Abstract
One Gram-negative, rod-shaped bacterial strain, isolated from an undescribed Heterorhabditis entomopathogenic nematode species was characterized to determine its taxonomic position. The 16S rRNA gene sequences indicate that it belongs to the class Gammaproteobacteria, to the family Morganellaceae, to the genus Photorhabdus, and likely represents a novel bacterial species. This strain, designated here as CRI-LCT, was therefore molecularly, biochemically, and morphologically characterized to describe the novel bacterial species. Phylogenetic reconstructions using 16S rRNA gene sequences show that CRI-LCT is closely related to P. laumondii subsp. laumondii TT01T and to P. laumondii subsp. clarkei BOJ-47T. The 16rRNA gene sequences between CRI-LCT and P. laumondii subsp. laumondii TT01T are 99.1% identical, and between CRI-LCT and P. laumondii subsp. clarkei BOJ-47T are 99.2% identical. Phylogenetic reconstructions using whole genome sequences show that CRI-LCT is closely related to P. laumondii subsp. laumondii TT01T and to P. laumondii subsp. clarkei BOJ-47T. Moreover, digital DNA-DNA hybridization (dDDH) values between CRI-LCT and its two relative species P. laumondii subsp. laumondii TT01T and P. laumondii subsp. clarkei BOJ-47T are 65% and 63%, respectively. In addition, we observed that average nucleotide identity (ANI) values between CRI-LCT and its two relative species P. laumondii subsp. laumondii TT01T and P. laumondii subsp. clarkei BOJ-47T are 95.8% and 95.5%, respectively. These values are below the 70% dDDH and the 95–96% ANI divergence thresholds that delimits prokaryotic species. Based on these genomic divergence values, and the phylogenomic separation, we conclude that CRI-LCT represents a novel bacterial species, for which we propose the name Photorhabdus africana sp. nov. with CRI-LCT (= CCM 9390T = CCOS 2112T) as the type strain. The following biochemical tests allow to differentiate P. africana sp. nov. CRI-LCT from other species of the genus, including its more closely related taxa: β-Galactosidase, citrate utilization, urease and tryptophan deaminase activities, indole and acetoin production, and glucose and inositol oxidation. Our study contributes to a better understanding of the taxonomy and biodiversity of this important bacterial group with great biotechnological and agricultural potential.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Species of the bacterial genus Photorhabdus are symbiotically associated with Heterorhabditis entomopathogenic nematodes (EPNs) [1,2,3,4]. Heterorhabditis EPNs are soil-dwelling microorganisms that parasitize, kill and reproduce inside insects. These nematodes are cosmopolitan, and have been isolated from different locations around the globe, except Antarctica [5]. These nematodes establish a close symbiotic association with Photorhabdus bacteria. The nematodes carry the symbionts inside their intestines, and release them immediately after colonizing a host [6]. Photorhabdus bacteria produce toxins and digestive enzymes that kill and pre-digest the infected host [7,8,9]. Nematodes and bacteria then proliferate in the cadavers until all resources are depleted [10]. Subsequently, nematodes and bacteria re-establish symbiosis and abandon the cadaver in search of a new host [11]. These lethal organisms are important biological control agents and are broadly used to control agricultural pests [12,13,14,15]. In addition, Photorhabdus produces different bioactive compounds, including antibiotics, and therefore these organisms are of high biotechnological and medical relevance [16].
The type strain of the genus Photorhabdus, HbT (= ATCC 29999T), was isolated from Heterorhabditis bacteriophora entomopathogenic nematodes collected from Brecon (Australia), and was initially classified in the genus Xenorhabdus, together with other bacterial species isolated from the Steinernema entomopathogenic nematodes [17, 18]. To harmonize the taxonomy of the bacteria symbiotically associated with both genera of entomopathogenic nematodes, Boemare et al. (1993) proposed to create the genus Photorhabdus to accommodate the bacterial species associated with Heterorhabditis nematodes, and to maintain the genus Xenorhabdus for the bacterial species associated with Steinernema nematodes [19]. Since its creation, several Photorhabdus species and subspecies have been described [18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36]. Currently, the Photorhabdus genus contains 30 valid taxa: 23 species, 6 of which are divided into different subspecies. The species with validly published names are: P. aegyptia, P. aballayi, P. akhurstii, P. antumapuensis, P. asymbiotica, P. australis, P. bodei, P. caribbeanensis, P. cinerea, P. hainanensis, P. heterorhabditis, P. hindustanensis, P. kayaii, P. khanii, P. kleinii, P. laumondii, P. luminescens, P. namnaonensis, P. noenieputensis, P. stackebrandtii, P. tasmaniensis, P. temperata, and P. thracensis. Several species are in turn divided into different subspecies. Photorhabdus akhurstii is divided into P. akhurstii subsp. akhurstii and P. akhurstii subsp. bharatensis; P. australis is divided into P. australis subsp. australis and P. australis subsp. thailandensis; P. heterorhabditis is divided into P. heterorhabditis subsp. heterorhabditis and P. heterorhabditis subsp. aluminescens; P. khanii is divided into P. khanii subsp. khanii and P. khanii subsp. guanajuatensis; P. laumondii is divided into P. laumondii subsp. laumondii and P. laumondii subsp. clarkei; and P. luminescens is divided into P. luminescens subsp. luminescens, P. luminescens subsp. mexicana, and P. luminescens subsp. venezuelensis [34,35,36,37].
The aim of this study was to characterize a novel Photorhabdus bacterial species isolated from an undescribed Heterorhabditis nematode species collected in South Africa. For this, we biochemically, morphologically, and molecularly characterized one representative strain of this species, designed here CRI-LCT. We propose to name this novel bacterial species Photorhabdus africana sp. nov. Our study, therefore, contributes to a better understanding of the taxonomy and biodiversity of a bacterial group of biotechnological and agricultural relevance, and thereby further advance our efforts toward developing more biocontrol tools for sustainable and environmentally friendly agriculture.
Materials and Methods
Nematode Isolation and Identification
Entomopathogenic nematodes (EPNs) were recovered from soil samples using Galleria mellonella larvae as baits [38, 39]. Soil samples were collected from a citrus orchard located in the Sundays River Valley, Eastern Cape province, South Africa (GPS coordinates: 33°37′09.9″S, 25°40′23.7″E. Altitude: 30 m.a.s.l.). One of the nematode strains isolated was designated here as CRI-LC. It was identified as previously described [36]. Briefly, genomic DNA from about 20 females was extracted using the genomic DNA isolation kit from QIAamp DNA Mini Kit (Qiagen, Valencia, CA) following the manufacturer’s instructions. Two genes/genomic regions were amplified by polymerase chain reaction (PCR): the internal transcribed spacer (ITS) region of the rRNA gene and the cytochrome oxidase subunit I (COI) gene. To amplify the ITS region, the following primers were used: 18S (5′-TTGATTACGTCCCTGCCCTTT-3′) and 26S (5′-TTTCACTCGCCGTTACTAAGG-3′) [40]. To amplify the cytochrome oxidase subunit I (COI), primers LCO-1490 (5′-GGTCAACAAATCATAAAGATATTGG-3′) and HCO-2198 (5′-TAAACTTCAGGGTGACCAAAAAATCA-3′) were used [41]. PCR reactions consisted of 12.5 µL of DreamTaq Green PCR Master Mix (Thermo Scientific), 0.5 µL of each forward and reverse primers at 10 µM, 1 µL of genomic DNA and 10.5 µL of nuclease-free distilled water. The PCR reactions were performed using a thermocycler with the following settings. For the ITS region: 1 cycle of 5 min at 94 °C followed by 40 cycles of 30 s at 94 °C, 30 s at 50 °C, 1 min 30 s at 72 °C, and by a single final elongation step at 72 °C for 10 min. For the COI gene, the PCR program was as follows: one cycle of 94 °C for 2 min, followed by 37 cycles of 94 °C for 30 s, 51 °C for 45 s, 72 °C for 2 min, and a final extension at 72 °C for 12 min. PCR was followed by electrophoresis (45 min, 100 V) of 10 µL of PCR products in a 1% TBA (Tris–boric acid–EDTA) buffered agarose gel stained with SYBR Safe DNA Gel Stain (Invitrogen, Carlsbad, California, USA). PCR products were purified using QIAquick PCR Purification Kit (Qiagen, Valencia, CA) and sequenced using forward and reverse primers by Sanger sequencing (Microsynth AG, Balgach, Switzerland). The obtained sequences were manually curated, trimmed, and deposited in the NCBI database under the accession numbers given in Table S1. To obtain ITS and COI gene sequences of nematodes that belong to all the currently described species of the genus Heterorhabditis, we searched the database of the National Center for Biotechnology Information (NCBI) using the Basic Local Alignment Search Tool (BLAST) and the accession numbers described previously [2, 36, 42]. The resulting sequences were used to reconstruct phylogenetic relationships by the Maximum Likelihood method based on the Kimura 2-parameter nucleotide substitution model [43]. To select the best substitution models, best-fit nucleotide substitution model analyses were carried out in MEGA 7 [44]. Sequences were aligned with MUSCLE (v3.8.31) [45]. The trees with the highest log likelihood are shown. The percentage of trees in which the associated taxa clustered together is shown next to the branches. Initial tree(s) for the heuristic search were obtained automatically by applying Neighbor–Join and BioNJ algorithms to a matrix of pairwise distances estimated using the Maximum Composite Likelihood (MCL) approach, and then selecting the topology with superior log likelihood value. The trees were drawn to scale, with branch lengths measured in the number of substitutions per site. Graphical representation and edition of the phylogenetic trees were performed with Interactive Tree of Life (v3.5.1) [46, 47].
Bacteria Isolation
To isolate the bacterial symbionts associated with as CRI-LC nematodes, Galleria mellonella larvae (Lepidoptera: Pyralidae) were infested with 150 infective juveniles. Three to four days later, several insect cadavers were dissected with a blade. Insect internal organs were spread onto Lysogeny Broth (LB) agar plates (Sigma-Aldrich, Switzerland) and incubated at 28 °C for 48–96 h. Based on colony morphology features, Photorhabdus-like colonies (yellow-orange colonies, bioluminescent) were sub-cultured until monocultures were obtained. Bacterial colonies that did not resemble Photorhabdus were not further sub-cultured or subjected to further characterization. Based on 16S rRNA gene sequences, seven bacterial monocultures were confirmed to belong to the Photorhabdus genus and to be conspecific. One of them was named CRI-LCT, designated the type strain of the species, and subjected to further characterization as described below.
Bacteria Molecular Characterization
To molecularly characterize CRI-LCT, phylogenetic relationships were reconstructed using 16S rRNA gene and whole genome sequences. In addition, sequence similarity scores were calculated as described below.
16S rRNA Gene Sequencing
16S rRNA gene sequences were obtained as described previously [36]. Briefly, genomic DNA was extracted and purified using the GenElute Bacterial Genomic DNA Kit (Sigma–Aldrich, Switzerland) following the manufacturer’s instructions, and then, the 16S rRNA gene was amplified by polymerase chain reaction (PCR) using the following universal primers: 27F (5'-AGAGTTTGATCMTGGCTCAG-3') and 1525R (5'-AAGGAGGTGWTCCARCC-3') and the following cycling conditions: 1 cycle at 94 °C for 10 min followed by 40 cycles at 94 °C for 60 s, 55 °C for 60 s, 72 °C for 60 s and a final extension at 72 °C for 5 min [48,49,50]. PCR products were separated by electrophoresis in a 1% TAE-agarose gel stained with GelRed nucleic acid gel stain (Biotium), gel-purified (QIAquick Gel Purification Kit, Qiagen) and sequenced by Sanger sequencing (Microsynth AG, Balgach, Switzerland). The obtained sequences were manually curated using Bioedit 7.2.5 [51]. In addition, 16S rRNA sequences were obtained directly from the whole genome sequences using the bacterial ribosomal RNA predictor Barrnap [52]. Phylogenetic relationships were reconstructed using the Maximum Likelihood method based on the Kimura 2-parameter model in MEGA7 as described above [44, 53, 54]. The accession numbers of the sequences used for these analyses are shown in Table S2.
Whole Genome Sequencing
Genome sequences were obtained as described previously [42, 55]. Briefly, genomic DNA was extracted and purified using the GenElute Bacterial Genomic DNA Kit (Sigma-Aldrich, Switzerland) following the manufacturer’s instructions. The resulting DNA was used for library preparation using the TruSeq DNA PCR–Free LT Library Prep (FC-121-3003) kit. Indexed libraries were then pooled at equimolar concentrations and sequenced (2 × 150 bp) on an Illumina HiSeq 3000 instrument. Genomes were assembled using the Bactopia pipeline [56]. To this end, the raw Illumina reads were quality trimmed using Trimmomatic 0.39 [57]. The resulting reads were assembled with SPAdes 3.14.1 (k-mer sizes of 31, 51, 71, 91, and 111 bp) [58]. Scaffolds with a mean read–depth smaller than 20% of the median read—depth of the longer scaffolds (≥ 5000 bp) as well as scaffolds that were shorter than 200 bp were removed. Minor assembly errors were corrected using Pilon 1.22 [59]. Completeness and contamination of the assembled genomes were assessed using checkM v1.2.2 with default parameters [60].
Core Genome-Based Phylogenetic Reconstructions and Sequence Comparisons
To reconstruct whole genome-based phylogenetic relationships, genomes were first aligned using Roary 3.13.0. Genes to be considered core had to be present in 85% of the genomes with an 85% protein identity. Obtained alignments were used to build phylogenetic trees using FastTree 2.1.10 based on the Generalized Time Reversible Model (GTR). Graphical representation and edition of the phylogenetic trees were performed with Interactive Tree of Life (v3.5.1) [46, 47]. Digital DNA–DNA hybridization (dDDH) scores were used to determine pairwise whole genome sequence similarities. These scores were calculated using the GBPD (Genome Blast Distance Phylogeny) method through the Genome-to-Genome Distance Calculator 2.1 and formula 2 of the Deutsche Sammlung von Mikroorganismen und Zellkulturen (DSMZ) web service (http://ggdc.dsmz.de) using default parameters [61,62,63,64]. Digital DNA-DNA hybridization (dDDH) values of 70% and 79% delimit species and subspecies boundaries, respectively [27, 61, 65]. Average nucleotide identify (ANI) values were calculated using FastANI [66]. The accession numbers of the sequences used for these analyses are shown in Table S2.
Genomic Comparative Analyses
Genomic comparative analyses to annotate and determine the presence/absence of genes that are involved in antibiotic resistance or in the production of specialized metabolites were carried out by aligning draft genome assemblies against the comprehensive antibiotic resistance database (“CARD”) [67,68,69,70,71,72] and against the antibiotics and secondary metabolite analysis shell (antiSMASH) database [73, 74]. Genes that passed the threshold values (antibiotic resistance: ≥ 70% nucleotide identity and ≥ 50% coverage; antiSMASH: ≥ 50% nucleotide identity) were considered as present in the genome [73, 74]. Below this threshold, genes were considered absent or nonfunctional.
Physiological, Biochemical and Morphological Characterization
To physiologically, biochemically, and morphologically characterize CRI-LCT, bacterial cultures from single primary form colonies were used. Bacterial primary forms were determined by examining colony consistency (mucoid), and bioluminescence and pigment production [75]. The selected colonies were further sub-cultured and maintained on Lysogeny Broth (LB) agar plates at 28–30 °C. Cell morphology was observed under a Kern transmitted light microscope at 1000 × magnification, with cells grown for two days at 28 °C on LB agar plates. The optimum temperature for bacterial growth was evaluated on LB plates at 18, 23, 28, 32, 37, and 42 °C. Growth in media with varying salt concentrations and pH levels was evaluated in 3 mL of LB medium using 14 mL Falcon tubes. Three NaCl concentrations were used: 1% (Regular LB medium), 2%, and 3% (w/v). Three pH levels were used: 5, 7, and 9. Each tube was inoculated with 0.3 mL (OD600 = 1) of an overnight bacterial culture, then incubated for 24 h at 28ºC and 180 rpm, and finally the OD600 was measured using a spectrophotometer. Four tubes per treatment were evaluated. Cytochrome oxidase production was tested on discs containing N,N-dimethyl-p-phenylenediamine oxalate and α-naphthol (Sigma-Aldrich, Switzerland). Catalase activity was determined by adding a drop of 10% (v/v) H2O2 into 50 µL of a 16 h-old liquid LB-bacterial culture. Biochemical characterization was carried out using the API20E system (bioMérieux, Inc. Durham, NC) according to the manufacturer’s instructions. To this end, bacteria were grown for 16 h at 28 °C in LB agar Petri plates. Then, one single colony was re-suspended in 5 mL of 0.85% (w/v) NaCl. The resulting bacterial solution was used to inoculate the different microtubes containing the biochemical tests. Samples were incubated at 28 °C. Results were evaluated after 24 h. Bioluminescence production was evaluated by making photographs of bioluminescence on 24 h-old LB agar-cultured bacteria using an Amersham Imager 600 instrument (Cytiva, US).
Ecological Characterization
To evaluate the entomopathogenic potential, bacteria were cultured overnight in LB liquid medium. Then, the bacterial cultures were collected and their optical densities at 600 nm (OD600) were measured. All cultures were then diluted to reach an OD600 = 1. The resulting cultures were serially diluted to obtain bacterial solutions with an OD600 = 0.01. 10 µL of the resulting bacterial solutions were injected into third-instar G. mellonella larvae. Eight larvae per bacterial strain were injected (n = 8). Control insects were injected with pure LB. Mortality was evaluated every 12 h for 3 days.
Results and Discussion
Nematode Molecular Identification
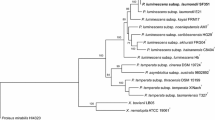
The bacterial strain, CRI-LCT, characterized in this study is hosted by entomopathogenic nematodes in their intestines (Fig. 1). Based on the sequences of the internal transcribed spacer (ITS) region of the rRNA gene and of the COI gene, these nematodes were found to belong to the Heterorhabditis genus, and likely represent a novel, undescribed species, closely related to H. ruandica and H. zacatecana (Fig. S1). Noteworthy, the symbiotic bacteria of H. ruandica nematodes is P. laumondii subsp. laumondii and the symbiotic bacteria of H. zacatecana nematodes is P. kleinii [42]. These two bacterial species are phylogenetically related to the symbiont of CRI-LC nematodes, showing certain degree of co-speciation (Fig. 2).
Phylogenetic reconstruction based on core genome sequences of Photorhabdus type strains with validly published names. 2237050 nucleotide positions (2228 core genes) were used in the analyses. Numbers at the nodes represent SH-like branch supports. Bar represents 0.05 nucleotide substitutions per sequence position. Accession numbers of the genome sequences used for the reconstruction are shown in Table S1
16S rRNA Gene-Based Phylogenetic Reconstruction and Sequence Comparisons
Phylogenetic reconstructions based on 16S rRNA gene sequences show that the bacterial strain isolated from Heterorhabditis CRI-LC nematodes, CRI-LCT, is closely related to P. laumondii subsp. laumondii TT01T and P. laumondii subsp. clarkei BOJ-47T (Fig. S2). 16S rRNA gene sequence similarity scores between CRI-LCT and these latest strains are 99.1% and 99.2%, respectively (Fig. S3). Using 16S rRNA and gyrB gene sequences as BLAST query, we found no records in the NCBI databank of other strains that could potentially be conspecific with CRI-LCT.
Core Genome-Based Phylogenetic Reconstructions and Sequence Comparisons
16S rRNA gene-based phylogenetic reconstructions and sequence comparisons suggest that CRI-LCT likely represents a new taxon, but they do not unambiguously resolve its phylogenetic relationships with other members of the genus or its taxonomic position. We therefore carried out a more detailed molecular characterization of CRI-LCT using core genome-based phylogenetic reconstructions and by calculating whole genome sequence similarity scores [61,62,63,64, 66]. In core genome phylogenies, we observed that CRI-LCT forms a distinct clade together with P. laumondii subsp. laumondii TT01T and P. laumondii subsp. clarkei BOJ-47T (Fig. 2). Due to the clear phylogenetic separations, CRI-LCT appears to represent a novel taxon. To test this hypothesis, we calculated digital DNA-DNA hybridization (dDDH) and average nucleotide identity (ANI) scores using the GBPD (Genome Blast Distance Phylogeny) method and FastANI, respectively (Figs. 3, S4). We observed that dDDH scores between CRI-LCT and P. laumondii subsp. laumondii TT01T, and between CRI-LCT and P. laumondii subsp. clarkei BOJ-47T are 65% and 63%, respectively. In addition, we observed that ANI values between CRI-LCT and P. laumondii subsp. laumondii TT01T, and between CRI-LCT and P. laumondii subsp. clarkei BOJ-47T are 95.8% and 95.5%, respectively. These values are below the 70% dDDH and the 95–96% ANI divergence thresholds that delimits prokaryotic species (Figs. 2, S4) [61, 62, 66]. Based on these genomic divergence values and the phylogenomic separations, CRI-LCT represents a new taxon, for which we propose the name Photorhabdus africana sp. nov. with CRI-LCT (= CCM 9390T = CCOS 2112 T) as the type strain.
Pairwise comparison of digital DNA-DNA Hybridization (dDDH) scores (%) of Photorhabdus type strains with validly published names. Accession numbers of gene sequences used are shown in Table S1
Genomic Features
Genome Size, Nucleotide Composition, and Number of Predicted Coding Genes
The genome of Photorhabdus africana sp. nov. CRI-LCT is predicted to contain 4560 protein-coding genes, a G + C content of 42.79%, and 5.2 Mbp (Table S3). These values are within the typical range observed for many of the species of the genus (Tables S3, S4). Using checkM (v1.2.2), the assembled genome is 96.42% complete, and has 2.29% of contamination.
Predicted Antibiotic Resistance Phenotypes
In silico analyses allow to predict that P. africana sp. nov. CRI-LCT and their most closely related species may be resistant to multiple antibiotics, which is a common trait in this bacterial genus (Tables S5). More specifically, the genome of P. africana sp. nov. CRI-LCT contain genes that confer resistance to different antibiotics, but P. africana sp. nov. CRI-LCT may be susceptible to elfamycins (Table S5).
Predicted Biosynthetic Capacity
In silico analyses using the antibiotics and secondary metabolite analysis shell (antiSMASH) database uncovers the presence of biosynthetic gene clusters dedicated to the production of several polyketides and non-ribosomal peptides in the genome of P. africana sp. nov. CRI-LCT and in the genomes of their more closely related taxa (Table S6). These metabolites are carotenoid, kolossin, luminmides, luminmycins, mevalagmapeptides, minimycins, odilorhabdins, piscibactins, putrebactins, ririwpeptides, syringopeptins, and tolaasins, which are typical compounds produced by this bacterial genus (Table S6). The production of kolossins, ririwpeptides, and syringopeptins differs between Photorhabdus africana sp. nov. CRI-LCT and its more closely related species (Table S6).
Physiological and Biochemical Characteristics
Biochemical tests show that Photorhabdus africana sp. nov. CRI-LCT exhibits biochemical capacities that are similar to those of its more closely related species (Table 1). However, P. africana sp. nov. CRI-LCT also exhibits unique biochemical capacities that differ from the biochemical capacities of their most closely related taxa, particularly, β-galactosidase, citrate utilization, urease and tryptophan deaminase activities, indole and acetoin production, and glucose and inositol oxidation (Table 1). Although, there are very few biochemical differences across the type strains of all the species/subspecies of this genus, additional biochemical tests potentially useful to differentiate the different taxa are presented in previous literature [26, 34]. Moreover, the three bacterial strains evaluated produce bioluminescence, which is a typical characteristic of this bacterial genus (Fig. S4).
Ecological Characterization
When injected into the hemocoel of G. mellonella larvae, all the three bacterial strains rapidly killed the infected insects (Fig. S5). Photorhabdus laumondii subsp. clarkei BOJ-47T was slightly more pathogenic than P. africana sp. nov. CRI-LCT, and P. africana sp. nov. CRI-LCT was slightly more pathogenic than P. laumondii subsp. laumondii TT01T within the first 24 h after infection (Fig. S5). However, all the three bacterial strains killed 100% of the infected insects within 48 h (Fig. S5).
Protologue
Description of Photorhabdus africana sp. nov.
(a.fri.ca'na. L. fem. adj. africana African, referring to the origin of the type strain). Cells are rod-shaped, approx. 2.0–2.2 µm wide and 4.0–5.1 µm long. Colonies are mucoid, circular, slightly irregular margins, yellow or orange in color, sometimes brownish (> 7-day-old cultures), with a diameter of approximately 1–2 mm after 48 h growth on LB agar. Produce bioluminescence. Bacterial growth in liquid LB occurs at temperatures between 18 °C and 37 °C. Optimal temperature for growth is 28–30 °C. Bacterial growth is strongly impaired at 37 °C, and no bacterial growth is observed at 42 °C. Bacteria grow in liquid LB with pH between 5 and 9 (optimum 7). Bacterial growth occurs in LB medium containing between 1% and 2% (w/v) NaCl (optimum 1%). Bacterial growth is inhibited in LB containing > 2% NaCl. Negative for cytochrome oxidase, β-galactosidase, arginine dihydrolase, lysine decarboxylase, ornithine decarboxylase, tryptophan deaminase, citrate utilization, urease, indole and acetoin production. Positive for gelatinase. Does not produce hydrogen sulfide. Oxidizes inositol. Does not oxidize glucose, mannitol, sorbitol, rhamnose, sucrose, melibiose, amygdalin or arabinose. Reduces nitrates. The type strain was isolated from an undescribed Heterorhabditis nematode species. Whole genome sequences of CRI-LCT were deposited in the National Center for Biotechnology Information (NCBI) databank under the accession numbers JAXBVE01; and the 16S rRNA gene sequence under the accession numbers OR835571. The assembled genome contains 5200517 base pairs, 4560 proteins, and a 42.79% G + C content (Tables S2, S3). The type strain of the species, CRI-LCT, was deposited in the Czech Collection of Microorganisms (CCM) and in the national Culture Collection of Switzerland (CCOS) under the following accession numbers: CCM 9390T and CCOS 2112T, respectively.
Code Availability
Whole genome sequences of CRI-LCT were deposited in the National Center for Biotechnology Information (NCBI) databank under the accession numbers JAXBVE01; and the 16S rRNA gene sequence under the accession numbers OR835571.
References
Ciche TA, Kim K, Kaufmann-Daszczuk B et al (2008) Cell invasion and matricide during Photorhabdus luminescens transmission by Heterorhabditis bacteriophora nematodes. Appl Environ Microbiol 74:2275–2287
Dhakal M, Nguyen KB, Hunt DJ et al (2020) Molecular identification, phylogeny and phylogeography of the entomopathogenic nematodes of the genus Heterorhabditis Poinar, 1976: a multigene approach. Nematology 1:1–17
Akhurst RJ (1983) Taxonomic study of Xenorhabdus, a genus of bacteria symbiotically associated with insect pathogenic nematodes. Int J Syst Evol Microbiol 33:38–45
Andaló V, Moino A, Nguyen K (2006) Heterorhabditis amazonensis n. sp. (Rhabditida: Heterorhabditidae) from Amazonas. Brazil Nematol 8:853–867
Bhat AH, Chaubey AK, Askary TH (2020) Global distribution of entomopathogenic nematodes, Steinernema and Heterorhabditis. Egypt J Biol Pest Control 30:1–15
Ciche TA, Ensign JC (2003) For the insect pathogen Photorhabdus luminescens, which end of a nematode is out? Appl Environ Microbiol 69:1890–1897
Bode HB (2009) Entomopathogenic bacteria as a source of secondary metabolites. Curr Opin Chem Biol 13:224–230
Tobias NJ, Mishra B, Gupta DK et al (2016) Genome comparisons provide insights into the role of secondary metabolites in the pathogenic phase of the Photorhabdus life cycle. BMC Genom 17:537
Tobias NJ, Shi Y-M, Bode HB (2018) Refining the natural product repertoire in entomopathogenic bacteria. Trends Microbiol 26:833–840
Stock SP (2015) Diversity, biology and evolutionary relationships. Nematode pathogenesis of insects and other pests. Springer, Berlin, pp 3–27
Somvanshi VS, Sloup RE, Crawford JM et al (2012) A single promoter inversion switches Photorhabdus between pathogenic and mutualistic states. Science 337:88–93
Zhang X, van Doan C, Arce CCM et al (2019) Plant defense resistance in natural enemies of a specialist insect herbivore. Proc Natl Acad Sci 116:23174–23181
Machado RAR, Thönen L, Arce CCM et al (2020) Engineering bacterial symbionts of nematodes improves their biocontrol potential to counter the western corn rootworm. Nat Biotechnol 38:600–608
Fallet P, Machado RA, Toepfer S et al (2020) A Rwandan survey of entomopathogenic nematodes that can potentially be used to control the fall armyworm. IOBC-WPRS Bulletin 150:87–90
Fallet P, de Gianni L, Machado RAR et al (2022) Comparative screening of mexican, rwandan and commercial entomopathogenic nematodes to be used against invasive fall armyworm. Spodoptera frugiperda Insect 13:205
Clarke DJ (2020) Photorhabdus: a tale of contrasting interactions. Microbiology 166:335–348
Poinar GO, Thomas GM, Hess R (1977) Characteristics of the specific bacterium associated with Heterorhabditis bacteriophora (Heterorhabditidae: Rhabditida). Nematologica 23:97–102
Thomas GM, Poinar GO (1979) Xenorhabdus gen. nov., a genus of entomopathogenic, nematophilic bacteria of the family Enterobacteriaceae. Int J Syst Bacteriol 29:352–360. https://doi.org/10.1099/00207713-29-4-352
Boemare NE, Akhurst RJ, Mourant RG (1993) DNA relatedness between Xenorhabdus spp. (Enterobacteriaceae), symbiotic bacteria of entomopathogenic nematodes, and a proposal to transfer Xenorhabdus luminescens to a new genus, Photorhabdus gen. nov. Int J Syst Bacteriol 43:249–255. https://doi.org/10.1099/00207713-43-2-249
Akhurst RJ, Boemare NE, Janssen PH et al (2004) Taxonomy of australian clinical isolates of the genus Photorhabdus and proposal of Photorhabdus asymbiotica subsp. asymbiotica subsp. nov. and P. asymbiotica subsp. australis subsp. nov. Int J Syst Evol Microbiol 54:1301–1310. https://doi.org/10.1099/ijs.0.03005-0
Ferreira T, van Reenen C, Pagès S et al (2013) Photorhabdus luminescens subsp. noenieputensis subsp. nov., a symbiotic bacterium associated with a novel Heterorhabditis species related to Heterorhabditis indica. Int J Syst Evol Microbiol 63:1853–1858. https://doi.org/10.1099/ijs.0.044388-0
Ferreira T, van Reenen CA, Endo A et al (2014) Photorhabdus heterorhabditis sp. nov., a symbiont of the entomopathogenic nematode Heterorhabditis zealandica. Int J Syst Evol Microbiol 64:1540–1545. https://doi.org/10.1099/ijs.0.059840-0
Fischer-Le Saux M, Viallard V, Brunel B, Normand P, Boemare NE (1999) Polyphasic classification of the genus Photorhabdus and proposal of new taxa: P. luminescens subsp. luminescens subsp. nov., P. luminescens subsp. akhurstii subsp. nov., P. luminescens subsp. laumondii subsp. nov., P. temperata sp. nov., P. temperata subsp. temperata subsp. nov. and P. asymbiotica sp. nov. Int J Syst Evol Microbiol 49:1645–1656
Glaeser SP, Tobias NJ, Thanwisai A et al (2017) Photorhabdus luminescens subsp. namnaonensis subsp. nov., isolated from Heterorhabditis baujardi nematodes. Int J Syst Evol Microbiol 67:1046–1051. https://doi.org/10.1099/ijsem.0.001761
Hazir S, Stackebrandt E, Lang E et al (2004) Two new subspecies of Photorhabdus luminescens, isolated from Heterorhabditis bacteriophora (Nematoda: heterorhabditidae): Photorhabdus luminescens subsp. Kayaii subsp. nov. and Photorhabdus luminescens subsp. Thracensis subsp. nov. Syst Appl Microbiol 27:36–42. https://doi.org/10.1078/0723-2020-00255
Machado RAR, Bruno P, Arce CCM et al (2019) Photorhabdus khanii subsp. guanajuatensis subsp. nov., isolated from Heterorhabditis atacamensis, and Photorhabdus luminescens subsp. mexicana subsp. nov., isolated from Heterorhabditis mexicana entomopathogenic nematodes. Int J Syst Evol Microbiol 69:652–661
Machado RAR, Wüthrich D, Kuhnert P et al (2018) Whole-genome-based revisit of Photorhabdus phylogeny: proposal for the elevation of most Photorhabdus subspecies to the species level and description of one novel species Photorhabdus bodei sp. nov., and one novel subspecies Photorhabdus laumondii subsp. clarkei subsp. nov. Int J Syst Evol Microbiol 68:2664–2681
Tailliez P, Laroui C, Ginibre N et al (2010) Phylogeny of Photorhabdus and Xenorhabdus based on universally conserved protein-coding sequences and implications for the taxonomy of these two genera. Proposal of new taxa: X. vietnamensis sp. nov., P. luminescens subsp. caribbeanensis subsp. nov., P. luminescens subsp. hainanensis subsp. nov., P. temperata subsp. khanii subsp. nov., P. temperata subsp. tasmaniensis subsp. nov., and the reclassification of P. luminescens subsp. thracensis as P. temperata subsp. thracensis comb. nov. Int J Syst Evol Microbiol 60:1921–1937. https://doi.org/10.1099/ijs.0.014308-0
Tóth T, Lakatos T (2008) Photorhabdus temperata subsp. cinerea subsp. nov., isolated from Heterorhabditis nematodes. Int J Syst Evol Microbiol 58:2579–2581. https://doi.org/10.1099/ijs.0.2008/000273-0
Szállás E, Koch C, Fodor A et al (1997) Phylogenetic evidence for the taxonomic heterogeneity of Photorhabdus luminescens. Int J Syst Bacteriol 47:402–407. https://doi.org/10.1099/00207713-47-2-402
Orozco RA, Hill T, Stock SP (2013) Characterization and phylogenetic relationships of Photorhabdus luminescens subsp. sonorensis (γ-Proteobacteria: Enterobacteriaceae), the bacterial symbiont of the entomopathogenic nematode Heterorhabditis sonorensis (Nematoda: Heterorhabditidae). Curr Microbiol 66:30–39. https://doi.org/10.1007/s00284-012-0220-6
An R, Grewal PS (2010) Photorhabdus temperata subsp. stackebrandtii subsp. nov. (Enterobacteriales: Enterobacteriaceae). Curr Microbiol 61:291–297. https://doi.org/10.1007/s00284-010-9610-9
An R, Grewal PS (2011) Photorhabdus luminescens subsp. kleinii subsp. nov. (Enterobacteriales: Enterobacteriaceae). Curr Microbiol 62:539–543. https://doi.org/10.1007/s00284-010-9741-z
Machado RAR, Muller A, Ghazal SM et al (2021) Photorhabdus heterorhabditis subsp. aluminescens subsp. nov., Photorhabdus heterorhabditis subsp. heterorhabditis subsp. nov., Photorhabdus australis subsp. thailandensis subsp. nov., Photorhabdus australis subsp. australis subsp. nov., and Photorhabdus aegyptia sp. nov. isolated from Heterorhabditis entomopathogenic nematodes. Int J Syst Evol Microbiol 71:4610
Castaneda-Alvarez C, Machado RAR, Morales-Montero P et al (2022) Photorhabdus antumapuensis sp. nov., a novel symbiotic bacterial species associated with Heterorhabditis atacamensis entomopathogenic nematodes. Int J Syst Evol Microbiol 72:5525
Machado RAR, Bhat AH, Castaneda-Alvarez C et al (2023) Photorhabdus aballayi sp. nov. and Photorhabdus luminescens subsp. venezuelensis subsp. nov., isolated from Heterorhabditis amazonensis entomopathogenic nematodes. Int J Syst Evol Microbiol 73:5872
Parte AC, Carbasse JS, Meier-Kolthoff JP et al (2020) List of prokaryotic names with standing in nomenclature (LPSN) moves to the DSMZ. Int J Syst Evol Microbiol 70:5607
White GF (1927) A method for obtaining infective nematode larvae from cultures. Science 66:302–303
Bedding RA, Akhurst RJ (1975) A simple technique for the detection of insect parasitic rhabditid nematodes in soil. Nematologica 21:109–110
Joyce SA, Reid A, Driver F et al (1994) Application of polymerase chain reaction (PCR) methods to identification of entomopathogenic nematodes. In: Burnell AM, Ehlers RU, Masson JP, (eds) COST 812 Biotechnology: genetics of entomopathogenic nematode-bacterium complexes. Proceedings of Symposium & workshop, St. Patrick’s College, Maynooth, Co. Kildare, Ireland, Luxembourg, European Commission, DG XII, pp 178–187
Folmer O, Black M, Hoeh W et al (1994) DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotech 3:294–299
Machado RAR, Bhat AH, Abolafia J et al (2021) Multi-locus phylogenetic analyses uncover species boundaries and reveal the occurrence of two new entomopathogenic nematode species, Heterorhabditis ruandica n. sp. and Heterorhabditis zacatecana n. sp. J Nematol 53:1–42
Kimura M (1980) A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16:111–120. https://doi.org/10.1007/bf01731581
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874
Edgar RC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32:1792–1797. https://doi.org/10.1093/nar/gkh340
Chevenet F, Brun C, Bañuls A-L et al (2006) TreeDyn: towards dynamic graphics and annotations for analyses of trees. BMC Bioinform 7:439. https://doi.org/10.1186/1471-2105-7-439
Letunic I, Bork P (2016) Interactive tree of life (iTOL) v3: an online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Res 44:W242–W245. https://doi.org/10.1093/nar/gkw290
Marchesi JR, Sato T, Weightman AJ et al (1998) Design and evaluation of useful bacterium-specific PCR primers that amplify genes coding for bacterial 16S rRNA. Appl Environ Microbiol 64:795–799
Hill V, Kuhnert P, Erb M et al (2020) Identification of Photorhabdus symbionts by MALDI-TOF MS. Microbiology 166:522–530. https://doi.org/10.1099/mic.0.000905
Lane D (1991) 16S/23S rRNA sequencing, 1st edn. John Wiley and sons, New York (USA)
Hall T (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp 41:95–98
Seemann T (2013) Barrnap 0.7: rapid ribosomal RNA prediction. https://github.com/tseemann/barrnap
Hasegawa M, Kishino H, Yano T (1985) Dating of the human-ape splitting by a molecular clock of mitochondrial DNA. J Mol Evol 22:160–174
Nei M, Kumar S (2000) Molecular evolution and phylogenetics. Oxford University Press, Oxford
Machado RAR, Somvanshi VS, Muller A et al (2021) Photorhabdus hindustanensis sp. nov., Photorhabdus akhurstii subsp. akhurstii subsp. nov., and Photorhabdus akhurstii subsp. bharatensis subsp. nov., isolated from Heterorhabditis entomopathogenic nematodes. Int J Syst Evol Microbiol 71:4998
Petit RA, Read TD (2020) Bactopia: a flexible pipeline for complete analysis of bacterial genomes. Msystems. https://doi.org/10.1128/mSystems.00190-20
Bolger AM, Lohse M, Usadel B (2014) Trimmomatic: a flexible trimmer for illumina sequence data. Bioinformatics 30:2114–2120. https://doi.org/10.1093/bioinformatics/btu170
Bankevich A, Nurk S, Antipov D et al (2012) SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol 19:455–477. https://doi.org/10.1089/cmb.2012.0021
Walker BJ, Abeel T, Shea T et al (2014) Pilon: an integrated tool for comprehensive microbial variant detection and genome assembly improvement. PLoS ONE 9:e112963. https://doi.org/10.1371/journal.pone.0112963
Parks DH, Imelfort M, Skennerton CT et al (2015) CheckM: assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Res 25:1043–1055
Meier-Kolthoff JP, Auch AF, Klenk H-P et al (2013) Genome sequence-based species delimitation with confidence intervals and improved distance functions. BMC Bioinform 14:60. https://doi.org/10.1186/1471-2105-14-60
Meier-Kolthoff JP, Hahnke RL, Petersen J et al (2014) Complete genome sequence of DSM 30083(T), the type strain (U5/41(T)) of Escherichia coli, and a proposal for delineating subspecies in microbial taxonomy. Stand Genom Sci 9:2. https://doi.org/10.1186/1944-3277-9-2
Auch AF, von Jan M, Klenk H-P et al (2010) Digital DNA-DNA hybridization for microbial species delineation by means of genome-to-genome sequence comparison. Stand Genom Sci 2:117–134. https://doi.org/10.4056/sigs.531120
Auch AF, Klenk H-P, Göker M (2010) Standard operating procedure for calculating genome-to-genome distances based on high-scoring segment pairs. Stand Genom Sci 2:142–148. https://doi.org/10.4056/sigs.541628
Wayne LG, Brenner DJ, Colwell RR et al (1987) Report of the ad hoc committee on reconciliation of approaches to bacterial systematics. Int J Syst Bacteriol 37:463–464
Jain C, Rodriguez-R LM, Phillippy AM et al (2018) High throughput ANI analysis of 90K prokaryotic genomes reveals clear species boundaries. Nat Commun 9:1–8
Chen C-Y, Clark CG, Langner S et al (2020) Detection of antimicrobial resistance using proteomics and the comprehensive antibiotic resistance database: a case study. Proteom Clin Appl 14:e1800182. https://doi.org/10.1002/prca.201800182
Alcock BP, Raphenya AR, Lau TTY et al (2020) CARD 2020: antibiotic resistome surveillance with the comprehensive antibiotic resistance database. Nucleic Acids Res 48:D517–D525. https://doi.org/10.1093/nar/gkz935
Guitor AK, Raphenya AR, Klunk J et al (2019) Capturing the resistome: a targeted capture method to reveal antibiotic resistance determinants in metagenomes. Antimicrob Agents Chemother. https://doi.org/10.1128/AAC.01324-19
Tsang K, Speicher D, McArthur A (2019) Pathogen taxonomy updates at the comprehensive antibiotic resistance database: implications for molecular epidemiology
McArthur AG, Waglechner N, Nizam F et al (2013) The comprehensive antibiotic resistance database. Antimicrob Agents Chemother 57:3348–3357. https://doi.org/10.1128/AAC.00419-13
Jia B, Raphenya AR, Alcock B et al (2017) CARD 2017: expansion and model-centric curation of the comprehensive antibiotic resistance database. Nucleic Acids Res 45:D566–D573. https://doi.org/10.1093/nar/gkw1004
Blin K, Shaw S, Kloosterman AM et al (2021) antiSMASH 6.0: improving cluster detection and comparison capabilities. Nucleic Acids Res 49:W29–W35
Blin K, Shaw S, Steinke K et al (2019) antiSMASH 5.0: updates to the secondary metabolite genome mining pipeline. Nucleic Acids Res 47:W81–W87
Eckstein S, Heermann R (2019) Regulation of phenotypic switching and heterogeneity in Photorhabdus luminescens cell populations. J Mol Biol 431:4559–4568
Acknowledgements
We thank the Swiss National Science Foundation and the Institute of Biology of the University of Neuchâtel (Switzerland) for their support and Luke Cousins (Citrus Research International, CRI, South Africa) for collecting the soil samples.
Funding
Open access funding provided by University of Neuchâtel. The work of RARM is supported by the Swiss National Science Foundation (Grant 186094 to RARM). The work of APM is supported by the Citrus Research International (CRI) South Africa. The work of RARM and AB is supported by the Gebert Rüf Foundation (Grant GRS-079/19).
Author information
Authors and Affiliations
Contributions
Conceptualization, Ricardo Machado; Data curation, Ricardo Machado; Formal analysis, Ricardo Machado; Funding acquisition, Ricardo Machado and Antoinette P. Malan; Investigation, Ricardo Machado, Anja Boss, Antoinette P. Malan, Nicholle J. Claasen, Aashaq Hussain Bhat, Joaquin Abolafia; Methodology, Ricardo Machado, Antoinette P. Malan; Project administration, Ricardo Machado and Antoinette P. Malan; Resources, Ricardo Machado and Antoinette P. Malan; Supervision, Ricardo Machado and Antoinette P. Malan; Validation, Ricardo Machado; Visualization, Ricardo Machado; Writing — original draft, Ricardo Machado; Writing — review & editing, Ricardo Machado, Antoinette P. Malan, Aashaq Hussain Bhat.
Corresponding author
Ethics declarations
Conflicts of Interest
The authors declare no conflict of interest and that no humans or animals were used for experimental purposes.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Machado, R.A.R., Malan, A.P., Boss, A. et al. Photorhabdus africana sp. nov. isolated from Heterorhabditis entomopathogenic nematodes. Curr Microbiol 81, 240 (2024). https://doi.org/10.1007/s00284-024-03744-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00284-024-03744-3