Abstract
Background
Neoadjuvant treatment of HER2-positive breast cancer frequently leads to a pathologic complete response (pCR), which is associated with favourable long-term outcome. Treatment regimens typically consist of 6–9 cycles of trastuzumab-based chemotherapy, although many patients achieve early radiologic complete response (rCR). If rCR accurately predicts pCR, the number of chemotherapy cycles can possibly be reduced.
Methods
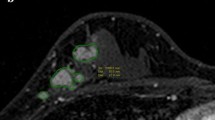
We performed a diagnostic accuracy study to determine the association between rCR and pCR in patients with stage II–III HER2-positive breast cancer treated with neoadjuvant trastuzumab-based chemotherapy at the Netherlands Cancer Institute. RCR was defined as the disappearance of pathologic contrast enhancement in the original tumour region on repeated magnetic resonance imaging (MRI). PCR was defined as the absence of invasive tumour cells in the resected breast specimen (ypT0/is). Diagnostic accuracy was estimated in the overall population and in subgroups based on hormone receptor (HR) status. The prognostic value of rCR for recurrence-free interval was evaluated as an exploratory analysis.
Results
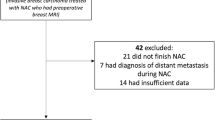
We identified 296 eligible patients with 297 HER2-positive tumours (154 HR-negative and 143 HR-positive) treated with neoadjuvant trastuzumab-based chemotherapy between 2004 and 2016. Overall, the rCR rate was 69% (206/297) and the pCR rate was 61% (181/297). Among 206 patients with rCR, 150 also had pCR (negative predictive value [NPV] = 150/206 = 73%). Among 91 patients without rCR, 60 had residual tumour at pathology (positive predictive value [PPV] = 60/91 = 66%). The NPV was better in HR-negative compared to HR-positive tumours (88 vs. 57%), while the PPV was better in HR-positive tumours (50 vs. 78%). Achieving rCR was associated with a 5-year recurrence-free interval of 88% compared to 68% without rCR (hazard ratio 0.34, 95% confidence interval 0.17–0.65, P = 0.001).
Conclusion
Achieving rCR corresponds well with pCR in HER2-positive breast cancer, particularly in the HR-negative subgroup. RCR is also associated with improved long-term outcome.

Similar content being viewed by others
References
Schneeweiss A, Chia S, Hickish T, Harvey V, Eniu A, Hegg R, Tausch C, Seo JH, Tsai YF, Ratnayake J, McNally V, Ross G, Cortes J (2013) Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA). Ann Oncol 24:2278–2284. doi:10.1093/annonc/mdt182
Broglio KR, Quintana M, Foster M, Olinger M, McGlothlin A, Berry SM, Boileau JF, Brezden-Masley C, Chia S, Dent S, Gelmon K, Paterson A, Rayson D, Berry DA (2016) Association of pathologic complete response to neoadjuvant therapy in HER2-positive breast cancer with long-term outcomes: a meta-analysis. JAMA Oncol 2:751–760. doi:10.1001/jamaoncol.2015.6113
Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, Bonnefoi H, Cameron D, Gianni L, Valagussa P, Swain SM, Prowell T, Loibl S, Wickerham DL, Bogaerts J, Baselga J, Perou C, Blumenthal G, Blohmer J, Mamounas EP, Bergh J, Semiglazov V, Justice R, Eidtmann H, Paik S, Piccart M, Sridhara R, Fasching PA, Slaets L, Tang S, Gerber B, Geyer CE Jr, Pazdur R, Ditsch N, Rastogi P, Eiermann W, von Minckwitz G (2014) Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 384:164–172. doi:10.1016/S0140-6736(13)62422-8
von Minckwitz G, Untch M, Nuesch E, Loibl S, Kaufmann M, Kummel S, Fasching PA, Eiermann W, Blohmer JU, Costa SD, Mehta K, Hilfrich J, Jackisch C, Gerber B, du Bois A, Huober J, Hanusch C, Konecny G, Fett W, Stickeler E, Harbeck N, Muller V, Juni P (2011) Impact of treatment characteristics on response of different breast cancer phenotypes: pooled analysis of the German neo-adjuvant chemotherapy trials. Breast Cancer Res Treat 125:145–156. doi:10.1007/s10549-010-1228-x
Ribeiro J, Cardoso MJ (2016) Highlights from the Tenth European Breast Cancer Conference (EBCC10), Amsterdam, 9–11 March 2016. Ecancermedicalscience 10:644. doi:10.3332/ecancer.2016.644
Chen JH, Feig B, Agrawal G, Yu H, Carpenter PM, Mehta RS, Nalcioglu O, Su MY (2008) MRI evaluation of pathologically complete response and residual tumors in breast cancer after neoadjuvant chemotherapy. Cancer 112:17–26. doi:10.1002/cncr.23130
Chen JH, Bahri S, Mehta RS, Carpenter PM, McLaren CE, Chen WP, Fwu PT, Hsiang DJ, Lane KT, Butler JA, Su MY (2014) Impact of factors affecting the residual tumor size diagnosed by MRI following neoadjuvant chemotherapy in comparison to pathology. J Surg Oncol 109:158–167. doi:10.1002/jso.23470
Bufi E, Belli P, Di Matteo M, Terribile D, Franceschini G, Nardone L, Petrone G, Bonomo L (2014) Effect of breast cancer phenotype on diagnostic performance of MRI in the prediction to response to neoadjuvant treatment. Eur J Radiol 83:1631–1638. doi:10.1016/j.ejrad.2014.05.002
Schaefgen B, Mati M, Sinn HP, Golatta M, Stieber A, Rauch G, Hennigs A, Richter H, Domschke C, Schuetz F, Sohn C, Schneeweiss A, Heil J (2016) Can routine imaging after neoadjuvant chemotherapy in breast cancer predict pathologic complete response? Ann Surg Oncol 23:789–795. doi:10.1245/s10434-015-4918-0
Fukuda T, Horii R, Gomi N, Miyagi Y, Takahashi S, Ito Y, Akiyama F, Ohno S, Iwase T (2016) Accuracy of magnetic resonance imaging for predicting pathological complete response of breast cancer after neoadjuvant chemotherapy: association with breast cancer subtype. SpringerPlus 5:152. doi:10.1186/s40064-016-1800-x
Bouzon A, Acea B, Soler R, Iglesias A, Santiago P, Mosquera J, Calvo L, Seoane-Pillado T, Garcia A (2016) Diagnostic accuracy of MRI to evaluate tumour response and residual tumour size after neoadjuvant chemotherapy in breast cancer patients. Radiol Oncol 50:73–79. doi:10.1515/raon-2016-0007
Marinovich ML, Houssami N, Macaskill P, Sardanelli F, Irwig L, Mamounas EP, von Minckwitz G, Brennan ME, Ciatto S (2013) Meta-analysis of magnetic resonance imaging in detecting residual breast cancer after neoadjuvant therapy. J Natl Cancer Inst 105:321–333. doi:10.1093/jnci/djs528
McGuire KP, Toro-Burguete J, Dang H, Young J, Soran A, Zuley M, Bhargava R, Bonaventura M, Johnson R, Ahrendt G (2011) MRI staging after neoadjuvant chemotherapy for breast cancer: does tumor biology affect accuracy? Ann Surg Oncol 18:3149–3154. doi:10.1245/s10434-011-1912-z
De Los Santos JF, Cantor A, Amos KD, Forero A, Golshan M, Horton JK, Hudis CA, Hylton NM, McGuire K, Meric-Bernstam F, Meszoely IM, Nanda R, Hwang ES (2013) Magnetic resonance imaging as a predictor of pathologic response in patients treated with neoadjuvant systemic treatment for operable breast cancer. Translational Breast Cancer Research Consortium trial 017. Cancer 119:1776–1783. doi:10.1002/cncr.27995
Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M, Fitzgibbons P, Hanna W, Jenkins RB, Mangu PB, Paik S, Perez EA, Press MF, Spears PA, Vance GH, Viale G, Hayes DF (2013) Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol 31:3997–4013. doi:10.1200/JCO.2013.50.9984
Loo CE, Rigter LS, Pengel KE, Wesseling J, Rodenhuis S, Peeters MJ, Sikorska K, Gilhuijs KG (2016) Survival is associated with complete response on MRI after neoadjuvant chemotherapy in ER-positive HER2-negative breast cancer. Breast Cancer Res 18:82. doi:10.1186/s13058-016-0742-0
Hayashi Y, Takei H, Nozu S, Tochigi Y, Ichikawa A, Kobayashi N, Kurosumi M, Inoue K, Yoshida T, Nagai SE, Oba H, Tabei T, Horiguchi J, Takeyoshi I (2013) Analysis of complete response by MRI following neoadjuvant chemotherapy predicts pathological tumor responses differently for molecular subtypes of breast cancer. Oncol Lett 5:83–89. doi:10.3892/ol.2012.1004
Ko ES, Han H, Han BK, Kim SM, Kim RB, Lee GW, Park YH, Nam SJ (2015) Prognostic significance of a complete response on breast MRI in patients who received neoadjuvant chemotherapy according to the molecular subtype. Korean J Radiol 16:986–995. doi:10.3348/kjr.2015.16.5.986
Price ER, Wong J, Mukhtar R, Hylton N, Esserman LJ (2015) How to use magnetic resonance imaging following neoadjuvant chemotherapy in locally advanced breast cancer. World J Clin Cases 3:607–613. doi:10.12998/wjcc.v3.i7.607
Mukhtar RA, Yau C, Rosen M, Tandon VJ, Hylton N, Esserman LJ (2013) Clinically meaningful tumor reduction rates vary by prechemotherapy MRI phenotype and tumor subtype in the I-SPY 1 trial (CALGB 150007/150012; ACRIN 6657). Ann Surg Oncol 20:3823–3830. doi:10.1245/s10434-013-3038-y
Santamaria G, Bargallo X, Fernandez PL, Farrus B, Caparros X, Velasco M (2016) Neoadjuvant systemic therapy in breast cancer: association of contrast-enhanced MR imaging findings, diffusion-weighted imaging findings, and tumor subtype with tumor response. Radiology. doi:10.1148/radiol.2016160176
Ko ES, Han BK, Kim RB, Ko EY, Shin JH, Hahn SY, Nam SJ, Lee JE, Lee SK, Im YH, Park YH (2013) Analysis of factors that influence the accuracy of magnetic resonance imaging for predicting response after neoadjuvant chemotherapy in locally advanced breast cancer. Ann Surg Oncol 20:2562–2568. doi:10.1245/s10434-013-2925-6
Hylton NM, Gatsonis CA, Rosen MA, Lehman CD, Newitt DC, Partridge SC, Bernreuter WK, Pisano ED, Morris EA, Weatherall PT, Polin SM, Newstead GM, Marques HS, Esserman LJ, Schnall MD (2016) Neoadjuvant chemotherapy for breast cancer: functional tumor volume by MR imaging predicts recurrence-free survival-results from the ACRIN 6657/CALGB 150007 I-SPY 1 trial. Radiology 279:44–55. doi:10.1148/radiol.2015150013
Schipper RJ, Moossdorff M, Beets-Tan RG, Smidt ML, Lobbes MB (2015) Noninvasive nodal restaging in clinically node positive breast cancer patients after neoadjuvant systemic therapy: a systematic review. Eur J Radiol 84:41–47. doi:10.1016/j.ejrad.2014.09.020
Acknowledgements
The authors thank M. van Loveren for her contributions to the manuscript.
Funding
No financial research support has been received for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest. Relevant financial activities outside the submitted work and other relationships or activities that readers could perceive to have influenced, or that give the appearance of potentially influencing: GSS received institutional research support funding from Roche, AstraZeneca, Merck and Novartis.
Informed consent
For this retrospective study formal consent was not required.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
van Ramshorst, M.S., Loo, C.E., Groen, E.J. et al. MRI predicts pathologic complete response in HER2-positive breast cancer after neoadjuvant chemotherapy. Breast Cancer Res Treat 164, 99–106 (2017). https://doi.org/10.1007/s10549-017-4254-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-017-4254-0