Abstract
Objectives
The study aimed to assess the efficiency of whole-body high-resolution compressed sensing-sensitivity encoding isotropic T1-Weighted Dixon (CSI-T1W-Dixon) scans in evaluating bone metastasis.
Methods
Forty-five high-risk prostate cancer patients with bone metastases were enrolled prospectively and underwent whole-body MRI sequences, which included the following: pre- and post-contrast CSI-T1W-Dixon and conventional multi-planar T1-Weighted Dixon (CMP-T1W-Dixon) (coronal, sagittal, and axial scans), short tau inversion recovery (STIR), and DWI. Comparison between the CMP-T1W-Dixon and CSI-T1W-Dixon images was done for the subjective image quality, the quantitative contrast-to-noise ratio (CNR), and signal-to-noise ratio (SNR). Furthermore, the diagnostic performance based on per-lesion and per-patient basis utilizing non-contrast T1-weighted (T1)/T1+ contrasted T1-weighted (T1C)/T1 + T1C + STIR + DWI sequences was compared between the CSI-T1W-Dixon and CMP-T1W-Dixon methods using reference standards (combining biopsy data and 6-month imaging follow-up).
Result
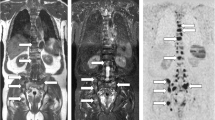
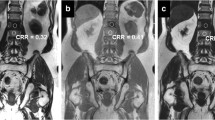
The CSI-T1W-Dixon images produced fewer image artifacts in the axial and coronal planes compared to the CMP-T1W-Dixon images. Also, the CSI-T1W-Dixon images provided better a CNR in fat-only images of all three planes and water-only images of the axial plane (p < 0.05). The CSI-T1W-Dixon showed a higher sensitivity than the CMP-T1W-Dixon techniques in analyzing T1-only images on a per-lesion basis (82.7% vs. 53.8% for sensitivity, p = 0.03). On a per-patient basis, no difference was found in the diagnostic capacity between the CSI-T1W-Dixon and CMP-T1W-Dixon sequences either alone or in combinations (p = 0.57–1).
Conclusion
High-resolution CSI-T1W-Dixon with higher image quality and diagnostic capacity can replace the CMP-T1W-Dixon method in evaluating bone metastasis in clinical practice.
Key Points
• Compressed sensing isotropic acquisition for 3D T1-weighted Dixon images can improve the image quality with fewer artifacts compared to the anisotropic multiplanar acquisition.
• Compressed sensing isotropic acquisition can save 67% of scanning time compared to anisotropic multiplanar acquisition.
• Compressed sensing isotropic 3D T1-weighted Dixon images can offer better diagnostic performance with higher sensitivity compared to anisotropic multiplanar images.





Similar content being viewed by others
Abbreviations
- AUC:
-
Area under the operating characteristic curve
- CMP:
-
Conventional multiplanar
- CMP-T1W-Dixon:
-
Conventional multi-planar T1-Weighted Dixon
- CNR:
-
Contrast-to-noise ratio
- Compressed SENSE:
-
Compressed sensing-sensitivity encoding
- CSI:
-
Compressed SENSE isotropic
- CSI-T1W-Dixon:
-
Compressed SENSE isotropic T1-Weighted Dixon
- CT:
-
Computed tomography
- DWI:
-
Diffusion-weighted imaging
- F:
-
Fat-only
- IP:
-
In-phase
- MRI:
-
Magnetic resonance imaging
- OP:
-
Out-of-phase
- PET:
-
Positron emission tomography
- PI:
-
Parallel imaging
- ROC:
-
Receiver operating characteristic
- ROI:
-
Region of interest
- SD:
-
Standard deviation
- SI:
-
Signal intensity
- SNR:
-
Signal-to-noise ratio
- STIR:
-
Short tau inversion recovery
- T1:
-
Noncontrast T1-weighted
- T1C:
-
Contrast T1-weighted
- T1W:
-
T1-Weighted
- W:
-
Water-only
References
Morone M, Bali MA, Tunariu N et al (2017) Whole-body MRI: current applications in oncology. AJR Am J Roentgenol 209:W336–W349
Petralia G, Padhani AR, Pricolo P et al (2019) Whole-body magnetic resonance imaging (WB-MRI) in oncology: recommendations and key uses. Radiol Med 124:218–233
Shen G, Deng H, Hu S, Jia Z (2014) Comparison of choline-PET/CT, MRI, SPECT, and bone scintigraphy in the diagnosis of bone metastases in patients with prostate cancer: a meta-analysis. Skeletal Radiol 43:1503–1513
Ma J, Costelloe CM, Madewell JE et al (2009) Fast dixon-based multisequence and multiplanar MRI for whole-body detection of cancer metastases. J Magn Reson Imaging 29:1154–1162
Costelloe CM, Madewell JE, Kundra V, Harrell RK, Bassett RL Jr, Ma J (2013) Conspicuity of bone metastases on fast Dixon-based multisequence whole-body MRI: clinical utility per sequence. Magn Reson Imaging 31:669–675
Lecouvet FE, Pasoglou V, Van Nieuwenhove S et al (2020) Shortening the acquisition time of whole-body MRI: 3D T1 gradient echo Dixon vs fast spin echo for metastatic screening in prostate cancer. Eur Radiol 30:3083–3093
Bray TJP, Singh S, Latifoltojar A et al (2017) Diagnostic utility of whole body Dixon MRI in multiple myeloma: A multi-reader study. PLoS One 12:e0180562
Goo HW (2015) Whole-body MRI in children: current imaging techniques and clinical applications. Korean J Radiol 16:973–985
Costelloe CM, Kundra V, Ma J et al (2012) Fast Dixon whole-body MRI for detecting distant cancer metastasis: a preliminary clinical study. J Magn Reson Imaging 35:399–408
Westerland O, Sivarasan N, Natas S et al (2018) Added value of contrast-enhanced T1-weighted and diffusion-weighted sequences for characterization of incidental findings on whole body magnetic resonance imaging in plasma-cell disorders. Clin Lymphoma Myeloma Leuk 18:822–828
Nam JG, Lee JM, Lee SM et al (2019) High acceleration three-dimensional T1-weighted dual echo dixon hepatobiliary phase imaging using compressed sensing-sensitivity encoding: comparison of image quality and solid lesion detectability with the standard T1-weighted Sequence. Korean J Radiol 20:438–448
Vranic JE, Cross NM, Wang Y, Hippe DS, de Weerdt E, Mossa-Basha M (2019) Compressed Sensing-Sensitivity Encoding (CS-SENSE) Accelerated brain imaging: reduced scan time without reduced image quality. AJNR Am J Neuroradiol 40:92–98
Liang D, Liu B, Wang J, Ying L (2009) Accelerating SENSE using compressed sensing. Magn Reson Med 62:1574–1584
Larbi A, Omoumi P, Pasoglou V et al (2019) Whole-body MRI to assess bone involvement in prostate cancer and multiple myeloma: comparison of the diagnostic accuracies of the T1, short tau inversion recovery (STIR), and high b-values diffusion-weighted imaging (DWI) sequences. Eur Radiol 29:4503–4513
Pasoglou V, Michoux N, Peeters F et al (2015) Whole-body 3D T1-weighted MR imaging in patients with prostate cancer: feasibility and evaluation in screening for metastatic disease. Radiology 275:155–166
Papageorgiou I, Dvorak J, Cosma I, Pfeil A, Teichgraeber U, Malich A (2020) Whole-body MRI: a powerful alternative to bone scan for bone marrow staging without radiation and gadolinium enhancer. Clin Transl Oncol 22:1321–1328
Zeilinger MG, Wiesmuller M, Forman C et al (2021) 3D Dixon water-fat LGE imaging with image navigator and compressed sensing in cardiac MRI. Eur Radiol 31:3951–3961
Zech CJ, Herrmann KA, Huber A et al (2004) High-resolution MR-imaging of the liver with T2-weighted sequences using integrated parallel imaging: comparison of prospective motion correction and respiratory triggering. J Magn Reson Imaging 20:443–450
Nael K, Ruehm SG, Michaely HJ et al (2006) High spatial-resolution CE-MRA of the carotid circulation with parallel imaging: comparison of image quality between 2 different acceleration factors at 3.0 Tesla. Invest Radiol 41:391–399
Bratke G, Rau R, Weiss K et al (2019) Accelerated MRI of the Lumbar Spine Using Compressed Sensing: Quality and Efficiency. J Magn Reson Imaging 49:e164–e175
Lecouvet FE, Simon M, Tombal B, Jamart J, Vande Berg BC, Simoni P (2010) Whole-body MRI (WB-MRI) versus axial skeleton MRI (AS-MRI) to detect and measure bone metastases in prostate cancer (PCa). Eur Radiol 20:2973–2982
Costelloe CM, Chuang HH, Madewell JE, Ueno NT (2010) Cancer response criteria and bone metastases: RECIST 1.1, MDA and PERCIST. J Cancer 1:80–92
Feng L, Benkert T, Block KT, Sodickson DK, Otazo R, Chandarana H (2017) Compressed sensing for body MRI. J Magn Reson Imaging 45:966–987
Stecco A, Trisoglio A, Soligo E, Berardo S, Sukhovei L, Carriero A (2018) Whole-body MRI with diffusion-weighted imaging in bone metastases: a narrative review. Diagnostics (Basel) 8:45
Carlbom L, Caballero-Corbalan J, Granberg D, Sorensen J, Eriksson B, Ahlstrom H (2017) Whole-body MRI including diffusion-weighted MRI compared with 5-HTP PET/CT in the detection of neuroendocrine tumors. Ups J Med Sci 122:43–50
Schmidt GP, Reiser MF, Baur-Melnyk A (2009) Whole-body MRI for the staging and follow-up of patients with metastasis. Eur J Radiol 70:393–400
Latifoltojar A, Duncan MKJ, Klusmann M et al (2020) Whole body 3.0 T magnetic resonance imaging in lymphomas: comparison of different sequence combinations for staging hodgkin’s and diffuse large B cell lymphomas. J Pers Med 10:284
Del Vescovo R, Frauenfelder G, Giurazza F et al (2014) Role of whole-body diffusion-weighted MRI in detecting bone metastasis. Radiol Med 119:758–766
Jambor I, Kuisma A, Ramadan S et al (2016) Prospective evaluation of planar bone scintigraphy, SPECT, SPECT/CT, 18F-NaF PET/CT and whole body 1.5T MRI, including DWI, for the detection of bone metastases in high risk breast and prostate cancer patients: SKELETA clinical trial. Acta Oncol 55:59–67
Lecouvet FE, El Mouedden J, Collette L et al (2012) Can whole-body magnetic resonance imaging with diffusion-weighted imaging replace Tc 99m bone scanning and computed tomography for single-step detection of metastases in patients with high-risk prostate cancer? Eur Urol 62:68–75
Gutzeit A, Doert A, Froehlich JM et al (2010) Comparison of diffusion-weighted whole body MRI and skeletal scintigraphy for the detection of bone metastases in patients with prostate or breast carcinoma. Skeletal Radiol 39:333–343
Acknowledgements
The authors thank Yong Zhang for his assistance in image analysis.
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Zhenhong Liao.
Conflict of interest
One of the authors of this manuscript (Xiaoyong Zhang) was an employee of Philips Medical Systems International. The remaining authors declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors (Zhenhong Liao) has significant statistical expertise.
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Study subjects or cohorts overlap
Whole-body compressed sensing isotropic T1-weighted Dixon has not been previously reported.
Methodology
• prospective
• diagnostic study
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(PDF 3.21 MB)
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liao, Z., Liu, G., Ming, B. et al. Evaluating prostate cancer bone metastasis using accelerated whole-body isotropic 3D T1-weighted Dixon MRI with compressed SENSE: a feasibility study. Eur Radiol 33, 1719–1728 (2023). https://doi.org/10.1007/s00330-022-09181-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-022-09181-9