Abstract
Plant endophytic microorganisms, which can enhance plant growth and resistance to biotic and abiotic stresses, are untapped resources with large potential applications for crop production. However, the endophytic community is influenced by multiple factors, such as host genetics, the environment, and other microbes. Thus, it is important to characterize well-adapted endophytes from native crops. We isolated 43 endophytic bacteria from sugarcane cultivar Yunzhe-99-91. All these 43 isolates were examined in vitro for nitrogenase activity and the ability to dissolve phosphorus and produce siderophore. One of these strains, B9, identified as Bacillus subtilis and showed maximum plant growth promotion, was selected for detailed studies. B9 promoted the production of organic acids such as propionic acid, acetic acid, malic acid and citric acid, and the production of phytohormones, including indole-3-acetic acid, cytokinin, 6-benzyladenine, and zeatin. Moreover, B9 significantly promoted the growth of sugarcane plantlets and increased the content of nitrogen, phosphorus, and potassium in the seedlings by 29.26%, 50.78%, and 15.49%, respectively. The photosynthetic rate, root development, and chlorophyll content were also improved with varying degrees compared to the non-inoculated control. The cotyledon and hypocotyl of sugarcane gems germinated faster when co-cultured with the B9 strain compared with control group. Colonization assay showed that B9 was mainly colonized in the roots, followed by the stems and leaves. In conclusion, the positive interaction between endophytic strain B9 and sugarcane may provide long-lasting benefits and a direction for developing and utilizing B9 as a biofertilizer for sugarcane cultivation to decrease fertilizer application.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plants can be observed as a complex micro-ecological system (Santoyo et al. 2015), and a variety of microorganisms co-exist with plants to form a nutrient-rich and competitive dynamic equilibrium system (Rahman et al. 2017). This system includes peripheral microorganisms that can infect the plants in vitro and “endophytes,” which can penetrate the plant tissues and systemically colonize (Schulz et al. 2006). Endophytic bacteria reside in healthy plant tissues, mainly in the vascular system of plant cells, cell gaps or xylem, and phloem, without causing obvious symptoms of infection (Ryan et al. 2010). Plants constitute vast and diverse niches for endophytic organisms. Compared with the microorganisms in other niches, such as the rhizosphere and phyllosphere, the specific niche insulates these endophytes from the adverse conditions of ultraviolet and stern climate, thereby allowing endophytes to stably synthesize and secrete metabolites, such as phytohormones and growth-regulating enzymes, which directly promote the plant growth and development at various stages of the plant life cycle through different mechanisms (Hallmann et al. 1999; Li and Wei 2020; Taghavi et al. 2009). Endophytes produce numerous bioactive compounds, which are widely used in agriculture and industry manufacturing of antibiotics and insecticides (Compant et al. 2005; Waller 2005). In addition, endophytic bacteria have the potential to remove the soil pollutants through enhanced bioremediation and may play a role in improving soil fertility (Sheng et al. 2008). Studies have revealed that most of the beneficial endophytic bacteria isolated from healthy plant roots have similar growth-promoting functions as plant growth-promoting rhizobacteria (PGPR) (Vessey 2003; Khalid et al. 2010; Lodewyckx et al. 2002; Lery et al. 2010).
Plant growth-promoting endophytes (PGPE) promote plant growth directly and indirectly. Direct mechanisms involve atmospheric nitrogen fixation (Reinhold et al. 1987; Boddey et al. 2003), phosphorus (P) and potassium (K) solubilization, making these readily available for plant growth (Egamberdiyeva 2007), siderophores production that can solubilize and sequester iron from the soil and protecting the plants from various diseases and production of several plant growth-regulating compounds, including plant hormones, profoundly influence the growth and differentiation of plant cells, tissues, and organs; the active metabolism-related enzymes in plants can promote the metabolism and improve the plant photosynthetic efficiency (Patten and Glick 1996). Indirect action refers to the inhibition or mitigation of the adverse effects of some plant diseases on the growth, development, and yield of plants, i.e., production of antibiotics, extracellular polymeric substances (EPS) such as polysaccharides, which activate the stress resistance of plants and to establish a harmonious symbiotic relationship with plants (Lodewyckx et al. 2002). In endophyte–plant mutualisms, several studies show that PGPE brings significant advantages to the plants, which have been widely studied for increasing agricultural productivity. Among the various plant growth-promoting bacteria (PGPB), Pseudomonas spp., Burkholderia, Azospirillum, and Bacillus have been extensively studied in several crops (John et al. 2017; Rondeau et al. 2019; Silva et al. 2018) including rice (Oryza sativa L.), maize (Zea mays L.), tomato (Solanum tuberosum L.), and sugarcane (Saccharum officinarum L.) (Boddey et al. 2003; Gyaneshwar et al. 2001; Verma et al. 2001; Olivares et al. 1996; Waweru et al. 2014).
Saccharum officinarum L. is one of the most important cash crops in Southern China. Yunnan province is the second-largest sugarcane growing area after Guangxi province. However, several problems persist in Yunnan, such as unreasonable fertilization and excessive or unscientific proportioning of sugarcane (Fan et al. 2018). It is well known that sugarcane needs much fertilizer during its growth, and the production of one ton of sugarcane requires 1.5–2 kg nitrogen (N), 1–1.5 kg phosphorus (P2O5), and 2–2.5 kg potassium (K2O) to be absorbed from the soil (Fen 2001). Consequently, large quantities of chemical fertilizers are applied to agricultural soil. This not only increases the cost for farmers but also brings environmental and public risks (Iqbal et al. 2020). Determining how to reduce the inputs of chemical fertilizer and identifying environmentally friendly alternatives to chemical fertilizer are important subjects in present scientific research. In Brazil, researchers are intensively working on further reducing the use of N-fertilizer application by one-half (< 125,000 t N/year) due to the biological nitrogen fixation, for producers to save approximately US$62.5 M annually (Oliveira et al. 2006). The first report on endophytic microorganisms in sugarcane began in Brazil in 1961 was published by Döbereiner (1961), which first demonstrated the link between the nitrogen-fixing bacteria and sugarcane. Afterward, the diversity of sugarcane microorganisms was reported one after another, such as Azospirillum, Burkholderia, Klebsiella, Enterobacter, Erwinia, Gluconacetobacter diazotrophicus, Herbaspirillum rubrisubalbicans, Herbaspirillum seropedicae, and Pseudomonas (Mirza et al. 2001; James and Olivares 1998; Boddey et al. 1991; Döbereiner 1997). Interestingly, the isolated microbial species and types slightly varied from place to place. According to the characteristics that can promote plant growth using endophytes, the endophyte communities and species were subjected to the regions and hosts (Shymanovich and Faeth 2019). Although there are many studies on endophytes (Silva et al. 2018; Tejera et al. 2005), few studies were conducted in Yunnan province, which is one of the major sugarcane production regions in China.
Therefore, this study was aimed to explore the resources of endophytic bacteria in Yunnan province. The isolation of native functional strains has great significance for the development of local agriculture due to the influence of the host genotype and environment on endophytic bacteria. Thus, the present research provides a systematic understanding and paves the way for further experimental exploration on the relationship between sugarcane with its endophytes, facilitating the development and utilization of microbial resources.
Materials and Methods
Isolation of Endophytic Bacteria
Endophytic bacteria were isolated from 6-month-old sugarcane plants (Yunzhe 99-91, YZ99), grown in the Field Gene Bank of Yunnan Agricultural University (YNAU), Kunming, Yunnan (N25.138726°, E102.758756°). The field has been in monoculture for more than ten years, and sugarcane grew in a typical subtropical environment. Healthy, non-flowering stalks and leaves were randomly collected from three independent plants of the same variety. Leaves, stems, and roots across these repeats were served as the main research material, which was collected for one gram from each repeat, respectively. Finally, one gram of these tissues of each sample were grinded separately, each seedling was treated as a single replicate. The operative procedures were as follows: (1) all of the above-mentioned materials were rinsed with tap water and gently wiped with a soft brush for two minutes to remove the surface impurities and dried with a paper towel to remove the water, (2) the above pre-treated stems, leaves, and roots tissue of 1.0 g were sampled and the stems and leaves were surface disinfected with 75% alcohol for 1 min and roots for 3 min, then sterilized in 1% NaClO solution for 3 min and 6 min, respectively, rinsed with sterile water six times to remove the disinfectant, and finally, dried using sterile filter paper, and (3) the sterilized tissue samples were placed in a sterile mortar that contained 9 mL of sterile water and uniformly grounded to homogenize the tissues. Then, the obtained suspension was serially diluted to concentrations of 10–3, 10–4, and 10–5. Next, 100 uL of the cell suspension described above were cultured on Luria–Bertani (LB) agar plate (g/L) (NaCl-10; bacto tryptone-5; yeast extract-5, and agar-20) at 37 °C for 1–2 days, and each gradient was set for three replicates. In addition, in order to determine the success of the surface sterilization procedure, 100 μL of water of the last rinsed explants was placed on LB agar and incubated at 25 °C for 7 days. No colonies appeared in the dish to thoroughly confirm the disinfection.
Molecular Identification
All isolates were cultured on LB solid medium at 28 °C for 3 days, and consistent morphological appearances were grouped as one category. The molecular identification of strains was performed. A total of 43 isolates were tested and grown overnight in LB medium for 24 h at 30 °C. After incubation, DNA extraction was performed according to Cheng and Jiang (2006) procedure. Three housekeeping genes (16S rRNA, gyrB, and rpoB) were amplified, respectively. The list of all primers was presented in Supplementary Table S1 (Additional file 1). The polymerase chain reaction (PCR) was performed in a thermocycler (Veriti 96-Well Thermal Cycler, ABI, USA) as follows: initial denaturation at 94 °C for 4 min and 30 s, followed by 30 cycles of denaturation at 94 °C for 45 s, annealing at 53 °C for 45 s, extension at 72 °C for 1 min, and final extension at 72 °C for 10 min. PCR products were sequenced by Qingke Company (ABI 3730xl Sanger). The sequencing results were spliced using DNAMAN V6, and the homology was compared with the National Center for Biotechnology Information (NCBI) database. The NCBI alignment search tool, BlastN, was used to identify homologous sequences in GenBank (http://blast.ncbi.nlm.nih.gov/Blast.cgi). Sequences that had ≥ 97% identity with several species of the same genus were identified up to the genus level, and homology (≥ 99%) identity was performed for single species (Drancourt et al. 2000).
Screening for Plant Growth-Promoting Traits
Isolates were screened for the plant growth-promoting (PGP) traits, such as nitrogen fixation, tricalcium phosphate [TCaP, Ca3 (PO4)2], calcium phytate (Ca-phy), and potassium (K) solubilization, and siderophore production. The screening was carried out using agar plate assays in Absinthe (Ashby) culture medium (g/L) (KH2PO4-0.2, MgSO4·7H2O-0.2, NaCl-0.2, CaCO3-5, mannitol-10, CaSO4·2H2O-0.1, and agar-18) for the nitrogen fixation assay (Xi et al. 2005). The culture medium of Pikovskaya (PVK) (g/L) (Nautiyal 1999) (glucose-10, (NH4)2SO4-0.5, NaCl-0.2, KCl-0.2, FeSO4·7H2O-0.002, MnSO4·4H2O-0.05, CaCO3-5, and agar-18.0) and the Ca-Phy medium (g/L) (Kumar et al. 2013) (glucose-15, Calcium phytate-5, (NH4)2SO4-0.5, MgSO4·7H2O-0.5, KCl-0.5, FeSO4·7H2O-0.01, MnSO4·4H2O-0.1, and agar-18.0) were used for the phosphate solubilization assay. The Pérez-Miranda method was used to detect the siderophore production (Pérez-Miranda et al. 2007). In general, the diameter of the measurable halo zone (D)-to-colony diameter (d) ratio was used to evaluate the ability of the strains to fix nitrogen, solubilize phosphorus, and produce siderophore (Wang et al. 2018). At this stage of the screening, there was no attempt to quantify the cell numbers, and reactions on the agar plates were visually scored. All isolated colonies were transferred into the medium, as described above, and incubated at 28 °C for 7 days. For the determination, clear halo zones were observed around the bacterial colonies. All assays described above were repeated thrice, with three replicates.
Plant Growth-Promoting Properties of Strain B9
According to the previous isolation and cultivation of bacteria, a functional strain was screened out, which was named B9, and this owned the traits of PGP. In order to investigate the biological activity, the quantitative determination of nitrogen fixation, potassium solubilization, organic and inorganic phosphate solubilization was carried out. The nitrogenase activity was assayed using a modified acetylene reduction assay (ARA)(Berman-Frank et al. 2001; Hardy et al. 1968). For the quantitative determination of phosphorus-dissolving capacity, the following mediums were used: PVK, the National Botanical Research Institute’s Phosphate (NBRIP) growth medium (g/L) (Shymanovich and Faeth 2019) (glucose-10, MgCl2·6H2O-5, MgSO4.H2O-0.25, KCI-0.2, (NH4)2SO4-0.1, and Ca3(PO4)2-5, pH-7), and the Ca-Phy and modified potassium medium (g/L) (NaH2PO4-2, MgSO4·7H2O-0.2, FeCl3-0.05-, sucrose-5, CaCO3-0.1, potassium feldspar-1, and agar-18, pH-7). A single colony of B9 was picked and incubated in the above medium, and the cultures were held in a shaking incubator at 36 °C, shaking at 160 r/min. The negative control (CK) is comprised of the culture filtrate that was not inoculated with the bacteria and incubated under the same conditions as the treatments. After culturing for 1, 3, 5, 7, and 9 days, the culture medium was centrifuged for five minutes (12,000 r/min), and the pH was determined using a calibrated Jenway 3510 pH meter. Then, the content of soluble phosphorus was determined using the molybdenum blue method (Maliha et al. 2004). Furthermore, for estimation of the potassium-dissolving capacity, the strain was also inoculated in the liquid potassium medium (g/L) (glucose-10, (NH4)2SO4-0.5, yeast extract-0.5, MgSO4·H2O-0.3, Na2HPO4-2, FeSO4·7H2O-0.03, MnSO4·4H2O-0.03, K-feldspar-2, pH-7) for 1, 3, 5, and 7 days and was centrifuged for five minutes (12,000 r/min) before detection (Hariprasad and Niranjana 2008). Then, the soluble potassium in the medium was determined using the flame photometry (4530F atomic absorption spectrophotometer (PC control), Shanghai, China). The experiments were repeated three times, with three replicates per sample.
Identification of Strain B9
Strain B9 was identified on the basis of morphological, physiological, biochemical, and molecular identification (Küpfer et al. 2006; Yamamoto and Harayama 1995). The citrate utilization test, amylohydrolysis (starch hydrolysis test), nitrate reduction test, indole test, gelatin liquefaction test, hydrogen sulfide test, and other physiological and biochemical indexes were performed according to the common bacterial system identification manual method (Young 1926). The website (www.bacterio.net) was used as an auxiliary tool to find the reference strain name, and the orthologous sequences used for multiple sequence alignments were downloaded from NCBI. Multiple nucleotide sequences were then aligned using ClustalW on MEGA 6 (Cummings 2014; Thompson et al. 1994). The phylogenetic tree was constructed using the maximum likelihood method with Kimura two-parameter model; the bootstrap analysis using 1000 replications was performed to assess the relative stability of the branches.
Production of Organic Acids (OAs) by B9 Strain
The OAs produced by the B9 strain was determined by High-performance liquid chromatography (HPLC). The OAs standard products including tartaric acid, succinic acid, malic acid, lactic acid, acetic acid, citric acid, and propionic acid were purchased from Macklin Biochemical Technology Co., Ltd., China and the NBRIP was used for measurements of the organic acids in the test medium. Strain B9 was transferred in the above medium and incubated in a shaker set at 160 r/min and 36 °C, the blank medium was used as the control. The determination of organic acid was carried out on the 9th day after inoculation of the B9 strain.
Production of Phytohormones by Strain B9
The phytohormones produced by the B9 strain were determined by High-Performance Liquid Chromatography Mass Spectrometry (HPLC–MS). The phytohormone standard products including indole-3-acetic acid (IAA), gibberellin (GA3), gibberellin (GA4 + 7), Zeatin (ZT), 6-Benzylaminopurine (6-BA), 6-furfurylaminopurine (6-KT), and other experimental reagents were purchased from Solarbio (Beijing, China). LB and Landy media (l-tryptophan loading 0.003 mol/L) (Landy et al. 1947; Glickmann and Dessaux 1995) were used for bacterial growth and the blank medium was used as the control. After the strain B9 inoculation at 37 °C, with shaking at 230 r/min, the samples of the culture broth were taken at intervals of 2 days, starting from the third to the seventh day. The harvested samples were analyzed by HPLC–MS, followed by above described protocol (Glickmann and Dessaux 1995). The varieties and contents of hormones in the LB medium and the content of IAA in the Landy medium were determined, respectively. For each outcome, the chromatogram of IAA, GA3, GA4 + 7, 6-BA, and 6-KT were processed using the software Xcilabur 3.0 (Thermo), and the chromatogram collection and integration of compounds in the determination of OAs were processed using the Agilent Chemstation software.
Preparation of Liquid Inoculum
Unless otherwise specified, LB broth was used for bacterial growth and proliferation. 1 mL of the B9 overnight culture was inoculated with 1000 mL of LB medium, and the cells were grown under agitation (160 r/min) at 37 °C for 3 days. Afterward, these cultures were centrifuged at 12,000 r/min for 15 min at 4 °C. The pellets were re-suspended in 500 mL of sterile water and repeated three times to remove the residual nutrients of medium and stored in the refrigerator at 4 °C. Before use, the cell concentration of B9 was adjusted to 108 CFU/mL.
Effect of Strain B9 on the Growth of Micropropagated Sugarcane Plantlets
A pot experiment was used to explore the growth-promoting ability of B9 strain on sugarcane. The potted soil consisted of nursery soil (alkali-hydrolyzable nitrogen 347.72 ± 3.41 mg/kg, available phosphorus 5.03 ± 0.43 mg/kg, total potassium 287.33 ± 2.65 mg/kg). This soil was filled in a growing basin (9 × 9 cm culture plastic cup with an average quality of 300 ± 20 g/pot).
To exclude the interference of other factors like vertical transmission properties of endophytes, the micropropagated sugarcane plantlets were developed from the sugarcane cultivar ROC22 and used in this experiment. After the process of differentiation, dedifferentiation, and redifferentiation for stem apical meristems, the generated plantlets were transferred to the nursery to obtain the complete plant with three leaves to prepare the strain inoculation. Twenty sugarcane seedlings (ROC22 cultivar) were transplanted into pots containing soil as mentioned above. Before the bacterial inoculation, the seedlings were watered as required until grown under normal conditions, with consistent growth. Bacteria treatment was applied to the sugarcane seedlings 10 days, 20 days, and 30 days after transplantation. During the inoculation, the inoculums mentioned above were diluted and slowly poured into the root of the plant and each pot was treated with strain B9 (~ 106 CFU/pot). The Nil treatment without inoculation (strain B9) served as the control and supplied the same amount of sterile water to ensure the consistency of the soil moisture. Pots were watered regularly and 15 mL sterile Hoagland dilution (tenfold dilution) was applied to each treatment pot at 20 days and 40 days after transplantation, to ensure that the essential nutrients for plant growth are provided (Hoagland and Arnon 1950). These plants were grown in a growth chamber with a 16-h light/8-h dark cycle and a constant temperature of 35 °C. Agronomic traits were used to measure at the time of phenotypic divergences appeared, which included the stem diameter and plant height, root length, root projected, photosynthesis, etc. Centimeter ruler, vernier caliper were used to measure the height, root length, leaf length, and stem; the traits related to the roots were measured using the MICROTEK scanner. A total of N in the plant was analyzed using the Kjeldahl digestion method (Alkali-hydrolyzable); the available P in the plant samples was estimated using the molybdovanadate method (Bray 1945), and the available K was analyzed using an atomic absorption spectrophotometer (AAS6300, Japan).
Effect of Strain B9 on the Growth of Six Sugarcane Genotypes
The present study aimed to compare the growth-promoting ability of strain B9 on different sugarcane genotypes. The six sugarcane varieties including ROC22, Yuetang 93-159 (YT93), Dianzhe11-728 (DZ11), Liucheng 09-182 (LC09), GuiTang11 (GT11), Dianzhe 01-58 (DZ01) were used for the treatment by dipping them in the liquid inoculum of B9 (~ 106 CFU/mL). With the same thickness, growth period, and position, the sugarcane gems were washed in running water to remove the dirt and cut into pieces, with a size of approximately 8 cm (younger and vigorous). Each variety was divided into two groups, inoculated with the strain B9 and no inoculation as control. Each group had no less than ten repeats. These gems were placed into the stainless steel disc (50 × 35 × 4.8 cm, length, width, and height). The inoculated group was poured with 2 L of B9 inoculum (~106 CFU/disc), and the non-inoculated group was poured with the 2 L of sterile water. Both treatments were cultured at a constant temperature chamber at 37 °C under 12-h light and 12-h darkness. The hypocotyl and cotyledon lengths were observed after 6 days.
The Construction of Green Fluorescent Protein-Tagged B9 (B9-gfp) and Plasmid Stability Under the Non-selective Condition
The B9-gfp was obtained through the conjugal transfer of the pHT01-P43GFPmut3a plasmid that carried the gene encoding green fluorescent protein (GFP) into the B9 cytoplasm, and this was used instead of its wild type in colonization preference test of the present study. To assess the stability of the B9-gfp strain without selection, B9-gfp was cultured overnight in LB medium (10 µg/mL chloramphenicol), followed by the adjustment to a suspension [optical density (Hallmann et al. 1999) 600 = 1.0] in LB broth. Subsequently, the stability was evaluated through continuous culturing in fresh LB broth (0.1% w/w, per five hours) without chloramphenicol (Cm) for 60 h at 37 °C under shaking (160 r/min). Morphological characterization was performed to compare the wild-type B9 and B9-gfp based on growth rate and morphology (sporulation rate and color). The single colony of B9-gfp and wild-type B9 was introduced to 100 mL of fresh LB broth and cultured under the aforementioned conditions. During the inoculation, the number of bacteria of the culture was measured every 2 days by the dilution plate method, and the number of the fluorescent colony-forming unit were counted under the blue laser source (488 nm) photomultiplier tube.
In Vitro Colonization Study of B9 in Different Sugarcane Tissues
The germinated sterile tissue seedlings of (ROC22) were transferred into 5 × 30 cm (diameter × height) sterile glass tubes containing 20 mL of 1:10 diluted hoagland nutrient solution. Before inoculation, strain B9-gfp was cultured in LB medium (10 µg/mL chloramphenicol) for 3 days. The bacteria was collected by centrifugation at 10,000 r/min for 15 min and then re-suspended in sterile distilled water to obtain a concentration of approximately 108 CFU/mL. Then, the cell suspension was transferred to plastic glass tubes containing B9-gfp at the concentration of 106 CFU/tube, or sterile water as control. The populations of B9-gfp in sugarcane tissues were monitored after 10 days of inoculation. The fresh samples of the roots and leaves of the plants were collected, and the colonization of B9-gfp bacteria in the tissue culture seedlings was observed by Laser Scanning Confocal Microscopy (LSCFM) after slicing.
Data Collection and Analysis
The difference between groups was compared by one-way analysis of variance (ANOVA) and least significant difference (LSD) test, where P ≤ 0.05 and P ≤ 0.01 were considered as significant and extremely significant, respectively. The analysis was performed with IBM SPSS 23.0 and GraphPad prism 8.0.1.
Results
Isolation and Identification of Endophytic Bacteria with Plant Growth Growth-Promoting Traits
A total of 43 bacterial strains were isolated from the leaves, stems, and roots tissues of sugarcane cultivar (Yunzhe 99-91). Among these, 35 were obtained from the root, 6 from the stem, and 2 from leaf tissues. From the results, we can observe that the distribution of the strains was influenced by the ecological niche of the plant and the number of strains was greater in the root as compared to stem and leaf tissues. Then, all isolates were grouped on basis of the colony shape and color and identified by rpoB and gyrB gene sequence analysis. As shown in Table 1, the most abundant genera were Bacillus, Burkholderia, and Acinetobacter, followed by Pseudomonas, Enterobacter, Pantoea, and Paenibacillus. Finally, all isolates were examined in vitro for their PGP traits including N2-fixation, inorganic phosphate solubilization, and the mineralization of organic phosphate. After one week of incubation on the selected medium, screening of all isolates was performed by comparing the presence and size of the halo zone formed around the isolates. Following an initial screening, one strain named B9, which had multiple PGP traits, was selected for the detailed study. The actual values were presented in Table S2 (Additional file 2). The formation of the halo zone on the Ashby solid medium was used as the parameter for the evaluation of the potential nitrogen fixation of the B9 strain (Fig. 1a). The presence of measurable halo zone on PVK (Fig. 1b) and Ca-Phy (Fig. 1c) represented the ability of the B9 strain to dissolve inorganic and organic phosphorus, respectively. The phosphate-solubilizing capacity of the strain was preliminarily assessed based on the size of the zone around each colony; it was speculated that the dissolved phosphorus content of B9 to the organic phosphorus source was higher than that of the inorganic phosphorus source. Siderophore-producing strains were detected by the CAS agar plate by removing Fe3+ from the chromazurol S, as indicated by the color changes, from blue to orange. The CAS plate indicated that isolate B9 was a potent siderophore-producing bacterium (Fig. 1d).
In vitro biological activity of strain B9 for nitrogen fixation, phosphate solubilization, and siderophore production. The colony morphology of B9 on a Ashby plates, b Pikovskaya, c calcium phytate, and d O-CAS. Visible halo zone formation confirms B9 is nitrogen-fixing, phosphate-solubilizing and siderophore-producing bacteria
Identification of Strain B9
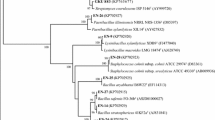
Strain B9 was isolated from the root of the sugarcane cultivar (Yunzhe 99-19). To identify its genus and species, phenotypic identification and housekeeping gene sequencing was performed (Tables 2 and 3; Fig. 2). Single colony morphology of strain B9 on LB medium shows light-orange, undulant round, flat, a bulge in the middle, a wrinkled surface with irregular edges and size of 0.5–1.5 cm. Strain was Gram-positive rod-shaped under the microscope. Physiological and biochemical identification revealed that B9 accorded with the Bacillus characteristics (Table 2). Further strain was identified based on 16S rRNA, rpoB and gyrB sequencing. Results showed that strain B9 was 99.8% similar with B. subtilis CICC10366 based on 16S rRNA sequencing and 99% and 98% similar with B. subtilis ATCC21228 on the basis of gyrB and rpoB sequencing, respectively. The 16S rRNA gene sequence of strain B9 was submitted to the GenBank database with accession number MH935511. Figure 2 presents the three independent evolutionary trees using housekeeping genes (a: gyrB, b: 16S rRNA, and c: rpoB). All the molecular phylogenetic analyses were inferred by using the Maximum Likelihood method based on the Kimura 2-parameter model; the parameters were set to default and the bootstrap values were set to 1000. The percentage of trees in which the associated taxa clustered together was shown next to the branches (bootstrap values). As shown in Fig. 2a, strains of Pseudomonas were used as an outgroup strains, the cluster tree in which strains of Bacillus genus participates, i.e., B. amyloliquefaciens, B. siamensis, B. velezensis, and B. safensis. Strain B9 and B. subtilis formed a cluster with high bootstrap supports value (100%). In Fig. 2b, Pantoea agglomerans strains were used as outgroup strains; strains of Bacillus genera including Bacillus safensis, B. cereus and B. halotolerans were used in clustering tree. Strain B9 was also clustered with B. subtilis. In Fig. 2c, the rpoB housekeeping gene was used to construct the clustering tree. Enterobacter were used as an outgroup, strains of Bacillus genus, i.e., B. altitudinis, B. glycinifermentans, and B. amyloliquefaciens were used, strain B9 was also clustered with B. subtilis with 100% bootstrap value. The phylogenetic analyses revealed that the B9 strain formed a clade with reference B. subtilis with relatively close genetic distance with high support values. According to the theory developed in Drancourt, it was proposed that this had a 99% and 97% sequence similarity as the threshold for defining species and genus identification, respectively (Drancourt et al. 2000). Therefore, the B9 strain was identified as B. subtilis. Meanwhile, the strain has been deposited in the Guangdong Provincial Center for Microbial Strains, with strain number GDMCC accession number: GDMCC 1.1665.
Quantitative Estimation of the Biological Activity of Strain B9
After the primary agar plate screening, quantitative estimation of nitrogen fixation, phosphorus and potassium solubilization detection of the strain B9 were tested. The acetylene reduction activity assay (ARA) was used to test nitrogen-fixing capability and nitrogenase activity of B9 was 1,126.93 ± 30.13 nmol/(mL h), indicating the nitrogen-fixing ability of strain B9. For the case of the P-solubilization test, the amount of soluble P released by strain B9 was measured in the liquid medium, and results showed the content of soluble P in the liquid culture filtrate after inoculating the B9 bacteria (Fig. 3). In the medium after 7 days of inoculation, the highest soluble P content was 290.25 ± 2.65 mg/L in the PVK medium (Fig. 3a) and 199.6 ± 9.72 mg/L in the NBRIP medium (Fig. 3b) with TCaP as the substrate. In the medium with Ca-Phy as the substrate, the content of soluble P reached the maximum at 9 days post-inoculation, and the amount of dissolved P was 1016.53 ± 14.11 mg/L (Fig. 3c). It was observed that the ability of B9 to mineralize organic phosphorus was stronger than solubilize in organic phosphorus, which was consistent with the above primary screening, i.e., formation of halo zone in the solid agar medium. The production of soluble P by B9 strain was significantly higher than that of control, which indicates that the strain has a certain ability to dissolve phosphorus. In the case of the K-solubilizing ability of B9, the highest content of soluble K was determined seventh day after inoculation, and the amount of soluble K was 5.455 ± 0.135 mg/L (Fig. 3d).
Phosphorus (P) and potassium (K)-solubilizing ability of B9 in a liquid medium. a Detection of soluble P content in PvK medium after inoculation of B9 strain. b Detection of soluble P content in NBRIP medium after inoculation of B9 strain. c Detection of soluble P content in Ca-Phy medium after inoculation of B9 strain. d Detection of soluble K content in Ca-Phy medium potassium medium after inoculation of B9 strain. Among them, inorganic phosphorus as the substrate (a, b), Organic phosphorus as the substrate (c) K-feldspar as the substrate (d). An asterisk (**) depicts significant between-group difference (P < 0.01)
Identification and Quantification of Organic Acids Produced by Strain B9
Interestingly, the pH of PVK, NBRIP two mediums, with Ca3(PO4)2 as the substrate, decreased after the inoculation with B9 and slightly increased after day 7. It was noteworthy that when the medium reached the lowest pH, the content of soluble phosphorus reached the highest level. As the content of soluble phosphorus decreased with the subsequent increase in pH, it was assumed that the mechanism of dissolved phosphorus correlated to the production of OAs and soluble phosphorus was assumed to originate from that the surface of Ca3(PO4)2, which was dissolved by OAs. The supernatant of strain B9 was used for HPLC analysis. On the ninth day, the exact types of OAs of acetic acid (306.51 ± 4.94 μg/μL), propionic acid (343.88 ± 19.21 μg/μL), malic acid (14.79 ± 1.02 μg/μL), and citric acid (5.07 ± 4.21 μg/μL) were observed in the fermentation broth (Table 4).
Identification and Quantification of Phytohormone Produced by B9 Bacteria
Plant hormones play an important role in the growth and development of plants. In order to assess the ability of strain B9 to produce hormones, the phytohormone content and composition of B9 culture broth were determined by high-performance liquid chromatography/mass spectrometry (HPLC/MS). The results were presented in Table 5. Among these, the production of IAA was the highest compared to other growth hormones, and the content of IAA produced by the B9 bacteria increased over time. The content of IAA increased by 188.65% from the third to the ninth day. Furthermore, the content of IAA was 66.87 times higher than that of ZT and 22.82 times higher than that of 6-BA after 9 days of inoculation. ZT, a naturally occurring cytokinin in plants, can promote lateral bud growth and stimulate cell division, callus growth, and seed germination. During the 9 days, ZT production initially increased and subsequently decreased. Then, ZT content continued to increase after 5 days. However, the total amount was small, which was maintained at 1.43 ± 0.20 ng/mL. In the case of the 6-BA, the trend of hormone content was the same as that of ZT, which reached the highest level after 9 days, with content of 4.19 ± 0.44 ng/mL (Table 5). At the same time, we measured the content of IAA in Landy medium. The content of IAA at 3, 5, 7, and 9 days of post-inoculation was 1.47, 1.96, 2.17, and 2.56 times higher than controls, respectively (Table 6). Our data showed that the B9 strain could produce more IAA under the premise of l-tryptophan in Landy medium by colorimetric assay, which was consistent with a previous study showing bacteria can produce more IAA under the premise of l-tryptophan in the Landy medium (Shao et al. 2015).
Effect of Strain B9 on Six Different Sugarcane Varieties
To test the effect of B9 on the growth of sugarcane, we inoculated 6 sugarcane genotypes with B9 strain and water as control. In the experiment, the ability of growth promotion of the B9 strain varied with different germplasm, which also confirms that the growth-promoting ability of the strain was affected by the host genotype. Data showed that the bacterial inoculation treatments presented higher hypocotyl length than that without bacterial inoculation; the hypocotyl lengths of these four varieties (DZ11, ROC22, YT93, GT11) were significantly higher than that of the non-inoculated (P < 0.01), for LC09 variety significant difference was observed (P < 0.05), and the variety (DZ01) had no different from the control group (Fig. 4j). Second, after inoculated with strain B9, the cotyledon length of all varieties (LC09, DZ11, ROC22, YT93, GT11, and DZ01) had significantly higher than un-inoculated controls (Fig. 4k) (P < 0.01). The above results revealed that B9 strain inoculation can promote the growth of sugarcane. It was worthwhile to note that the B9 strain has a significant effect on the growth of hypocotyl of sugarcane variety DZ11. While hypocotyl was not germinated in the control group, the B9 strain promoted the growth of hypocotyl with a length of 3.87 ± 1.68 cm. After inoculating the B9 strain, the cotyledon growth of the YT93 treatment was increased by 183.50%, when compared to the control group, and this was followed by DZ11, which increased by 173.33%. There was also a significant increase in hypocotyl length and the number after inoculation.
Growth-promoting ability of B9 strain in six different sugarcane varieties. We recorded the differences in morphology of the sugarcane gems for six different sugarcane cultivar genotypes (the left plot is control and the right for B9 strain treatment respectively) and the ROC22 variety culture seedlings after B9 strain inoculation. a The morphological appearance of the sugarcane gems for the genotypes of sugarcane cultivar YT93. b The morphological appearance of the sugarcane gems for the genotypes of sugarcane cultivar DZ11. c The morphological appearance of the sugarcane gems for the genotypes of sugarcane cultivar LC09. d The morphological appearance of the sugarcane gems for the genotypes of sugarcane cultivar ROC22. e The morphological appearance of the sugarcane gems for the genotypes of sugarcane cultivar DZ01. f The morphological appearance of the sugarcane gems for the genotypes of sugarcane cultivar GT11. g The differences in morphology of sugarcane culture seedlings, the plantlet on the left represent the non-inoculation group, and the plantlet on the right represents the inoculation group. h The root scan results for the inoculation of strain B9 group for sugarcane sterile seedlings at 50 days. i The root scan results for the non-inoculation group for sugarcane sterile seedlings at 50 days. j The effects of hypocotyl length of B9 inoculation in 6 days on different varieties of sugarcane seedlings. k The effects of cotyledon length of B9 inoculation in 6 days on different varieties of sugarcane seedlings. All pictures of the root were performed using a MICROTEK scanner
Effect of Strain B9 on the Growth of Sugarcane Seedlings
All growth parameters of sugarcane seedlings were observed at 55 days post-inoculation of strain B9. Results showed a significant increase in all growth parameters of sugarcane seedlings inoculated with strain B9 as compared to control (Fig. 4g–i). The indexes of seedlings between the treatment group and the control group were measured, including leaf number, leaf width, plant height, photosynthesis, stem diameter, stem length, and the indicators of the root for the root projection area, root area index, root volume, root average diameter, and root length. As the data showed (Table 7), the B9 inoculation treatment was better than the control in all respects. The leaf number, leaf width, and plant height of the B9 treatment group were 5.37%, 66.7%, and 68.52% higher than those in the control group, respectively. Meanwhile, there were significant differences in the photosynthesis rate between the treatment and control groups. The net photosynthesis rate of an indicator of material productivity per unit leaf area was 16.24% higher (15.03 ± 1.32 μmol/m2/s) in the treatment group as compared to the control group. Hence, this could be inferred to promote the efficiency of photosynthesis and increase the accumulation of photosynthetic products after inoculation. The leaf transpiration rate (Trmmol) for the treatment group was 1.99 ± 0.25 mmol/m2/s. This reflects the intensity of the plant transpiration rate, which is the physical quantity of the plant water metabolism status and water use efficiency. The data showed that the treatment group had a high evaporation rate relative to the control group, but there was no significant difference between these two. The total amount of chlorophyll in the treated group (2.95 ± 0.06 mg/g) was 44.61% higher than that in the control group. These data showed that the B9 strain increases the chlorophyll content of plants and contributes to the prior increase in photosynthesis rate. The chlorophyll, photosynthetic rate, and transpiration rate of the leaves were all determined through the photosynthetic level of the plant, and the intensity of the photosynthetic capacity was determined by the accumulation of biological quality. During the sugarcane production, the length and thickness of the stem that determines the yield of the treatment group were 18.77 ± 1.76 cm and 3.76 ± 0.11 mm, respectively. These were 74.44% and 90.86% significantly higher, respectively, when compared to the control group. When weighing the above-ground and underground parts of the plant, it was revealed that the above-ground and underground values were 3.69 ± 0.26 g and 1.31 ± 0.34 g, respectively, in the treatment group, respectively, and these were 284.38% and 445.83% higher than those in the control group. The data were collected from the roots using MICROTEK Scanmaker, in order to induce B9 to be more visible and intuitive for the root growth-promoting changes. In the treatment group, the projection area was 25.52 ± 12.35 cm2, the root area index and root volume was 80.14 ± 38.79 cm2 and 1.17 ± 0.50 cm3, and the scanning root length was 982.28 ± 588.78 cm. These above data indexes were high as 166.67%, 166.78%, 185.36% and 190.64%, when compared to the control (Fig. 4h–i). In order to verify whether inoculation can promote the nutrient uptake by seedlings, the contents of total N, P and K in sugarcane seedlings was measured, and it was clarified that the contents of N, P and K after the B9 treatment were significantly higher when compared to the controls (P < 0.01). These increased by 29.26%, 50.78%, and 15.49%, respectively.
In Situ Colonization of GFP-Tagged B9 on Sugarcane
The growth rates and morphology of the transconjugant cells and the wild-type B9 cells in the antibiotic-free LB medium were not significantly different, suggesting little growth burden from the plasmid to the transconjugant cells. However, it was found that the number of colonies formed by the B9-gfp was lower than that of wild-types (Fig. S1, Additional file 3). At 10 days after inoculation, the root, stem, and leaves samples were assessed using both fluorescence and confocal microscopy. Green fluorescent gfp-tagged B9 cells were found to attach, individually or in groups, to the root surfaces predominantly at root maturation zones. The roots had the highest number of bacteria, followed by the stems and leaves. Cells were visualized under laser confocal microscopy. The viable counts of the test strain in root stabilized at approximately 105–107 CFU/g, 103–105 CFU/g in the stem, and 103–104 CFU/g in the leaves. It can also be observed that the B9 strain has a strong colonization capacity in different tissues of sugarcane (Fig. 5).
Microscopic images show that the gfp-tagged B9 cells colonized on and in the roots, stems, and leaves of the micropropagated ROC22. The bars present the 50 μm. The white arrows denote the location of the B9-gfp, and the arrows point to the representative aggregated bacteria. a Strain B9 colonization on plant root epidermis. b Strain B9 colonization on plant epidermal cells of the stem. c Strain B9 colonization in mesophyll and vascular tissues of the leaf. All images were taken 10 days after inoculation under a laser confocal microscope
Discussion
Yunnan province is one of the largest sugarcane producing regions of China. However, isolation and characterization of endophytic bacteria from sugarcane in Yunnan, China is very less explored (Muήoz-Rojas and Caballero-Mellado 2003). Hence, it is required to isolate and identify an effective endophytic diazotroph from a main agricultural crop like sugarcane. In the present study, several endophytic bacterial strains were isolated from the Yunnan native sugarcane cultivar YZ99-91 and characterized into six different genera. Previously, Wang et al. (2013) isolated 20 endophytic bacteria from the stem tissues of sugarcane species (Badila) planted in Guangzhou Park, and classified into 16 genera. In contrast, we observed less genera as compared to earlier studies might be due to the less number and difference in sugarcane variety and types of endophytes, i.e., obligate or facultative, respectively. The facultative strains were able to colonize on both the surface and plant interior, and survived well in soil, when compared to obligate bacteria that did not survive well in in vitro condition, but colonized the plant interior and aerial parts. Compared with the bacterial species identified by molecular sequencing, traditional isolation methods are insufficient to detect these non-separated microorganisms, but the tangible isolation also provides a material basis for more biological activity research, such as the ability of the strain to produce a variety of hormones and polysaccharides, which can be determined in vitro. Based on the bacterial isolation results presented in this study, the number of endophytic bacterial species was higher in the roots followed by stems and leaves. Endophytic bacteria that promote plant development enter plant tissues in a variety of ways; the roots are the most common entry point for endophytic (Passari et al. 2015). Endophytic bacteria enter different plant tissues in several ways. The most common way to entry is through the roots, through primary and lateral root hair cells, root cracks and wounds, as well as hydrolysis of root cells. Other sites include stomata, particularly on leaves and young stems, lenticels, and germinating radicles (Sørensen and Sessitsch 2006). These results are consistent with the previous findings, which displayed roots have very rich microbial resources as compared to other plant tissues (Araújo et al. 2001; Bai et al. 2002).
In this study, we selected strain B9 because of its high nitrogen-fixing and phosphate and potassium-solubilizing ability. Then, these characteristics were quantitatively studied, and the reasons and mechanisms for this series of biological activities were explored. Currently, the main method for the initial screening of functional strains depends on the production of transparent circle, which is used to determine the biological activity of the strains by comparing the ratio of the clear circle produced by each bacterium to the diameter of the colony itself, and then to quantify the indicators of interest after screening the functional strains with excellent performance. This method has been used for the screening of nitrogen-fixing and phosphate and potassium-solubilizing strains. In this study, the transparent circles produced by strain B9 on Ashby medium indicated its nitrogen-fixing ability. As well as, nitrogenase activity of B9 was 1126.93 ± 30.13 nmol/mL h (565.47 ± 15.04 nmol/gh) by ARA method, which was relatively higher compared to the results of Wang et al. (2020) and Zhang (2016). To avoid the screening omissions caused by inappropriate culture medium as much as possible during the screening process, three selection culture mediums are commonly used for screening phosphate-releasing microorganisms and selected for the detection of phosphorus-releasing characteristics of the strain. In this study, strain B9, a gram-positive bacteria did not obvious to dissolve phosphorus plate compared to the other bacteria, but in the quantitative determination of soluble phosphorus content in liquid culture, the phosphate-solubilizing ability of strain B9 was stronger than our hypothesis. The results are consistent with the results of Nautiyal’s study, i.e., a single clear circle ratio cannot truly reflect the amount of soluble phosphorus released by the strains in the phosphate-solubilizing medium. Nevertheless, the plate screening method is still used as the preferred method for the initial screening test of functional strains due to its simplicity and speed (Nautiyal 1999). Currently, the main mechanisms involved in the phosphorolysis process by phosphate-solubilizing strains are the sink theory (Drancourt et al. 2000), the organic acid (OA) theory (Glickmann and Dessaux 1995), the exopolysaccharide theory, and the acidification theory caused by H+ exocytosis (Illmer and Schinner 1995). Among them, the OA secretion theory is generally recognized as one of the most important mechanisms by which phosphate-solubilizing strains exert phosphate-solubilizing activity: the strain first synthesizes low-molecular-weight organic acids; then the organic acids are secreted extracellularly, causing acidification of its cells and their surroundings through the chelation effect that leads a decrease to the concentration of surrounding metal ions or causes a decrease in the pH of the substrate; finally, H+ replaces various metal elements or Ca2+, leading to the release of phosphorus. At the same time, organic acids secreted by the strains also play an important role in the function of potassium solubilization. In this study, the organic acids produced by the strains were detected as propionic acid, acetic acid, malic acid and citric acid. Among them, propionic acid accounted for the largest amount of all secreted organic acids, but the reason is not yet known and will continue to be studied in depth in subsequent experiments. At this stage, it can be speculated that the phosphate-solubilizing characteristics of the strains are related to its own production of large amounts of organic acids. Although the potassium-solubilizing capacity of strain B9 was found to be weak compared to other rhizospheric potassium-solubilizing strains (4.12–13.95 mg/L), in the later stage, total nitrogen, total phosphorus, and total potassium of plantlets were determined. These treated seedlings after B9 strain inoculation had higher levels of nitrogen, phosphorus, and potassium when compared to the non-bacterial control group. This confirms that the B9 strain can help sugarcane seedlings to obtain more nitrogen, phosphorus and potassium in the growth process.
To explore the growth-promoting characteristics of B9 strains, the promoting effect of the strains on sugarcane aseptic tissue culture seedlings and different sugarcane cultivated varieties gems were studied. According to the experimental results, after the inoculation of B9 strain, the phenotypic and physiological indexes of the plants were better than those of the non-sterile treatment, including more leaves and a larger leaf area. This increased photosynthesis products and the further increase of the biomass level of sugarcane. In the process of sugarcane planting, biomass has always been an important parameter index. Increasing the biomass of sugarcane can increase the income of sugarcane farmers and increase the yield of sucrose. Endophytes can effectively promote plant growth, but it still depends on the local environment and host genotype, and this is where the novelty and implication of our investigation resides (Wei 2016). In the study of plant growth-promoting mechanisms by bacteria, the content of plant hormones produced by the strains was determined (Velázquez et al. 2008; He et al. 2004). For substances that directly promote plant growth, the phytohormones secreted by endophytes affect plants in small amounts and are efficient. The phytohormones produced by B9 were IAA, ZT, and 6-BA. Hence, the hormones produced were non-single, and importantly, auxin accounts for the largest proportion of the phytohormone secretions. In comparison with the IAA content produced by the rhizosphere growth-promoting bacteria SQR9, the B9 strain produced a higher IAA content (Shao et al. 2015). In addition, the auxin-to-cytokinins ratios regulate the root and shoot meristem. This phenomenon can also explain the promoting effect of B9 strains on sugarcane hypocotyl elongation and new shoots sprouting. IAA, during plant growth, promotes cell differentiation and enhances both the elongation and development of lateral roots in plants. Furthermore, this also supports its host during stress conditions, such as drought and pathogenic attacks. In sugarcane production, at the early time of seedling emergence, more numbers of hypocotyl and the length of the hypocotyl, which are helpful to improve the adaptability to the environment, prolong the growth period as soon as possible, promote the quality of sugarcane, and allows this to be harvested ahead of time. Hence, this has great application value to its production. At the same time, the healthier seedlings could enhance the stress resistance, cost-efficient and increase plant yield. It was also found that B9 strains had the largest number of colonization in the root system by plate counting. Furthermore, through the colonization assay, it was proven that a series of morphological changes in the root growth of plants is due to the existence of endophytes. The colonization also reflects the preference of the strain, providing a theoretical basis inoculation method for the future.
Exploring the entire process of plant growth cannot be interpreted from a single level, but involves a complex regulatory network. However, it is clear that the absorption and utilization of mineral elements are indispensable in the process of plant growth (Yu et al. 2020). Compared with barren nutrient supply, relatively well-funded NPK content is of great significance to crop growth and increased yield (Zhang et al. 2019). This also explains why the screening of growth-promoting strains is mainly related to whether they can have the characteristics of nitrogen fixation, phosphorus solubilization, and potassium dissolution. At the same time, the organic acids produced by microorganisms in the process of their own metabolism are closely related to plant nutrient absorption, which leads to the study of the growth-promoting mechanism of microorganisms and plants; determination of the secretion of organic acids is an important work in the study of the growth-promoting mechanism of strains, because it mediates better plant uptake of mineral nutrients from the soil. In addition, a large number of literatures also prove that the exogenous plant hormones produced by the strain can promote the growth of crops. These growth hormones include auxin and gibberellins (Sun et al. 2019). However, although we know from the above research that the strain can assist sugarcane seedlings to absorb more mineral elements and promote their growth, the growth-promoting process is more combined with the setting of the thesis topic, focusing on the perspective of exploring the biological activity of the strain. The lack of some feedback data within the plant is the shortcoming of this study. For example, when sugarcane seedlings were inoculated with B9 strain, the photosynthetic efficiency was increased, and how these more photosynthetic products will be distributed and utilized in sugarcane, and which genes and proteins are regulated and involved in the process of material transformation will continue to be reported in follow-up research.
In the present study, the multiple PGP traits of the B9 strain provide a direction for further research on the plan of increasing yield, reducing the use of fertilizer for sugarcane production. The growth-promoting effects and functions of sugarcane endophytes were confirmed in the field of exploring the interaction between sugarcane and microorganisms. The present study also opens new lines of investigation, where the strain affects sugarcane growth by changing the transcriptional and metabolic levels of sugarcane.
Data Availability
All data during this study are included in this published article and its supplementary information files.
Abbreviations
- OAs:
-
Organic acids
- IAA:
-
Indole-3-acetic acid
- 6-BA:
-
Cytokinin 6-benzyladenine
- ZT:
-
Zeatin
- KT:
-
6-Furfurylaminopurine
- YT93:
-
Yuetang 93-159
- DZ11:
-
Dianzhe11-728
- LC09:
-
Liucheng 09-182
- GT11:
-
GuiTang11
- DZ01:
-
Dianzhe 01-58
- YZ99:
-
Yunzhe-99-91
- h:
-
Hour
- PGPR:
-
Plant growth-promoting rhizosphere
- P:
-
Phosphorus
- K:
-
Potassium
- PGPE:
-
Plant growth-promoting endophytes
- EPS:
-
Extracellular polymeric substances
- N:
-
Nitrogen
- m:
-
Million
- YNAU:
-
Yunnan Agricultural University
- Absinthe:
-
Ashby
- Ca-Phy:
-
Calcium phytate
- PVK:
-
Pikovskaya
- D:
-
Halo diameter
- d:
-
Colony diameter
- LB:
-
Luria–Bertani broth
- NBRIP:
-
National Botanical Research Institute’s Phosphate
- OAs:
-
Organic acids
- ARA:
-
Acetylene reduction activity
- HPLC/MS:
-
High-performance liquid chromatography/mass spectrometry
- LC–MS:
-
Liquid chromatography mass spectrometry
- HPLC:
-
High-performance liquid chromatography
- Cm:
-
Chloramphenicol
- Tab.:
-
Table
- Fig.:
-
Figure
- CK:
-
Control
- YNAU:
-
Yunnan Agricultural University
- PGP:
-
Plant growth-promoting
- r:
-
Revolution
- min:
-
Minute
- ARA:
-
Acetylene reduction assay
References
Araújo W, Maccheroni W, Aguilar-Vildoso C, Barroso P, Saridakis H, Azevedo J (2001) Variability and interactions between endophytic bacteria and fungi isolated from leaf tissues of citrus rootstocks. Can J Microbiol 47(3):229. https://doi.org/10.1139/cjm-47-3-229
Bai Y, D’Aoust F, Smith DL, Driscoll BT (2002) Isolation of plant-growth-promoting Bacillus strains from soybean root nodules. Can J Microbiol 48(3):230–238. https://doi.org/10.1139/w02-014
Berman-Frank I, Cullen JT, Shaked Y, Falkowski SPG (2001) Iron availability, cellular iron quotas, and nitrogen fixation in Trichodesmium. Limnol Oceanogr 46(6):1249–1260. https://doi.org/10.2307/2670974
Boddey RM, Urquiaga S, Reis V, Döbereiner J (1991) Biological nitrogen fixation associated with sugar cane. Plant Soil 137(1):111–117. https://doi.org/10.1007/BF02187441
Boddey RM, Urquiaga S, Alves BJR, Reis V (2003) Endophytic nitrogen fixation in sugarcane: present knowledge and future applications. Plant Soil 252(1):139–149. https://doi.org/10.1023/A:1024152126541
Bray R (1945) Determination of total, organic and available forms of phosphorus in soils. Soil Sci 59(1):39–46
Cheng HR, Jiang N (2006) Extremely rapid extraction of DNA from bacteria and yeasts. Biotechnol Lett 28(1):55–59. https://doi.org/10.1007/s10529-005-4688-z
Compant S, Reiter B, Sessitsch A, Nowak J, Clement C, Ait Barka E (2005) Endophytic colonization of Vitis vinifera L. by plant growth-promoting bacterium Burkholderia sp. strain PsJN. Appl Environ Microbiol 71(4):1685–1693. https://doi.org/10.1128/AEM.71.4.1685-1693.2005
Cummings MP (2014) MEGA (Molecular evolutionary genetics analysis). Dictionary of Bioinformatics and Computational Biology American Cancer Society
Döbereiner J (1961) Nitrogen-fixing bacteria of the genus Beijerinckia Derx in the rhizosphere of sugar cane. Plant Soil 15(3):211–216
Döbereiner J (1997) Biological nitrogen fixation in the tropics: Social and economic contributions. Soil Biol Biochem 29(5):771–774. https://doi.org/10.1016/S0038-0717(96)00226-X
Drancourt M, Bollet C, Carlioz A, Martelin RC, Raoult D (2000) 16S Ribosomal DNA sequence analysis of a large collection of environmental and clinical unidentifiable bacterial isolates. J Clin Microbiol 38(10):3623–3630. https://doi.org/10.1002/1097-4660(200010)75:10%3c933::AID-JCTB301%3e3.3.CO;2-3
Egamberdiyeva D (2007) The effect of plant growth promoting bacteria on growth and nutrient uptake of maize in two different soils. Appl Soil Ecol 36(2–3):184–189. https://doi.org/10.1016/j.apsoil.2007.02.005
Fan X, Guo JW, Deng J, Zhang YB, Zhang XX, Yang SL, Li RD (2018) Evaluationon the fertilization of sugarcane in the main ecological areas of Yunnan Province. J Plant Nutr Fertil (Chin) 118(1):249–258
Fen S (2001) Characteristics of sugarcane fertilizer demand and fertilization technology. Appl Technol Rural Areas 12(2):15. https://doi.org/10.3969/j.issn.1007-7103.2001.02.015
Glickmann E, Dessaux Y (1995) A critical examination of the specificity of the salkowski reagent for indolic compounds produced by phytopathogenic bacteria. Appl Environ Microbiol 61(2):793–796. https://doi.org/10.1002/bit.260450314
Gyaneshwar P, James EK, Mathan N, Reddy PM, Reinhold-Hurek B, Ladha JK (2001) Endophytic colonization of rice by a diazotrophic strain of Serratia marcescens. J Bacteriol 183(8):2634–2645. https://doi.org/10.1128/JB.183.8.2634-2645.2001
Hallmann J, Rodrı́Guez-Kábana RR, Kloepper JW (1999) Chitin-mediated changes in bacterial communities of the soil, rhizosphere and within roots of cotton in relation to nematode control. Soil Biol Biochem 31(4):551–560. https://doi.org/10.1016/S0038-0717(98)00146-1
Hardy RW, Holsten RD, Jackson EK, Burns RC (1968) The acetylene-ethylene assay for n(2) fixation: laboratory and field evaluation. Plant Physiol 43(8):1185–1207. https://doi.org/10.1104/pp.43.8.1185
Hariprasad P, Niranjana SR (2008) Isolation and characterization of phosphate solubilizing rhizobacteria to improve plant health of tomato. Plant Soil 316(1):13–24. https://doi.org/10.1007/s11104-008-9754-6
He H, Cai XQ, Lan CZ (2004) Colonization, promotion growth and biocontrol for anthracnose of endophytic bacterium BS-2 from Capsicum annuum in cabbage. J Plant Protect 31(4):347–352. https://doi.org/10.1300/J064v24n01_09
Hoagland DR, Arnon DI (1950) The water culture method for growing plants without soil. Calif Agr Exp Stn Circ 347:1–32. https://doi.org/10.1016/S0140-6736(00)73482-9
Illmer P, Schinner F (1995) Solubilization of inorganic calcium phosphates-solubilization mechanisms. Soil Boil Biochem 27(3):257–263. https://doi.org/10.1016/0038-0717(94)00190-C
Iqbal A, Dong Q, Wang X, Gui H, Zhang H, Zhang X, Song M (2020) Variations in nitrogen metabolism are closely linked with nitrogen uptake and utilization efficiency in cotton genotypes under various nitrogen supplies. Plants (Basel) 9(2):250. https://doi.org/10.3390/plants9020250
James EK, Olivares FL (1998) Infection and colonization of sugar cane and other graminaceous plants by endophytic diazotrophs. Crit Rev Plant Sci 17(1):77–119. https://doi.org/10.1080/07352689891304195
John JC, Jishma P, Karthika NR, Nidheesh KS, Ray JG, Mathew J, Radhakrishnan EK (2017) Pseudomonas fluorescens R68 assisted enhancement in growth and fertilizer utilization of Amaranthus tricolor (L.). Biotech 7(4):256. https://doi.org/10.1007/s13205-017-0887-2
Khalid A, Arshad M, Zahir Z (2010) Screening plant growth-promoting rhizobacteria for improving growth and yield of wheat. J Appl Microbiol 96(3):473–480. https://doi.org/10.1046/j.1365-2672.2003.02161.x
Kumar V, Singh P, Jorquera MA, Sangwan P, Kumar P, Verma AK, Agrawal S (2013) Isolation of phytase-producing bacteria from Himalayan soils and their effect on growth and phosphorus uptake of Indian mustard (Brassica juncea). World J Microb Biot 29(8):1361–1369. https://doi.org/10.1007/s11274-013-1299-z
Küpfer M, Kuhnert P, Korczak B, Peduzzi R, Demarta A (2006) Genetic relationships of Aeromonas strains inferred from 16S rRNA, gyrB and rpoB gene sequences. Int J Syst Evol Micr 56(12):2743–2751. https://doi.org/10.1099/ijs.0.63650-0
Landy M, Rosenman SB, Warren GH (1947) An antibiotic from Bacillus subtilis active against pathogenic fungi. J Bacteriol 54(1):24. https://doi.org/10.3181/00379727-67-16367
Lery LMS, Hemerly AS, Nogueira EM, Krüger WMAV, Bisch PM (2010) Quantitative proteomic analysis of the interaction between the endophytic plant-growth-promoting bacterium. Mol Plant Microbe Interact 24(5):562–576. https://doi.org/10.1094/MPMI-08-10-0178
Li Y, Wei K (2020) Comparative functional genomics analysis of cytochrome P450 gene superfamily in wheat and maize. BMC Plant Biol 20(1):93. https://doi.org/10.1186/s12870-020-2288-7
Lodewyckx C, Vangronsveld J, Porteous F, Moore E, Taghavi S, Mezgeay M, Lelie D (2002) Taylor & Francis online: endophytic bacteria and their potential applications. Crit Rev Plant Sci 21(6):583–606
Maliha R, Samina K, Najma A, Sadia A, Farooq L (2004) Organic acids production and phosphate solubilization by phosphate solubilizing microorganisms (PSM) under in vitro conditions. Pak J Biol Sci 7(2):2. https://doi.org/10.3923/pjbs.2004.187.196
Mirza MS, Ahmad W, Latif F, Haurat J, Bally R, Normand P, Malik KA (2001) Isolation, partial characterization, and the effect of plant growth-promoting bacteria (PGPB) on micro-propagated sugarcane in vitro. Plant Soil 237(1):47–54. https://doi.org/10.1007/s11676-018-0723-5
Muήoz-Rojas J, Caballero-Mellado J (2003) Population dynamics of Gluconacetobacter diazotrophicus in sugarcane cultivars and its effect on plant growth. Microb Ecol 46(4):454–464. https://doi.org/10.2307/4251831
Nautiyal CS (1999) An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol Lett 170(1):265–270
Olivares FL, Baldani V, Reis VM, Baldani JI, Dӧbereiner J (1996) Occurrence of the endophytic diazotrophs Herbaspirillum spp. in roots, stems, and leaves, predominantly of Gramineae. Biol Fertil Soils 21(3):197–200. https://doi.org/10.1007/BF00335935
Oliveira ALM, Canuto EL, Urquiaga S, Reis VM, Baldani JI (2006) Yield of micropropagated sugarcane varieties in different soil types following inoculation with diazotrophic bacteria. Plant Soil 284:23–32. https://doi.org/10.1007/s11104-006-0025-0
Passari AK, Mishra VK, Gupta VK, Yadav MK, Saikia R, Singh BP (2015) In vitro and in vivo plant growth promoting activities and DNA fingerprinting of antagonistic endophytic actinomycetes associates with medicinal plants. PLoS ONE 10:1–10. https://doi.org/10.1371/journal.pone.0139468
Patten CL, Glick BR (1996) Bacterial biosynthesis of indole-3-acetic acid. Can J Microbiol 42(3):207–220. https://doi.org/10.1139/m96-032
Pérez-Miranda S, Cabirol N, George-Téllez R, Zamudio-Rivera LS, Fernández FJ (2007) O-CAS, a fast and universal method for siderophore detection. J Microbiol Meth 70(1):127–131. https://doi.org/10.1016/j.mimet.2007.03.023
Rahman L, Shinwari ZK, Iqrar I, Rahman L, Tanveer F (2017) An assessment on the role of endophytic microbes in the therapeutic potential of Fagonia indica. Ann Clin Microbiol Antimicrob 16(1):53. https://doi.org/10.1186/s12941-017-0228-7
Reinhold B, Hurek T, Fendrik I (1987) Cross-reaction of predominant nitrogen-fixing bacteria with enveloped, round bodies in the root interior of kallar grass. Appl Environ Microbiol 53(4):889–891. https://doi.org/10.1002/bit.260290516
Rondeau M, Esmaeel Q, Crouzet J, Blin P, Gosselin I, Sarazin C, Pernes M, Beaugrand J, Wisniewski-Dyé F, Vial L, Faure D, Clément C, Ait BE, Jacquard C, Sanchez L (2019) Biofilm-constructing variants of Paraburkholderia phytofirmans PsJN outcompete the wild-type form in free-living and static conditions but not in planta. Appl Environ Microbiol 85(11):e02670-e12618. https://doi.org/10.1128/AEM.02670-18
Ryan RP, Germaine K, Franks A, Ryan D, Dowling DN (2010) Bacterial endophytes: recent developments and applications. FEMS Microbiol Lett 278(1):1–9. https://doi.org/10.1111/j.1574-6968.2007.00918.x
Santoyo G, Moreno-Hagelsieb G, Orozco-Mosqueda MDC, Glick BR (2015) Plant growth-promoting bacterial endophytes. Microbiol Res 183:92–99. https://doi.org/10.1016/j.micres.2015.11.008
Schulz BJE, Boyle CJC, Sieber TN (2006) Microbial root endophytes volume 9 || mutualistic interactions with fungal root endophytes. Soil Biol 9:261–279
Shao J, Xu Z, Zhang N, Shen Q, Zhang R (2015) Erratum to: contribution of indole-3-acetic acid in the plant growth promotion by the rhizospheric strain bacillus amyloliquefaciens SQR9. Biol Fertil Soils 51(3):331. https://doi.org/10.1007/s00374-014-0984-x
Sheng X, Xia J, Jiang C, He L, Qian M (2008) Characterization of heavy metal-resistant endophytic bacteria from rape (Brassica napus) roots and their potential in promoting the growth and lead accumulation of rape. Environ Pollut 156(3):1164–1170. https://doi.org/10.1016/j.envpol.2008.04.007
Shymanovich T, Faeth SH (2019) Environmental factors affect the distribution of two Epichloë fungal endophyte species inhabiting a common host grove bluegrass (Poa alsodes). Ecol Evol 9(11):6624–6642. https://doi.org/10.1002/ece3.5241
Silva PRAD, Vidal MS, Soares CP, Polese V, Tadra-Sfeir MZ, Souza EM, Simões-Araújo JL, Baldani JI (2018) Sugarcane apoplast fluid modulates the global transcriptional profile of the diazotrophic bacteria Paraburkholderia tropica strain Ppe8. PLoS ONE 13(12):e0207863. https://doi.org/10.1371/journal.pone.0207863
Sørensen J, Sessitsch A (2006) Plant-associated bacteria-lifestyle and molecular interactions. Modern Soil Microbiol Second Ed 01:211–236
Sun H, Feng F, Liu J, Zhao Q (2019) Nitric oxide affects rice root growth by regulating auxin transport under nitrate supply. Front Plant Sci 9:659. https://doi.org/10.3389/fpls.2018.00659 (Erratum in: Front Plant Sci. 2019 Sep 17;10:1123)
Taghavi S, Garafola C, Monchy S, Newman L, Hoffman A, Weyens N, Barac T, Vangronsveld J, van der Lelie D (2009) Genome survey and characterization of endophytic bacteria exhibiting a beneficial effect on growth and development of poplar trees. Appl Environ Microbiol 75(3):748–757. https://doi.org/10.1128/AEM.02239-08
Tejera N, Lluch C, Martìnez-Toledo MV, Gonzàlez-López J (2005) Isolation and characterization of azotobacter and azospirillum strains from the sugarcane rhizosphere. Plant Soil 270(1):223–232. https://doi.org/10.1007/s11104-004-1522-7
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22(22):1673–1680. https://doi.org/10.1093/nar/22.22.4673
Velázquez E, Rojas M, Lorite MJ, Rivas R, Zurdo-Piñeiro JL, Heydrich M, Bedmar EJ (2008) Genetic diversity of endophytic bacteria which could be find in the apoplastic sap of the medullary parenchym of the stem of healthy sugarcane plants. J Basic Microbiol 48(2):118–124. https://doi.org/10.1002/jobm.200700161
Verma SC, Ladha JK, Tripathi AK (2001) Evaluation of plant growth promoting and colonization ability of endophytic diazotrophs from deep water rice. J Biotechnol 91(2–3):127–141. https://doi.org/10.1016/s0168-1656(01)00333-9
Vessey JK (2003) Plant growth promoting rhizobacteria as biofertilizers. Plant Soil 255(2):571–586. https://doi.org/10.1023/A:1026037216893
Waller F (2005) The endophytic fungus Pirifomospora indica reprograms barley to salt-stress tolerance, disease resistance, and higher yield. Proc Natl Acad Sci USA 102(38):13386–13391. https://doi.org/10.1073/pnas.0504423102
Wang L, Zhang JL, Wang JH, Zheng JR, Li ZJ, Han FG, Cao G (2013) Isolation and molecular identification of endophytic bacteria in sugarcane stem (In Chinese). Chin J Trop Crops 34(11):2227–2232
Wang X, Wang C, Sui J, Liu Z, Li Q, Ji C, Song X, Hu Y, Wang C, Sa R (2018) Isolation and characterization of phosphofungi, and screening of their plant growth-promoting activities. AMB Express 8(1):63. https://doi.org/10.1186/s13568-018-0593-4
Wang J, Li R, Zhang H, Wei G, Li Z (2020) Beneficial bacteria activate nutrients and promote wheat growth under conditions of reduced fertilizer application. BMC Microbiol 20(1):38. https://doi.org/10.1186/s12866-020-1708-z
Waweru B, Turoop L, Kahangi E, Coyne D, Dubois T (2014) Non-pathogenic Fusarium oxysporum endophytes provide field control of nematodes, improving yield of banana (Musa sp.). Biol control 74(Complete):82–88
Wei CY (2016) Studies on physiologycial and molecular foundation of sugarcane-endophytic diazotroph DX120E interaction. Guangxi University, Guangxi
Xi LQ, Yao T, Yang J, Han WX, Zhang DG (2005) Porperty of associative nitrogen-fixing bacteria producing IAA and its promoting growth of oat. Grassl Turf 4(111):25
Yamamoto S, Harayama S (1995) PCR amplification and direct sequencing of gyrB genes with universal primers and their application to the detection and taxonomic analysis of Pseudomonas putida strains. Appl Environ Microbiol 61(3):1104–1109. https://doi.org/10.1016/j.ijms.2010.08.029
Young CC (1926) Bergey’s manual of determinative bacteriology. Am J Public Health (N Y) 16(5):520–521. https://doi.org/10.1002/jps.3030470235
Yu YY, Xu JD, Huang TX, Zhong J, Yu H, Qiu JP, Guo JH (2020) Combination of beneficial bacteria improves blueberry production and soil quality. Food Sci Nutr 8(11):5776–5784. https://doi.org/10.1002/fsn3.1772
Zhang HB (2016) Studies on the isolation and characterization of diazotrophic endophytesfrom sugarcane and their rhizosphere soil (In Chinese). South China Agricultural University, Guangzhou
Zhang H, Zeng Z, Zou Z, Zeng F (2019) Climate, life form and family jointly control variation of leaf traits. Plants (Basel) 8(8):286. https://doi.org/10.3390/plants8080286
Acknowledgements
We would like to thank Prof. YQH for the technical assistance. We are also grateful to Shahzad Munir and YML for their assistance in the experiments and writing language.
Funding
This project was supported by the Fujian Agricultural and Forestry University National Sugarcane Engineering Technology Research Center Open Project (NER02018.6.1) and Yunnan Province Modern Agricultural Sugarcane Industry Technical System Construction Project (2020-2021).
Author information
Authors and Affiliations
Contributions
FSL and LLH conceived this study design. YND, LK, PS, LFL, and LYX performed the laboratory experiments. YND, PS, and LK conceptualized the statistical analyses. YND, FSL, and LLH participated in data interpreting. All authors approve the final version of the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interest.
Ethical Approval
All procedures were performed approved by the local Ethics Committee, and the experimental protocols were performed according to the guidelines of Yunnan Agricultural University.
Consent for Publication
Not applicable.
Additional information
Handling Editor: Mikihisa Umehara.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Di, Yn., Kui, L., Singh, P. et al. Identification and Characterization of Bacillus subtilis B9: A Diazotrophic Plant Growth-Promoting Endophytic Bacterium Isolated from Sugarcane Root. J Plant Growth Regul 42, 1720–1737 (2023). https://doi.org/10.1007/s00344-022-10653-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-022-10653-x