Abstract
Purpose
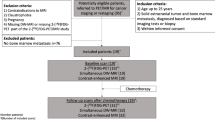
Whole-body magnetic resonance imaging (WB-MRI) is a radiation-free alternative to the 99mTc-HDP bone scan (BS) for the detection of bone metastasis. The major drawback is the long examination time and application of gadolinium enhancer. The aim of this study is to analyze (i) the performance of WB-MRI versus the BS and (ii) the diagnostic benefit of gadolinium (WB-MRI + Gd) compared to a non-enhanced protocol (NE WB-MRI).
Methods and materials
1256 eligible WB-MRI scans were analyzed retrospectively with a single inclusion criterion, a clinical 12-month follow-up or a biopsy as ground truth. N = 285 patients received both a WB-MRI and a BS within 12 months. All the patients were imaged with a coronal T1w and a STIR, and n = 528 (42%) received an additional T1w-mDixon with gadoteridol (0.1 mmol Gd-DTPA/kg).
Results
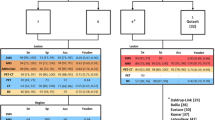
From 1256 eligible patients, n = 884 (70%) had breast cancer as a primary disease, n = 101(8%) prostate cancer, and n = 77(6%) lung cancer. The sensitivity (Se) and negative predictive value (NPV) of the WB-MRI was 98/99%, significantly higher compared to BS with 82/89%, P < 0.001 Mc Nemar’s test. The specificity (Spe) and positive predictive value (PPV) of the WB-MRI and BS was 85/82% and 91/86%, respectively. The interobserver agreement between WB-MRI and BS was 71%, Cohen’s kappa 0.42. Analysis of the added diagnostic value of gadolinium revealed Se/Spe/PPV/NPV of 98/93/92/98% for the NE WB-MRI and 99/93/85/100% for the WM-MRI + Gd, P > 0.05 binary logistic regression with Fischer’s exact test.
Conclusion
WB-MRI exceeds the sensitivity of BS without compromising the specificity, even after omitting the gadolinium enhancer.






Similar content being viewed by others
Abbreviations
- 1.5 T:
-
1.5 Tesla
- 3 T:
-
3 Tesla
- 18F-FDG PET/CT:
-
2-Deoxy-2-[fluorine-18]fluoro-d-glucose integrated with computed tomography
- BS:
-
Bone scan, 99mTc-hydrohydiphosphonate bone scintigraphy
- CI:
-
Confidence interval
- FFE:
-
Fast field echo
- MRI:
-
Magnetic resonance imaging
- NE WB-MRI:
-
Non-enhanced whole body MRI
- NPV:
-
Negative predictive value
- PPV:
-
Positive predictive value
- Se:
-
Sensitivity
- Spe:
-
Specificity
- STIR:
-
Short tau inversion recovery
- T1w:
-
T1-weighted imaging
- TSE:
-
Time spin echo
- WB-MRI:
-
Whole body MRI
- WB-MRI + Gd:
-
Whole body MRI with gadoteridol
References
Wu L-M, Gu H-Y, Zheng J, Xu X, Lin L-H, Deng X, et al. Diagnostic value of whole-body magnetic resonance imaging for bone metastases: a systematic review and meta-analysis. J Magn Reson Imaging. 2011;34:128–35.
Costelloe CM, Madewell JE, Kundra V, Harrell RK, Bassett RL, Ma J. Conspicuity of bone metastases on fast Dixon-based multisequence whole-body MRI: clinical utility per sequence. Magn Reson Imaging. 2013;31:669–75.
Cardoso F, Senkus E, Costa A, Papadopoulos E, Aapro M, André F, et al. 4th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 4)†. Ann Oncol Off J Eur Soc Med Oncol. 2018;29:1634–57.
Parker C, Gillessen S, Heidenreich A, Horwich A. ESMO Guidelines Committee Cancer of the prostate: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol Off J Eur Soc Med Oncol. 2015;26(Suppl 5): v69–v77.
Planchard D, Popat S, Kerr K, Novello S, Smit EF, Faivre-Finn C, et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol Off J Eur Soc Med Oncol. 2018;29:iv192–iv237.
Kosmin M, Makris A, Joshi PV, Ah-See M-L, Woolf D, Padhani AR. The addition of whole-body magnetic resonance imaging to body computerised tomography alters treatment decisions in patients with metastatic breast cancer. Eur J Cancer Oxf Engl. 1990;2017(77):109–16.
Di Gioia D, Stieber P, Schmidt GP, Nagel D, Heinemann V, Baur-Melnyk A. Early detection of metastatic disease in asymptomatic breast cancer patients with whole-body imaging and defined tumour marker increase. Br J Cancer. 2015;112:809–18.
Lecouvet FE, Talbot JN, Messiou C, Bourguet P, Liu Y, de Souza NM. Monitoring the response of bone metastases to treatment with Magnetic Resonance Imaging and nuclear medicine techniques: A review and position statement by the European Organisation for Research and Treatment of Cancer imaging group. Eur J Cancer. 2014;50:2519–31.
Larbi A, Dallaudière B, Pasoglou V, Padhani A, Michoux N, Vande Berg BC, et al. Whole body MRI (WB-MRI) assessment of metastatic spread in prostate cancer: Therapeutic perspectives on targeted management of oligometastatic disease. Prostate. 2016;76:1024–33.
Goldvaser H, Ribnikar D, Fazelzad R, Seruga B, Templeton AJ, Ocana A, et al. Influence of non-measurable disease on progression-free survival in patients with metastatic breast cancer. Cancer Treat Rev. 2017;59:46–53.
Cohen JF, Korevaar DA, Altman DG, Bruns DE, Gatsonis CA, Hooft L, et al. STARD 2015 guidelines for reporting diagnostic accuracy studies: explanation and elaboration. BMJ Open. 2016;6:e012799.
Cook C, Cleland J, Huijbregts P. Creation and critique of studies of diagnostic accuracy: use of the STARD and QUADAS methodological quality assessment tools. J Man Manip Ther. 2007;15:93–102.
Lenkinski RE. Gadolinium retention and deposition revisited: how the chemical properties of gadolinium-based contrast agents and the use of animal models inform us about the behavior of these agents in the human brain. Radiology. 2017;285:721–4.
Kanda T, Nakai Y, Hagiwara A, Oba H, Toyoda K, Furui S. Distribution and chemical forms of gadolinium in the brain: a review. Br J Radiol. 2017;90:20170115.
Gulani V, Calamante F, Shellock FG, Kanal E, Reeder SB. International Society for Magnetic Resonance in Medicine Gadolinium deposition in the brain: summary of evidence and recommendations. Lancet Neurol. 2017;16:564–70.
O’Sullivan GJ, Carty FL, Cronin CG. Imaging of bone metastasis: An update. World J Radiol. 2015;7:202–11.
Suva LJ, Griffin RJ, Makhoul I. Mechanisms of bone metastases of breast cancer. Endocr Relat Cancer. 2009;16:703–13.
Barchetti F, Stagnitti A, Megna V, Al Ansari N, Marini A, Musio D, et al. Unenhanced whole-body MRI versus PET-CT for the detection of prostate cancer metastases after primary treatment. Eur Rev Med Pharmacol Sci. 2016;20:3770–6.
Nakanishi K, Kobayashi M, Takahashi S, Nakata S, Kyakuno M, Nakaguchi K, et al. Whole body MRI for detecting metastatic bone tumor: comparison with bone scintigrams. Magn Reson Med Sci MRMS Off J Jpn Soc Magn Reson Med. 2005;4:11–7.
Yang H-L, Liu T, Wang X-M, Xu Y, Deng S-M. Diagnosis of bone metastases: a meta-analysis comparing 18FDG PET, CT. MRI and bone scintigraphy. Eur Radiol. 2011;21:2604–17.
Liu T, Wang S, Liu H, Meng B, Zhou F, He F, et al. Detection of vertebral metastases: a meta-analysis comparing MRI, CT, PET, BS and BS with SPECT. J Cancer Res Clin Oncol. 2017;143:457–65.
Yilmaz MH, Ozguroglu M, Mert D, Turna H, Demir G, Adaletli I, et al. Diagnostic value of magnetic resonance imaging and scintigraphy in patients with metastatic breast cancer of the axial skeleton: a comparative study. Med Oncol Northwood Lond Engl. 2008;25:257–63.
Liu T, Cheng T, Xu W, Yan W-L, Liu J, Yang H-L. A meta-analysis of 18FDG-PET, MRI and bone scintigraphy for diagnosis of bone metastases in patients with breast cancer. Skeletal Radiol. 2011;40:523–31.
Shen G, Deng H, Hu S, Jia Z. Comparison of choline-PET/CT, MRI, SPECT, and bone scintigraphy in the diagnosis of bone metastases in patients with prostate cancer: a meta-analysis. Skeletal Radiol. 2014;43:1503–13.
Jambor I, Kuisma A, Ramadan S, Huovinen R, Sandell M, Kajander S, et al. Prospective evaluation of planar bone scintigraphy, SPECT, SPECT/CT, 18F-NaF PET/CT and whole body 15T MRI, including DWI, for the detection of bone metastases in high risk breast and prostate cancer patients: SKELETA clinical trial. Acta Oncol Stockh Swed. 2016;55:59–67.
Qu X, Huang X, Yan W, Wu L, Dai K. A meta-analysis of 18FDG-PET-CT, 18FDG-PET, MRI and bone scintigraphy for diagnosis of bone metastases in patients with lung cancer. Eur J Radiol. 2012;81:1007–155.
Liu T, Xu J-Y, Xu W, Bai Y-R, Yan W-L, Yang H-L. Fluorine-18 deoxyglucose positron emission tomography, magnetic resonance imaging and bone scintigraphy for the diagnosis of bone metastases in patients with lung cancer: which one is the best?—A meta-analysis. Clin Oncol R Coll Radiol G B. 2011;23:350–8.
Layne KA, Dargan PI, Archer JRH, Wood DM. Gadolinium deposition and the potential for toxicological sequelae—a literature review of issues surrounding gadolinium-based contrast agents. Br J Clin Pharmacol. 2018;84:2522–34.
Elbeshlawi I, AbdelBaki MS. Safety of gadolinium administration in children. Pediatr Neurol. 2018;86:27–322.
Walker R, Kessar P, Blanchard R, Dimasi M, Harper K, DeCarvalho V, et al. Turbo STIR magnetic resonance imaging as a whole-body screening tool for metastases in patients with breast carcinoma: preliminary clinical experience. J Magn Reson Imaging. 2000;11:343–50.
Acknowledgements
The authors are thankful to Mr. Stefan Stein and Mr. Sven Winzler for excellent and consequent IT support. Mrs. Gabriele Kaps leaded the group of radiographers that guaranteed the seamless and flawless MRI workflow. Finally, special accreditation goes to Mrs. Ines Lischka for her proficiency in MRI and her tireless dedication in daily problem-tackling.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
AM and IP conceived and design the study, IP, JD, IC and AM acquired and interpreted data, IP, IC and AP analyzed and interpreted data. All authors participated in drafting the article, AM an UT revised the article critically for important intellectual content. All authors approved the final manuscript version to be published.
Corresponding author
Ethics declarations
Conflicts of interest
Authors declare no conflicts of interest in the manuscript, including financial, consultant, institutional and other relationships that might lead to bias.
Ethical approval
All the patient data were derived from the database of our institution. Data were analyzed retrospectively, fully anonymized, in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its amendments, the European regulation 536/2014 and its latest addendum ICH GCP E6(R2)/2017, as well as with the guidelines of the local Institutional Review Board for clinical studies (Ref. number 2019-1288).
Informed consent
No informed consent is required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Papageorgiou, I., Dvorak, J., Cosma, I. et al. Whole-body MRI: a powerful alternative to bone scan for bone marrow staging without radiation and gadolinium enhancer. Clin Transl Oncol 22, 1321–1328 (2020). https://doi.org/10.1007/s12094-019-02257-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-019-02257-x