Abstract
Purpose
To compare the diagnostic accuracy of whole-body T1, short tau inversion recovery (STIR), high b-value diffusion-weighted imaging (DWI), and sequence combinations to detect bone involvement in prostate cancer (PCa) and multiple myeloma (MM) patients.
Materials and methods
We included 50 consecutive patients with PCa at high risk for metastasis and 47 consecutive patients with a histologically confirmed diagnosis of MM who received whole-body MRI at two institutions from January to December 2015. Coronal T1, STIR, and reconstructed coronal high b-values DWI were obtained for all patients. Two musculoskeletal radiologists read individual sequences, pairs of sequences (T1-DWI, T1-STIR, and STIR-DWI), and all combined (T1-STIR-DWI) to detect bone involvement. Receiver operating characteristic curve analysis was used to assess diagnostic performance according to a “best valuable comparator” combining baseline and 6-month imaging and clinical and biological data. Interobserver agreement was calculated.
Results
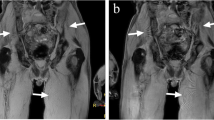
Interobserver agreement for individual and combined MRI sequences was very good in the PCa group and ranged from good to very good in the MM group (0.76–1.00). In PCa patients, T1-DWI, T1-STIR, and T1-STIR-DWI showed the highest performance (sensitivity = 100% [95% CI = 90.5–100%], specificity = 100% [75.3–100%]). In MM patients, the highest performance was achieved by T1-STIR-DWI (sensitivity = 100% [88.4–100%], specificity = 94.1% [71.3–100%]). T1-STIR-DWI significantly outperformed all sequences (p < 0.05) except T1-DWI (p = 0.49).
Conclusion
In PCa patients, a combination of either T1-DWI or T1-STIR sequences is not inferior to a combination of three sequences to detect bone metastases. In MM, T1-STIR-DWI and T1-DWI had the highest diagnostic performance for detecting bone involvement.
Key Points
• The sequences used in Whole Body MRI studies to detect bone involvement in prostate cancer and myeloma were evaluated.
• In prostate cancer, any pairwise combinations of T1, STIR, and DWI have high diagnostic value.
• In myeloma, the combinations T1-STIR-DWI or T1-DWI sequences should be used.





Similar content being viewed by others
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- AUC:
-
Area under the receiver operating characteristic curve
- BVC:
-
Best valuable comparator
- CI:
-
Confidence interval
- DWI:
-
Diffusion-weighted imaging
- MM:
-
Multiple myeloma
- PCa:
-
Prostate cancer
- PET:
-
Positron emission tomography
- PSA:
-
Prostate-specific antigen
- ROC:
-
Receiver operating characteristic
- STIR:
-
Short tau inversion recovery
References
Heusner TA, Kuemmel S, Koeninger A et al (2010) Diagnostic value of diffusion-weighted magnetic resonance imaging (DWI) compared to FDG PET/CT for whole-body breast cancer staging. Eur J Nucl Med Mol Imaging 37:1077–1086
Walker R, Kessar P, Blanchard R et al (2000) Turbo STIR magnetic resonance imaging as a whole-body screening tool for metastases in patients with breast carcinoma: preliminary clinical experience. J Magn Reson Imaging 11:343–350
Kwee TC, Fijnheer R, Ludwig I et al (2010) Whole-body magnetic resonance imaging, including diffusion-weighted imaging, for diagnosing bone marrow involvement in malignant lymphoma. Br J Haematol 149:628–630
Messiou C, Kaiser M (2015) Whole body diffusion weighted MRI--a new view of myeloma. Br J Haematol 171:29–37
Dimopoulos MA, Hillengass J, Usmani S et al (2015) Role of magnetic resonance imaging in the management of patients with multiple myeloma: a consensus statement. J Clin Oncol 33:657–664
Lee SY, Kim HJ, Shin YR, Park HJ, Lee YG, Oh SJ (2017) Prognostic significance of focal lesions and diffuse infiltration on MRI for multiple myeloma: a meta-analysis. Eur Radiol 27:2333–2347
Padhani AR, Koh DM, Collins DJ (2011) Whole-body diffusion-weighted MR imaging in cancer: current status and research directions. Radiology 261:700–718
Wu LM, Gu HY, Zheng J et al (2011) Diagnostic value of whole-body magnetic resonance imaging for bone metastases: a systematic review and meta-analysis. J Magn Reson Imaging 34:128–135
Lauenstein TC, Goehde SC, Herborn CU et al (2004) Whole-body MR imaging: evaluation of patients for metastases. Radiology 233:139–148
Lecouvet FE (2016) Whole-body MR imaging: musculoskeletal applications. Radiology 279:345–365
Padhani AR, Lecouvet FE, Tunariu N et al (2017) METastasis reporting and data system for prostate Cancer: practical guidelines for acquisition, interpretation, and reporting of whole-body magnetic resonance imaging-based evaluations of multiorgan involvement in advanced prostate Cancer. Eur Urol 71:81–92
Pasoglou V, Michoux N, Tombal B, Jamar F, Lecouvet FE (2015) wbMRI to detect bone metastases: critical review on diagnostic accuracy and comparison to other imaging modalities. Clin Transl Imaging 3:141–157
Eustace S, Tello R, DeCarvalho V et al (1997) A comparison of whole-body turboSTIR MR imaging and planar 99mTc-methylene diphosphonate scintigraphy in the examination of patients with suspected skeletal metastases. AJR Am J Roentgenol 169:1655–1661
Lecouvet FE, El Mouedden J, Collette L et al (2012) Can whole-body magnetic resonance imaging with diffusion-weighted imaging replace Tc 99m bone scanning and computed tomography for single-step detection of metastases in patients with high-risk prostate cancer? Eur Urol 62:68–75
Daldrup-Link HE, Franzius C, Link TM et al (2001) Whole-body MR imaging for detection of bone metastases in children and young adults: comparison with skeletal scintigraphy and FDG PET. AJR Am J Roentgenol 177:229–236
Takenaka D, Ohno Y, Matsumoto K et al (2009) Detection of bone metastases in non-small cell lung cancer patients: comparison of whole-body diffusion-weighted imaging (DWI), whole-body MR imaging without and with DWI, whole-body FDG-PET/CT, and bone scintigraphy. J Magn Reson Imaging 30:298–308
Ohno Y, Koyama H, Onishi Y et al (2008) Non-small cell lung cancer: whole-body MR examination for M-stage assessment--utility for whole-body diffusion-weighted imaging compared with integrated FDG PET/CT. Radiology 248:643–654
Engelhard K, Hollenbach HP, Wohlfart K, von Imhoff E, Fellner FA (2004) Comparison of whole-body MRI with automatic moving table technique and bone scintigraphy for screening for bone metastases in patients with breast cancer. Eur Radiol 14:99–105
Takahara T, Imai Y, Yamashita T, Yasuda S, Nasu S, Van Cauteren M (2004) Diffusion weighted whole body imaging with background body signal suppression (DWIBS): technical improvement using free breathing, STIR and high resolution 3D display. Radiat Med 22:275–282
Pearce T, Philip S, Brown J, Koh DM, Burn PR (2012) Bone metastases from prostate, breast and multiple myeloma: differences in lesion conspicuity at short-tau inversion recovery and diffusion-weighted MRI. Br J Radiol 85:1102–1106
Han SN, Amant F, Michielsen K et al (2018) Feasibility of whole-body diffusion-weighted MRI for detection of primary tumour, nodal and distant metastases in women with cancer during pregnancy: a pilot study. Eur Radiol 28:1862–1874
Shen G, Deng H, Hu S, Jia Z (2014) Comparison of choline-PET/CT, MRI, SPECT, and bone scintigraphy in the diagnosis of bone metastases in patients with prostate cancer: a meta-analysis. Skeletal Radiol 43:1503–1513
NICE (2016) https://www.nice.org.uk/guidance/ng35/chapter/recommendations. National Institute for Health and Care Excellence
Messiou C, Collins DJ, Morgan VA, Desouza NM (2011) Optimising diffusion weighted MRI for imaging metastatic and myeloma bone disease and assessing reproducibility. Eur Radiol 21:1713–1718
Tombal B, Alcaraz A, James N, Valdagni R, Irani J (2014) Can we improve the definition of high-risk, hormone naive, non-metastatic prostate cancer? BJU Int 113:189–199
Lecouvet FE, Geukens D, Stainier A et al (2007) Magnetic resonance imaging of the axial skeleton for detecting bone metastases in patients with high-risk prostate cancer: diagnostic and cost-effectiveness and comparison with current detection strategies. J Clin Oncol 25:3281–3287
Heidenreich A, Bastian PJ, Bellmunt J et al (2014) EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent-update 2013. Eur Urol 65:124–137
Vanel D, Dromain C, Tardivon A (2000) MRI of bone marrow disorders. Eur Radiol 10:224–229
Baur-Melnyk A, Buhmann S, Durr HR, Reiser M (2005) Role of MRI for the diagnosis and prognosis of multiple myeloma. Eur J Radiol 55:56–63
Padhani AR, Koh DM (2011) Diffusion MR imaging for monitoring of treatment response. Magn Reson Imaging Clin N Am 19:181–209
Koh DM, Blackledge M, Padhani AR et al (2012) Whole-body diffusion-weighted MRI: tips, tricks, and pitfalls. AJR Am J Roentgenol 199:252–262
Lecouvet FE, Simon M, Tombal B, Jamart J, Vande Berg BC, Simoni P (2010) Whole-body MRI (WB-MRI) versus axial skeleton MRI (AS-MRI) to detect and measure bone metastases in prostate cancer (PCa). Eur Radiol 20:2973–2982
Libshitz HI, Malthouse SR, Cunningham D, MacVicar AD, Husband JE (1992) Multiple myeloma: appearance at MR imaging. Radiology 182:833–837
Moulopoulos LA, Varma DG, Dimopoulos MA et al (1992) Multiple myeloma: spinal MR imaging in patients with untreated newly diagnosed disease. Radiology 185:833–840
Maeder Y, Dunet V, Richard R, Becce F, Omoumi P (2017) Bone marrow metastases: T2-weighted Dixon spin-Echo fat images can replace T1-weighted spin-Echo images. Radiology. https://doi.org/10.1148/radiol.2017170325:170325
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33:159–174
DeLong ER, DeLong DM, Clarke-Pearson DL (1988) Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44:837–845
Lecouvet FE, Vande Berg BC, Malghem J, Omoumi P, Simoni P (2009) Diffusion-weighted MR imaging: adjunct or alternative to T1-weighted MR imaging for prostate carcinoma bone metastases? Radiology 252:624
Latifoltojar A, Hall-Craggs M, Bainbridge A et al (2017) Whole-body MRI quantitative biomarkers are associated significantly with treatment response in patients with newly diagnosed symptomatic multiple myeloma following bortezomib induction. Eur Radiol 27:5325–5336
Winfield JM, Poillucci G, Blackledge MD et al (2018) Apparent diffusion coefficient of vertebral haemangiomas allows differentiation from malignant focal deposits in whole-body diffusion-weighted MRI. Eur Radiol 28:1687–1691
Park SY, Shin SJ, Jung DC et al (2017) PI-RADS version 2: quantitative analysis aids reliable interpretation of diffusion-weighted imaging for prostate cancer. Eur Radiol 27:2776–2783
Messiou C, Giles S, Collins DJ et al (2012) Assessing response of myeloma bone disease with diffusion-weighted MRI. Br J Radiol 85:e1198–e1203
Funding
This study has received support by Fondation Contre le Cancer, Fondation Saint Luc, and Fonds de Recherche Clinique des Cliniques universitaires Saint Luc (Belgian non-profit organizations).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Prof Frédéric Lecouvet.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors has significant statistical expertise.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• Retrospective
• Cross-sectional study
• Multicentre study (two)
Rights and permissions
About this article
Cite this article
Larbi, A., Omoumi, P., Pasoglou, V. et al. Whole-body MRI to assess bone involvement in prostate cancer and multiple myeloma: comparison of the diagnostic accuracies of the T1, short tau inversion recovery (STIR), and high b-values diffusion-weighted imaging (DWI) sequences. Eur Radiol 29, 4503–4513 (2019). https://doi.org/10.1007/s00330-018-5796-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-018-5796-1