Abstract
Purpose
A series of new 68Ga-labeled tracers based on [68Ga]Ga-PSMA-617 were developed to augment the tumor-to-kidney ratio and reduce the activity accumulation in bladder, ultimately minimize radiation toxicity to the urinary system.
Methods
We introduced quinoline group, phenylalanine and decanoic acid into different tracers to enhance their lipophilicity, strategically limiting their metabolic pathway through the urinary system. Their binding affinity onto LNCaP cells was determined through in vitro saturation assays and competition binding assays. In vivo metabolic study, PET imaging and biodistribution experiment were performed in LNCaP tumor-bearing B-NSG male mice. The most promising tracer was selected for first-in-human study.
Results
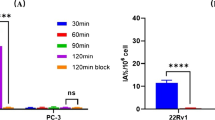
Four radiotracers were synthesized with radiochemical purity (RCP) > 95% and molar activity in a range of 20.0-25.5 GBq/μmol. The binding affinities (Ki) of TWS01, TWS02 to PSMA were in the low nanomolar range (< 10 nM), while TWS03 and TWS04 exhibited binding affinities with Ki > 20 nM (59.42 nM for TWS03 and 37.14 nM for TWS04). All radiotracers exhibited high stability in vivo except [68Ga]Ga-TWS03. Micro PET/CT imaging and biodistribution analysis revealed that [68Ga]Ga-TWS02 enabled clear tumor visualization in PET images at 1.5 h post-injection, with higher tumor-to-kidney ratio (T/K, 0.93) and tumor-to-muscle ratio (T/M, 107.62) compared with [68Ga]Ga-PSMA-617 (T/K: 0.39, T/M: 15.01) and [68Ga]Ga-PSMA-11 (T/K: 0.15, T/M: 24.00). In first-in-human study, [68Ga]Ga-TWS02 effectively detected PCa-associated lesions including primary and metastatic lesions, with lower accumulation in urinary system, suggesting that [68Ga]Ga-TWS02 might be applied in the detection of bladder invasion, with minimized radiation toxicity to the urinary system.
Conclusion
Introduction of quinoline group, phenylalanine and decanoic acid into different tracers can modulate the binding affinity and pharmacokinetics of PSMA in vivo. [68Ga]Ga-TWS02 showed high binding affinity to PSMA, excellent pharmacokinetic properties and clear imaging of PCa-associated lesions, making it a promising radiotracer for the clinical diagnosis of PCa. Moreover, TWS02 with a chelator DOTA could also label 177Lu and 225Ac, which could be used for PCa treatment without significant side effects.
Trial registration
The clinical evaluation of this study was registered On October 30, 2021 at https://www.chictr.org.cn/ (No: ChiCTR2100052545).





Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Bergengren O, Pekala KR, Matsoukas K, Fainberg J, Mungovan SF, Bratt O, et al. 2022 update on prostate cancer epidemiology and risk factors-a systematic review. Eur Urol. 2023;84:191–206. https://doi.org/10.1016/j.eururo.2023.04.021.
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA: a cancer journal for clinicians. 2021;71:7–33. https://doi.org/10.3322/caac.21654.
Sekhoacha M, Riet K, Motloung P, Gumenku L, Adegoke A, Mashele S. Prostate cancer review: genetics, diagnosis, treatment options, and alternative approaches. Molecules. 2022;27. https://doi.org/10.3390/molecules27175730.
Rebello RJ, Oing C, Knudsen KE, Loeb S, Johnson DC, Reiter RE, et al. Prostate cancer. Nat Rev Dis Primers. 2021;7:9.https://doi.org/10.1038/s41572-020-00243-0
Daryanani A, Turkbey B. Recent advancements in CT and MR imaging of prostate cancer. Semin Nucl Med. 2022;52:365–73. https://doi.org/10.1053/j.semnuclmed.2021.11.013.
Fischer BM, Siegel BA, Weber WA, von Bremen K, Beyer T, Kalemis A. PET/CT is a cost-effective tool against cancer: synergy supersedes singularity. Eur J Nucl Med Mol Imaging. 2016;43:1749–52. https://doi.org/10.1007/s00259-016-3414-5.
Catana C, Guimaraes AR, Rosen BR. PET and MR imaging: the odd couple or a match made in heaven? J Nucl Med. 2013;54:815–24. https://doi.org/10.2967/jnumed.112.112771.
Basu S. The scope and potentials of functional radionuclide imaging towards advancing personalized medicine in oncology: emphasis on PET-CT. Discov Med. 2012;13:65–73.
Cermik TF, Ergul N, Yilmaz B, Mercanoglu G. Tumor imaging with 68Ga-DOTA-FAPI-04 PET/CT: comparison with 18F-FDG PET/CT in 22 different cancer types. Clin Nucl Med. 2022;47:e333–9. https://doi.org/10.1097/RLU.0000000000004073.
Cornford P, van den Bergh RCN, Briers E, Van den Broeck T, Cumberbatch MG, De Santis M, et al. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer. Part II-2020 update: treatment of relapsing and metastatic prostate cancer. Eur Urol. 2021;79:263–82. https://doi.org/10.1016/j.eururo.2020.09.046.
Carter RE, Feldman AR, Coyle JT. Prostate-specific membrane antigen is a hydrolase with substrate and pharmacologic characteristics of a neuropeptidase. Proc Natl Acad Sci USA. 1996;93:749–53. https://doi.org/10.1073/pnas.93.2.749.
Farolfi A, Calderoni L, Mattana F, Mei R, Telo S, Fanti S, et al. Current and emerging clinical applications of PSMA PET diagnostic imaging for prostate cancer. J Nucl Med. 2021;62:596–604. https://doi.org/10.2967/jnumed.120.257238.
Wester HJ, Schottelius M. PSMA-targeted radiopharmaceuticals for imaging and therapy. Semin Nucl Med. 2019;49:302–12. https://doi.org/10.1053/j.semnuclmed.2019.02.008.
Souvatzoglou M, Eiber M, Martinez-Moeller A, Fürst S, Holzapfel K, Maurer T, et al. PET/MR in prostate cancer: technical aspects and potential diagnostic value. Eur J Nucl Med Mol Imaging. 2013;40(Suppl 1):S79-88. https://doi.org/10.1007/s00259-013-2445-4.
Krohn T, Verburg FA, Pufe T, Neuhuber W, Vogg A, Heinzel A, et al. [(68)Ga]PSMA-HBED uptake mimicking lymph node metastasis in coeliac ganglia: an important pitfall in clinical practice. Eur J Nucl Med Mol Imaging. 2015;42:210–4. https://doi.org/10.1007/s00259-014-2915-3.
Iravani A, Hofman MS, Mulcahy T, Williams S, Murphy D, Parameswaran BK, et al. (68)Ga PSMA-11 PET with CT urography protocol in the initial staging and biochemical relapse of prostate cancer. Cancer Imaging. 2017;17:31. https://doi.org/10.1186/s40644-017-0133-5.
Hofman MS, Lawrentschuk N, Francis RJ, Tang C, Vela I, Thomas P, et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): a prospective, randomised, multicentre study. Lancet. 2020;395:1208–16. https://doi.org/10.1016/s0140-6736(20)30314-7.
Afshar-Oromieh A, Babich JW, Kratochwil C, Giesel FL, Eisenhut M, Kopka K, et al. The rise of PSMA ligands for diagnosis and therapy of prostate cancer. J Nucl Med. 2016;57:79s–89s. https://doi.org/10.2967/jnumed.115.170720.
Lütje S, Heskamp S, Cornelissen AS, Poeppel TD, van den Broek SA, Rosenbaum-Krumme S, et al. PSMA ligands for radionuclide imaging and therapy of prostate cancer: clinical status. Theranostics. 2015;5:1388–401. https://doi.org/10.7150/thno.13348.
Eder M, Schäfer M, Bauder-Wüst U, Hull WE, Wängler C, Mier W, et al. 68Ga-complex lipophilicity and the targeting property of a urea-based PSMA inhibitor for PET imaging. Bioconjug Chem. 2012;23:688–97. https://doi.org/10.1021/bc200279b.
Sachpekidis C, Afshar-Oromieh A, Kopka K, Strauss DS, Pan L, Haberkorn U, et al. (18)F-PSMA-1007 multiparametric, dynamic PET/CT in biochemical relapse and progression of prostate cancer. Eur J Nucl Med Mol Imaging. 2020;47:592–602. https://doi.org/10.1007/s00259-019-04569-0.
Hong JJ, Liu BL, Wang ZQ, Tang K, Ji XW, Yin WW, et al. The value of (18)F-PSMA-1007 PET/CT in identifying non-metastatic high-risk prostate cancer. EJNMMI Res. 2020;10:138. https://doi.org/10.1186/s13550-020-00730-1.
Mease RC, Kang CM, Kumar V, Banerjee SR, Minn I, Brummet M, et al. An improved (211)At-labeled agent for PSMA-targeted alpha-therapy. J Nucl Med. 2022;63:259–67. https://doi.org/10.2967/jnumed.121.262098.
Benesova M, Umbricht CA, Schibli R, Muller C. Albumin-binding PSMA ligands: optimization of the tissue distribution profile. Mol Pharm. 2018;15:934–46. https://doi.org/10.1021/acs.molpharmaceut.7b00877.
Potemkin R, Strauch B, Kuwert T, Prante O, Maschauer S. Development of (18)F-Fluoroglycosylated PSMA-ligands with improved renal clearance behavior. Mol Pharm. 2020;17:933–43. https://doi.org/10.1021/acs.molpharmaceut.9b01179.
Siebinga H, Hendrikx J, Huitema ADR, de Wit-van der Veen BJ. Predicting the effect of different folate doses on [(68)Ga]Ga-PSMA-11 organ and tumor uptake using physiologically based pharmacokinetic modeling. EJNMMI Res. 2023;13:60. https://doi.org/10.1186/s13550-023-01008-y
Benešová M, Bauder-Wüst U, Schäfer M, Klika KD, Mier W, Haberkorn U, et al. Linker modification strategies to control the Prostate-Specific Membrane Antigen (PSMA)-targeting and pharmacokinetic properties of DOTA-conjugated PSMA inhibitors. J Med Chem. 2016;59:1761–75. https://doi.org/10.1021/acs.jmedchem.5b01210.
Kopka K, Benešová M, Bařinka C, Haberkorn U, Babich J. Glu-ureido-based inhibitors of prostate-specific membrane antigen: lessons learned during the development of a novel class of low-molecular-weight theranostic radiotracers. J Nucl Med. 2017;58:17s–26s. https://doi.org/10.2967/jnumed.116.186775.
Zhang X, Wu Y, Zeng Q, Xie T, Yao S, Zhang J, et al. Synthesis, preclinical evaluation, and first-in-human PET study of quinoline-containing PSMA tracers with decreased renal excretion. J Med Chem. 2021;64:4179–95. https://doi.org/10.1021/acs.jmedchem.1c00117.
Hennrich U, Eder M. [(68)Ga]Ga-PSMA-11: The first FDA-approved (68)Ga-Radiopharmaceutical for PET imaging of prostate cancer. Pharmaceuticals (Basel). 2021;14. https://doi.org/10.3390/ph14080713.
Chen Y, Zhang X, Ni M, Gao X, Wang X, Xie Q, et al. Synthesis, preclinical evaluation, and first-in-human pet study of [(68)Ga]-labeled biphenyl-containing PSMA tracers. J Med Chem. 2023;66:13332–45. https://doi.org/10.1021/acs.jmedchem.3c01475.
Gao F, Sihver W, Jurischka C, Bergmann R, Haase-Kohn C, Mosch B, et al. Radiopharmacological characterization of (6)(4)Cu-labeled alpha-MSH analogs for potential use in imaging of malignant melanoma. Amino Acids. 2016;48:833–47. https://doi.org/10.1007/s00726-015-2131-x.
Gao F, Sihver W, Bergmann R, Belter B, Bolzati C, Salvarese N, et al. Synthesis, characterization, and initial biological evaluation of [(99m) Tc]Tc-Tricarbonyl-labeled DPA-alpha-MSH peptide derivatives for potential melanoma imaging. ChemMedChem. 2018;13:1146–58. https://doi.org/10.1002/cmdc.201800110.
Yang H, Zhang C, Yuan Z, Rodriguez-Rodriguez C, Robertson A, Radchenko V, et al. Synthesis and evaluation of a macrocyclic actinium-225 chelator, quality control and in vivo evaluation of (225) Ac-crown-alphaMSH peptide. Chemistry. 2020;26:11435–40. https://doi.org/10.1002/chem.202002999.
Benesova M, Schafer M, Bauder-Wust U, Afshar-Oromieh A, Kratochwil C, Mier W, et al. Preclinical evaluation of a tailor-made DOTA-conjugated PSMA inhibitor with optimized linker moiety for imaging and endoradiotherapy of prostate cancer. J Nucl Med. 2015;56:914–20. https://doi.org/10.2967/jnumed.114.147413.
Ceci F, Rovera G, Iorio GC, Guarneri A, Chiofalo V, Passera R, et al. Event-free survival after (68) Ga-PSMA-11 PET/CT in recurrent Hormone-Sensitive Prostate Cancer (HSPC) patients eligible for salvage therapy. Eur J Nucl Med Mol Imaging. 2022;49:3257–68. https://doi.org/10.1007/s00259-022-05741-9.
Aboagye EO, Barwick TD, Haberkorn U. Radiotheranostics in oncology: Making precision medicine possible. CA: a cancer journal for clinicians. 2023;73:255–74. https://doi.org/10.3322/caac.21768.
Lisney AR, Leitsmann C, Strauss A, Meller B, Bucerius JA, Sahlmann CO. The role of PSMA PET/CT in the primary diagnosis and follow-up of prostate cancer-a practical clinical review. Cancers. 2022;14. https://doi.org/10.3390/cancers14153638.
Giesel FL, Hadaschik B, Cardinale J, Radtke J, Vinsensia M, Lehnert W, et al. F-18 labelled PSMA-1007: biodistribution, radiation dosimetry and histopathological validation of tumor lesions in prostate cancer patients. Eur J Nucl Med Mol Imaging. 2017;44:678–88. https://doi.org/10.1007/s00259-016-3573-4.
Piron S, Verhoeven J, Vanhove C, De Vos F. Recent advancements in (18)F-labeled PSMA targeting PET radiopharmaceuticals. Nucl Med Biol. 2022;106–107:29–51. https://doi.org/10.1016/j.nucmedbio.2021.12.005.
Georgakopoulos A, Bamias A, Chatziioannou S. Current role of PSMA-PET imaging in the clinical management of prostate cancer. Ther Adv Med Oncol. 2023;15:17588359231208960. https://doi.org/10.1177/17588359231208960.
Sheikhbahaei S, Afshar-Oromieh A, Eiber M, Solnes LB, Javadi MS, Ross AE, et al. Pearls and pitfalls in clinical interpretation of prostate-specific membrane antigen (PSMA)-targeted PET imaging. Eur J Nucl Med Mol Imaging. 2017;44:2117–36. https://doi.org/10.1007/s00259-017-3780-7.
Hofman MS, Hicks RJ, Maurer T, Eiber M. Prostate-specific membrane antigen PET: clinical utility in prostate cancer, normal patterns, pearls, and pitfalls. Radiographics. 2018;38:200–17. https://doi.org/10.1148/rg.2018170108.
Kuo HT, Pan J, Zhang Z, Lau J, Merkens H, Zhang C, et al. Effects of linker modification on tumor-to-kidney contrast of (68)Ga-labeled PSMA-targeted imaging probes. Mol Pharm. 2018;15:3502–11. https://doi.org/10.1021/acs.molpharmaceut.8b00499.
Benesova M, Bauder-Wust U, Schafer M, Klika KD, Mier W, Haberkorn U, et al. Linker modification strategies to control the Prostate-Specific Membrane Antigen (PSMA)-targeting and pharmacokinetic properties of DOTA-conjugated PSMA inhibitors. J Med Chem. 2016;59:1761–75. https://doi.org/10.1021/acs.jmedchem.5b01210.
Acknowledgements
We would like to thank Translational Medicine Core Facility of Shandong University for consultation and instrument availability that supported this work. We would like to thank the School of Basic Medical Science Core Facility, Shandong University for technician support. We would like to thank the School of Pharmaceutical sciences, Shandong University for technician support.
Funding
This study was financially supported by the National Natural Science Foundation of China (22376125), and the Natural Science Foundation of Shandong Province (ZR2019BA015, ZR2023MH004).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Haodong Hou, Yanzhi Wang, Yuze Ma, Xiaobing Niu, and Suan Sun. The first draft of the manuscript was written by Haodong Hou, Yuan Pan, Guihua Hou, Weijing Tao, and Feng Gao. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval
Animal experiments were conducted following the regulations approved by the Animal Ethics Committee of Shandong University (ECSBMSSDU2021-2–67). Human studies were approved by the Ethics Committee of Huai’an First People’s Hospital (Approval No.: YX-2020–168-01).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent to publish
The authors affirm that human research participants provided informed consent for publication of the images in Fig. 5a and b.
Competing interests
All authors declare they have no relevant financial or non-financial interests that are directly or indirectly related to the work submitted for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hou, H., Pan, Y., Wang, Y. et al. Development and first-in-human study of PSMA-targeted PET tracers with improved pharmacokinetic properties. Eur J Nucl Med Mol Imaging (2024). https://doi.org/10.1007/s00259-024-06726-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00259-024-06726-6