Abstract
Sm0.95Ho0.05FeO3 was successfully prepared in a single phase using the citrate combustion method. The investigated sample was characterized using X-ray diffraction (XRD), a high-resolution transmission electron microscope (HRTEM) and X-ray photoemission spectroscopy (XPS). XRD confirmed that the sample was synthesized in an orthorhombic phase with an average crystallite size of 12 nm. HRTEM indicated that the sample was prepared in the nanoscale with an average particle size of 18 nm. XPS was used to identify the chemical bonds, binding energies and core levels of Sm0.95Ho0.05FeO3. The magnetic properties were studied using a vibrating sample magnetometer and DC magnetic susceptibility using Faraday’s method. The sample has an antiferromagnetic behavior with weak ferromagnetic components. The presence of magnetic Ho3+ ions in the SmFeO3 sample causes the magnetic exchange interaction between the 2p orbital of Fe3+ and the 4d sub-shell of Ho3+ ions. The dependence of pH value on the removal efficiency of Pb2+ from water was studied. The maximum Pb2+ removal efficiency of the Ho-doped SmFeO3 nano perovskite is 26% at pH = 5 and 99% at pH = 8. The Freundlich, Langmuir and Temkin isotherms were studied to understand the adsorption mechanism. The Temkin adsorption isotherm best fitted with the experimental data.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Rare earth orthoferrite (RFeO3) has attracted attention due to its interesting physical and chemical properties, such as multiferroicity, magnetic properties, spin–phonon coupling, catalytic properties, an abnormal dielectric constant, magnetization reversal at very low temperatures, a high Neel temperature (TN) above room temperature and a high dielectric constant [1,2,3,4,5,6]. Many applications for ABO3 orthoferrites have been developed, including energy storage devices, spintronics, heavy metal and dye removal from wastewater, and chemical sensors [7,8,9,10,11]. Rare earth orthoferrites usually have a distorted orthorhombic structure with the Pbnm space group [6, 8, 12]. In this structure, the rare earth cation R3+ has 12 coordination numbers, while Fe ions coordinate with six O2− anions, forming FeO6 octahedra [13, 14]. The tilting and distortion of FeO6 affect the ionic radii of rare earth cations. The distortion of FeO6 increases by decreasing the rare earth ionic radii due to the R–O bond length and bond angle change.
SmFeO3 is one of the rare earth orthoferrite materials. It has attracted great attention from researchers due to its properties, such as the antiferromagnetic properties with weak ferromagnetism, the highest spin reorientation transition temperature (470 K), the particle size-affected dielectric constant and piezoelectricity at room temperature [14]. The magnetic properties of SmFeO3 originate from three magnetic interactions, i.e., rare earth–rare earth (R3+–R3+), rare earth–iron (R3+–Fe3+) and iron–iron (Fe3+–Fe3+) interactions [1, 15].
The change in A-site cation radius affects the distortion of the perovskite structure and the tilting of the FeO6 octahedron. The doping of rare earth ions at the A site has an impact on the magnetic properties of SmFeO3. Liu [16] studied the effect of nonmagnetic La ion doping on the magnetic properties of SmFeO3. Because the Sm spins are in a frustrated state, the magnetic anisotropy energy and exchange bias of Sm1−xLaxFeO3 rise slightly for x = 0.05 and then fall by increasing the La content by more than 0.05. Praveena et al. [15] prepared Sm1−xCaxFeO3 where x = 0, 0.25, 0.5, 0.75 and 1 using the sol–gel method at low temperatures. As Ca2+ increases, a significant enhancement in remnant magnetization (Mr) and coercivity (Hc) is observed. The magnetic properties of the Sm1−xCaxFeO3 samples were improved due to the destruction of the spin cycloid with Sm.
Heavy metal pollution is an international issue that is growing with the development of modern industry. The sources of lead in the environment are ceramic printing industries, battery manufacturing, ammunition and acid metal plating [17]. The presence of the toxic heavy metal lead in water has great harmful effects on the human body, so it is one of the most worrying problems [18, 19]. High lead exposure has been reported to have a number of negative health impacts on children, including neurotoxicity [20], low IQ [21, 22], poor mental development [23], low quantitative skills [24], and learning difficulties. For adults, lead exposure has health consequences such as diabetes, renal disease [25] and cognitive dysfunction from early childhood exposure [26].
There are many conventional methods for the removal of heavy metal ions, such as membrane filtration, chemical precipitation, reverse osmosis, ultrafiltration and flotation. The adsorption method is more effective for heavy metal ion removal due to its properties such as simplicity, cost-effectiveness, no sludge formation and good regeneration capacity [27,28,29]. Adsorption occurs due to the physical or chemical attraction of lead ions to the surface of the adsorbent [30].
Perovskites are effective materials for heavy metal removal owing to their properties such as high stability, low price, high surface energy, and absorptivity, catalytic, electronic, magnetic and optical characteristics [28]. One of the main advantages of using magnetic materials (i.e., perovskite) for heavy metal removal is the easy separation from the solution by an external magnet. Some of the perovskite samples that were used for heavy metal removal are La0.85Ce0.15FeO3 [8], CeCoO3 [31], LaFeO3 [32], and LaAlO3 [33]. Meanwhile, the removal of lead ions from water by using Sm0.95Ho0.05FeO3 nanocomposites has not been reported yet. Therefore, this work demonstrated the use of Ho-doped SmFeO3 nanoparticles to eliminate lead from aqueous solutions.
In this work, Sm0.95Ho0.05FeO3 was synthesized using a simple citrate combustion method. The aim of this study is to study the phase formation, morphology and magnetic behavior of Sm0.95Ho0.05FeO3. The other goal is to examine the ability of the investigated sample to remove lead from water. The effect of pH value on the removal efficiency of pb2+ ions from water was studied.
2 Experimental work
2.1 Preparation of nanocomposites
Sm0.95Ho0.05FeO3 was synthesized by the citrate combustion method. The precursor metal nitrates (purity 99.9% Sigma-Aldrich) were mixed in stoichiometric ratios with citric acid as a fuel. The citric acid/nitrate ratio is equal to 1. The pH of the solution was adjusted to 7 using the ammonia solution. The solution was heated on the hot plate until a highly viscous liquid was formed. The viscous liquid swelled and auto-ignited as it was heated further, resulting in a fluffy powder. This preparation method is low cost, simple, and quick. The sample was ground well for 1 h using an agate mortar to obtain a powder. The sample was sintered at 500 °C for 2 h with a heating/cooling rate of 4 degrees/min in air. Figure 1 illustrates the flowchart of the preparation of Ho-doped SmFeO3.
2.2 Sample characterizations and measurements
The sample was well ground into powder before being subjected to the following characterizations. X-ray diffraction (XRD, Bruker advance D8 diffractometer, λ = 1.5418 Å) was used to study the structure of the investigated sample, Sm0.95Ho0.05FeO3. The crystalline phases of the sample were identified using the International Centre for Diffraction Data (ICDD) card number 74-1474.
A certain amount of the sample was suspended in deionized water by using an ultrasonicator for 30 min before the characterization using HRTEM. The surface morphology of Ho-doped SmFeO3 was characterized using a transmission electron microscope (HRTEM, JEOL-2100) operated at 200 kV. The surface chemical composition of the investigated sample was measured using the X-ray photoelectron spectrometer (XPS) via K-ALPHA (Thermo Fisher Scientific, USA).
The magnetic behavior of the Ho-doped perovskite SmFeO3 was studied using a vibrating sample magnetometer and DC magnetic susceptibility using the Faraday method [34]. The magnetic susceptibility of the investigated sample was measured by introducing a cylindrical glass tube with a small amount of powdered sample at the point of maximum gradient.
2.3 Heavy metal removal
2.3.1 Effect of pH value
A lead ion solution of concentration (100 ppm) was prepared from pure lead nitrate to determine the heavy metal ion removal efficiency of the Sm0.95Ho0.05FeO3 sample. A working solution (50 ppm) was prepared by diluting the above solution. A few 250-mL flasks containing 50 ppm lead ion solution and 0.02 g of the investigated sample were used. By using ammonia and diluted nitric acid solutions, the pH values of lead solutions were adjusted from 3 to 8. The resultant solutions were stirred at 25 °C using an electric shaker (Orbital Shaker SO1) at 200 rpm for 1 h. By using a 0.2-μm syringe filter, 10 mL of the supernatant solutions was filtered. Inductively coupled plasma spectrometry (ICP, Prodigy7) was used to calculate the concentration of pb2+ ions in the filtrated solution.
The metal ion removal efficiency (η) and the equilibrium adsorption capacity (q) were calculated according to the following equations [35]:
where Ci and Ce are the initial and final concentrations (mg/L) of the metal ion solution, respectively, while m is the mass of the adsorbent and V is the volume of Pb (II) solution.
3 Results and discussion
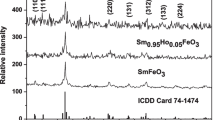
XRD was used to examine the crystalline structure of the prepared sample, Sm0.95Ho0.05FeO3. Figure 2 shows the XRD pattern of the investigated sample. All the diffraction peaks are indexed with the ICCD card number 74-1474. Sm0.95Ho0.05FeO3 has been synthesized in a single-phase orthorhombic structure with space group Pbnm. Major diffracted peaks (110), (111), (112), (220), (131), (312), (133) and (224) were observed in the XRD pattern at 2θ values of 22.96, 25.66, 32.51, 47.05, 53.38, 58.88, 63.97 and 69.88, which indicates the sample has an orthorhombic phase. The high-intensity peak (112) observed at 2θ indicates the growth direction of Sm0.95Ho0.05FeO3. The values of lattice parameters were calculated on the basis of the orthorhombic structure according to the following equation:
The average crystallite size was estimated using Scherer’s equation [36] and is reported in Table 1. The value of the crystallite size (12.1 nm) indicates that the sample was synthesized in the nano scale.
The theoretical density of the investigated sample was calculated using the following equation:
where Z = 4 is the number of molecules per unit cell, N is Avogadro's number, M is the molecular weight, and V is the unit cell volume.
The Goldsmith perovskite tolerance factor (t) measures the lattice mismatch between the ions of the perovskite materials [37]. The highest value of t is equal to 1 for a cubic perovskite structure. The value of the tolerance factor was calculated according to the following equation:
where rA, rB, and rO are the ionic radii of the A, B, and oxygen ions, respectively. The A-site ionic radius can be calculated according to the equation \(r_{{\text{A}}} = \, 0.95r_{{{\text{Sm}}}}^{3 + } + \, 0.05 r_{{{\text{Ho}}}}^{3 + }\). The tolerance factor value of the investigated sample is 0.8786, which indicates that the sample has an orthorhombic structure. The tolerance factor is less than one due to the lattice distortion arising from the tilting of <FeO6> octahedral structure.
Figure 3 illustrates the EDX energy spectra of Sm0.95Ho0.05FeO3. The atomic percentages (at%) and weight percentages (wt%) of the Fe, O, Sm, and Ho elements are illustrated in the inset table in Fig. 3. The experimental at% and wt% were obtained from EDX, while the theoretical values were calculated from the chemical formula Sm0.95Ho0.05FeO3. The small variation of weight and atomic percentages between the experimental and theoretical values is due to oxygen deficiency, which can be advantageous for heavy metal removal from water.
Figure 4a, b illustrates the HRTEM images of the investigated nanoparticles at different scales. The average particle size of Sm0.95Ho0.05FeO3 is 18 nm, which confirms that the sample was synthesized in the nanoscale. The nanoparticles are agglomerated due to the magnetic properties of the sample. Figure 4b includes an inset of the selected area electron diffraction (SAED) pattern. The bright rings in the SAED indicate that the sample was synthesized in good crystalline form. The SAED pattern also illustrated that the prepared Sm0.95Ho0.05FeO3 nanoparticles are polycrystalline in nature.
The XPS characterization was carried out to verify the chemical composition and valence state of the Sm0.95Ho0.05FeO3 nanoparticles. The wide-range XPS survey spectra of Sm0.95Ho0.05FeO3 nanoparticles are illustrated in Fig. 5a. The peaks of different elements such as Sm, Ho, Fe, O, and C have been identified. The C 1s peak is observed at 287.21 eV, which is used as a charge reference to rectify the binding energy of the XPS spectra [38]. Figure 5a illustrates that there is no secondary phase from holmium metal or oxide. Therefore, Ho was substituted at the expense of the Sm site and maintained the perovskite structure in a single phase as a result obtained from XRD data. The XPS peak of Ho 4d at a binding energy of 160.79 eV due to the presence of Ho3+ is shown in Fig. 5b [39,40,41]. The Ho 4d spectrum is obvious in a small range of scan spectra as a result of the very small quantity of Ho in Sm0.95Ho0.05FeO3 perovskite.
The magnetic parameters of orthoferrite depend on many factors, such as the composition, the cation preference occupation, purity, homogeneity and surface to volume ratio [1]. The magnetic properties of Sm0.95Ho0.05FeO3 were studied by using the hysteresis loop and Faraday’s law. The magnetic hysteresis loop of the investigated perovskite is illustrated in Fig. 6 at room temperature. The values of the maximum magnetization (Ms), the coercive field (Hc) and the remnant magnetization (Mr) are listed in Table 2. The hysteresis loop has a small area with nonlinearity, a very small coercive field, and remnant magnetization. The non-saturation behavior of the magnetic loop shows the dominance of its antiferromagnetic character. The hysteresis loop has S-shape, which indicates the presence of an antiferromagnetic order with weak ferromagnetic components [1]. The super exchange interactions are presented between the magnetic ions Fe3+, Ho3+ and Sm3+ ions, while the weak ferromagnetism originates from canted antiferromagnetic spins in the investigated sample. The anisotropy constant value is calculated from the following equation [42] and reported in Table 2.
where Hc is the coercivity, K is the magnetic anisotropy constant and Ms denotes saturation magnetization. The weak anisotropy of Sm0.95Ho0.05FeO3 is due to the non-collinearity (canting) of spins on their surface [42].
Ateia et al. [14] used the citrate auto-combustion method to prepare SmFeO3 and found that the saturation magnetization was 0.639 emu/g. In the present study, the maximum magnetization of Ho-doped SmFeO3 increased four times that of SmFeO3 as a result of the substitution of Ho3+ ions (10.3 μB) instead of Sm3+ ions (1.6 μB) [43]. Due to lattice distortion caused by the difference in ionic radii of Ho3+ (1.015 Å) and Sm3+ (1.079 Å), the magnetization of SmFeO3 is increased by substituting Ho3+ ions for Sm3+ ions [44].
The dependence of the molar magnetic susceptibility (χM) on the absolute temperature T (K) is illustrated in Fig. 7 for the investigated sample Sm0.95Ho0.05FeO3. The χM values decrease as the temperature increases to the Néel temperature. Figure 7 confirms the AFM behavior of the Ho-doped SmFeO3 sample with weak ferromagnetic components [45, 46]. The values of magnetic constants such as Curie temperature (Tc), Curie–Weiss constant (θ), Curie constant (C) and the effective magnetic moment (μeff) are calculated and reported in Table 3. Introducing the Ho3+ ions into the sample increases the magnetization due to the presence of new magnetic interactions between Ho3+–Ho3+ and Ho3+–Sm3+ interactions. By introducing Ho3+ ions into SmFeO3, the distortion increased, as illustrated in the tolerance factor, leading to changes in the bond lengths of Fe–O and Sm–O. The structure distortion, the bond angle and the bond length all strongly affect the super exchange interaction [47]. The presence of magnetic Ho3+ ions in the investigated sample causes the magnetic exchange interaction between the 2p orbital of Fe3+ and the 4d sub-shell of Ho3+ ions [48].
The interaction between the adsorbent and the adsorbate depends on many factors, such as the pH value of the solution [8]. The pH value determines the adsorbent's net surface charge, the type of adsorbed species and the degree of ionization [49]. In aqueous solutions, iron oxides are hydrated and form FeOH groups, which cover their surfaces. Depending on the pH value of the solution, the hydroxylated sites on the surface can react with H+ or OH− ions from dissolved acids or bases, and positive (FeOH2+) or negative (FeO−) charges are produced on the surface of perovskite [17].
Figure 8 shows the dependence of the adsorption efficiency of the removal of Pb2+ heavy metal ions on the pH solution. Since there are more H+ ions in the solution when the pH is very low, H+ and heavy metal ions compete for the same active sites on the adsorbent, which results in a low removal efficiency. As pH rises, the sample surface acquires a negative sign and the adsorption of lead ions increases. In this case, electrostatic interactions dominate the adsorption process. Figure 8 illustrates that the optimum pH value for precipitate Pb2+ is pH = 5. At a high pH value (pH = 8), Pb2+ ions precipitate as hydroxide, which is unfavorable for the adsorption process [50].
The adsorption isotherm gives more information about the mechanism of adsorption between the investigated sample and the lead ion liquid phase. In the present work, Langmuir, Freundlich and Temkin isotherm models were studied for the absorption efficiency of lead ions on Sm0.95Ho0.05FeO3.
The Langmuir, Freundlich and Temkin isotherm models [51, 52] were applied to explain the equilibrium adsorption of the pb2+ ion on Sm0.95Ho0.05FeO3 nanoparticles. Equations (8)–(10) express the Langmuir, Freundlich and Temkin isotherm models, respectively.
where Ce is the equilibrium metal concentration (mg/L), qm and KL are the Langmuir constants associated with maximum adsorption capacity (mg/g), Kf is the Freundlich constant, \(\frac{1}{n}\) is the adsorption intensity, T is the absolute temperature (K), R is the common gas constant (0.008314 kJ/mol K), \(\frac{1}{{b_{{\text{T}}} }}\) is the Temkin constant related to the heat of sorption (kJ/mol) and \(K_{{\text{T}}}\) is the Temkin constant related to adsorption capacity (L/g).
Figure 9 illustrates the relationship between ln qe and ln Ce which agrees with the Freundlich isotherm model. The values of 1/n and Kf were calculated from the slope and the intercept of the best fit line in Fig. 9. The dependence of Ce/q on Ce according to the Langmuir isotherm model is illustrated in Fig. 10. Adsorbent–adsorbate interactions are taken into account in the Temkin isotherm. Figure 11 illustrates the linear plot between qe and lnCe according to the Temkin isotherm model. The constants \(\frac{1}{{b_{{\text{T}}} }}\) and \(K_{{\text{T}}}\) can be determined from the slope and intercept, respectively.
Table 4 shows the values of the correlation coefficient (R2) for the Langmuir, Freundlich and Temkin isotherm models. The Temkin isotherm has an R2 of 0.9453. Thus, the Temkin adsorption isotherm best fitted with the experimental data.
4 Conclusions
The X-ray diffraction analysis ensures Sm0.95Ho0.05FeO3 was synthesized in a single-phase orthorhombic structure. The average particle size of the investigated sample is 18 nm. The bright rings in the SAED indicate that the sample was synthesized with good crystalline nature. XPS illustrated that Ho was substituted at the expense of Sm and maintained the perovskite structure in a single phase. Sm0.95Ho0.05FeO3 has antiferromagnetic properties with a maximum magnetization equal to 2.5467 emu g−1. Introducing the Ho3+ ions into the sample increases the magnetization due to the presence of new magnetic interactions between Ho3+–Ho3+ and Ho3+–Sm3+ interactions. Ho-doped SmFeO3 can act as a heavy metal pb2+ adsorber from wastewater. The optimum removal efficiency of lead ions from water is 26% by Sm0.95Ho0.05FeO3. The Temkin adsorption isotherm is the best description of the adsorption of the Pb2+ ion from water.
References
R. Shukla, R. Dhaka, S. Dash, S.C. Sahoo, B. Bahera, P.D. Babu, R. Choudhary, A.K. Patra, Structural, dielectric and magnetic properties of Bi–Mn doped SmFeO3. Ceram. Int. 46(7), 8730–8744 (2020)
Z. Cheng, F. Hong, Y. Wang, K. Ozawa, H. Fujii, H. Kimura, Y. Du, X. Wang, S. Dou, Interface strain-induced multiferroicity in a SmFeO3 film. ACS Appl. Mater. Interfaces 6(10), 7356–7362 (2014)
A.V. Kimel, A. Kirilyuk, A. Tsvetkov, R.V. Pisarev, T. Rasing, Laser-induced ultrafast spin reorientation in the antiferromagnet TmFeO3. Nature 429(6994), 850–853 (2004)
M.M. Arman, M.A. Ahmed, Effects of vacancy co-doping on the structure, magnetic and dielectric properties of LaFeO3 perovskite nanoparticles. Appl. Phys. A 128(7), 1–9 (2022)
M. Reda, S.I. El-Dek, M.M. Arman, Improvement of ferroelectric properties via Zr doping in barium titanate nanoparticles. J. Mater. Sci.: Mater. Electron. 33, 1–24 (2022)
S. Chaturvedi, P. Shyam, A. Apte, J. Kumar, A. Bhattacharyya, A.M. Awasthi, S. Kulkarni, Dynamics of electron density, spin-phonon coupling, and dielectric properties of SmFeO3 nanoparticles at the spin-reorientation temperature: role of exchange striction. Phys. Rev. B 93(17), 174117 (2016)
J.T. Mefford, W.G. Hardin, S. Dai, K.P. Johnston, K.J. Stevenson, Anion charge storage through oxygen intercalation in LaMnO3 perovskite pseudocapacitor electrodes. Nat. Mater. 13(7), 726–732 (2014)
M.M. Arman, Synthesis, characterization, magnetic properties, and applications of La0.85Ce0.15FeO3 perovskite in heavy metal Pb2+ removal. J. Supercond. Novel Magn. 2022, 1–9 (2022)
Z.A. Elsiddig, H. Xu, D. Wang, W. Zhang, X. Guo, Y. Zhang, Z. Sun, J. Chen, Modulating Mn4+ ions and oxygen vacancies in nonstoichiometric LaMnO3 perovskite by a facile sol–gel method as high-performance supercapacitor electrodes. Electrochim. Acta 253, 422–429 (2017)
Z. Anajafi, M. Naseri, G. Neri, Acetone sensing behavior of p-SmFeO3/n-ZnO nanocomposite synthesized by thermal treatment method. Sens. Actuators B Chem. 304, 127252 (2020)
P. Song, H. Zhang, D. Han, J. Li, Z. Yang, Q. Wang, Preparation of biomorphic porous LaFeO3 by sorghum straw biotemplate method and its acetone sensing properties. Sens. Actuators B Chem. 196, 140–146 (2014)
M.M. Arman, N.G. Imam, R.L. Portales, S.I. El-Dek, Synchrotron radiation X-ray absorption fine structure and magnetization improvement of A-site Ce3+ doped LaFeO3. J. Magn. Magn. Mater. 513, 167097 (2020)
M.M. Arman, M.A. Ahmed, S.I. El-Dek, Influence of vacancy co-doping on the physical features of NdFeO3 nanostructure perovskites. Appl. Phys. A 126(1), 1–10 (2020)
E.E. Ateia, M.M. Arman, E. Badawy, Role of coupling divalent cations on the physical properties of SmFeO3 prepared by citrate auto-combustion technique. Appl. Phys. A 125(8), 1–7 (2019)
K. Praveena, P. Bharathi, H.L. Liu, K.B.R. Varma, Structural, multiferroic properties and enhanced magnetoelectric coupling in Sm1−xCaxFeO3. Ceram. Int. 42(12), 13572–13585 (2016)
Q. Liu, X.X. Wang, C. Song, J.X. Sui, X. Yan, J.C. Zhang, H.S. Zhao, F. Yuan, Y.Z. Long, Magnetic properties of La doped SmFeO3. J. Magn. Magn. Mater. 469, 76–80 (2019)
M.A. Ahmed, S.M. Ali, S.I. El-Dek, A. Galal, Magnetite–hematite nanoparticles prepared by green methods for heavy metal ions removal from water. Mater. Sci. Eng. B 178(10), 744–751 (2013)
B. Daus, R. Wennrich, H. Weiss, Sorption materials for arsenic removal from water: a comparative study. Water Res. 38(12), 2948–2954 (2004)
R. Bondu, V. Cloutier, E. Rosa, M. Benzaazoua, A review and evaluation of the impacts of climate change on geogenic arsenic in groundwater from fractured bedrock aquifers. Water Air Soil Pollut. 227(9), 1–14 (2016)
H.L. Needleman, C. McFarland, R.B. Ness, S.E. Fienberg, M.J. Tobin, Bone lead levels in adjudicated delinquents: a case control study. Neurotoxicol. Teratol. 24(6), 711–717 (2002)
H.W. Mielke, J.L. Adams, P.L. Reagan, P.W. Mielke Jr., Soil-dust lead and childhood lead exposure as a function of city size and community traffic flow: the case for lead abatement in Minnesota. Environ. Geochem. Health 9, 253–271 (1989)
H.W. Mielke, Lead dust contaminated USA communities: comparison of Louisiana and Minnesota. Appl. Geochem. 8, 257–261 (1993)
L. Agwaramgbo, E. Agwaramgbo, C. Mercadel, S. Edwards, E. Buckles, Lead remediation of contaminated water by charcoal, LA red clay, spinach, and mustard green. J. Environ. Prot. 2(9), 1240 (2011)
R.L. Canfield, C.R. Henderson Jr., D.A. Cory-Slechta, C. Cox, T.A. Jusko, B.P. Lanphear, Intellectual impairment in children with blood lead concentrations below 10 μg per deciliter. N. Engl. J. Med. 348(16), 1517–1526 (2003)
J.L. Lin, D.T. Lin-Tan, K.H. Hsu, C.C. Yu, Environmental lead exposure and progression of chronic renal diseases in patients without diabetes. N. Engl. J. Med. 348(4), 277–286 (2003)
R.A. Shih, H. Hu, M.G. Weisskopf, B.S. Schwartz, Cumulative lead dose and cognitive function in adults: a review of studies that measured both blood lead and bone lead. Environ. Health Perspect. 115(3), 483–492 (2007)
J. Rubio, M.L. Souza, R.W. Smith, Overview of flotation as a wastewater treatment technique. Miner. Eng. 15(3), 139–155 (2002)
G. Jayanthi, S. Sumathi, V. Andal, Synthesis and applications of perovskite in heavy metal ions removal—a brief perspective. Mater. Today Proc. 55, 201–211 (2022)
M. Czikkely, E. Neubauer, I. Fekete, P. Ymeri, C. Fogarassy, Review of heavy metal adsorption processes by several organic matters from wastewaters. Water 10(10), 1377 (2018)
M.I. Sabela, K. Kunene, S. Kanchi, N.M. Xhakaza, A. Bathinapatla, P. Mdluli, D. Sharma, K. Bisetty, Removal of copper(II) from wastewater using green vegetable waste derived activated carbon: an approach to equilibrium and kinetic study. Arab. J. Chem. 12(8), 4331–4339 (2019)
C.B. Njoku, E. Oseghe, T.A. Msagati, Synthesis and application of perovskite nanoparticles for the adsorption of ketoprofen and fenoprofen in wastewater for sustainable water management. J. Mol. Liq. 346, 118232 (2022)
M.P. Rao, S. Musthafa, J.J. Wu, S. Anandan, Facile synthesis of perovskite LaFeO3 ferroelectric nanostructures for heavy metal ion removal applications. Mater. Chem. Phys. 232, 200–204 (2019)
W. Haron, A. Wisitsoraat, U. Sirimahachai, S. Wongnawa, Removal of toxic heavy metal ions from water with LaAlO3 perovskite. Songklanakarin J. Sci. Technol. 40(5), 993–1001 (2018)
A. Rais, Absolute method of calibration of a Faraday microbalance. Measurement 35(3), 289–292 (2004)
R. Ramadan, S.I. El-Dek, M.M. Arman, Enhancement of Mn-doped magnetite by mesoporous silica for technological application. Appl. Phys. A 126(11), 1–13 (2020)
M.M. Arman, Structural, morphological and magnetic properties of hexaferrite BaCo2Fe16O27 nanoparticles and their efficient lead removal from water. Appl. Phys. A 128(12), 1–10 (2022)
M.M. Arman, S.I. El-Dek, Role of annealing temperature in tailoring Ce-doped LaFeO3 features. J. Phys. Chem. Solids 152, 109980 (2021)
P.W. Wang, M. Guttag, C.-S. Tu, Surface modification of multiferroic BiFeO3 ceramic by argon sputtering. J. Surf. Eng. Mater. Adv. Technol. 4, 295–308 (2014)
S.K. Ray, Y.K. Kshetri, T. Yamaguchi, T.H. Kim, S.W. Lee, Characterization and multicolor upconversion emission properties of BaMoO4: Yb3+, Ln3+ (Ln = Tm, Ho, Tm/Ho) microcrystals. J. Solid State Chem. 272, 87–95 (2019)
T. Han, S. Ma, P. Yun, H. Sheng, X. Xu, P. Cao, S. Pei, A. Alhadi, Synthesis and characterization of Ho-doped SmFeO3 nanofibers with enhanced glycol sensing properties. Vacuum 191, 110378 (2021)
A. Manzoor, M.A. Khan, T. Alshahrani, M.H. Alhossainy, M. Sharif, T. Munir, M.I. Arshad, M.A. Iqbal, Effect of Ho3+ ions on microwave losses and hightemperature electrical behavior of Li-based magnetic oxides. Ceram. Int. 47, 4633–4642 (2021)
E.E. Ateia, M.A. Ateia, M.M. Arman, Assessing of channel structure and magnetic properties on heavy metal ions removal from water. J. Mater. Sci.: Mater. Electron. 33(11), 8958–8969 (2022)
H. Lueken, B.G. Magnetochemie, Teubner Stuttgart (B. G. Teubner, Leipzig, 1999)
R.D. Shannon, Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr Sect A Cryst Phys Diffr Theor Gener Crystallogr 32(5), 751–767 (1976)
E. Dagotto, T. Hotta, A. Moreo, Colossal magnetoresistant materials: the key role of phase separation. Phys. Rep. 344(1–3), 1–153 (2001)
A. Urushibara, Y. Moritomo, T. Arima, A. Asamitsu, G. Kido, Y. Tokura, Insulator-metal transition and giant magnetoresistance in La1−xSrxMnO3. Phys. Rev. B 51(20), 14103 (1995)
A. Kumar, K.L. Yadav, J. Rani, Low temperature step magnetization and magnetodielectric study in Bi0.95La0.05Fe1−xZrxO3 ceramics. Mater. Chem. Phys. 134(1), 430–434 (2012)
S. Sharif, G. Murtaza, T. Meydan, P.I. Williams, J. Cuenca, S.H. Hashimdeen, F. Shaheen, R. Ahmad, Structural, surface morphology, dielectric and magnetic properties of holmium doped BiFeO3 thin films prepared by pulsed laser deposition. Thin Solid Films 662, 83–89 (2018)
M.K. Ahmed, R. Ramadan, M. Afifi, A.A. Menazea, Au-doped carbonated hydroxyapatite sputtered on alumina scaffolds via pulsed laser deposition for biomedical applications. J. Mark. Res. 9(4), 8854–8866 (2020)
X. Liu, R. Ma, X. Wang, Y. Ma, Y. Yang, L. Zhuang, S. Zhang, R. Jehan, J. Chen, X. Wang, Graphene oxide-based materials for efficient removal of heavy metal ions from aqueous solution: a review. Environ. Pollut. 252, 62–73 (2019)
Q. Liu, Y. Liu, Distribution of Pb(II) species in aqueous solutions. J. Colloid Interface Sci. 268(1), 266–269 (2003)
T.M. Elmorsi, Equilibrium isotherms and kinetic studies of removal of methylene blue dye by adsorption onto miswak leaves as a natural adsorbent. J. Environ. Prot. 2(06), 817 (2011)
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares no conflict of interest regarding the publication of this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Arman, M.M. Preparation, characterization and magnetic properties of Sm0.95Ho0.05FeO3 nanoparticles and their application in the purification of water. Appl. Phys. A 129, 38 (2023). https://doi.org/10.1007/s00339-022-06320-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-022-06320-3