Abstract
Objectives
To compare the diagnostic accuracy of a single T2 Dixon sequence to the combination T1+STIR as anatomical sequences used for detecting tumoral bone marrow lesions in whole-body MRI (WB-MRI) examinations.
Methods
Between January 2019 and January 2020, seventy-two consecutive patients (55 men, 17 women, median age = 66 years) with solid (prostate, breast, neuroendocrine) cancers at high risk of metastasis or proven multiple myeloma (MM) prospectively underwent a WB-MRI examination including coronal T1, STIR, T2 Dixon and axial diffusion-weighted imaging sequences. Two radiologists independently assessed the combination of T1+STIR sequences and the fat+water reconstructions from the T2 Dixon sequence. The reference standard was established by consensus reading of WB-MRI and concurrent imaging available at baseline and at 6 months. Repeatability and reproducibility of MRI scores (presence and semi-quantitative count of lesions), image quality (SNR: signal-to-noise, CNR: contrast-to-noise, CRR: contrast-to-reference ratios), and diagnostic characteristics (Se: sensitivity, Sp: specificity, Acc: accuracy) were assessed per-skeletal region and per-patient.
Results
Repeatability and reproducibility were at least good regardless of the score, region, and protocol (0.67 ≤ AC1 ≤ 0.98). CRR was higher on T2 Dixon fat compared to T1 (p < 0.0001) and on T2 Dixon water compared to STIR (p = 0.0128). In the per-patient analysis, Acc of the T2 Dixon fat+water was higher than that of T1+STIR for the senior reader (Acc = +0.027 [+0.025; +0.029], p < 0.0001) and lower for the junior reader (Acc = −0.029 [−0.031; −0.027], p < 0.0001).
Conclusions
A single T2 Dixon sequence with fat+water reconstructions offers similar reproducibility and diagnostic accuracy as the recommended combination of T1+STIR sequences and can be used for skeletal screening in oncology, allowing significant time-saving.
Key Points
• Replacement of the standard anatomic T1 + STIR WB-MRI protocol by a single T2 Dixon sequence drastically shortens the examination time without loss of diagnostic accuracy.
• A protocol based on fat + water reconstructions from a single T2 Dixon sequence offers similar inter-reader agreement and a higher contrast-to-reference ratio for detecting lesions compared to the standard T1 + STIR protocol.
• Differences in the accuracy between the two protocols are marginal (+ 3% in favor of the T2 Dixon with the senior reader; −3% against the T2 Dixon with the junior reader).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over recent years, whole-body magnetic resonance imaging (WB-MRI) has demonstrated a high diagnostic performance and is now recommended in clinical guidelines for skeletal lesion detection and follow-up in patients with metastases from solid cancers and multiple myeloma (MM) [1,2,3,4,5,6]. Standard WB-MRI examinations combine anatomic T1 and short-Tau inversion recovery (STIR) sequences and functional diffusion-weighted imaging (DWI) sequences [7,8,9]. T1 is the reference sequence for marrow lesion detection and characterization; STIR increases the sensitivity for lesion detection [10, 11]. DWI sequences add diagnostic value to anatomic sequences thanks to a high lesion to background contrast and extend cancer screening to lymph nodes and extraskeletal organs [12,13,14,15,16]. A limitation of WB-MRI is its duration and various initiatives are undertaken to accelerate the different sequences [17,18,19].
The Dixon technique relies on the chemical shift between protons of water and fat and decomposes the signal from these two components in the same voxel. A Dixon sequence generates four types of images: in-phase (IP) (equivalent to non-fat-suppressed anatomic images), out-of-phase (OP), water images (equivalent to fat-suppressed), and fat images (equivalent to water-suppressed) [20,21,22]. A single T2 Dixon sequence combines STIR-like information thanks to water images, and T1-like information thanks to fat images [23,24,25]. The diagnostic performance of the T2 Dixon sequence for detecting metastatic and MM lesions and its ability to replace the T1+STIR sequences has been demonstrated in spinal MRI examinations [26,27,28].
Herein, we hypothesized that a faster WB-MRI protocol using a single T2 Dixon sequence may be used without loss of diagnostic accuracy for detecting neoplastic bone marrow lesions. This study compares the (i) repeatability and reproducibility, (ii) image quality (signal-to-noise ratio (SNR); contrast-to-noise ratio (CNR); contrast-to-reference ratio (CRR), and (iii) diagnostic accuracy of combined fat + water reconstructions from a single T2 Dixon sequence with those of the combined T1+STIR sequences.
Materials and methods
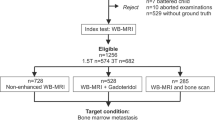
Patient population (Fig. 1)
The study included consecutive adult (≥ 18 years old) patients with either solid cancers at high risk for metastases or biopsy-proven newly diagnosed multiple myeloma (MM).
The indication for WB-MRI in newly diagnosed patients with solid cancers was validated in multidisciplinary tumor boards using cancer-specific indications.
In prostate cancer, high risk for metastasis was defined according to the National Comprehensive Cancer Network (NCCN) guidelines: cancer with ≥ 20 ng/ml prostate-specific antigen, Gleason score ≥ 8, and Union for International Cancer Control clinical T stage 3 or 4 [29]. In breast cancer, high risk was defined according to the European Society of Medical Oncology (ESMO) criteria: clinically positive axillary nodes, tumors > 5 cm, aggressive biology and clinical signs, and symptoms or laboratory values suggesting the presence of metastases [30]. In neuroendocrine cancer, high risk for metastasis was defined as follows: tumor grade ≥ III, tumor size ≥ 3 cm in diameter, T4 stage, N1 stage, histology of neuroendocrine carcinoma, and esophagus as the primary site [31].
Exclusion criteria were previous history of treated cancer, patients with more than one primary cancer, and contraindications to MRI.
All patients underwent a WB-MRI examination for the assessment of bone involvement from January 2019 to January 2020. This single-center study was approved by the institutional ethics committee. No informed consent was required for the retrospective reading of prospectively acquired data.
MRI protocol
All acquisitions were performed on a 3.0-T magnet (Ingenia, Philips Healthcare). Patients were imaged head first in the supine position, from head to proximal femurs, covered with head, neck, spine, and two 6-element body matrix coils. 3DT1, STIR, T2 Dixon and DWI sequences were performed. Synthetic water, fat, IP, and OP images were automatically reconstructed from the Dixon acquisition. After acquiring three stacks of T1, STIR, and T2 Dixon images in the coronal plane and four stacks of DWI in the axial plane, a single stack of pasted reformatted coronal images was constructed for each sequence. The total acquisition time was 49 min. Imaging parameters are detailed in Table 1.
MRI readings
All images were stored and read on the institutional Picture Archiving and Communication System (Carestream Vue). The combinations of T1+STIR sequences and of fat+water reconstructions from the T2 Dixon sequence were assessed by two radiologists with 2- and 15-years’experience in WB-MRI. Readings were performed independently, randomly, and blinded to clinical information and to DWI. Images were assessed twice by the junior reader at a 3-month interval for measuring the repeatability. IP and OP images derived from the Dixon acquisition were not considered for analysis as they did not add diagnostic value for lesion detection based on preliminary evaluation and literature [26]. DWI was used during the consensus session for determining the reference standard.
Bone involvement
Four patterns of bone marrow involvement were considered, as previously described: the normal, focal, diffuse, and “salt-and-pepper” patterns [6, 12, 32,33,34,35,36].
Normal marrow was defined as showing a homogeneous high signal intensity on T1 and fat images, and homogeneous low signal intensity on STIR and water images. A focal bone marrow lesion (focal metastasis in solid cancers or focal plasmocytoma in MM, with a minimal diameter of 5 mm) was defined as a low signal intensity area on T1 and fat images (similar to or lower than the signal intensity of discs and muscles on T1, very low signal on fat), intermediate to high signal intensity on STIR and water images, and high signal intensity on high b-value DW images. Diffuse marrow infiltration (diffuse metastatic disease in solid cancers and diffuse bone involvement in MM) was defined as homogeneous low signal intensity of the bone marrow on T1 and fat images (similar to or lower than the signal intensity of discs and muscles on T1, very low signal on fat), an intermediate to high signal intensity of the marrow on STIR and water images, and high signal intensity of the marrow on high b-value images. The fourth “salt-and-pepper” pattern of infiltration was observed in MM, defined by the presence of innumerable unmeasurable tiny foci with low signal intensity on T1 and fat and intermediate to high signal intensity on STIR and water images.
Eight skeletal regions were studied: skull, thoracic cage, cervical, thoracic, lumbar spine, pelvis, humerus, and femurs. In the per-region analysis, two scores were assessed: a categorical score (presence of lesion = yes/no) and a semi-quantitative score corresponding to the count of lesions (0 = no lesion; 1 = 1 to 5 lesions; 2 = 6 to 10 lesions; 3 = more than10 lesions; 4 = diffuse disease). In the per-patient analysis, two similar scores were assessed: a total categorical score (patient positive if at least one positive region = yes/no) and a total semi-quantitative score corresponding to the total count of lesions in all regions considered (0 = no lesion; 1 = 1 to 5 lesions; 2 = 6 to 10 lesions; 3 = more than10 lesions; 4 = diffuse disease).
Reference standard and adjudication of readings
In the absence of a systematic pathologic gold standard, a best valuable comparator (BVC) was used as the reference standard for tumoral bone marrow involvement. This BVC was constructed during a consensus session by the readers along with a third reader (radiologist with 15 years’experience in WB-MRI) and clinicians, relying on the concurrent study of all baseline WB-MRI sequences (T1, STIR, T2 fat and water, DWI), clinical data, and other available imaging studies [37, 38]. At least one systematic 6-month follow-up WB-MRI examination was performed in all patients. A radiographic skeletal survey was performed at diagnosis and repeated after 6 months in all MM patients. In solid cancer patients, baseline and follow-up evaluations using other techniques (bone scintigraphy, thoraco-abdomino-pelvic (TAP) CT, positron-emission tomography (PET)-CT) were available, depending on the primary cancer. The causes of false positive (FP) / false negative (FN) were determined for each protocol and reader during the consensus session.
Image quality
As the value of an imaging sequence used for lesion detection depends on the contrast between lesions and their environment, SNR (SNR=SI/σbackground), CNR (CNR=(SIlesion–SIreference)/σbackground), and CRR (CRR = (SIlesion–SIreference)/SIreference) were assessed (SI: mean signal intensity in the region of interest (ROI), σbackground: standard deviation of SI in the image background). Measurements were performed by the junior reader after verification of the true pathologic nature of the measured lesions according to the reference standard. A single ROI, the largest possible, was drawn within bone lesions without including bone cortices, with a maximum of five lesions per patient and a minimal diameter of 10 mm. For spine lesions, the reference ROI was chosen in an unequivocally non-involved bone marrow area of the involved vertebra or in case of large lesions in the bone marrow of the closest uninvolved vertebra. For other bone lesions, the reference ROI was chosen in an unequivocally non-involved area. Two ROIs, the largest possible, were drawn in the image background to assess the noise. All measurements were performed on the same coronal slice.
Statistical analysis
Due to the non-normality of data distributions (according to the Shapiro-Wilk test at p < 0.05), the comparison of SNR, CNR, and CRR measurements between protocols was performed using Wilcoxon’s signed-rank test [39].
Repeatability and reproducibility of MRI (categorical and semi-quantitative) scores were assessed using Gwet’s AC1 agreement coefficient [40]. Strength of intra-(repeatability) and inter-reader agreement (reproducibility) was interpreted according to the Landis-Koch’s scale: AC1 < 0.20 = poor; 0.21 ≤ AC1 < 0.40 = fair; 0.41 ≤ AC1 < 0.60 = moderate; 0.61 ≤ AC1 < 0.80 = good and AC1 ≥ 0.81 = very good [41].
Diagnostic characteristics and agreement between each protocol and the reference standard were assessed. True positives (TP), false positives (FP), false negatives (FN), true negatives (TN), sensitivity (Se), specificity (Sp), accuracy (Acc=(TP + TN)/(TP + FP + FN + TN)), and AC1 were reported for the per-region/per-patient analyses (for both readers). A two-sided exact test was used for comparing the proportion of lesions/patients correctly detected in the per-region/per-patient analyses by each protocol compared to the reference standard (significance level after Bonferroni-like correction p < 0.0083). This analysis was performed for the whole cohort of patients, and for two subgroups (N = 20 patients with MM and N = 52 patients with metastases; see Supplementary Materials).
Difference in Acc between protocols in the per-patient analysis was assessed using a resampling procedure (without replacement) based on 300 samples of N = 54 patients randomly drawn from the whole cohort of N = 72 patients (respectively based on samples of N = 15/N = 38 patients randomly drawn from the subgroups of patients with MM/metastases). A paired t-test was then performed from which the mean difference in Acc was reported for each of the three groups that were studied (significance level p < 0.0083; see Supplementary Materials).
Finally, an agreement between each protocol and the reference standard on the semi-quantitative score in the per-patient analysis was assessed using the AC1 coefficient.
All calculations were done with Statsdirect Statistical Software v3.3.5 and with Matlab R2021b.
Results
Patient characteristics
Seventy-two patients were included (55 men, 17 women, median age = 66 years [64 years; 68 years]). Twenty were examined for staging biopsy-proven MM. Fifty-two had newly diagnosed solid cancers at high risk for metastases (30 prostate cancers; 10 breast cancers; 12 neuroendocrine cancers) (Fig. 1). According to the reference standard, 35 (5 MM; 30 solid cancer patients) had focal bone lesions; 8 (6 MM; 2 solid cancer patients) had diffuse marrow involvement; and 29 had normal marrow (9 MM; 20 solid cancer patients). Due to the semi-quantitative scoring, the exact number of focal lesions cannot be provided. There were at least 3, 91, 26, 80, 64, 220, 8, and 27 focal lesions in the skull, thorax, cervical, dorsal, lumbar spine, pelvis, humerus, and femurs, respectively; in total, at least 519 tumoral bone marrow lesions were observed.
Repeatability and reproducibility
In the per-region analysis, repeatability of readings was at least good regardless of the region, score (categorical or semi-quantitative), and protocol (T1+STIR or fat+water) (Table 2). Reproducibility was at least good regardless of the region, score, and protocol. In the per-patient analysis, considering the worst level of agreement measured in both analyses (intra- and inter-reader agreement), reproducibility was at least good regardless of both the score and protocol.
Image quality
T1 images had significantly higher SNR compared to T2 Dixon fat images for both tissue types (plesion < 0.0001; preference < 0.0093). T2 Dixon fat images had significantly higher CRR compared to T1 images (p < 0.0001), and T2 Dixon water images had a significantly higher CRR compared to STIR images (p = 0.0128) (Table 3).
Diagnostic characteristics
In the per-region analysis, Se of T1+STIR was ≥ 93% for the senior reader (≥ 89% for the junior) regardless of the region (Table 4). Sp was ≥ 94% for the senior reader (≥ 82% for the junior) regardless of the region. Se of T2 Dixon fat+water was ≥ 93% for the senior reader (≥ 93% for the junior) regardless of the region. Sp was ≥ 91% for the senior reader (≥ 75% for the junior).
In the per-patient analysis, two trends were observed. First, the junior reader achieved a similar Se but a lower Sp compared to the senior, regardless of the protocol. Second, the senior reader achieved a slightly higher Se with the T2 Dixon protocol (Se = 98% vs Se = 93% with T1+STIR) while the junior achieved a slightly lower Sp with that protocol (Sp = 72% vs Sp = 79% with T1+STIR).
Compared to the reference standard, no significant difference was observed in the proportion of lesions correctly detected in the per-region analysis, or in the proportion of positive patients correctly detected in the per-patient analysis, regardless of both the reader and protocol (Figs. 2 and 3). A non-significant trend suggesting that the junior reader detected more humeral lesions (corresponding to FP) using T1+STIR compared to the reference standard was observed (proportion difference +8.33% [+ 1.28%; + 15.9%], p = 0.0313).
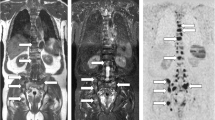
Agreement between sequences on metastatic lesions: WB-MRI study in a 46-year-old man with metastatic neuroendocrine cancer. Two metastases are observed in the L1 vertebral body (arrow) and in the right wing of the sacrum (arrowhead). Coronal T1 (A) and STIR (B) WB-MRI images show both lesions. Fat (C) and water (D) reconstructions of the TSE T2 Dixon acquisition show the same lesions. The reference standard, based on reading of all MR images and concurrent imaging studies, confirmed the presence of two metastases. E Concurrent DWI image (b = 1000 s mm−2, inverted gray scale) confirms the presence of both lesions and shows no additional lesion. F Gallium-68 dotatate PET/CT fused image shows the same two lesions presenting tracer uptake
Agreement between sequences on myeloma lesions: WB-MRI study in a 65-year-old man with newly diagnosed multiple myeloma. Several lesions are observed in the lumbar spine, iliac bones, and left femur (arrows). Coronal T1 (A) and STIR (B) WB-MRI images show four lesions. Fat (C) and water (D) reconstructions of the TSE T2 Dixon acquisition show the same lesions. The reference standard, based on reading of all MR images and concurrent imaging studies, confirmed the presence of these lesions. E Concurrent DWI image (b = 1000 s mm−2, inverted gray scale) confirms the presence of lesions. F [18F]F-FDG PET/CT image shows iliac and left femur lesions presenting tracer uptake
In the per-patient analysis, the resampling procedure demonstrated a significantly higher Acc of T2 Dixon fat+water compared to T1+STIR when assessed by the senior reader (AccDixon Fat+Water = 0.957, AccT1+STIR = 0.930, mean difference in Acc = +0.027 [+ 0.025; + 0.029], p < 0.0001). Conversely, it demonstrated a significantly lower Acc of T2 Dixon fat+water compared to T1+STIR when assessed by the junior (AccDixon Fat+Water = 0.860, AccT1+STIR = 0.889, mean difference in Acc = −0.029 [−0.031; −0.027], p < 0.0001).
When assessing differences in Acc according to the patient subgroups, the following observations were made (Supplementary Tables 1 and 2): the senior reader demonstrated a slightly higher Acc using the T2 Dixon protocol in the metastatic patient subgroup only (mean difference in Acc = + 0.036 [+ 0.033; + 0.038], p < 0.0001), while the junior reader demonstrated a slightly lower Acc using that protocol, regardless of the patient subgroup (MM: mean difference in Acc = −0.052 [−0.058; −0.046], p < 0.0001, metastatic patients: mean difference in Acc= −0.019 [−0.021; −0.017], p < 0.0001).
Semi-quantitative score
In the per-patient analysis, agreement between the protocols and the reference standard was good for the junior reader (regardless of the protocol), and very good for the senior reader (regardless of the protocol) (T1+STIR vs reference standard: AC1junior = 0.76 [0.65; 0.87], AC1senior = 0.86 [0.77; 0.95]; T2 Dixon fat+water vs reference standard: AC1junior = 0.78 [0.68; 0.89], AC1senior= 0.93 [0.87; 1.00]).
False-negative and false-positive readings
FP readings resulted from benign conditions: degenerative disease (subchondral cysts, juxta-articular/discal changes), enchondromas, focal marrow hyperplasia, vertebral hemangiomas, enostosis, and benign fractures. FN readings resulted from missed lesions (Table 5) (Fig. 4).
False-negative observation (detection error): WB-MRI in a 71-year-old man with newly diagnosed prostate cancer. Coronal T1 (A) and STIR (B) WB-MRI images show single centimetric bone metastasis within the right ischio-pubic ramus, with low signal intensity on T1 and high signal intensity on STIR (arrow). The lesion was missed by one observer on these T1 and STIR images. Fat (C) and water (D) reconstructions of the T2 Dixon acquisition show the same lesion with low signal intensity on the FAT image and high signal intensity on the WATER image (arrows). The reference standard, based on reading of all MR images and concurrent imaging studies, confirmed the presence of a solitary right ischio-pubic lesion. E Concurrent DWI image (b = 1000 s mm−2, inverted gray scale) confirms the presence of the lesion (arrow). F Gallium-68 PSMA fused PET/CT image confirms the presence of the lesion
Discussion
This study compared the diagnostic accuracy of a single T2 Dixon sequence including fat+water reconstructions to the guidelines-recommended combination of T1+STIR sequences used as morphological sequences in WB-MRI examinations performed to detect skeletal metastases or MM lesions [7,8,9]. Our results showed that the accuracy (Acc) of the combination of T2 Dixon fat+water reconstructions was similar (−2.9%; +2.7%) to that of the reference protocol combining T1+STIR sequences.
These results are in line with published studies that compared the performance of a T2 Dixon sequence and of the combination of T1 and fat-suppressed fluid-sensitive sequences in MRI studies limited to the spine or to a spine segment for the detection of metastases or MM [26,27,28]. These three studies concluded that the T2 Dixon protocol had similar diagnostic performance compared to the standard protocol, providing STIR-like (fluid sensitive) information with the water image, and T1-like (fat sensitive) information with the fat image, and that its use significantly reduces the acquisition time of spine MRI in oncologic indications.
The present study extends this conclusion to WB-MRI examinations used for skeletal screening.
Both repeatability and reproducibility of readings were at least good for both the T2 Dixon and the T1+STIR protocols, for both the categorical (disease present/absent) and semi-quantitative scores (lesion count), and in both the regional and global (per-patient) analyses. Of note, all quantitative evaluations of repeatability/reproducibility (Gwet’s coefficient) were higher for the T2 Dixon evaluation compared to T1+STIR.
Regarding image quality, the T1 sequence had higher SNR compared to the T2 Dixon fat images in lesions and reference areas. This difference is not unexpected as the T1 sequence is acquired in 3D mode, which intrinsically provides a higher signal than 2D sequences [42]. This differs from the studies of Maeder and Danner, where a higher SNR for the T2 Dixon fat images compared to the T1 images acquired in 2D mode was found [26, 28]. In our study, the T1 and the T2 Dixon fat sequences had similar CNR. Interestingly, T2 Dixon fat images had significantly higher CRR compared to T1 images, and T2 Dixon water images had significantly higher CRR compared to STIR images. In practice, this higher CRR results in an improved detectability of the lesions.
Regarding the diagnostic performance, both protocols showed similar levels of sensitivity and specificity in the per-region analysis, with no significant difference between them in the proportion of correctly detected lesions, compared to the reference standard. Some FP and FN findings were observed, presenting similar causes for both protocols and both readers.
The junior reader had a higher number of FP observations compared to the senior, resulting in a lower specificity and accuracy, in both the per-region and per-patient analyses. FP observations were errors of interpretation of degenerative changes or benign conditions, already reported as pitfalls in previous studies of the diagnostic performance of WB-MRI for the detection of bone marrow lesions [11, 43]. These causes of FP should be taken into account during the learning phase of less-experienced readers to avoid overdiagnosis.
Both readers had a low number of FN observations, leading to high sensitivity of both the T2 Dixon and T1+STIR protocols. FNs were due to errors of detection, i.e. lesions missed by the reader or lesions not seen on the available sequences but detected by the reference standard.
Considering the high level of accuracy achieved in this study and the shorter acquisition time, the T2 Dixon protocol represents a realistic alternative to the classical T1+STIR combination for the skeletal screening and follow-up of patients with metastases from solid cancers and MM. In our center, its implementation allows sparing 9 min 12s in the acquisition time of morphological sequences. The total acquisition time of WB-MRI including T2 Dixon and DWI sequences is 27 min 03 s.
This study has several limitations. First, it was performed in a single center and on a single MRI magnet. However, the cohort of patients was large and representative of current WB-MRI indications. Enlarging the cohort of patients should allow refining the evaluation of the difference in accuracy between protocols. Extending our protocol in a multi-centric study including patients imaged on different MRI magnets and multiple readings should allow the generalization of our observations.
Second, only the fat and water reconstructions derived from the T2 Dixon sequence were used, excluding IP and OP images. This choice relies on previous observations showing the lack of added value of IP and OP images to detect bone lesions [26]. However, the availability of IP and OP images may be an additional advantage of the T2 Dixon protocol. Indeed, IP images are equivalent to non-fat-suppressed T2-weighted images and provide anatomic information that may be useful in the evaluation of the spinal canal and spinal cord, in the characterization of vertebral fractures, and in the detection of visceral and lymph node metastases without additional imaging time [44]. Moreover, the availability of IP and OP images and their comparisons may help in the determination of the neoplastic nature of an ambiguous bone marrow lesion and in the recognition of focal marrow hyperplasia and acute benign vertebral fractures, which are frequent pitfalls causing FP observations. Benign lesions indeed show a signal dropout on OP images due to the intravoxel coexistence of microscopic fatty components and hydrated normal cells within the bone marrow [45, 46]. Besides this qualitative approach, the T2 Dixon sequence allows fat fraction (FF) quantification, which can be used to differentiate benign and malignant lesions [24]. Further studies should assess the added value of IP and OP images and of FF measurements.
Third, this study only assessed the diagnostic performance of morphological sequences, in a time-saving perspective. Although the DWI sequence was systematically available, we did not compare its diagnostic accuracy to that of anatomic sequences. The high accuracy (close to 1.00) of the T2 Dixon fat+water protocol suggests that this morphologic sequence alone may be sufficient for skeletal screening, with no need for DWI. This high performance of the anatomic T2 Dixon for bone screening, making DWI superfluous in this indication, has been suggested in a study in 5 patients with bone metastases of renal cancer [47]. We did not question the recommended systematic combination of anatomic and functional DWI sequences. Indeed, DWI sequences allow optimization of bone lesion detection, screening of lymph nodes and visceral lesions, and provide ADC measurements useful for lesion characterization and assessment of treatment response [7, 9, 48,49,50].
Conclusion
This study in patients with bone metastases or MM shows that a shorter anatomical WB-MRI protocol relying on a single T2 Dixon sequence with fat and water reconstructions may replace the reference T1+STIR sequences for skeletal screening, shortening examination duration, without loss of diagnostic accuracy.
Abbreviations
- AC1:
-
Gwet’s agreement coefficient
- Acc:
-
Accuracy
- BVC:
-
Best valuable comparator
- CNR:
-
Contrast-to-noise ratio
- CRR:
-
Contrast-to-reference ratio
- CT TAP:
-
Computed tomography of thorax, abdomen, and pelvis
- DWI:
-
Diffusion-weighted imaging
- FF:
-
Fat fraction
- FN:
-
False negative
- FP:
-
False positive
- IP:
-
In-phase imaging from the T2 Dixon sequence
- MM:
-
Multiple myeloma
- OP:
-
Out-of-phase imaging from the T2 Dixon sequence
- PET:
-
Positron emission tomography
- ROI:
-
Region of interest
- Se:
-
Sensitivity
- SI:
-
Signal intensity
- SNR:
-
Signal-to-noise ratio
- Sp:
-
Specificity
- STIR:
-
Short-Tau inversion recovery
- TN:
-
True negative
- TP:
-
True positive
- TSE:
-
Turbo spin echo
- WB-MRI:
-
Whole-body magnetic resonance imaging
References
Lauenstein TC, Goehde SC, Herborn CU et al(2004) Whole-body MR imaging: evaluation of patients for metastases. Radiology 233:139–148
Walker R, Kessar P, Blanchard R et al (2000) Turbo STIR magnetic resonance imaging as a whole-body screening tool for metastases in patients with breast carcinoma: preliminary clinical experience. J Magn Reson Imaging 11:343–350
Kwee TC, Kwee RM, Verdonck LF, Bierings MB, Nievelstein RA (2008) Magnetic resonance imaging for the detection of bone marrow involvement in malignant lymphoma. Br J Haematol 141:60–68
Ghanem N, Lohrmann C, Engelhardt M et al(2006) Whole-body MRI in the detection of bone marrow infiltration in patients with plasma cell neoplasms in comparison to the radiological skeletal survey. Eur Radiol 16:1005–1014
Dimopoulos MA, Hillengass J, Usmani S et al (2015) Role of magnetic resonance imaging in the management of patients with multiple myeloma: a consensus statement. J Clin Oncol 33:657–664
Lecouvet FE, Boyadzhiev D, Collette L et al (2019) MRI versus [18F]F-FDG-PET/CT for detecting bone marrow involvement in multiple myeloma: diagnostic performance and clinical relevance. Eur Radiol. https://doi.org/10.1007/s00330-019-06469-1
Padhani AR, Lecouvet FE, Tunariu N et al (2017) METastasis reporting and data system for prostate cancer: practical guidelines for acquisition, interpretation, and reporting of whole-body magnetic resonance imaging-based evaluations of multiorgan involvement in advanced prostate cancer. Eur Urol 71:81–92
Messiou C, Hillengass J, Delorme S et al (2019) Guidelines for acquisition, interpretation, and reporting of whole-body MRI in myeloma: Myeloma Response Assessment and Diagnosis System (MY-RADS). Radiology 291:5–13
Lecouvet FE (2016) Whole-body MR imaging: musculoskeletal applications. Radiology 279:345–365
Mirowitz SA, Apicella P, Reinus WR, Hammerman AM (1994) MR imaging of bone marrow lesions: relative conspicuousness on T1-weighted, fat-suppressed T2-weighted, and STIR images. AJR Am J Roentgenol 162:215–221
Larbi A, Omoumi P, Pasoglou V et al (2019) Whole-body MRI to assess bone involvement in prostate cancer and multiple myeloma: comparison of the diagnostic accuracies of the T1, short tau inversion recovery (STIR), and high b-values diffusion-weighted imaging (DWI) sequences. Eur Radiol 29:4503–4513
Padhani AR, Koh DM, Collins DJ (2011) Whole-body diffusion-weighted MR imaging in cancer: current status and research directions. Radiology 261:700–718
Takenaka D, Ohno Y, Matsumoto K et al (2009) Detection of bone metastases in non-small cell lung cancer patients: comparison of whole-body diffusion-weighted imaging (DWI), whole-body MR imaging without and with DWI, whole-body FDG-PET/CT, and bone scintigraphy. J Magn Reson Imaging 30:298–308
Takahara T, Imai Y, Yamashita T, Yasuda S, Nasu S, Van Cauteren M (2004) Diffusion weighted whole body imaging with background body signal suppression (DWIBS): technical improvement using free breathing, STIR and high resolution 3D display. Radiat Med 22:275–282
Pearce T, Philip S, Brown J, Koh DM, Burn PR (2012) Bone metastases from prostate, breast and multiple myeloma: differences in lesion conspicuity at short-tau inversion recovery and diffusion-weighted MRI. Br J Radiol 85:1102–1106
Sivesgaard K, Johnk ML, Larsen LP et al (2017) Comparison of four MRI protocols for detection of extrahepatic colorectal cancer metastases. J Magn Reson Imaging 46:1619–1630
Lecouvet FE, Pasoglou V, Van Nieuwenhove S et al (2020) Shortening the acquisition time of whole-body MRI: 3D T1 gradient echo Dixon vs fast spin echo for metastatic screening in prostate cancer. Eur Radiol 30:3083–3093
Zormpas-Petridis K, Tunariu N, Curcean A et al (2021) Accelerating whole-body diffusion-weighted MRI with deep learning-based denoising image filters. Radiol Artif Intell 3:e200279
Pasoglou V, Michoux N, Peeters F et al (2015) Whole-body 3D T1-weighted MR imaging in patients with prostate cancer: feasibility and evaluation in screening for metastatic disease. Radiology 275:155–166
Dixon WT (1984) Simple proton spectroscopic imaging. Radiology 153:189–194
Ma J (2008) Dixon techniques for water and fat imaging. J Magn Reson Imaging 28:543–558
Bley TA, Wieben O, Francois CJ, Brittain JH, Reeder SB (2010) Fat and water magnetic resonance imaging. J Magn Reson Imaging 31:4–18
Zanchi F, Richard R, Hussami M, Monier A, Knebel JF, Omoumi P (2020) MRI of non-specific low back pain and/or lumbar radiculopathy: do we need T1 when using a sagittal T2-weighted Dixon sequence? Eur Radiol 30:2583–2593
Bacher S, Hajdu SD, Maeder Y, Dunet V, Hilbert T, Omoumi P (2021) Differentiation between benign and malignant vertebral compression fractures using qualitative and quantitative analysis of a single fast spin echo T2-weighted Dixon sequence. Eur Radiol 31:9418–9427
Guerini H, Omoumi P, Guichoux F et al (2015) Fat suppression with dixon techniques in musculoskeletal magnetic resonance imaging: a pictorial review. Semin Musculoskelet Radiol 19:335–347
Maeder Y, Dunet V, Richard R, Becce F, Omoumi P (2018) Bone marrow metastases: T2-weighted Dixon spin-echo fat images can replace T1-weighted spin-echo images. Radiology 286:948–959
Hahn S, Lee YH, Suh JS (2018) Detection of vertebral metastases: a comparison between the modified Dixon turbo spin echo T(2) weighted MRI and conventional T(1) weighted MRI: a preliminary study in a tertiary centre. Br J Radiol 91:20170782
Danner A, Brumpt E, Alilet M, Tio G, Omoumi P, Aubry S (2019) Improved contrast for myeloma focal lesions with T2-weighted Dixon images compared to T1-weighted images. Diagn Interv Imaging 100:513–519
Mohler JL, Kantoff PW, Armstrong AJ et al (2014) Prostate cancer, version 2.2014. J Natl Compr Canc Netw 12:686–718
Cardoso F, Paluch-Shimon S, Senkus E et al (2020) 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann Oncol 31:1623–1649
Cai W, Tan Y, Ge W, Ding K, Hu H (2018) Pattern and risk factors for distant metastases in gastrointestinal neuroendocrine neoplasms: a population-based study. Cancer Med 7:2699–2709
Vanel D, Dromain C, Tardivon A (2000) MRI of bone marrow disorders. Eur Radiol 10:224–229
Baur-Melnyk A, Buhmann S, Durr HR, Reiser M (2005) Role of MRI for the diagnosis and prognosis of multiple myeloma. Eur J Radiol 55:56–63
Lecouvet FE, Simon M, Tombal B, Jamart J, Vande Berg BC, Simoni P (2010) Whole-body MRI (WB-MRI) versus axial skeleton MRI (AS-MRI) to detect and measure bone metastases in prostate cancer (PCa). Eur Radiol 20:2973–2982
Koh DM, Blackledge M, Padhani AR et al (2012) Whole-body diffusion-weighted MRI: tips, tricks, and pitfalls. AJR Am J Roentgenol 199:252–262
Moulopoulos LA, Varma DG, Dimopoulos MA et al (1992) Multiple myeloma: spinal MR imaging in patients with untreated newly diagnosed disease. Radiology 185:833–840
Sanal SM, Flickinger FW, Caudell MJ, Sherry RM (1994) Detection of bone marrow involvement in breast cancer with magnetic resonance imaging. J Clin Oncol 12:1415–1421
Lecouvet FE, Geukens D, Stainier A et al (2007) Magnetic resonance imaging of the axial skeleton for detecting bone metastases in patients with high-risk prostate cancer: diagnostic and cost-effectiveness and comparison with current detection strategies. J Clin Oncol 25:3281–3287
Conover WJ (1999) Practical Nonparametric Statistics. John Wiley & Sons Inc., New York
Gwet KL (2008) Computing inter-rater reliability and its variance in the presence of high agreement. Br J Math Stat Psychol 61:29–48
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33:159–174
McRobbie DW, Moore EA, Graves MJ, Prince MR (2017) Acronyms anonymous. In: McRobbie DW, Moore EA, Graves MJ, Prince MRMRI from Picture to Proton. Cambridge University Press, pp 185–206
Ohno Y, Koyama H, Onishi Y et al (2008) Non-small cell lung cancer: whole-body MR examination for M-stage assessment--utility for whole-body diffusion-weighted imaging compared with integrated FDG PET/CT. Radiology 248:643–654
Low RN, Austin MJ, Ma J (2011) Fast spin-echo triple echo dixon: Initial clinical experience with a novel pulse sequence for simultaneous fat-suppressed and nonfat-suppressed T2-weighted spine magnetic resonance imaging. J Magn Reson Imaging 33:390–400
Douis H, Davies AM, Jeys L, Sian P (2016) Chemical shift MRI can aid in the diagnosis of indeterminate skeletal lesions of the spine. Eur Radiol 26:932–940
Zajick DC Jr, Morrison WB, Schweitzer ME, Parellada JA, Carrino JA (2005) Benign and malignant processes: normal values and differentiation with chemical shift MR imaging in vertebral marrow. Radiology 237:590–596
Wang X, Pirasteh A, Brugarolas J et al (2018) Whole-body MRI for metastatic cancer detection using T2 -weighted imaging with fat and fluid suppression. Magn Reson Med 80:1402–1415
Giles SL, Messiou C, Collins DJ et al (2014) Whole-body diffusion-weighted MR imaging for assessment of treatment response in myeloma. Radiology 271:785–794
Perez-Lopez R, Mateo J, Mossop H D et al (2017) Diffusion-weighted imaging as a treatment response biomarker for evaluating bone metastases in prostate cancer: a pilot study. Radiology 283:168–177
Zhang L, Wang Q, Wu X et al (2021) Baseline bone marrow ADC value of diffusion-weighted MRI: a potential independent predictor for progression and death in patients with newly diagnosed multiple myeloma. Eur Radiol 31:1843–1852
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Frederic E. Lecouvet.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors (Nicolas Michoux) has significant statistical expertise.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• retrospective
• cross-sectional study
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(PDF 215 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chiabai, O., Van Nieuwenhove, S., Vekemans, MC. et al. Whole-body MRI in oncology: can a single anatomic T2 Dixon sequence replace the combination of T1 and STIR sequences to detect skeletal metastasis and myeloma?. Eur Radiol 33, 244–257 (2023). https://doi.org/10.1007/s00330-022-09007-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-022-09007-8