Abstract
W-hexaferrite BaCo2Fe16O27 was prepared using the citrate nitrate combustion method. The sample was characterized using XRD, SEM, EDX and elemental mapping. XRD confirmed that the sample was synthesized in a single phase hexagonal structure with an average crystallite size 37.39 nm. SEM images of the sample show a spongy morphology with the agglomerated grain owing to dipole interaction between the crystallites. The magnetic properties of BaCo2Fe16O27 were studied using H-M hysteresis loop and the DC magnetic susceptibility. The sample has a ferrimagnetic behavior with saturation magnetization 64.133 emu/g. The magnetic properties of BaCo2Fe16O27 are originated from the Fe3+–O–Fe3+ superexchange. The synthesized sample is used as an adsorbent to remove the heavy metal Pb2+ from water. BaCo2Fe16O27 has Pb2+ removal efficiency 99% and 28% at pH 8 and 7 respectively. The Langmuir and Freundlich isotherms were used to analyze the experimental data. The Freundlich adsorption isotherm fitted the experimental data well.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The hexaferrites have six main types (M-, U-, W-, X-, Y- and Z-types) according to the chemical formulas and stacking sequences of these building blocks. W-type hexaferrites have a general formula AMe2Fe16O27, where A is an alkaline earth ion (i.e., Ba and Sr) and Me is a divalent transition metal ion such as Mg, Zn, Co, Ni, Fe. They are characterized by ferrimagnetic behavior with high Curie temperatures. The magnetic properties of Ba(Sr)M2+Fe16O27 vary with the type divalent cation. The hexagonal ferrites are used in a wide range of devices, such as magnetic recording media, high-performance microwave-absorbing, permanent magnets, shielding materials in electronic devices operating at GHz frequencies and bubble memories and microwave devices [1,2,3,4].
To unravel the verities of application, various divalent and trivalent cations are frequently inserted in the sub lattices of W-type barium hexagonal ferrites (BHF). Several methods of preparation were used to prepare BHF compounds with different nanocrystal sizes, shapes, and characteristics [5]. The synthesize routes such as the conventional solid-state reaction method [6], the sol–gel method [7], the micro-emulsion route [8], the ball milling [9], auto-combustion [10] and co-precipitation method [11] were used for preparation nanoparticles of BHF systems. The citrate combustion method is a fast, simple and easy technique for the synthesis of a variety of advanced nanomaterials and ceramics [12]. In this method, the thermal redox reaction occurs between an oxidant and a fuel (as citric acid) [13]. The monophasic nanopowders were prepared with a homogeneous microstructure in short reaction times using a citrate combustion method [14, 15].
The structural, morphological and magnetic properties of Ba W- type hexaferrite (BHFs) were strongly influenced by the divalent cations and rare-earth substitution. The enhancement of zinc in Ba1Cu2-xZnxFe16O27 (x = 0.0 and 0.4) has been studied by R. Sagayaraj [5]. The pure and doped samples have a semiconductor like behavior.
The SEM images reveal that the Ba hexaferrites gained the hexagonal structure with spongy morphology. Ba1Cu2-xZnxFe16O27 nanoparticles may be utilized for treating bacterial infection in the clinical sector. Kai Huang et al. [16] studied the effect of Ca ions on the structure and magnetic properties of BHFs. The samples Ba1-xCaxCo2Fe16O27 (x = 0, 0.1, 0.3, 0.4 and 0.5) were prepared using a sol–gel method. The coercive field and the saturation magnetization were increased by increasing the amount of Ca substitution. The Ca2+ doped W- type hexaferrite improved microwave absorbency. M.A. Ahmed et al. [17] studied the effect of the influence of rare-earth ions on the magnetic properties of barium hexaferrite Ba0.95R0.05Mg0.5Zn0.5CoFe16O27 (R = Y, Er, Ho, Sm, Nd, Gd, and Ce ions). The curie temperature (TC) and effective magnetic moment increased for the Sm doped sample. Jin Tang et al. [18] were prepared \({\mathrm{Ba}}_{1-x}{\mathrm{La}}_{x}{\mathrm{Fe}}_{2}^{2+}{\mathrm{Fe}}_{16}^{3+}{\mathrm{O}}_{27}\) using the standard ceramic method. The values of the lattice constant c decreased with the increase of the doped La3+ in the samples. There is a monotonic dependence of the coercivity (Hc) and the magnetic anisotropy field (Ha) on the La3+ amount.
Heavy metals in aqueous solutions, such as Cr(VI), Pb(II), and Cd(II), are poisonous even in low amounts and have caused serious health effects on humans. As a result, it is critical to remove these heavy metals from the aqueous environment to protect biodiversity, hydrosphere ecosystems, and humans. To remove these harmful heavy metals from wastewater, several techniques such as chemical precipitation, electrolytic separation, membrane separation, ion exchange, and adsorption have been used [19].
For the removal of heavy metals, the adsorption technique has been widely used. Nanomaterials have been considered an excellent adsorbent for the removal of heavy metal ions from wastewater [20,21,22]. Nanomaterials were used for removal of heavy metals from water using adsorption techniques based on the physical interaction between metal ions and nanomaterials [23].
In this present work, the author synthesized BaCo2Fe16O27 nanoparticles using the citrate combustion method as a fast, simple and easy technique. The aim of this study is understanding the crystallite structure, morphology and magnetic properties of W-type hexaferrites, additionally, studying the heavy metal removal efficiency using BaCo2Fe16O27 nanoparticles. To identify the best conditions for heavy metal Pb2+ ion removal from water, the removal efficiency of Pb2+ ion was studied as a function of contact time and pH.
2 Experimental techniques
2.1 Preparation of nanoparticles
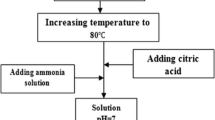
The hexaferrite BaCo2Fe16O27 sample was prepared by citrate nitrate combustion method as illustrated in Fig. 1. The metal nitrates (purity 99.9%, Sigma–Aldrich) were mixed in stoichiometric ratios.
2.2 Measurements
The X-ray diffraction (XRD) was carried out using X-ray diffraction (XRD, Bruker advance D8 diffractometer, λ = 1.5418 Å). The sample was characterized by scanning electron microscopy (SEM) and energy dispersive spectroscopy (EDS) to study the surface morphology and the chemical structure using OXFORD INCA PentaFETX3-England. The magnetic properties of the hexaferrite BaCo2Fe16O27 sample were assessed using two techniques: the first is the H-M hysteresis loop using the vibrating sample magnetometer (VSM; 9600-1 LDJ, USA). While the other is the measurement of the DC magnetic susceptibility as a function of absolute temperature using the Faraday method [24].
2.3 Heavy metal removal
2.3.1 Effect of pH value
The heavy metal Pb2+ ion removal efficiency of BaCo2Fe16O27 sample was studied. Pb2+ ions solution of concentration (50 ppm) was prepared as an initial concentration. 0.02 g of BaCo2Fe16O27 nanoparticles was added to five beakers (250-mL) containing the above solution. The pH values of the solutions were adjusted from 3 to 8 and they were stirred using an electric shaker (ORBITAL SHAKER SO1) at 200 rpm for 1 h. A 0.2-m syringe filter was used to filter 10 ml of the supernatant solutions at regular intervals. Inductively coupled plasma (ICP) spectrometry (Prodigy7) was used to determine the concentration of heavy metals in the filtrate.
2.3.2 Effect of contact time
The 50 ppm Pb2+ solution was pipetted into a beaker containing 0.10 g of BaCo2Fe16O27, with the pH value adjusted to its optimal value. After varying contact times, the concentration of Pb2+ in the solution was determined. The following equations were used to calculate the metal ion removal efficiency \((\eta \)) and the equilibrium adsorption capacity (q) [25]:
where Ci and Ce are the initial and final concentrations (mg/L) of metal ion solution, respectively. While m is the mass of adsorbent and V is the volume of Pb2+ solution.
3 Results and discussion
Figure 2 illustrates the XRD pattern of BaCo2Fe16O27 nanoparticles. The sample has a single phase hexagonal structure with the space group P63/ mmc [5]. The data were indexed with ICDD card number 019–0098. The average crystallite size was calculated using the well-known Debye‐Scherer equation formula [26]
where D is the average crystallite size, λ is the wavelength of X-ray radiation, θ is the Bragg angle, and β is the full-width at half-maximum intensity of the powder pattern peak. The average crystallite size is 37.39 nm. The lattice parameters were calculated based on the hexagonal symmetry according to Eq. (4).
The value of theoretical density was calculated from Eq. (5) and reported in Table 1
where Z = 2 is the number of molecules per unit cell, N is Avogadro’s number, M is the molecular weight and V is the unit cell volume [27].
The crystal axis ratio c/a is normally 4.0 for W type Ba hexaferrites. While the value of c/a ratio is 4.6733 for the investigated sample, owing to the migration of divalent cations in the voids causes the John–Teller effect [5].
The morphology and the grain size of the investigated sample were studied using SEM [28]. Fig. 3 illustrates SEM micrographs of BaCo2Fe16O27 nanoparticles. SEM images show a spongy morphology with the agglomerated grains. The average grain size of the sample is 73.7 nm. A reason for agglomeration is dipole interaction between the crystallites [29]. The grains have an irregular distribution with a hexagonal shape, which is the basic crystal cell of W- type ferrites. The EDX energy spectra of BaCo2Fe16O27 HFs are presented in Fig. 4. The inset table in Fig. 4 illustrates the weight percentage (wt. %) and atomic percentage (at.%) of the O, Ba, Fe and Co elements theoretically and experimentally. The theoretical wt. % and at.% were calculated from the chemical formula BaCo2Fe16O27 while the experimental values were obtained from EDX. The weight and atomic percentages clearly illustrate a small variation between the experimental and theoretical values due to oxygen deficiency, which can be advantageous for heavy metal removal from water. Figure 5 illustrates the elemental mapping of BaCo2Fe16O27 sample. The elements barium, cobalt, iron and oxygen are present in a homogenous distribution.
The magnetic properties of the investigated sample were studied using a vibrating sample magnetometer (VSM). Figure 6 (a) illustrates the curve of magnetic hysteresis for BaCo2Fe16O27 nanoparticles at room temperature. The values of coercive field Hc, saturation magnetization Ms and remanent magnetization Mr were listed in Table 2. The saturation magnetization (Ms) was determined also by the law of approach to saturation (LAS) [30, 31]:
where Ms is the saturation magnetization of the domains per unit volume, A is a constant associated with microstress, B is a constant representing the magneto-crystalline anisotropy contribution, and χH is the forced magnetization term. Figure 6 (b) illustrates the plotting of M versus 1/H2 in the high field range given a straight line. The saturation magnetization Ms of BaCo2Fe16O27 nanoparticle can also be detected by extrapolating the plot of magnetization versus 1/H2 to approach zero [32,33,34]. In this method, the Ms value is 67.28 emu/g. The obtained value is very comparable to the experimental value, signifying that an applied field of ± 20 kOe is appropriate to saturate the investigated sample. The factors affected on the magnetization of W-type hexaferrite are the composition of ferrite, the distance of the Fe–O bond and the superexchange interaction through Fe3+–O–Fe3+ [35].
Moreover, the coercivity of W-type hexaferrite is affected by many factors such as magneto-crystalline anisotropy, shape anisotropy, and saturation magnetization [36]. The magneto-crystalline anisotropy can be determined by the Stoner-Wohlfarth equation as follows [37]:
where K is the magneto-crystalline anisotropy constant, Ms is the saturation magnetization and Hc is the coercive field. The saturation magnetization and the net magnetic moment of the sample depend on the presence of the magnetic ions such as Co2+ (3.7 μB) and Fe3+ (5 μB) [38].
The squareness ratio R of the remanence to the saturation magnetization (Mr/Ms) indicates the domain structure of the investigated sample. The squareness ratio R value = 0.5 is indicative of a single domain and the lower value is associated with a multidomain structure and the particles interact by magneto-static interactions [39]. In the present work, the sample BaCo2Fe16O27 has R > 0.5, indicating the exchange coupled interaction between the domains.
Figure 7 illustrates the dependence of the molar magnetic susceptibility on absolute temperature as a function of the magnetic field intensities. As shown in the figure, three distinct regions were obtained. With increasing temperature, χM increases steadily in the first region, rapidly increased in the second region, and decreases rapidly in the third region.
A deeper examination of the first region reveals the sample to be pure ferrimagnetic material in which the thermal energy was insufficient to disturb the aligned moments of the spins. In the second region, the thermal energy is enough to make the dipoles freely align in the direction of the applied field. In the last region, the sample transfers from ferrimagnetic to paramagnetic behavior after the Curie temperature where χM decreases drastically.
Figure 8 shows the relation between the reciprocal of magnetic susceptibility χM−1 and the absolute temperature at different magnetic field intensities in the paramagnetic region. The magnetic parameters, such as the Curie constant (C) and the Curie–Weiss constant (θ), were calculated from the extrapolation of the linear part χM−1 in the paramagnetic region. The Curie constant (C) equal to the reciprocal of the slope of a straight line in the paramagnetic region. The values of effective magnetic moments (μeff) were calculated from the following relationship
where C is the Curie constant. These magnetic parameters were reported in Table 3. The data obey the well-known Curie–Weiss law [40].
where χM is the molar magnetic susceptibility, T is the absolute temperature and θ is the Curie–Weiss constant. The magnetic properties of the sample BaCo2Fe16O27 are originated from the Fe3+–O–Fe3+ superexchange interactions, which are mutually antiparallel, and also the Fe3+–Fe3+ direct exchange interactions.
Table 4 illustrates the comparative study between the present results and those reported earlier. The author noticed that my sample is the largest in the remanence magnetization and the coercive field, compared with the results obtained by other authors [16, 41,42,43,44]. The saturation magnetization (Ms) of the present sample is smaller than the sample obtained by Mohammad K. Dmour [42]. The variation in the values of Ms, Mr and Hc for the samples is due to the different preparation methods and ionic radii.
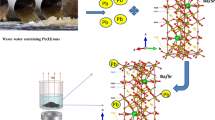
The investigated sample was prepared in nano scale (crystallite size = 37.39 nm) with a spongy morphology so the surface to volume ratio is large, which increases the number of active sites to trap Pb2+ ions and increases the removal efficiency. One of the main advantages of using Baco2Fe16O27 for Pb2+ adsorption is the easy separation from the solution using an external magnetic field due to its large magnetization.
The heavy metal Pb2+ ion removal from the wastewater was studied with different parameters such as pH value and contact time. Figure 9 illustrates the effect of pH solution on the heavy metal adsorption process. The adsorption of Pb2+ ion increased by increasing the pH value. It is clear that at lower values of pH, the adsorption of heavy metal ions is low. This is due to competition between H+ and Pb2+ on the active sites of adsorbent [45]. At pH = 7, the amount of H+ decreases in the solution and Pb2+ can be easily adsorbed on the active sites. Otherwise, at pH = 8, the solution contains oH−. Consequently, the heavy metal Pb2+ can be precipitated as lead hydroxide [46]. The precipitation of Pb2+ at pH 8 is not only a result of the adsorption of Pb2+ on BaCo2Fe16O27, but also the formation of lead hydroxide. So, the optimum pH value is 7.
The effect of contact time on the Pb2+ ion efficiency illustrates in Fig. 10 over a range (20–140) min. The adsorption of heavy metal Pb2+ increases by increasing the contact time. At the beginning of adsorption, a large number of active sites are available [47]. Finally, the optimum conditions for Pb2+ removal efficiency are pH 7 for 140 min using BaCo2Fe16O27 nano particles.
The mechanism of Pb2+ adsorption process can be studied using the adsorption isotherm. In the present work, two isotherm models that have been studied are the Langmuir and Freundlich models.
The multilayer adsorption on a heterogeneous surface can be described by Freundlich isotherm, which is commonly used to describe heavy metal adsorption on various adsorbents. The empirical model was proven to be consistent with a heterogeneous surface's exponential distribution of active centers. The relation between the amount of solute adsorbed (qe) and the equilibrium concentration of solute in solution (Ce) is given by the following equation:
This equation can be linearized to give the following expression:
Where, Kf is a constant for the Freundlich system, depends on the quantity of metal ion adsorbed onto adsorbent at an equilibrium concentration. Figure 11 illustrates the dependence of ln qe on ln Ce. The values of Kf and 1/n are calculated from the intercept and slope of the best fit line in Fig. 11.
The Langmuir isotherm model assumes that maximal adsorption occurs in a saturated monolayer of adsorbate molecules on the adsorbent surface. Many ground water effluent treatment procedures employ the Langmuir adsorption isotherm, which has also been used to explain the adsorption of heavy metals by various adsorbents. The Langmuir isotherm is considered that once a metal ion occupies an active site, no further adsorption occurs, and the adsorbed layer is unimolecular. The following equation describes the Langmuir isotherm model:
where qm is the adsorption capacity at maximum adsorption. Figure 12 shows the relation between (Ce/qe) and (Ce) for heavy metal Pb2+ to determine the Langmuir constants.
By comparing the correlation coefficient R2, the applicability of the isotherm equations is determined. The inset tables in both Figs. 11 and 12 illustrate that R2 = 0.91393 for Freundlich isotherm, while R2 = 0.7348 for Langmuir isotherm. Thus, the Freundlich adsorption isotherm fitted the experimental data well.
4 Conclusion
The barium hexaferrites was synthesized using citrate nitrate combustion method. XRD revealed that the sample BaCo2Fe16O27 was crystallized in a single phase hexagonal structure with space group P63/mmc. The average crystallite size is 37.39 nm. The morphology and the grain size of the investigated sample were studied using SEM. The sample has a spongy morphology with the agglomerated grains. The average grain size of the sample is 73.7 nm. The magnetic properties of BaCo2Fe16O27 were studied using two techniques: H–M hysteresis loop and the DC magnetic susceptibility. The sample has antiferromagnetic properties. The values of the saturation magnetization (Ms) and remanence magnetization (Mr) are 64.133 and 33.099 emu.g−1 respectively. The squareness ratio (Mr/Ms) is greater than 0.5, indicating the exchange coupled interaction between the magnetic domains. The heavy metal Pb2+ ion removal from the wastewater was studied with different parameters such as pH value and contact time. BaCo2Fe16O27 has Pb2+ removal efficiency 99% and 28% at pH 8 and 7 respectively. The Langmuir and Freundlich isotherms were used to analyze the experimental data. The Freundlich adsorption isotherm fitted the experimental data well.
References
Z.W. Li, G.Q. Lin, L. Chen, Y.P. Wu, C.K. Ong, J. Appl. Phys. 98, 94310 (2005)
Z. Su, Y. Chen, B. Hu, A.S. Sokolov, S. Bennett, L. Burns et al., J. Appl. Phys. 113, 1 (2013)
Z.H. Yang, Z.W. Li, Y.H. Yang, Mater. Chem. Phys. 144, 568 (2014)
H. Cho, S. Kim, Ceram. Int. 45, 9406 (2019)
R. Sagayaraj, T. Dhineshkumar, A. Prakash, S. Aravazhi, G. Chandrasekaran, D. Jayarajan et al., Chem. Phys. Lett. 759, 137944 (2020)
S.K. Nandi, S.K. Nath, A.K.M.A. Hossain, J.U. Khan, J. Supercond. Nov. Magn. 27, 2655 (2014)
X.F. Yang, Q. Jin, Z. Chen, Q. Li, B. Liu, J. Magn. Magn. Mater. 367, 64 (2014)
M.A. Khan, F. Hussain, M. Rashid, A. Mahmood, S.M. Ramay, A. Majeed, J. Mag. Mag. Mater. 452, 73 (2018)
A. Awadallah, S.H. Mahmood, Y. Maswadeh, I. Bsoul, M. Awawdeh, Q.I. Mohaidat et al., Mater. Res. Bull. 74, 192 (2016)
T. Koutzarova, S. Kolev, I. Nedkov, K. Krezhov, D. Kovacheva, B. Blagoev et al., J. Supercond. Nov. Magn. 25, 2631 (2012)
M.J.P. Asl, A. Ghasemi, G.R. Gordani, J. Supercond. Nov. Magn. 28, 109 (2015)
K.C. Patil, S.T. Aruna, T. Mimani, Solid State. Mater. Sci. 6, 507–512 (2002)
S. R. Jain, K. C. Adiga, V. R. Pai Verneker, Combustion and flame 40, 71–79 (1981).
A. Majid, J. Tunney, S. Argue, D. Wang, M. Post, J. Alloys Compd. 398, 48–54 (2005)
A. Mali, A. Ataie, Ceram. Int. 30, 1979–1983 (2004)
K. Huang, X. Liu, S. Feng, Z. Zhang, J. Yu, Niu, X. Huang et al., J. Magn. Magn. Mater. 379, 16–21 (2015).
M.A. Ahmed, N. Okasha, R.M. Kershi, J. Magn. Magn. Mater. 320, 1146–1150 (2008)
J. Tang, X. Liu, K.M.U. Rehman, D. Li, M. Li, Y. Yang, J. Magn. Magn. Mater. 452, 354–359 (2018)
T. Ahamad, M. Naushad, B. M. Al-Maswari, J. Ahmed, Z. A. ALOthman, S. M. Alshehri et al. J. Ind. Eng. Chem. 53, 268 (2017).
H. Sadegh, G.A.M. Ali, V.K. Gupta, A.S.H. Makhlouf, R.S. ghoshekandi, M.N. Nadagouda et al. J. Nanostruct. Chem. 7, 1 (2017).
D. Pal, S.K. Maiti, Environ. Saf. 187, 109833 (2020)
X. Wang, Y. Guo, L. Yang, M. Han, J. Zhao, X. Cheng et al., J. Anal. Toxicol. 2, 154 (2012)
A. Tripathi, M.R. Ranjan, J. Bioremed. Biodeg. 6, 315 (2015)
M.M. Arman, N.G. Imam, R.L. Portales, S.I. El-Dek, J. Magn. Magn. Mater. 513, 167097 (2020)
R. Ramadan, S.I. El-Dek, M.M. Arman, Appl. Phys. A. 126, 900 (2020)
M.M. Arman, S.I. El-Dek, J. Phys. Chem. Solids 152, 109980 (2021)
M. Reda, S.I. El-Dek, M.M. Arman, J. Mater. Sci. 33(21), 16753–16776 (2022)
E.E. Ateia, M.A. Ateia, M.G. Fayed, S. El-Hout, S.G. Mohamed, M.M. Arman, Appl. Phys. A 128, 1–10 (2022)
A. Ghasemi, S. Ekhlasi, M. Mousavinia, J. Mag. Mag. Mater. 354, 136 (2014)
S.H. Mahmood, G.H. Dushaq, I. Bsoul et al., J. Appl. Math. Phys. 2, 77 (2014)
D. Jiles, Introduction to Magnetism and Magnetic Materials, 3rd ediition. (CRC Press, 2015)
A. Baykal, S. Esir, A. Demir, S. Güner, Ceram. Int. 41, 231 (2015)
M.A. Almessiere, Y. Slimani, A. Baykal, Ceram. Int. 44, 9000 (2018)
S. Güner, M. Amir, M. Geleri, M. Sertkol, A. Baykal, Ceram. Int. 41, 10915 (2015)
X.S. Liu, W. Zhong, S. Yang, Z. Yu, B.X. Gu, Y.W. Du, J. Magn. Magn. Mater. 238, 207 (2002)
B. K. Rai, S. R. Mishra, V. V. Nguyen, J. P. Liu J. Alloys Compd. 550, 198 (2013).
E.E. Ateia, M.M. Arman, E. Badawy, Appl. Phys. A 125, 499 (2019)
R.A. Khan, S. Mizukami, A.M. Khan, B. Ismail, A.R. Khan, T. Miyazaki, J. Alloys Compd. 637, 197 (2013)
J.C. Jiles, Acta Materiala. 51, 5907 (2003)
M.M. Arman, M.A. Ahmed, Appl. Phys. A 1–9, 128 (2022)
S. Anand, S. Pauline, Adv. Mater. Interf. 8(3), 2001810 (2021)
M. K. Dmour, E. S. Al-Hwaitat, I. Bsoul, S. H. Mahmood J. Superconductivity Novel Magn. 33(2), 473–482 (2020).
F. Guo, X. Wu, G. Ji, J. Xu, L. Zou, S. Gan, J. Supercond. Novel Magn. 27(2), 411–420 (2014)
M.M. Hessien, D.A. Rayan, M.H.H. Mahmoud, A. Alhadhrami, M.M. Rashad, J. Mater. Sci. 29(12), 9771–9779 (2018).
Y. Kikuchi, Q. Qian, M. Machida, H. Tatsumoto, Carbon 44, 195 (2006)
M.A. Ahmed, S.T. Bishay, S.M. Abd-Elwahab, R. Ramadan, J. Mol. Liq. 240, 604 (2017)
S.Z.N. Ahmad, W.N.W. Salleh, A.F. Ismail, N. Yusof, M.Z.M. Yusop, F. Aziz, Chemosphere 248, 126008 (2020)
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that he has no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Arman, M.M. Structural, morphological and magnetic properties of hexaferrite BaCo2Fe16O27 nanoparticles and their efficient lead removal from water. Appl. Phys. A 128, 1103 (2022). https://doi.org/10.1007/s00339-022-06244-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-022-06244-y