Abstract
Novel multiferroic nanoparticles Sm1−xHoxFeO3 have been prepared using a simple citrate auto-combustion method. According to the X-ray diffraction (XRD) data, the multiferroic samples had an orthorhombic single phase structure. The field emission scanning electron microscope (FESEM) illustrated that the nanoparticles possessed a cotton-like morphology with a porous nature. The vibrating sample magnetometer (VSM) demonstrates the antiferromagnetic (AFM) behavior of the samples. The maximum magnetization (Mm) of Sm0.95Ho0.05FeO3 is five times greater than that of SmFeO3. The ferroelectricity of Sm1−xHoxFeO3 has Г2-spin structure. The antiferromagnetic (AFM) spin canting has a great effect on both the magnetization and the ferroelectricity of the investigated perovskites. Sm1−xHoxFeO3; 0.0 ≤ x ≤ 0.10 samples are concluded to be novel single-phase multiferroic materials capable of adsorbing toxic chromium (Cr6+) metal ions from water. A hydrogen potential of pH 6 is the best condition for precipitating chromium ions. The adsorption of Cr6+ ions on Sm1−xHoxFeO3 was studied using the Langmuir and Freundlich isotherm models.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Multiferroics (MF) are materials that have more than one ferroic order at the same time, such as ferroelectric and antiferromagnetic. Off-center structural distortions and local spins cause ferroelectricity and magnetism, respectively. Multiferroics have a wide range of potential applications, including data storage, sensors, spintronics, and photovoltaic devices [1,2,3]. Multiferroics are prepared in a single phase or as composite materials. Because the ferroelectric and ferromagnetic requirements for transition-metal ions are incompatible, only a few MF materials with a single phase have been discovered. Most current multiferroic materials have a lower TC than the room temperature [4, 5]. Therefore, the challenge for the researchers is the development of new multiferroic materials with high performance at room temperature.
Rare-earth orthoferrites (RFeO3) have become the hot spots in materials science because of their unique physical and chemical properties, such as multiferroicity, spin reorientation (SR), and antiferromagnetism [6, 7]. Multiferroics are insulating materials that have both magnetic and ferroelectric (FE) orders [8, 9]. The magnetic properties of RFeO3 originate from the electrons in both the 4f-orbitals of the rare earth cation R3+ and the 3d-orbitals of Fe3+. Due to the strong Fe3+–Fe3+ interaction, RFeO3 exhibits antiferromagnetic properties. While the magnetic coupling between the electrons of 4f R and 3d Fe is responsible for the spin reorientation transition (SR) interaction [10].
SmFeO3 is a rare earth orthoferrite with a space group of Pbnm. SmFeO3 has antiferromagnetic properties with weak ferromagnetism. SFO has the greatest SR temperature (TSR = 450–480 K), and at this temperature, the magnetic axis rotation occurs [11]. Re3+ ions occupy the A-site position, whereas Fe3+ ions occupy the B-site position and are surrounded by the oxygen ions to create the FeO6 octahedron.
In the present work, the Sm1−xHoxFeO3 nanoparticles were prepared using a simple wet combustion method. The role of Ho doping on the structure, morphology, ferroelectricity and magnetic characteristics of SmFeO3 was studied. The capacity of Sm1-xHoxFeO3 multiferroic to remove the harmful Cr6+ ion from an aqueous solution was examined.
2 Experimental
2.1 Synthesis of nanoparticles
The citrate auto-combustion method [12,13,14] was used to prepare the Sm1−xHoxFeO3 samples. In distilled water, the stoichiometric ratios of Sm nitrate, Ho nitrate, Fe nitrate (Sigma-Aldrich company, purity: 99.9%), and citric acid are dissolved. The nitrate to citric acid ratio was 1:1. The mixed solution was heated on a hot plate till the liquid became a viscous liquid. After further heating, the fluid became a fluffy powder. The prepared perovskites were annealed at 500 °C for two hours at a rate of 4 °C/min for heating and cooling.
2.2 Characterizations and measurements
A Bruker Advance D8 diffractometer was used to study the crystallinities of Ho-doped SmFeO3. For phase identification, the XRD data was compared with the International Centre for Diffraction Data (ICDD) card number 74–1474. The particle shape of the samples was studied via the high-resolution transmission electron microscope (JEOL-2100). The particle size distribution for the samples was performed using the software ImageJ Fiji and Origin Lab. The surface morphology of Sm1−xHoxFeO3 multiferroic was studied using the field-emission scanning electron microscope attached to energy dispersive X-ray spectroscopy (FESEM, EDX model Quanta 250).
The magnetic behavior of the prepared multiferroic was studied via its magnetic susceptibility and a vibrating sample magnetometer (VSM). The M–E hysteresis of the investigated multiferroic was estimated using the VSM (9600-1 LDJ) at a high magnetic field of up to 20 kOe. The relationship between the DC magnetic susceptibility (χM) and the temperature was studied by the Faraday method [15, 16]. In this experiment, the sample powder was put into a glass tube and placed at the maximum gradient of the field.
A uniaxial press was used to pressurize the powdered perovskites into pellets at a pressure of 5 × 103 N/m2. The two pellet surfaces were coated with silver paste. The samples were subjected to P–E loop measurement using a handmade Sawyer Tower circuit.
2.3 Toxic Cr6+ metal removal
The efficiency of Sm1−xHoxFeO3 samples to remove heavy metal (Cr6+) from water was studied. A solution of chromium (Cr6+) ions with a concentration of 50 ppm was prepared. Then, the above solution was introduced to a set of 250 mL beakers together with 0.02 g of Sm1−xHoxFeO3 samples. The ammonia solution and diluted sulfuric acid were used to adjust the pH of Cr6+ solutions. Using an electric shaker (Orbital Shaker SO1), the resulting solutions were shaken at room temperature for 1 h at a speed of 200 rpm. A 0.2-μm syringe filter filtered 10 mL of the solutions. The content of Cr6+ in the filtrate was determined using the inductively coupled plasma (ICP) spectrometer Prodigy7. The following equations were used to compute the equilibrium adsorption capacity (q) as well as the efficiency of metal ion removal (\(\eta\)).
where Ci and Ce are the heavy metal Cr6+ ion solution’s initial and final concentrations, respectively. While m is the adsorbent mass and V denotes the volume of the Cr6+ solution.
3 Results and discussion
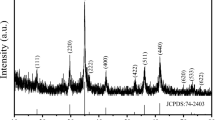
XRD was used to study the phase structure of Ho doped SmFeO3. In comparison to the ICDD card 74-1474, the perovskites were synthesized in a single phase, as shown in Fig. 1. The X-ray photoemission spectroscopy (XPS) of Sm0.95Ho0.05FeO3 revealed that there are no impurities in the sample [17]. The samples have an orthorhombic structure with the Pbnm space group. The following equation is used to compute the lattice parameters.
The Debye-Sherrer's equation was used to calculate the crystallite sizes (D) of the perovskites [17,18,19].
where λ is the X-ray wavelength of (λ = 1.5406 Å), θ is the Bragg angle and β is the full width at half maximum intensity. Table 1 lists the crystallite sizes of the prepared perovskites and indicates that they were prepared in the nanoscale.
The unit cell volume decreases as the Ho content increases due to the substitution of a small ionic radius (RHo = 1.072 Å) at the expense of a large ionic radius (RSm = 1.132 Å) [20]. The density was computed according to the following equation:
where Z (4) represents the number of molecules in a unit cell, V denotes the volume of a single cell, and M denotes the molecular weight. N represents Avogadro’s number. The values of Dx were increased by increasing the Ho content, as shown in Table 1.
The tolerance factor (t) is a geometrical parameter that distinguishes off-center symmetry from central symmetry. The following equation was utilized to determine the values of t [21].
where rA, rFe, and rO are the radii of the A, Fe, and oxygen ions, respectively. Because Ho3+ has a smaller ionic radius (RHo = 1.072 Å) than Sm3+ (RSm = 1.132 Å), a small amount of Ho3+ doping changes the SmFeO3 unit cell parameters and cell volume. By increasing the Ho content, t decreases, as shown in Table 1. The tolerance factor data show that the samples have distorted orthorhombic structures.
The presence of the noises in the XRD pattern indicates that the samples were prepared in nanoscale. It is well known that the XRD of nanomaterials is characterized by broadening peaks with noise [22]. The factors that affect the broadening of peaks and the signal-to-noise ratios are the crystallite size, lattice strains, and particle morphology of the samples [23]. The substitution of the large A site cation in perovskite structure by the Ho3+ ions leads to an increase in the distortion in the structure [24]. In the investigated samples Sm1−xHoxFeO3, by increasing the Ho concentration (x = 0.10), the distortion increases and the noise in the XRD pattern increases. The values of the tolerance factor in Table 1 indicate that the tolerance factor decreases with increasing Ho concentration in the samples, leading to increased distortion in the samples. The bond angle and the bond length of Fe–O and Sm–O are strongly dependent on the distortion in the crystal structure. These data agree well with the data reported by Liu et al. [24], who prepared Bi1−xHoxFeO3, 0.0 ≤ x ≤ 0.2, and reported that at high Ho doping concentrations, x > 0.1, the broadening of the XRD peaks, lattice distortion, and phase transformation occurred.
The EDX spectrum for the Sm0.90Ho0.10FeO3 sample is shown in Fig. 2, while the EDX of the sample Sm0.95Ho0.05FeO3 was reported in my previous study [17]. The EDX spectra illustrate the presence of the elements Fe, O, Ho, and Sm in the sample without any impurities. The resultant chemical compositions agree with the stoichiometric ratio used during synthesis.
As shown in Fig. 3, HRTEM was used to examine the morphology and form of Ho doped SmFeO3 nanoparticles. The shape of the particles of the prepared samples is close to orthorhombic, but the particles are agglomerated due to their magnetic properties. The size distribution histograms of the investigated samples are illustrated in Fig. 3d–f. The particle size values of the multiferroic are reported in Table 1. The particle size is in the range of 15–23 nm, which means that the samples were prepared on a nanoscale. The insets in Fig. 3 illustrate the selected area electron diffraction (SAED) images of the prepared perovskites. The SAED pattern illustrates the bright rings, which indicate that the synthesized nanoparticles are polycrystalline in nature.
a–c HRTEM images of the samples; a SmFeO3, b Sm0.95Ho0.05FeO3, and c Sm0.90Ho0.10FeO3 (Fig. 3). d–f The size distribution histograms for the samples
Figure 4 depicts the surface topography of samples of Ho-doped SmFeO3 using a FESEM. Due to their magnetic characteristics, the produced samples are tightly packed with irregular elliptical grains. The magnetic properties of Sm0.95Ho0.05FeO3 are impacted by the increase in grain size. The pores in the samples increase the surface area to volume ratio and therefore encourage their use in gas sensors, Li-ion batteries, and water treatment.
The perovskite AFeO3 has two magnetic sublattices: the first is the electron of 4f rare earth ions, and the second is the electron of 3d Fe ions [25]. The presence of the magnetic ions Fe, Sm, and Ho causes the magnetic behavior of Sm1−xHoxFeO3. Figure 5 illustrates the room temperature magnetic hysteresis loops of Sm1−xHoxFeO3, 0.0 ≤ x ≤ 0.2. The magnetic hysteresis loops exhibit S-shape and non-saturation behavior, which illustrates the antiferromagnetic (AFM) properties of the investigated perovskites with weak ferromagnetism. The AFM properties of the samples originate from the Fe–Fe superexchange interaction via the oxygen ions [26]. According to the Dzyaloshinskii–Moriya (DM) exchange interaction, the coercive field values illustrate the presence of weak ferromagnetic components due to the Fe3+ spin canting in the samples [27]. SmFeO3 has a G-type antiferromagnetic behavior in which its spins are arranged with a small canting antiparallel. As a result of the DM interaction, the components of the moments along the c axis cannot cancel each other out, leading to the presence of weak ferromagnetic properties in the samples [28]. Many researchers assured the antiferromagnetic behavior with weak ferromagnetic components for the perovskite structure [29,30,31,32].
The weak ferromagnetic properties in the SmFeO3 perovskite oxides originate from two reasons:
-
1.
The first reason is the structure distortion due to the replacement of Ho3+ ions with a small ionic radius (RHo = 1.072 Å) instead of Sm3+ ions with a large ionic radius (RSm = 1.132 Å). In the present work, the doping Ho3+ ions in SmFeO3 with 5% and 10% do not cause a structural transformation. While the lattice distortion increases, which changes the bond length of Sm–O and Fe–O bonds. The superexchange interaction is strongly dependent on the bond lengths of Sm–O and Fe–O bonds and the bond angles of Fe–O–Fe.
-
2.
The second reason is the superexchange interaction between the magnetic ions Re3+ and Fe3+ ions. The samples Sm1−xHoxFeO3 have three types of magnetic interactions: the strongest interaction is Fe3+–Fe3+ interaction, the intermediate interaction is Re3+–Fe3+ (i.e., Ho3+–Fe3+ and Sm3+–Fe3+), and the weakest interaction is Re3+–Re3+ interaction (Sm3+–Sm3+ and Ho3+–Ho3+). The holmium ions are characterized by a large magnetic moment (10.3 μB). At the small Ho concentration, the superexchange interaction occurs between the 4d orbital of Ho3+ ions and the 2p orbital of Fe3+ ions and enhances the ferromagnetic properties of Sm0.95Ho0.05FeO3. While at high concentrations of Ho doping, for the sample Sm0.90Ho0.10FeO3, the maximum magnetization (Mm) and remanence magnetization (Mr) decrease due to increasing the lattice distortion in the sample, decreasing the strength of superexchange interaction, and suppressing the collinearity of AFM spin canting of Fe3+ ions [33, 24]. Liu et al. [24] reported that the magnetization of BiFeO3 increased with a small concentration of Ho3+ doping and also decreased at high Ho3+ doping concentration. These results agree well with the investigated samples, Sm1−xHoxFeO3.
Table 2 shows the values of coercivity (Hc), remanence magnetization (Mr), and maximum magnetization (Mm) determined from magnetic hysteresis loops. With 5% Ho doping, the maximum magnetization (Mm = 0.50 emu/g for SmFeO3) increases dramatically (Mm = 2.55 emu/g for Sm0.95Ho0.05FeO3). The replacement of Ho ions in the orthoferrites leads to distortion of the lattice, tilts of the FeO6 octahedron, and alteration of the Fe3+–Fe3+ interactions. Since Ho occupies the Sm site, the magnetization was increased due to the contribution of Ho ions in the magnetic couplings like Ho–O–Ho, Ho–O–Sm and Fe–O–Ho. The magnetic moment of the Ho3+ ion (10.3 μB) is greater than that of the Sm3+ ion (1.6 μB). Therefore, the doped SmFeO3 samples exhibit a higher value of magnetization [34].
The variation of the lattice parameters and unit cell volume of the investigated samples is a result of the difference in the values of the ionic radii of Sm3+ (RSm = 1.132 Å) and Ho3+ ions (RHo = 1.072 Å). However, there are no serious structural phase changes for the samples Sm0.95Ho0.05FeO3 and Sm0.90Ho0.10FeO3. The concentration of Ho doping strongly affects the bond length and the bond angle of Sm–O and Fe–O bonds and is also responsible for the values of magnetization [32]. The superexchange interactions depend on the bond angle and bond length of Fe–O–Fe [35].
The values of the magnetic anisotropy constants were determined using Eq. (7) and listed in Table 2 [36].
where Ms is the saturation magnetization, Hc is the coercivity, and K is the anisotropy constant. The non-collinearity of spins on the surface of the perovskites is the cause of their weak anisotropy [36]. Table 3 shows the comparative study of the Mm, Mr and Hc of the perovskite materials. The magnetic properties are strongly dependent on the type of A-site cation, crystallite size, and preparation techniques.
Figure 6 depicts the relationship between χM and the absolute temperature at different magnetic field strengths for SmFeO3 nanoparticles. χM values rise with increasing temperature until TSR = 463 K, when they begin to fall slowly until they reach the Neel temperature (TN). After TN, the prepared sample loses its antiferromagnetic properties and begins to exhibit paramagnetic behavior. Because of the AFM coupling between the Fe3+ ions (Fe–O–Fe), the investigated samples have antiferromagnetic properties. By increasing the temperature, the magnetization of the sample decreases as a result of the decrease in the magnetocrystalline anisotropy until TN, at which the magnetic disorder occurs completely [39, 40]. At a temperature greater than TN, the sample exhibits paramagnetic properties. According to the following equation, the χM decreases as the applied magnetic field is increased.
Figure 7 shows the dependence of χM−1 on the absolute temperature. The data complies with the Curie–Weiss law. The magnetic parameters like the Curie–Weiss constant (θ), the Curie constant (C), and the effective magnetic moment (μeff) were determined from the slope in the paramagnetic zone as illustrated in Fig. 7 using the following equations:
Table 4 shows the C, θ and μeff values for the investigated samples. The values of TN were determined from the differentiation of molar magnetic susceptibility with respect to temperature (dχM/dT) as an accurate value and listed in Table 4. μeff values increase for the Ho doped samples due to the Ho3+ ions having a larger magnetic moment than the Sm3+ ions. When Ho3+ ions are introduced into SmFeO3, magnetic couplings like Ho–O–Ho, Ho–O–Sm, and Ho–O–Fe are formed.
The comparative study between the investigated nanoparticles is shown in Fig. 8. All samples exhibit AFM properties and weak ferromagnetic components. The SR transition of perovskite materials depends on the doping amount of Ho3+ ions owing to the exchange interactions between the 4f electrons of Sm3+ and Ho3+ ions and the 3d-electrons of Fe3+ ions. The TSR of Sm0.90Ho0.10FeO3 is 363 K lower than that of SmFeO3 because, as Sm3+ ions are replaced by Ho3+ ions, the strength of the magnetic exchange interaction between Sm3+ and Fe3+ decreases, and thus the SR decreases. The substitution of Sm3+ by Ho3+ affects the angle and the length of the Fe–O bond, which leads to the tilting < FeO6 > octahedron. Magnetic interactions between Sm–O–Ho, Ho–O–Ho and Fe–O–Ho are another reason for the increased magnetic behavior.
Figure 9 depicts the P–E loops of Ho doped SmFeO3 perovskites. The ferroelectric properties of the samples originate from the shift of Fe ions from the center of the oxygen octahedron < O6 > to form strong bonds [41] and also from the tilting of the FeO6 octahedron, which influences the crystal lattice symmetry [42]. Because of leakage current, there is no saturation polarization [43, 44]. The values of saturation polarization (Ps), electric coercivity (Ec) and remnant polarization (Pr), are listed in Table 5. The saturation polarization increased slightly with Ho doping due to the tilting of the BO6 octahedron, which increases the ferroelectric properties of the samples. The ferroelectric properties of the samples were changed by different factors such as dopant concentration, phase change [35].
The investigated samples, Sm1−xHoxFeO3, have multiferroic behavior at room temperature. The magnetic properties are derived from the spin canting antiferromagnetic Fe3+ ions with Dzyaloshinskii-Moriya (DM) exchange interactions. The ferroelectricity of the investigated samples has Г2—spin structure, which originated from the reverse Dzyaloshinskii-Moriya interaction between pairs of nonequivalent canted AFM spins [45, 46]. The induced spin canting has a great influence in both the magnetization and the ferroelectricity of the samples. The ferroelectricity of the samples is magnetically induced. Therefore, with the small Ho doping concentration in Sm0.95Ho0.05FeO3, both Mm and Ps increased than that of SmFeO3, and also both Mm and Ps decreased for the sample Sm0.90Ho0.10FeO3.
The adsorption of harmful Cr6+ ions on the adsorbent depends on the pH value of the solution because its value controls the adsorbent’s surface charge [47]. The iron oxide particles in the perovskite Sm1−xHoxFeO3 are hydrated in aqueous solutions, and FeOH surrounds the sample surface [48]. Depending on the pH level, the FeOH groups on the absorbent's surface react with the OH− or H+ ions of dissolved bases or acids to form negative (FeO−) or positive (FeOH2+) charges.
The dependence of the adsorption of Cr6+ ions on the solution pH is shown in Fig. 10. According to the graph, pH 6 is the best condition for precipitating chromium ions. Although the removal efficiency of SmFeO3 is greater than that of Ho doped samples at pH 6, the magnetization of the samples Sm0.95Ho0.05FeO3 and Sm0.90Ho0.10FeO3 is greater than that of SmFeO3, and so the Ho doped SmFeO3 can be easily separated from the solution to be reused again. At low values of pH, i.e., pH 4 and 5, the Sm1−xHoxFeO3 surface adsorbs both the H+ and Cr6+ ions. So the removal efficiency of Cr6+ ions is low. At pH 8, Cr6+ ions precipitate as hydroxide, which is unfavorable for adsorption [49].
The adsorption isotherm gives most information about the adsorption process between the Sm1−xHoxFeO3 and heavy metal ions. The adsorption of Cr6+ ions on Sm1−xHoxFeO3 was studied by Langmuir and Freundlich isotherm models.
The multilayer adsorption of Cr6+ ions on an adsorbent surface was studied using the Freundlich isotherm, which is expressed using Eq. (11).
where qe denotes the solute adsorbed and Ce denotes the solute equilibrium concentration. This equation can be written in linear form, as illustrated in the following equation:
where Kf is the Freundlich constant. Figure 11 shows the relation between ln qe and ln Ce. The values of 1/n and Kf are the slope and intercept of the best-fit line in Fig. 11.
The adsorption of Cr6+ ions on the Ho doped SmFeO3 was studied using the Langmuir [50] isotherm model, which can be described using the following equation:
where qm denotes the maximum adsorption capacity (mg g−1), Ce is the equilibrium Cr6+ concentration (mg L−1), KL is the Langmuir constant (L mg−1), and qe is the equilibrium adsorption capacity (mg g−1) which was calculated using the Eq. (14).
where Ci denotes the Cr6+ initial concentration (mg L−1) in the solution, m is the adsorbent mass, and V refers to the volume of the Cr6+ solution. Figure 12 depicts the fitting of the experimental data with the Langmuir model.
Table 6 contains the values of the correlation coefficient (R2) of the two isotherm models (Langmuir and Freundlich) for the Sm1−xHoxFeO3 samples. The Freundlich isotherm effectively matches the experimental data, as shown by the R2 values.
4 Conclusions
In summary, the citrate auto-combustion method is used to synthesize Ho-doped SmFeO3 multiferroic in a single phase. XRD illustrated that the samples have an orthorhombic structure, and their crystallite sizes are 18–20 nm. The SAED pattern illustrates that the investigated perovskites have a polycrystalline nature. FESEM showed the porous, cotton-like morphology of Ho doped SmFeO3 nanoparticles. The sample Sm0.95Ho0.05FeO3 has the largest anisotropy constant and the maximum magnetization. Sm0.95Ho0.05FeO3 is therefore suggested as an optimum sample for numerous applications such as sensors, catalysis, and heavy metal removal. The ferroelectricity of the investigated multiferroic is magnetically induced. For the first time, novel multiferroic materials, Sm1−xHoxFeO3; 0.0 ≤ x ≤ 0.10 are presented and recommended for heavy metal removal from water. The perovskites Sm1−xHoxFeO3 are effective water absorbers for heavy metals like Cr6+. The Freundlich isotherm best describes the adsorption isotherm of the Cr6+ ion from water using Ho doped SmFeO3.
Data availability
The data is available when it is required.
References
L. Cao, Z. Ding, X. Liu, J. Ren, Y. Chen, M. Ouyang, F. Yang, J. Alloy. Compd. 800, 134–139 (2019)
L. Luo, Y. Guo, W. Yan, K. Liang, Q. Sun, J. Su, J. Zhu, J. Mater. Sci.: Mater. Electron. 30, 17872 (2019)
Y. Li, X.X. Cui, N.N. Sun, J.H. Du, X.W. Li, G.X. Jia, X.H. Hao, Adv. Opt. Mater. 7, 1801105 (2019)
J.A. Mundy, C.M. Brooks, M.E. Holtz, J.A. Moyer, H. Das, A.F. Rébola, J.T. Heron, J.D. Clarkson, S.M. Disseler, Z. Liu, Nature 537, 523–527 (2016)
S. Dong, J. Liu, S. Cheong, Z. Ren, Adv. Phys. 64, 519–629 (2015)
G. Song, J. Su, S. Fang, J. Tong, X. Xu, H. Yang, N. Zhang, Physica B 589, 412185 (2020)
J.F. Scott, J. Gardner, Mater. Today 21, 553–561 (2018)
S.W. Cheong, M. Mostovoy, Nat. Mater. 6, 13–20 (2007)
R. Ramesh, N.A. Spaldin, Nat. Mater. 6, 21–29 (2007)
J. Shen, H. Zhao, B. Song, R. Liu, J. Du, Q. Xu, Q. Li, J. Magn. Magn. Mater. 527, 167724 (2021)
R.L. White, J. Appl. Phys. 40, 1061 (1969)
G.V. Priya, N. Murali, M.K. Raju, B. Krishan, D. Parajuli, P. Choppara, P.L. Narayana, Appl. Phys. A 128, 663 (2022)
D. Parajuli, P. Taddesse, N. Murali, K. Samatha, Appl. Phys. A 128, 58 (2022)
E.E. Ateia, M.M. Arman, A.T. Mohamed, Sci. Rep. 13, 3141 (2023)
H. Shen, J. Xu, A. Wu, J. Zhao, M. Shi, Mater. Sci. Eng. B 157, 77 (2009)
M.A. Ateia, E.E. Ateia, M. Mosry, M.M. Arman, Appl. Phys. A 128, 1–14 (2022)
M.M. Arman, Appl. Phys. A 129, 1–10 (2023)
S.Y. Mulushoa, N. Murali, M.T. Wegayehu, V. Veeraiah, K. Samatha, Materials Today: Proceedings 5, 26460–26468 (2018)
D. Parajuli, P. Taddesse, N. Murali, K. Samatha, J. Indian Chem. Soc. 99, 100380 (2022)
R.D. Shannon, Acta Crystallogr. Sect. A Crystal Phys. Diffr. Theoret. General Crystallogr. 32, 751–767 (1976)
M.M. Arman, N.G. Imam, R. Loredo Portales, S.I. El-Dek, J. Magn. Magn Mater. 513, 167097 (2020)
C.F. Holder, R.E. Schaak, ACS Nano 13, 7359–7365 (2019)
S. Mourdikoudis, R.M. Pallares, N.T. Thanh, Nanoscale 10, 12871–12934 (2018)
Y.Q. Liu, Y.J. Wang, J. Zhang, M. Gao, Y.J. Zhang, M.B. Wei, J.H. Yang, Mater. Sci. Semicond. Process. 40, 787–795 (2015)
S.X. Cao, H.Z. Zhao, B.J. Kang, J.C. Zhang, W. Ren, Sci. Rep. 4, 5960 (2014)
M.A. Ahmed, N.G. Imam, M.K. Abdelmaksoud, Y.A. Saeid, J. Rare Earths 33, 965–971 (2015)
J.S. Zhou, L.G. Marshall, Z.Y. Li, X. Li, J.M. He, Phys. Rev. B 102, 104420 (2020)
Q. Liu, X.X. Wang, C. Song, J.X. Sui, X. Yan, J.C. Zhang, Y.Z. Long, J. Magn. Magn. Mater. 469, 76–80 (2019)
A.T. Apostolov, I.N. Apostolova, S. Trimper, J.M. Wesselinowa, Solid State Commun. 300, 113692 (2019)
K. Bouziane, A. Yousif, I.A. Abdel-Latif, K. Hricovini, C. Richter, J. Appl. Phys. 97, 10A504 (2005)
E.E. Ateia, M.M. Arman, E. Badawy, Appl. Phys. A 125, 1–7 (2019)
S. Sharif, G. Murtaza, T. Meydan, P.I. Williams, J. Cuenca, S.H. Hashimdeen, R. Ahmad, Thin Solid Films 662, 83–89 (2018)
A.A. Khan, A. Ahlawat, S.M. Faisal, M.K. Singh, A.K. Karnal, S. Satapathy, J. Alloy. Compd. 808, 151603 (2019)
H. Ueken, B.G. Magnetochemie, Teubner Stuttgart. (Wiesbaden, Leipzig, 1999)
A. Kumar, K.L. Yadav, J. Rani, Mater. Chem. Phys. 134, 430–434 (2012)
E.E. Ateia, M.A. Ateia, M.M. Arman, J. Mater. Sci.: Mater. Electron. 33, 8958–8969 (2022)
Q. Lin, X. Yang, J. Lin, Z. Guo, Y. He, Int. J. Hydrogen Energy 43, 12720–12729 (2018)
E.E. Ateia, B. Hussein, C. Singh, M.M. Arman, The European Physical Journal Plus 137, 443 (2022)
S. Tekeli, M. Erdogan, B. Aktas, J. Matér. Sci. Eng. B 386, 1–9 (2004)
R. Ramadan, S.I. El-Dek, M.M. Arman, Appl. Phys. A 126, 1–13 (2020)
M.M. Arman, M.A. Ahmed, S.I. El-Dek, J. Supercond. Novel Magn. 31, 1867–1879 (2018)
H. Liu, X. Yang, Ferroelectrics 507, 69–85 (2017)
M.M. Arman, S.I. El-Dek, J. Supercond. Novel Magn. 30, 1221–1229 (2017)
J. Liu, M. Niu, L. Wang, G. Chen, D. Xu, J. Mater. Sci. Mater. Electron. 31, 3479–3491 (2020)
C.N.R. Rao, A. Sundaresan, R. Saha, J. Phys. Chem. Lett. 3, 2237–2246 (2012)
J.H. Lee, Y.K. Jeong, J.H. Park, M.A. Oak, H.M. Jang, J.Y. Son, J.F. Scott, Phys. Rev. Lett. 107, 117201 (2011)
M.K. Ahmed, R. Ramadan, M. Afifi, A.A. Menazea, J. Market. Res. 9, 8854–8866 (2020)
M.A. Ahmed, S.M. Ali, S.I. El-Dek, A. Galal, Mater. Sci. Eng., B 178, 744–751 (2013)
M.M. Arman, J. Supercond. Novel Magn. 35, 1241–1249 (2022)
W. Haron, J. Sci. Technol. 9, 544–551 (2017)
Acknowledgements
The author acknowledges the effort of Dr. A. Kamel Tamman (Faculty of Science, Cairo University) in the preparation of the P–E loop experiment.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
MMA put the idea on paper, prepared the samples, and discussed the characterization, physical properties, and application of the samples.
Corresponding author
Ethics declarations
Conflict of interest
The author declares that he has no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Arman, M.M. Novel multiferroic nanoparticles Sm1−xHoxFeO3 as a heavy metal Cr6+ ion removal from water. Appl. Phys. A 129, 400 (2023). https://doi.org/10.1007/s00339-023-06666-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-023-06666-2