Abstract
Objectives
The aim of this study is to investigate the added value of diffusion-weighted imaging (DWI) to dynamic-contrast enhanced (DCE)-MRI to identify a pathological complete response (pCR) in patients with HER2-positive breast cancer and radiological complete response (rCR).
Materials and methods
This is a single-center observational study of 102 patients with stage I-III HER2-positive breast cancer and real-world documented rCR on DCE-MRI. Patients were treated between 2015 and 2019. Both 1.5 T/3.0 T single-shot diffusion-weighted echo-planar sequence were used. Post neoadjuvant systemic treatment (NST) diffusion-weighted images were reviewed by two readers for visual evaluation and ADCmean. Discordant cases were resolved in a consensus meeting. pCR of the breast (ypT0/is) was used to calculate the negative predictive value (NPV). Breast pCR-percentages were tested with Fisher’s exact test. ADCmean and ∆ADCmean(%) for patients with and without pCR were compared using a Mann-Whitney U-test.
Results
The NPV for DWI added to DCE is 86% compared to 87% for DCE alone in hormone receptor (HR)-/HER2-positive and 67% compared to 64% in HR-positive/HER2-positive breast cancer. Twenty-seven of 39 non-rCR DWI cases were false positives. In HR-negative/HER2-positive breast cancer the NPV for DCE MRI differs between MRI field strength (1.5 T: 50% vs. 3 T: 81% [p = 0.02]). ADCmean at baseline, post-NST, and ∆ADCmean were similar between patients with and without pCR.
Conclusion
DWI has no clinically relevant effect on the NPV of DCE alone to identify a pCR in early HER2-positive breast cancer. The added value of DWI in HR-positive/HER2-positive breast cancer should be further investigated taken MRI field strength into account.
Clinical relevance statement
The residual signal on DWI after neoadjuvant systemic therapy in cases with early HER2-positive breast cancer and no residual pathologic enhancement on DCE-MRI breast should not (yet) be considered in assessing a complete radiologic response.
Key Points
-
Radiologic complete response is associated with a pathologic complete response (pCR) in HER2+ breast cancer but further improvement is warranted.
-
No relevant increase in negative predictive value was observed when DWI was added to DCE.
-
Residual signal on DW-images without pathologic enhancement on DCE-MRI, does not indicate a lower chance of pCR.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Treatment with neoadjuvant chemotherapy plus anti-HER2-directed agents results in high pathological complete response (pCR) rates and excellent survival outcomes [1,2,3,4,5,6,7,8]. Patients who do not reach a pCR of the breast and/or lymph nodes after neoadjuvant systemic treatment (NST) face an inferior prognosis and are candidates for adjuvant treatment with trastuzumab emtansine [9]. Conversely, patients with a complete early treatment response during NST are candidates for image-guided trials to de-escalate systemic or surgical treatment [10,11,12,13,14]. Such de-escalation trials need a high negative predictive value (NPV) since residual invasive disease requires extended treatment.
In clinical practice, dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) is widely used for response evaluation of breast tumors during and after NST. However, the NPV of a radiological complete response (rCR) on DCE-MRI for identifying a pCR breast after NST in HER2-positive patients remains suboptimal and differs between hormone-receptor (HR)-positive (42–78%) and HR-negative breast cancer (69–88%) [15,16,17,18,19,20].
DWI is a functional MRI-technique based on the random motion of water molecules in tissue microstructures. Within oncologic imaging, DWI can be used for identifying hyper-cellular structures (i.e., tumors) by showing hindered diffusion. To quantify this, different b-value images can be used to calculate the apparent diffusion coefficient (ADC)-map. DWI can also be used to identify longitudinal microstructural changes in diffusivity due to NST. ADC difference over time (∆ADC) is reported as a potential imaging biomarker [21]. However, no externally validated thresholds for ADC values or ∆ADC percentages are currently available for post-NST-scans in HER2-positive patients specifically [22]. Also, the role of both quantitative and qualitative DWI evaluation in HER2-positive breast cancer remains unclear since most studies do not differ between breast cancer subtypes despite the difference in accuracy for identifying residual disease of DCE-MRI [23,24,25,26].
A residual signal on DW-images after NST is sometimes observed in breast cancer when DCE shows no residual enhancement. To be able to use MRI breast in image-guided systemic treatment de-escalation in patients with a pathological complete response, a high NPV is essential. Therefore, we investigated if adding DWI to standalone DCE-MRI increases the NPV in stage I-III HER2-positive breast cancer patients with no residual pathologic enhancement after NST.
Materials and methods
Patients
All patients with stage I-III HER2-positive breast cancer treated in the Netherlands Cancer Institute between January 2015 and March 2019 were identified. Inclusion criteria for this observational study were: (1) treatment with trastuzumab-based NST, (2) baseline and post-NST breast MRI available, (3) rCR on DCE-MRI breast after NST, and (4) breast surgery. This study was approved by the institutional review board without the need to obtain informed consent.
All included patients had confirmed HER2-positive invasive carcinoma on biopsy prior to treatment. HER2 positivity is defined as a score 3 + in immunohistochemistry, or scored as 2 + together with the amplification of the HER2-encoding gene assessed with in situ hybridization. Tumors with ≥ 10% estrogen and/or progesterone receptor expression were considered HR-positive. A breast MRI-scan was performed at baseline and after NST for response evaluation. After neoadjuvant treatment, all patients underwent breast-conserving surgery or a mastectomy and axillary staging with sentinel lymph node biopsy, MARI procedure [27, 28], or axillary lymph node dissection.
MRI acquisition
Patients were scanned in a prone position using a 1.5-T or 3.0-T MRI scanner (Achieva, Philips Medical Systems) with a dedicated breast array coil. The MRI protocol consisted of DCE and DWI acquisitions. DCE was based on gradient echo sequences, acquiring several 3D-T1s (T1W High-Resolution Isotropic Volume Examination (THRIVE)), starting with one pre-contrast (i.e., unenhanced) scan, followed by a series of post-contrast scans. Fifteen (15) mL gadolinium-based contrast agent (Dotarem®, Guerbet) was administered to provide extra T1 contrast. From the DCE-scan series, subtracted wash-in and wash-out images were calculated on the scanner workstation. Moreover, between the pre-contrast and first post-contrast 3D-T1, dynamic ultrafast (4D-perfusion) scans were performed for characterizing lesions’ inflow curve characteristics.
DWI preceded the DCE acquisition, using a single shot-echo planar imaging (SS-EPI) sequence using SPectral Attenuated Inversion Recovery (SPAIR) fat suppression and SENSitivity Encoding (SENSE) for parallel imaging, with b values varying from b0 to b1500. To minimize pseudo-diffusion/perfusion effects, corresponding ADC maps were calculated based on b = 150 s/mm2 and b = 800 s/mm2, if available [29]. Sequence parameters are reported in the Supplementary Material (Supplementary Material, page 2).
Image analysis
Baseline and post-NST DCE-MRI scans were evaluated as part of routine clinical practice. rCR as observed on the DCE scans was retrieved from the radiology reports as previously described by Van Ramshorst et al [15]. After reviewing all reports, ambiguous cases were revised by a dedicated breast radiologists (R.W., reader 1). The definition used for rCR on DCE-MRI was the absence of pathological contrast enhancement in the original tumor region. Therefore, minimal remaining contrast enhancement in the original tumor region similar to or less than surrounding normal breast tissue was considered physiological. All cases with rCR on DCE-MRI were subsequently assessed by two dedicated readers. Reader 1 had 4 years of experience in reading MRI breast and reader 2 had 16 years of experience in MRI breast. A standardized reading form was used, endorsed by both readers who evaluated the available images using the picture archiving and communication system.
The reading protocol consisted of the following items on the pre-treatment scans: quality of DWI-MRI (good/moderate/bad), region of interest (ROI), and ADCmean [mm2/s] (Supplementary Material, page 4). The quality of the DW-images was good when the image was reasonable free of distortion, ghosting, and chemical shift artifacts, and had adequate fat suppression. When there were evident artifacts, but interpretation could be performed, the image quality was labeled moderate. The readers were instructed to select an oval ROI on the ADC-map, corresponding to the most pronounced appearing hindered diffusion region on the high DW-image (Fig. 1). In case lesions covered multiple slices, the middle cross-sectional area on the ADC-map was used. Subsequently, also post-NST DWI-MRI scan quality was scored, followed by the items: high signal at b0 (yes/no), high signal at high DW-image ( ≥ b800) (yes/no), tumor visible at ADC-map (yes/no), characteristics, DWI-MRI rCR conclusion (Supplementary Material, page 4). The ROI for the ADC values on post-NST scans was placed within the former tumor region that still showed a relative high signal on high b-value images (≥ b800, using the baseline MRI as a reference). If the tumor was no longer visible, a landmark (marker or anatomic structure) could be used to support post-NST identification on DWI. The final conclusion for the DWI-MRI rCR reading was scored as follows: definitely rCR, probably rCR, possible rCR, or definitely no rCR. Disagreements on the final DWI-MRI conclusion were resolved in a consensus meeting. Only cases scored with definitely rCR by both readers after the consensus meeting were considered rCR on DCE and DWI.
Assessment of ADC value in a patient with radiological complete response at contrast-enhanced MRI breast. A–D show pre-NST images and E–H show the post-NST images. A, E are subtracted images showing the tumor area (the dark spot in E is due to the clip inserted at the tumor site). B, F show the corresponding area at the b800 images. C, G show the corresponding ADC maps. D, H show the ROIs used with the corresponding ADC values in 10−6 mm2/s
Endpoints
The primary endpoint for this study was pCR defined as the absence of residual invasive cancer (ypT0/is) on microscopic evaluation of the resected breast specimen. After surgery, all resection specimens were evaluated for any residual invasive disease by a dedicated breast cancer pathologist according to guideline-driven routine clinical practice. In case of doubt, immunohistochemistry for keratins (CAM 5.2) was performed to check whether remaining epithelial cancer cells were detected or not. As a secondary endpoint, we evaluated pCR breast defined as the absence of residual invasive cancer (ypT0). The NPV for DCE with and without DWI was calculated as the percentage of true negative patients (rCR plus pCR) within all patients with rCR. The change in ADCmean after NST was calculated as the percentage difference between post-NST and baseline ADCmean \(\left(\frac{{ADCmean}\,{{{\mbox{post}}}}\mbox{-}{{{\mbox{NST}}}}\mbox{\mbox{-}}{ADC}{{{{{\rm{mean}}}}}} \, {{\mbox{pre}}}\mbox{-}{{\mbox{NST}}}}{{ADCmean}\,{{\mbox{pre}}}\mbox{-}{{\mbox{NST}}}}\times 100 \%\right)\). For ADCmean analysis only values within the range 0.50 × 10−3 mm2/s–3.0 × 10−3 mm2/s were included in the analysis to minimize the effects of unrealistic outliers.
Statistical analysis
All statistical analyses were performed using SPSS version 27.0. Differences in clinical and treatment characteristics between patients with and without pCR were analyzed using an independent t-test for continuous and normally distributed data, a Mann-Whitney U test for continuous and not normally distributed data, and a Fisher’s exact test for the categorical variables. Pre- and post-NST ADCmean distributions and ∆ADCmean (%) were compared for pCR/non-pCR using a Mann-Whitney U test. A Cohen’s kappa (κ) was used to test inter-reader variability. For all statistical analyses a p-value < 0.05 was considered statistically significant.
Results
Patients
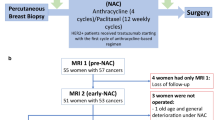
We identified 177 patients with stage I-III HER2-positive breast cancer treated from January 2015 until March 2019 (Fig. 2). Of these, 123 had no residual pathological enhancement on post-NST DCE-MRI and were therefore considered as radiologic complete responders. Of them, 102 had MRI scans available at baseline and post-NST.
Study flow chart. Abbreviations: DCE, dynamic contrast-enhanced; pCR, pathological complete response; NST, neoadjuvant systemic therapy; ADC, apparent diffusion coefficient. *Numbers do not add up because in 7 cases for Reader 1, both pre-treatment ADCmean and post-treatment ADCmean were not available, and in 5 cases for Reader 2, both pre-treatment and post-treatment ADC mean were not available
Most patients (N = 71) received a combination of paclitaxel, carboplatin, trastuzumab and pertuzumab, as standard-of-care treatment [5]. Table 1 shows the patient and tumor characteristics.
Three-quarters of all included patients (76%) underwent breast-conserving surgery, and 24% mastectomy as definite surgery. The median time between the baseline MRI and the post-NST MRI was 196 days (interquartile range [IQR] 177–208 days), and 27 days (IQR 19–33 days) between post-NST MRI and surgery. After NST 76 patients had a confirmed pCR and 26 had not, resulting in a NPV for DCE-MRI of 75%. Residual invasive tumor in the resection specimen was mostly smaller than 2 cm (ypT1: 77%). HR-positive breast cancer was associated with a reduced likelihood of pCR between HR-negative and positive cohorts (87% vs. 64%, p = 0.01, Table 1). Also, patients with rCR on a 3-T DCE-MRI scan had higher chance of pCR than patients with rCR on 1.5 T MRI scan (86 vs 65%, p = 0.02), more pronounced within the HR-positive subgroup (50 vs. 81%, p = 0.03) than in HR-negatives (88% vs. 91%). Therefore, we present the qualitative analyses separately for the two HR-subgroups and for MRI field strength.
Imaging characteristics
The majority of the post-NST DWI-MRI scans was of good quality (Supplementary Material, page 3). Reader 1 scored 98 out of 102 cases (96%) as good quality, and reader 2 89 of 102 (87%). The level of inter-reader variability on the scored elements was high, with a poor to slight agreement score except for visibility at the b0-image (κ = 0.38) and tumor characteristics (κ = 0.20) on which the level was fair. Reader 2 scored more non-pCR cases than pCR cases as visible on b0-images. A hyper-intense residual signal at high b-value images did not differentiate between pCR and non-pCR cases as scored by both readers. For most patients the readers used (> 70%) b800 as the high b-value for determining residual signal at the original tumor location.
Qualitative DWI evaluation
We found a definite rCR on combined DCE- and DWI-MRI in 63 patients. The corresponding overall NPV for combined DCE-DWI is 78% (Table 2). Twenty-seven of 39 non-rCR DWI cases (69%) were false positives. Twelve of 39 non-rCR DWI cases had residual invasive disease (31%) and were true positives. Within the HER2-positive/HR-negative subgroup the NPV is 87% for DCE-only and 86% for combined DCE-DWI. In HER2+ /HR+ breast cancer patients the NPV for DCE alone is 64% and combined with DWI the NPV is 67%. Results for subgroups based on the MRI field strength are also shown in Table 2. The NPV at 1.5 T increases from 50% with DCE-only to 56% when DWI is added to the response evaluation, but remains lower than 3-T MRI evaluation for both DCE and DCE plus DWI. The pCR-percentages for rCR and non-rCR cases on DWI-MRI for both pCR breast definitions (ypT0/is, N = 76 and ypT0, N = 71) are presented in Fig. 3. Results for the alternative pCR definition without in situ disease (ypT0) show great overlap with the primary endpoint, only three cases of the 27 false positive DWI cases had residual ductal or lobular carcinoma in situ.
Pathological complete response (pCR) percentages per hormone receptor (HR)-subgroup in patients with and without a radiologic complete response (rCR) based on qualitative DWI evaluation. A results for a pCR defined as the absence of invasive residual disease (ypT0/is). B results for pCR defined as the absence of residual invasive and in situ disease (ypT0). pCR, pathological complete response; HR, hormone receptor; DWI, diffusion-weighted imaging
Quantitative DWI evaluation
Baseline ADCmean could not be determined in nine cases by reader 1 due to technical issues and seven were non-realistic outliers and therefore excluded. Baseline ADCmean could not be determined in 13 cases by reader 2 due to technical issues. Post-NST ADCmean could be determined in patients in which the readers could identify the original tumor location and were able to place a ROI (Fig. 2). If the former tumor location could not be determined due to no residual signal on DWI and no valid landmark the readers were not able to draw a ROI. Furthermore, 2 cases had non-realistic outlier ADC values and were excluded in the quantitative analysis. From the included 102 patients a ∆ADC(%) could be calculated in 42 cases by reader 1 and in 41 cases by reader 2. Baseline ADCmean were 0.84 ± 0.21 × 10−3 mm2/s and 0.89 ± 0.33 × 10−3 mm2/s in patients with and without pCR for reader 1 (p = 0.88) and respectively 0.95 ± 0.24 × 10−3 mm2/s and 0.89 ± 0.28 × 10−3 mm2/s (p = 0.15) for reader 2 (Table 3). Post-NST ADCmean were respectively 1.55 ± 0.51 × 10−3 mm2/s versus 1.37 ± 0.44 × 10−3 mm2/s (p = 0.22) by reader 1 and 1.62 ± 0.43 × 10−3 mm2/s versus 1.55 ± 0.39 × 10−3 mm2/s (p = 0.56) by reader 2. Overall, most patients showed an increase in ADCmean after NST, reflected in a positive ∆ADCmean percentage. However, no statistically significant differences were found between patients with or without pCR. DeltaADCmean percentages are also shown by MRI field strength and show no significant differences between pCR and non-pCR cases, but numbers are low since only 48 patients were scanned using the same field strength pre-and post-NST. The variance between the measurements for pCR/non-pCR, quantified by the percentage change in ADCmean after NST, is demonstrated in Fig. 4.
Discussion
We investigated the added value of qualitative and quantitative DWI-MRI in patients with stage I-III HER2-positive breast cancer with a complete radiologic remission on DCE-MRI for identifying a pCR of the breast after NST. We found that 1.5-T DCE-MRI has a lower chance of detecting residual disease than 3-T DCE-MRI in HR-positive/HER2-positive disease. We did not find a clinically relevant increase in NPV after adding qualitative DWI assessment to the standard DCE evaluation. While 39 of 102 cases had a residual signal on DWI, 27 of these were false positives and showed no residual invasive disease on pathology examination. Furthermore, we did not find a difference between pCR and non-pCR patients in terms of baseline ADCmean, post-NST ADCmean or ∆ADCmean percentage, and we observed a high inter-reader variability.
DWI in standard breast cancer evaluation protocols is generally recommended to increase the specificity of DCE-MRI alone [30]. This multi-parametric MRI approach provides the opportunity to combine vascularity and cellularity-related measurements, which helps to distinguish benign breast lesions from malignant tissue and may prevent unnecessary biopsies. We hypothesized that DWI would complement DCE to detect residual invasive disease after NST, especially in HR-positive tumors. As HR-positive tumors are less likely to reach pCR [1,2,3,4,5], and because the NPV of DCE-MRI post-NST in HR-positive/HER2-positive breast cancers is only around 60% [15, 17]. Also, the tumor micro-environments (TMEs) and types of responses differ between HR-subtypes with for example higher percentage of immune cells in more HER2-driven tumors. Therefore, also cellularity could differ with corresponding varying ADCs between HER2-subgroups at response evaluation [31,32,33]. However, we found no clinically relevant increase in NPV for HR-positive breast cancer patients by adding DWI. In addition a NPV of 67% remains not adequate. This prompts us with the question how we can further improve response evaluation for this specific subgroup. For this subgroup, we observed, despite a small subgroup size, a large difference in NPVs between 1.5 T and 3.0 T. It might be, therefore, desirable to further investigate the influence of different field strengths on pCR prediction in specifically HR-positive/HER2-positive breast cancer, in DCE as well as in DWI.
Our research does not stand on its own. A recent meta-analysis showed a high area under the curve of 0.82 for pCR detection with DWI after NST, but the performance of the combination of DWI and DCE was not investigated [24]. Hahn et al observed an increase in accuracy, specificity and positive predictive value when DWI was added to DCE in response evaluation. However, in line with our results, no increase in NPV was observed [23]. This study did not stratify their results based on breast cancer subgroups, MRI field strength or provided data on inter-reader variability. Our findings show a difference in NPV between HR subgroups as well as a difference between MRI field strengths, which importance is also reported in another recent meta-analysis [34]. These results underline the essence of analyzing the effect of magnetic field strengths in breast MRI response evaluation since a higher field strength (1.5 T vs. 3.0 T) will also significantly increase the signal-to-noise ratio, thereby not neglecting other challenges when using a higher field strength [35].
Besides visual evaluation, ADC values hold a promise of high reproducibility if measured correctly [36]. However, we observed variability in the obtained results of quantitative DWI. In literature the baseline ADCmean differs among breast cancer subtypes, and does not seem to be highly predictive for pCR within HER2-positive breast cancer [21, 26, 37,38,39]. Our study supports this finding in patients with a rCR on DCE-MRI after NST. Using manually assessed ADCmean alone before the start of NST, as a purely predictive biomarker, is therefore unsupported in stage I-III HER2-positive breast cancer. Nevertheless, the use of longitudinal ADCmean changes seems more promising in identifying pCR. Liu et al found higher post-NST ADCmean in pCR patients compared to patients with residual disease with a corresponding high NPV of ranging from 87% to 95% within subgroups with HER2-positive patients [38]. Two differences in study design might be attributing to the difference with our study. First, the readers in other studies were instructed to draw a ROI in visual normal breast tissue if the tumor was not visible anymore [21, 26, 38]. Our readers were instructed to draw a ROI if they could determine the original tumor area. This was mostly limited to patients who had residual high signal on b800-b1500-images resulting in less than 40% of patients in which a ADCmean difference could be calculated. In practice, we encountered that determining the ROI-location for rCR on DWI can be very challenging, especially in multi-focal disease and in patients with excellent response (complete rCR on DCE-MRI) due to distortion in the DWI scan, as inclusion of healthy unaffected fibro-glandular tissue needs to be avoided. Secondly, our analysis was performed within a highly selected group of patients with already a rCR on DCE. This implies that the post-NST-ADCmean or ADCmean difference could potentially differentiate complete responders from non-complete responders independently, but the added value to rCR on DCE-MRI seems limited.
The I-SPY and ACRIN consortiums conducted multi-center multi-scanner studies investigating the role of DWI in response prediction. Partridge et al showed that ∆ADC-percentage was predictive for pCR at early treatment scans and post-systemic treatment scans [21]. This difference was not specifically found in the HER2-positive subgroups, but only 67 HER2-positive patients were included in this analysis. Therefore, a small difference could possibly not be detected. Li et al reported functional tumor volume (FTV), and longest diameter to be the most predictive DCE features [40]. The same author group investigated the predictive value of ∆ADC combined with the quantitative DCE-feature percentage change in functional tumor volume (∆FTV%) and found a small but significant added value of ∆ADCmean(%) within HER2-positive and HR-positive patients but also did not take inter-reader variance nor MRI field strength into account [22].
Limitations
Our study has some limitations. First, this was a retrospective study where the MRI protocols were not identical for all patients, including variability in the magnitude and b-values used. Furthermore, we selected patients with rCR on DCE-MRI. Therefore, we cannot report on accuracy, specificity or sensitivity for all women with stage I-III HER2-positive breast cancer. Second, the inter-reader variability was high for almost all scored imaging characteristics, despite organizing a pre-reading meeting in which both readers provided consent for the reading -protocol. However, this does not affect the outcomes for the qualitative analysis since a consensus reading was performed. Furthermore, the ROI-selection technique could possibly be optimized. Since manually selecting ROIs with low diffusivity showed excellent agreement and sensitivity in early response evaluation, we used a practical approach with a manually 2D-ROI selection method [41]. For future studies, (semi)automatic or 3D whole-tumor ROI selection methods could potentially improve the reproducibility of ADCs [42, 43].
Conclusions
We did not find evidence that DWI-MRI provides a clinically relevant increase in the NPV of DCE-MRI to identify pathologic complete responders in stage I-III HER2-positive breast cancer after NST. Further studies using (semi-)automated delineations of (3D-)ROIs and including non-radiologic complete responders on DCE-MRI should be conducted to further assess the role of DWI in response evaluation after NST in HER2-positive breast cancer. Hormone-receptor subgroups and MRI field strength should always be taken into account in those future studies.
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- DCE:
-
Dynamic contrast-enhanced
- DWI:
-
Diffusion-weighted imaging
- HER2:
-
Human epidermal growth factor receptor 2
- HR:
-
Hormone receptor
- MRI:
-
Magnetic resonance imaging
- NPV:
-
Negative predictive value
- NST:
-
Neoadjuvant systemic treatment
- pCR:
-
Pathological complete response
- rCR:
-
Radiological complete response
- ROI:
-
Region of interest
- SD:
-
Standard deviation
References
Gianni L, Pienkowski T, Im YH et al (2012) Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol 13:25–32
Baselga J, Bradbury I, Eidtmann H et al (2012) Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet 379:633–640
Schneeweiss A, Chia S, Hickish T et al (2013) Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA). Ann Oncol 24:2278–2284
de Azambuja E, Holmes AP, Piccart-Gebhart M et al (2014) Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): survival outcomes of a randomised, open-label, multicentre, phase 3 trial and their association with pathological complete response. Lancet Oncol 15:1137–1146
van Ramshorst MS, van der Voort A, van Werkhoven ED et al (2018) Neoadjuvant chemotherapy with or without anthracyclines in the presence of dual HER2 blockade for HER2-positive breast cancer (TRAIN-2): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 19:1630–1640
Gianni L, Pienkowski T, Im YH et al (2016) 5-year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer (NeoSphere): a multicentre, open-label, phase 2 randomised trial. Lancet Oncol 17:791–800
Schneeweiss A, Chia S, Hickish T et al (2018) Long-term efficacy analysis of the randomised, phase II TRYPHAENA cardiac safety study: Evaluating pertuzumab and trastuzumab plus standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer. Eur J Canc 89:27–35
van der Voort A, van Ramshorst MS, van Werkhoven ED et al (2021) Three-Year Follow-up of Neoadjuvant Chemotherapy With or Without Anthracyclines in the Presence of Dual ERBB2 Blockade in Patients With ERBB2-Positive Breast Cancer: a secondary analysis of the TRAIN-2 randomized, Phase 3 Trial. JAMA Oncol 7:978–984
von Minckwitz G, Huang CS, Mano MS et al (2019) Trastuzumab emtansine for residual invasive HER2-positive breast cancer. New Eng J Med. 380:617–628
Pérez-García JM, Gebhart G, Ruiz Borrego M et al (2021) Chemotherapy de-escalation using an (18)F-FDG-PET-based pathological response-adapted strategy in patients with HER2-positive early breast cancer (PHERGain): a multicentre, randomised, open-label, non-comparative, phase 2 trial. Lancet Oncol 22:858–871
Gluz O, Nitz U, Christgen M et al (2023) Efficacy of endocrine therapy plus trastuzumab and pertuzumab vs de-escalated chemotherapy in patients with hormone receptor-positive/ERBB2-positive early breast cancer: the neoadjuvant WSG-TP-II randomized clinical trial. JAMA Oncol 9:946–954
van der Voort A, Louis FM, van Ramshorst MS et al (2024) MRI-guided optimisation of neoadjuvant chemotherapy duration in stage II-III HER2-positive breast cancer (TRAIN-3): a multicentre, single-arm, phase 2 study. Lancet Oncol. https://doi.org/10.1016/S1470-2045(24)00104-9
Heil J, Sinn P, Richter H et al (2018) RESPONDER - diagnosis of pathological complete response by vacuum-assisted biopsy after neoadjuvant chemotherapy in breast Cancer - a multicenter, confirmative, one-armed, intra-individually-controlled, open, diagnostic trial. BMC Cancer 18:851
van Loevezijn AA, van der Noordaa MEM, van Werkhoven ED et al (2021) Minimally invasive complete response assessment of the breast after neoadjuvant systemic therapy for early breast cancer (MICRA trial): interim analysis of a multicenter observational cohort study. Ann Surg Oncol 28:3243–3253
van Ramshorst MS, Loo CE, Groen EJ et al (2017) MRI predicts pathologic complete response in HER2-positive breast cancer after neoadjuvant chemotherapy. Breast Cancer Res Treat 164:99–106
Hylton N (2013) MR imaging for the prediction of breast cancer response to neoadjuvant chemotherapy. Radiology 266:367
Bufi E, Belli P, Di Matteo M et al (2014) Effect of breast cancer phenotype on diagnostic performance of MRI in the prediction to response to neoadjuvant treatment. Eur J Radiol 83:1631–1638
De Los Santos JF, Cantor A, Amos KD et al (2013) Magnetic resonance imaging as a predictor of pathologic response in patients treated with neoadjuvant systemic treatment for operable breast cancer. Translational Breast Cancer Research Consortium trial 017. Cancer 119:1776–1783
Hayashi Y, Takei H, Nozu S et al (2013) Analysis of complete response by MRI following neoadjuvant chemotherapy predicts pathological tumor responses differently for molecular subtypes of breast cancer. Oncol Lett 5:83–89
Weber JJ, Jochelson MS, Eaton A et al (2017) MRI and prediction of pathologic complete response in the breast and axilla after neoadjuvant chemotherapy for breast cancer. J Am Coll Surg 225:740–746
Partridge SC, Zhang Z, Newitt DC et al (2018) Diffusion-weighted MRI findings predict pathologic response in neoadjuvant treatment of breast cancer: the ACRIN 6698 multicenter trial. Radiology 289:618–627
Li W, Newitt DC, Wilmes LJ et al (2019) Additive value of diffusion-weighted MRI in the I-SPY 2 TRIAL. J Magn Reson Imaging 50:1742–1753
Hahn SY, Ko EY, Han BK, Shin JH, Ko ES (2014) Role of diffusion-weighted imaging as an adjunct to contrast-enhanced breast MRI in evaluating residual breast cancer following neoadjuvant chemotherapy. Eur J Radiol 83:283–288
Li Z, Li J, Lu X, Qu M, Tian J, Lei J (2021) The diagnostic performance of diffusion-weighted imaging and dynamic contrast-enhanced magnetic resonance imaging in evaluating the pathological response of breast cancer to neoadjuvant chemotherapy: a meta-analysis. Eur J Radiol 143:109931
Gao W, Guo N, Dong T (2018) Diffusion-weighted imaging in monitoring the pathological response to neoadjuvant chemotherapy in patients with breast cancer: a meta-analysis. World J Surg Oncol 6:145
Woodhams R, Kakita S, Hata H et al (2010) Identification of residual breast carcinoma following neoadjuvant chemotherapy: diffusion-weighted imaging–comparison with contrast-enhanced MR imaging and pathologic findings. Radiology 254:357–366
van Loevezijn AA, van der Noordaa MEM, Stokkel MPM et al (2022) Three-year follow-up of de-escalated axillary treatment after neoadjuvant systemic therapy in clinically node-positive breast cancer: the MARI-protocol. Breast Cancer Res Treat 193:37–48
Koolen BB, Donker M, Straver ME et al (2017) Combined PET-CT and axillary lymph node marking with radioactive iodine seeds (MARI procedure) for tailored axillary treatment in node-positive breast cancer after neoadjuvant therapy. Br J Surg 104:1188–1196
Le Bihan D (2019) What can we see with IVIM MRI? Neuroimage 187:56–67
Baltzer P, Mann RM, Iima M et al (2020) Diffusion-weighted imaging of the breast-a consensus and mission statement from the EUSOBI International Breast Diffusion-Weighted Imaging working group. Eur Radiol 30:1436–1450
Sammut SJ, Crispin-Ortuzar M, Chin SF et al (2022) Multi-omic machine learning predictor of breast cancer therapy response. Nature 601:623–629
Lee HJ, Lee JE, Jeong WG et al (2022) HER2-positive breast cancer: association of MRI and clinicopathologic features with tumor-infiltrating lymphocytes. AJR Am J Roentgenol 218:258–269
García-Figueiras R, Padhani AR, Baleato-González S (2016) Therapy Monitoring with Functional and Molecular MR Imaging. Magn Reson Imaging Clin N Am 24:261–288
Janssen LM, den Dekker BM, Gilhuijs KGA, van Diest PJ, van der Wall E, Elias SG (2022) MRI to assess response after neoadjuvant chemotherapy in breast cancer subtypes: a systematic review and meta-analysis. NPJ Breast Cancer 8:107
Rahbar H, Partridge SC, DeMartini WB, Thursten B, Lehman CD (2013) Clinical and technical considerations for high quality breast MRI at 3 Tesla. J Magnet Resonance Imaging 37:778–790
Newitt DC, Zhang Z, Gibbs JE et al (2019) Test-retest repeatability and reproducibility of ADC measures by breast DWI: Results from the ACRIN 6698 trial. JMRI J Magnet Resonance Imaging 49:1617–1628
Bufi E, Belli P, Costantini M et al (2015) Role of the Apparent Diffusion Coefficient in the Prediction of Response to Neoadjuvant Chemotherapy in Patients With Locally Advanced Breast Cancer. Clin Breast Cancer 15:370–380
Liu S, Ren R, Chen Z et al (2015) Diffusion-weighted imaging in assessing pathological response of tumor in breast cancer subtype to neoadjuvant chemotherapy. JMRI J Magnet Resonance Imaging 42:779–787
Richard R, Thomassin I, Chapellier M et al (2013) Diffusion-weighted MRI in pretreatment prediction of response to neoadjuvant chemotherapy in patients with breast cancer. Eur Radiol 23:2420–2431
Li W, Newitt DC, Gibbs J et al (2020) Predicting breast cancer response to neoadjuvant treatment using multi-feature MRI: results from the I-SPY 2 TRIAL. NPJ Breast Cancer 6:63
Le NN, Li W, Onishi N et al (2022) Effect of inter-reader variability on diffusion-weighted MRI apparent diffusion coefficient measurements and prediction of pathologic complete response for breast cancer. Tomography 8:1208–1220
Ahlawat S, Khandheria P, Del Grande F et al (2016) Interobserver variability of selective region-of-interest measurement protocols for quantitative diffusion weighted imaging in soft tissue masses: Comparison with whole tumor volume measurements. JMRI J Magnet Resonance Imaging 43:446–454
Rahbar H, Kurland BF, Olson ML et al (2016) Diffusion-weighted breast magnetic resonance imaging: a semiautomated voxel selection technique improves interreader reproducibility of apparent diffusion coefficient measurements. J Comput Assist Tomogr 40:428–435
Acknowledgements
We thank M. Lopez-Yurda from the Netherlands Cancer Institute for her contributions to the manuscript.
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is R.M. Mann.
Conflict of Interest
R.M. Mann received research funding for studies unrelated to this work from: Siemens Healthineers, Bayer healthcare, Beckton & Dickinson, Screenpoint medical, Koning, PA Imaging. R.M. Mann is advisory editorial board member of European Radiology, associate editor for breast imaging of Radiology and member of the executive board of the European Society of Breast Imaging (EUSOBI). G.S. Sonke received institutional research funding from AstraZeneca, Merck, Novartis, Roche and Seagen and consulting fees from Biovica and Seagen, all unrelated to this work. All other authors declare no competing interests.
Statistics and Biometry
M. Lopez-Yurda kindly provided statistical advice for this manuscript.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
The Institutional Review Board approval from the Review Board of the Netherlands Cancer Institute was obtained.
Study subjects or cohorts overlap
A subset of 45 patients have been previously reported in Van Ramshorst MS, Loo CE, Groen EJ et al (2017) MRI predicts pathologic complete response in HER2-positive breast cancer after neoadjuvant chemotherapy. Breast Cancer Res Treat. 164:99-106. https://doi.org/10.1007/s10549-017-4254-0.
Methodology
-
Retrospective
-
Diagnostic
-
Single-centre
Additional information
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
van der Voort, A., van der Hoogt, K.J.J., Wessels, R. et al. Diffusion-weighted imaging in addition to contrast-enhanced MRI in identifying complete response in HER2-positive breast cancer. Eur Radiol (2024). https://doi.org/10.1007/s00330-024-10857-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00330-024-10857-7