11104-007-9249-x
Li H-F, McGrath SP, Zhao F-J (2008) Selenium uptake, translocation and speciation in wheat supplied with selenate or selenite. New Phytol 178:92–102. https://doi.org/10.1111/j.1469-8137.2007.02343.x
Li N, Wang J, Song W-Y (2015) Arsenic uptake and translocation in plants. Plant Cell Physiol 57:4–13. https://doi.org/10.1093/pcp/pcv143
Li S-M, Glombitza K-W (1991) Carmalols and phlorethofuhalols from the brown alga Carpophyllum maschalocarpum. Phytochemistry 30:3417–3421. https://doi.org/10.1016/0031-9422(91)83220-F
Li Y-X, Wijesekara I, Li Y, Kim S-K (2011) Phlorotannins as bioactive agents from brown algae. Process Biochem 46:2219–2224. https://doi.org/10.1016/j.procbio.2011.09.015
Li Y, Fu X, Duan D, Liu X, Xu J, Gao X (2017) Extraction and identification of phlorotannins from the brown alga, Sargassum fusiforme (Harvey) Setchell. Mar Drugs. https://doi.org/10.3390/md15020049
Limmer M, Burken J (2016) Phytovolatilization of organic contaminants. Environ Sci Technol 50:6632–6643. https://doi.org/10.1021/acs.est.5b04113
Liu L, Li W, Song W, Guo M (2018) Remediation techniques for heavy metal-contaminated soils: principles and applicability. Sci Total Environ 633:206–219. https://doi.org/10.1016/j.scitotenv.2018.03.161
Lomartire S, Gonçalves AMM (2022) Antiviral activity and mechanisms of seaweeds bioactive compounds on enveloped viruses—A review. Mar Drugs 20:385. https://doi.org/10.3390/md20060385
Lu H, Sun J, Zhu L (2017) The role of artificial root exudate components in facilitating the degradation of pyrene in soil. Sci Rep 7:7130. https://doi.org/10.1038/s41598-017-07413-3
Ma JF, Yamaji N, Mitani N, Xu X-Y, Su Y-H, McGrath SP, Zhao F-J (2008) Transporters of arsenite in rice and their role in arsenic accumulation in rice grain. Proc Natl Acad Sci 105:9931–9935. https://doi.org/10.1073/pnas.0802361105
Ma Y, Oliveira RS, Freitas H, Zhang C (2016) Biochemical and molecular mechanisms of plant-microbe-metal interactions: Relevance for phytoremediation. Front Plant Sci 7:918. https://doi.org/10.3389/fpls.2016.00918
Małecka A, Konkolewska A, Hanć A, Ciszewska L, Staszak AM, Jarmuszkiewicz W, Ratajczak E (2021) Activation of antioxidative and detoxificative systems in Brassica juncea L. plants against the toxicity of heavy metals. Sci Rep 11:22345. https://doi.org/10.1038/s41598-021-01827-w
Masondo NA, Kulkarni MG, Finnie JF, van Staden J (2018) Influence of biostimulants-seed-priming on Ceratotheca triloba germination and seedling growth under low temperatures, low osmotic potential and salinity stress. Ecotoxicol Environ Saf 147:43–48. https://doi.org/10.1016/j.ecoenv.2017.08.017
Meier IC, Tückmantel T, Heitkötter J, Müller K, Preusser S, Wrobel TJ, Kandeler E, Marschner B, Leuschner C (2020) Root exudation of mature beech forests across a nutrient availability gradient: the role of root morphology and fungal activity. New Phytol 226:583–594. https://doi.org/10.1111/nph.16389
Mesa-Marín J, Pérez-Romero JA, Redondo-Gómez S, Pajuelo E, Rodríguez-Llorente ID, Mateos-Naranjo E (2020) Impact of plant growth promoting bacteria on Salicornia ramosissima ecophysiology and heavy metal phytoremediation capacity in estuarine soils. Front Microbiol 11:553018. https://doi.org/10.3389/fmicb.2020.553018
Meslet-Cladière L, Delage L, Leroux CJ-J, Goulitquer S, Leblanc C, Creis E, Gall EA, Stiger-Pouvreau V, Czjzek M, Potin P (2013) Structure/function analysis of a type III polyketide synthase in the brown alga Ectocarpus siliculosus reveals a biochemical pathway in phlorotannin monomer biosynthesis. Plant Cell 25:3089–3103. https://doi.org/10.1105/tpc.113.111336
Mitton FM, Miglioranza KSB, Gonzalez M, Shimabukuro VM, Monserrat JM (2014) Assessment of tolerance and efficiency of crop species in the phytoremediation of DDT polluted soils. Ecol Eng 71:501–508. https://doi.org/10.1016/j.ecoleng.2014.07.069
Murata N, Azuma M, Yamauchi K, Miyake H, Tanaka R, Shibata T (2020) Phlorotannins remarkably suppress the formation of Nε-(Carboxymethyl)lysine in a collagen-glyoxal environment. Nat Prod Commun 15:1–6. https://doi.org/10.1177/1934578X20941655
Múzquiz de la Garza AR, Tapia-Salazar M, Maldonado-Muñiz M, de la Rosa-Millán J, Gutiérrez-Uribe JA, Santos-Zea L, Barba-Dávila BA, Ricque-Marie D, Cruz-Suárez LE (2019) Nutraceutical potential of five mexican brown seaweeds. Biomed Res Int 2019:1–15. https://doi.org/10.1155/2019/3795160
Ogra Y, Awaya Y, Anan Y (2015) Comparison of accumulation of four metalloids in Allium Sativum. Bull Environ Contam Toxicol 94:604–608. https://doi.org/10.1007/s00128-015-1508-6
Olguín EJ, Sánchez-Galván G (2011) Phytofiltration of heavy metals: Assessment of the key factors involved in the design of a sustainable process. In: Moo-Young M (ed) Comprehensive Biotechnology (Second Edition), 2nd edn. Academic Press, Burlington, pp 207–213
Olguín EJ, Sánchez-Galván G (2012) Heavy metal removal in phytofiltration and phycoremediation: the need to differentiate between bioadsorption and bioaccumulation. New Biotechnol 30:3–8. https://doi.org/10.1016/j.nbt.2012.05.020
Omoarelojie LO, Kulkarni MG, Finnie JF, van Staden J (2019) Strigolactones and their crosstalk with other phytohormones. Ann Bot 124:749–767. https://doi.org/10.1093/aob/mcz100
Oves M, Khan MS, Zaidi A (2013) Chromium reducing and plant growth promoting novel strain Pseudomonas aeruginosa OSG41 enhance chickpea growth in chromium amended soils. Eur J Soil Biol 56:72–83. https://doi.org/10.1016/j.ejsobi.2013.02.002
Park JM, Trevor Sewell B, Benedik MJ (2017) Cyanide bioremediation: the potential of engineered nitrilases. Appl Microbiol Biotechnol 101:3029–3042. https://doi.org/10.1007/s00253-017-8204-x
Parshad B, Duraisamy A, Saini S, Yadav P, Vats P, Sharma S (2016) Synthesis and SAR study of antioxidant potential of polyhydroxy coumarin derivatives. Med Chem 6:506–514. https://doi.org/10.4172/2161-0444.1000391
Philippot L, Raaijmakers JM, Lemanceau P, van der Putten WH (2013) Going back to the roots: the microbial ecology of the rhizosphere. Nat Rev Microbiol 11:789–799. https://doi.org/10.1038/nrmicro3109
Pilon-Smits EAH (2017) Mechanisms of plant selenium hyperaccumulation. In: Pilon-Smits EAH, Winkel LHE, Lin Z-Q (eds) Selenium in plants: Molecular, Physiological, Ecological and Evolutionary Aspects. Springer International Publishing, Cham, pp 53–66
Pilon-Smits EAH, Quinn CF (2010) Selenium Metabolism in Plants. In: Hell R, Mendel R-R (eds) Cell Biology of Metals and Nutrients. Springer, Berlin, pp 225–241
Radziemska M, Vaverková MD, Baryła A (2017) Phytostabilization—management strategy for stabilizing trace elements in contaminated soils. Int J Environ Res Public Health. https://doi.org/10.3390/ijerph14090958
Ragan MA, Smidsrød O, Larsen B (1979) Chelation of divalent metal ions by brown algal polyphenols. Mar Chem 7:265–271. https://doi.org/10.1016/0304-4203(79)90043-4
Rajkumar M, Sandhya S, Prasad MNV, Freitas H (2012) Perspectives of plant-associated microbes in heavy metal phytoremediation. Biotechnol Adv 30:1562–1574. https://doi.org/10.1016/j.biotechadv.2012.04.011
Raklami A, Meddich A, Oufdou K, Baslam M (2022) Plants-microorganisms-based bioremediation for heavy metal cleanup: recent developments, phytoremediation techniques, regulation mechanisms, and molecular responses. Int J Mol Sci 23:5031. https://doi.org/10.3390/ijms23095031
Rasool F, Uzair M, Naeem MK, Rehman N, Afroz A, Shah H, Khan MR (2021) Phenylalanine ammonia-lyase (PAL) genes family in wheat (Triticum aestivum L.): Genome-wide characterization and expression profiling. Agronomy 11:2511. https://doi.org/10.3390/agronomy11122511
Reeves RD, Baker AJM, Jaffré T, Erskine PD, Echevarria G, van der Ent A (2018) A global database for plants that hyperaccumulate metal and metalloid trace elements. New Phytol 218:407–411. https://doi.org/10.1111/nph.14907
Rengasamy KRR, Aderogba MA, Amoo SO, Stirk WA, van Staden J (2013) Potential antiradical and alpha-glucosidase inhibitors from Ecklonia maxima (Osbeck) Papenfuss. Food Chem 141:1412–1415. https://doi.org/10.1016/j.foodchem.2013.04.019
Rengasamy KRR, Kulkarni MG, Stirk WA, van Staden J (2015) Eckol - a new plant growth stimulant from the brown seaweed Ecklonia maxima. J Appl Phycol 27:581–587. https://doi.org/10.1007/s10811-014-0337-z
Rezania S, Taib SM, Md Din MF, Dahalan FA, Kamyab H (2016) Comprehensive review on phytotechnology: heavy metals removal by diverse aquatic plants species from wastewater. J Hazard Mater 318:587–599. https://doi.org/10.1016/j.jhazmat.2016.07.053
Riaz M, Kamran M, Fang Y, Wang Q, Cao H, Yang G, Deng L, Wang Y, Zhou Y, Anastopoulos I, Wang X (2021) Arbuscular mycorrhizal fungi-induced mitigation of heavy metal phytotoxicity in metal contaminated soils: a critical review. J Hazard Mater 402:123919. https://doi.org/10.1016/j.jhazmat.2020.123919
Richards SL, Wilkins KA, Swarbreck SM, Anderson AA, Habib N, Smith AG, McAinsh M, Davies JM (2015) The hydroxyl radical in plants: from seed to seed. J Exp Bot 66:37–46. https://doi.org/10.1093/jxb/eru398
Rizwan M, Ali S, Qayyum MF, Ok YS, Zia-ur-Rehman M, Abbas Z, Hannan F (2017) Use of maize (Zea mays L.) for phytomanagement of Cd-contaminated soils: a critical review. Environ Geochem Health 39:259–277. https://doi.org/10.1007/s10653-016-9826-0
Roy D, Sreekanth D, Pawar D, Mahawar H, Barman K (2021) Phytoremediation of arsenic contaminated water using aquatic, semi-aquatic and submerged weeds. In: Mendes KF, de Sousa R, Mielke KC (eds) Biodegradation technology of organic and inorganic pollutants. IntechOpen, London
Sabir M, Waraich EA, Hakeem KR, Öztürk M, Ahmad HR, Shahid M (2015) Phytoremediation: Mechanisms and adaptations. In: Hakeem KR, Sabir M, Öztürk M, Mermut AR (eds) Soil Remediation and Plants. Academic Press, San Diego, pp 85–105
Sánchez-Camargo AP, Montero L, Cifuentes A, Herrero M, Ibáñez E (2016) Application of Hansen solubility approach for the subcritical and supercritical selective extraction of phlorotannins from Cystoseira abies-marina. RSC Adv 6:94884–94895. https://doi.org/10.1039/C6RA16862K
Schwitzguébel J-P (2017) Phytoremediation of soils contaminated by organic compounds: hype, hope and facts. J Soils Sediments 17:1492–1502. https://doi.org/10.1007/s11368-015-1253-9
Sheoran V, Sheoran AS, Poonia P (2016) Factors affecting phytoextraction: A review. Pedosphere 26:148–166. https://doi.org/10.1016/S1002-0160(15)60032-7
Shibata T, Kawaguchi S, Hama Y, Inagaki M, Yamaguchi K, Nakamura T (2004) Local and chemical distribution of phlorotannins in brown algae. J Appl Phycol 16:291–296. https://doi.org/10.1023/B:JAPH.0000047781.24993.0a
Shrestha P, Bellitürk K, Görres JH (2019) Phytoremediation of heavy metal-contaminated soil by switchgrass: A comparative study utilizing different composts and coir fiber on pollution remediation, plant productivity, and nutrient leaching. Int J Environ Res Public Health 16:1261. https://doi.org/10.3390/ijerph16071261
Shrestha, S., Zhang, W., Smid, S.D. 2021. Phlorotannins: A review on biosynthesis, chemistry and bioactivity. Food Bioscience, 39, 100832. doi:https://doi.org/10.1016/j.fbio.2020.100832
Singh, B.S.M., Singh, D., Dhal, N.K. 2022. Enhanced phytoremediation strategy for sustainable management of heavy metals and radionuclides. Case Studies in Chemical and Environmental Engineering, 5, 100176. doi:https://doi.org/10.1016/j.cscee.2021.100176
Singh G, Pankaj U, Chand S, Verma RK (2019) Arbuscular mycorrhizal fungi-assisted phytoextraction of toxic metals by Zea mays L. from tannery sludge. Soil and Sediment Contamination: an International Journal 28:729–746. https://doi.org/10.1080/15320383.2019.1657381
Singh R, Kesavan AK, Landi M, Kaur S, Thakur S, Zheng B, Bhardwaj R, Sharma A (2020) 5-aminolevulinic acid regulates Krebs cycle, antioxidative system and gene expression in Brassica juncea L. to confer tolerance against lead toxicity. J Biotechnol 323:283–292. https://doi.org/10.1016/j.jbiotec.2020.09.004
Sridharan R, Krishnaswamy VG, Archana KM, Rajagopal R, Thirumal Kumar D, Doss GP, C. (2021) Integrated approach on azo dyes degradation using laccase enzyme and Cul nanoparticle. SN Applied Sciences 3:370. https://doi.org/10.1007/s42452-021-04164-9
Sugiura, Y., Katsuzaki, H., Imai, K., Amano, H. 2021. The anti-allergic and anti-inflammatory effects of phlorotannins from the edible brown algae, Ecklonia sp. and Eisenia sp. Natural Product Communications, 16, 1934578X211060924. doi:https://doi.org/10.1177/1934578X211060924
Suman J, Uhlik O, Viktorova J, Macek T (2018) Phytoextraction of heavy metals: A promising tool for clean-up of polluted environment? Front Plant Sci 9:1476. https://doi.org/10.3389/fpls.2018.01476
Sut-Lohmann, M., Jonczak, J., Raab, T. 2020. Phytofiltration of chosen metals by aquarium liverwort (Monosoleum tenerum). Ecotoxicology and Environmental Safety, 188, 109844. doi:https://doi.org/10.1016/j.ecoenv.2019.109844
Sylvain B, Mikael M-H, Florie M, Emmanuel J, Marilyne S, Sylvain B, Domenico M (2016) Phytostabilization of As, Sb and Pb by two willow species (S. viminalis and S. purpurea) on former mine technosols. CATENA 136:44–52. https://doi.org/10.1016/j.catena.2015.07.008
Tahara K, Hashida K, Otsuka Y, Ohara S, Kojima K, Shinohara K (2013) Identification of a hydrolyzable tannin, oenothein b, as an aluminum-detoxifying ligand in a highly aluminum-resistant tree, Eucalyptus camaldulensis. Plant Physiol 164:683–693. https://doi.org/10.1104/pp.113.222885
Tang J, Wang W, Chu W (2020) Antimicrobial and anti-quorum sensing activities of phlorotannins from seaweed (Hizikia fusiforme). Front Cellular Infect Microbiol 10:586750. https://doi.org/10.3389/fcimb.2020.586750
Thakur S, Singh L, Wahid ZA, Siddiqui MF, Atnaw SM, Din MFM (2016) Plant-driven removal of heavy metals from soil: uptake, translocation, tolerance mechanism, challenges, and future perspectives. Environ Monit Assess 188:206. https://doi.org/10.1007/s10661-016-5211-9
Tian S, Xie R, Wang H, Hu Y, Hou D, Liao X, Brown PH, Yang H, Lin X, Labavitch JM, Lu L (2017) Uptake, sequestration and tolerance of cadmium at cellular levels in the hyperaccumulator plant species Sedum alfredii. J Exp Bot 68:2387–2398. https://doi.org/10.1093/jxb/erx112
Toth G, Pavia H (2000) Lack of phlorotannin induction in the brown seaweed Ascophyllum nodosum in response to increased copper concentrations. Mar Ecol Prog Ser 192:119–126. https://doi.org/10.3354/meps192119
Tu C, Teng Y, Luo Y, Sun X, Deng S, Li Z, Liu W, Xu Z (2011) PCB removal, soil enzyme activities, and microbial community structures during the phytoremediation by alfalfa in field soils. J Soils Sediments 11:649–656. https://doi.org/10.1007/s11368-011-0344-5
Ullah A, Heng S, Munis MFH, Fahad S, Yang X (2015) Phytoremediation of heavy metals assisted by plant growth promoting (PGP) bacteria: a review. Environ Exp Bot 117:28–40. https://doi.org/10.1016/j.envexpbot.2015.05.001
Virk AL, Lin B-J, Kan Z-R, Qi J-Y, Dang YP, Lal R, Zhao X, Zhang H-L (2022) Simultaneous effects of legume cultivation on carbon and nitrogen accumulation in soil. In: Sparks DL (ed) Advances in Agronomy. Academic Press, Cambridge, pp 75–110
Wang T, Jónsdóttir R, Liu H, Gu L, Kristinsson HG, Raghavan S, Ólafsdóttir G (2012) Antioxidant capacities of phlorotannins extracted from the brown algae Fucus vesiculosus. J Agric Food Chem 60:5874–5883. https://doi.org/10.1021/jf3003653
Wei S, Pan S (2010) Phytoremediation for soils contaminated by phenanthrene and pyrene with multiple plant species. J Soils Sediments 10:886–894. https://doi.org/10.1007/s11368-010-0216-4
Wolfe NL, Hoehamer CF (2003) Enzymes used by plants and microorganisms to detoxify organic compounds. In: McCutcheon SC, Schnoor JL (eds) Phytoremediation: Transformation and control of contaminants. John Wiley & Sons Inc, New Jersey, pp 159–187
Wyrwicka A, Steffani S, Urbaniak M (2014) The effect of PCB-contaminated sewage sludge and sediment on metabolism of cucumber plants (Cucumis sativus L.). Ecohydrol Hydrobiol 14:75–82. https://doi.org/10.1016/j.ecohyd.2014.01.003
Xing H, Zhang J, Tan L, Li R, Chen J, Lan J, Zhang J, Liu X, Li Z (2022) Degradation of bisphenol A enhanced by dialysis membrane enclosed laccase catalysis. Environ Technol Innov 27:102435. https://doi.org/10.1016/j.eti.2022.102435
Xu Z, Wu Y, Xiao Z, Ban Y, Belvett N (2019) Positive effects of Funneliformis mosseae inoculation on reed seedlings under water and TiO2 nanoparticles stresses. World J Microbiol Biotechnol 35:81. https://doi.org/10.1007/s11274-019-2656-3
Yadav R, Singh S, Kumar A, Singh AN (2022) Phytoremediation: A wonderful cost-effective tool. In: Kathi S, Devipriya S, Thamaraiselvi K (eds) Cost Effective Technologies for Solid Waste and Wastewater Treatment. Elsevier, Amsterdam, pp 179–208
Yan A, Wang Y, Tan SN, Mohd Yusof ML, Ghosh S, Chen Z (2020) Phytoremediation: a promising approach for revegetation of heavy metal-polluted land. Front Plant Sci 11:359. https://doi.org/10.3389/fpls.2020.00359
Yan L, Riaz M, Liu J, Yu M, Cuncang J (2022) The aluminum tolerance and detoxification mechanisms in plants; recent advances and prospects. Crit Rev Environ Sci Technol 52:1491–1527. https://doi.org/10.1080/10643389.2020.1859306
Yuan M, He H, Xiao L, Zhong T, Liu H, Li S, Deng P, Ye Z, Jing Y (2014) Enhancement of Cd phytoextraction by two Amaranthus species with endophytic Rahnella sp. JN27. Chemosphere 103:99–104. https://doi.org/10.1016/j.chemosphere.2013.11.040
Zhalnina K, Louie KB, Hao Z, Mansoori N, da Rocha UN, Shi S, Cho H, Karaoz U, Loqué D, Bowen BP, Firestone MK, Northen TR, Brodie EL (2018) Dynamic root exudate chemistry and microbial substrate preferences drive patterns in rhizosphere microbial community assembly. Nat Microbiol 3:470–480. https://doi.org/10.1038/s41564-018-0129-3
Zhan F, Li B, Jiang M, Li T, He Y, Li Y, Wang Y (2019) Effects of arbuscular mycorrhizal fungi on the growth and heavy metal accumulation of bermudagrass [Cynodon dactylon (L.) Pers.] grown in a lead–zinc mine wasteland. Int J Phytorem 21:849–856. https://doi.org/10.1080/15226514.2019.1577353
Zhan X, Zhu M, Shen Y, Yue L, Li J, Gardea-Torresdey JL, Xu G (2018) Apoplastic and symplastic uptake of phenanthrene in wheat roots. Environ Pollut 233:331–339. https://doi.org/10.1016/j.envpol.2017.10.056
Zhao FJ, Ma JF, Meharg AA, McGrath SP (2009) Arsenic uptake and metabolism in plants. New Phytol 181:777–794. https://doi.org/10.1111/j.1469-8137.2008.02716.x
Zhao S, Rogers MJ, Ding C, He J (2018) Reductive debromination of polybrominated diphenyl ethers-microbes, processes and dehalogenases. Front Microbiol 9:1292. https://doi.org/10.3389/fmicb.2018.01292
Acknowledgements
The authors thank Kelp Products (Pty) Ltd., Simon's Town, South Africa, for providing research funds for this work.
Funding
Open access funding provided by University of KwaZulu-Natal.
Author information
Authors and Affiliations
Contributions
LOO and JvS conceptualized the review and proposed the scope of the paper. LOO wrote the original manuscript and designed the graphical diagrams. JvS reviewed and edited the manuscript. Both authors have read, edited and approved the manuscript. JvS acquired the financial support.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Handling Editor: Vijay kumar.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Omoarelojie, L.O., van Staden, J. Perspectives on the Potentials of Phlorotannins in Enhancing Phytoremediation Performance. J Plant Growth Regul (2023). https://doi.org/10.1007/s00344-023-11075-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00344-023-11075-z



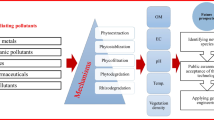









 organic pollutant;
organic pollutant;  metalloid;
metalloid;  volatile organic compound;
volatile organic compound;  root exudate
root exudate
 organic pollutant;
organic pollutant;  metal(loid);
metal(loid);  volatile organic compound;
volatile organic compound;  root exudate
root exudate