Abstract
Spent coffee ground (SCG) oil is an ideal substrate for the biosynthesis of polyhydroxyalkanoates (PHAs) by Cupriavidus necator. The immiscibility of lipids with water limits their bioavailability, but this can be resolved by saponifying the oil with potassium hydroxide to form water-soluble fatty acid potassium salts and glycerol. Total saponification was achieved with 0.5 mol/L of KOH at 50 °C for 90 min. The relationship between the initial carbon substrate concentration (C0) and the specific growth rate (µ) of C. necator DSM 545 was evaluated in shake flask cultivations; crude and saponified SCG oils were supplied at matching initial carbon concentrations (C0 = 2.9–23.0 g/L). The Han-Levenspiel model provided the closest fit to the experimental data and accurately described complete growth inhibition at 32.9 g/L (C0 = 19.1 g/L) saponified SCG oil. Peak µ-values of 0.139 h−1 and 0.145 h−1 were obtained with 11.99 g/L crude and 17.40 g/L saponified SCG oil, respectively. Further improvement to biomass production was achieved by mixing the crude and saponified substrates together in a carbon ratio of 75:25% (w/w), respectively. In bioreactors, C. necator initially grew faster on the mixed substrates (µ = 0.35 h−1) than on the crude SCG oil (µ = 0.23 h−1). After harvesting, cells grown on crude SCG oil obtained a total biomass concentration of 7.8 g/L and contained 77.8% (w/w) PHA, whereas cells grown on the mixed substrates produced 8.5 g/L of total biomass and accumulated 84.4% (w/w) of PHA.
Key points
• The bioavailability of plant oil substrates can be improved via saponification.
• Cell growth and inhibition were accurately described by the Han-Levenpsiel model.
• Mixing crude and saponified oils enable variation of free fatty acid content.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the 2018/2019 crop year, around 20.26 million tonnes of green coffee were produced globally (International Coffee Organisation 2020). However, approximately 90% (dry w/w) of the whole coffee fruit is discarded as solid waste in the form of pulp, husk, silver skin, and spent coffee grounds (SCGs) (Murthy and Naidu 2012; Salazar-López et al. 2020). Coffee is predominantly either used to produce soluble (instant) coffee or directly brewed (boiled, filtered or high-pressured) into the coffee beverage (Mussatto et al. 2011; Gómez-de la Cruz et al. 2015). These processes generate vast volumes of SCGs as a by-product, which are of fine particle size, high humidity (50–85%, w/w), and organic load. Considering that around 650 kg of wet SCG are obtained from every tonne of green coffee (Murthy and Naidu 2012), approximately 13.2 million tonnes of wet SCG were produced from the coffee grown in the 2018/2019 crop year. Despite being of rich chemical composition with minimal contamination, the vast majority of SCG are discarded into landfills, incinerated, or used as animal feed (Machado et al. 2012).
SCGs consist predominantly of lignocellulose—a covalently bonded network of lignin (25–33%, w/w) and hemicellulose (30–40%) polymers surrounding a crystalline cellulose core (8.6–13.3%), as well as lipids (10–20%), proteins (6.7–13.6%), and phenolic compounds (2.5%) (Obruca et al. 2015). Owing to this, as well as their high availability and low cost, the utilization of SCGs has garnered increasing interest in recent years. SCGs could potentially be used in a wide range of applications, including directly as a fuel, as an adsorption material, and in the production of biodiesel, bioethanol, and other higher-value products, including polyhydroxyalkanoates (PHAs) (Karmee 2018; Kovalcik et al. 2018; McNutt and He 2019). The concept of an SCG-based biorefinery, in which feedstocks undergo a series of integrated processes to convert them into value-added products, has gathered significant attention in recent years (Aristizábal-Marulanda et al. 2017; Caetano et al. 2017; Kourmentza et al. 2018; Girotto et al. 2018; Mata et al. 2018; Karmee 2018; Kovalcik et al. 2018; Zabaniotou and Kamaterou 2019; Atabani et al. 2019; Massaya et al. 2019; Rajesh Banu et al. 2020; Battista et al. 2020).
PHAs are biodegradable, bio-based, and biocompatible polymers with similar physical properties to certain petroleum-derived plastics, such as polypropylene. PHAs are synthesized and accumulated by several bacteria and archaea as discrete granules in the cell cytoplasm. Organisms primarily use them as a form of carbon and energy storage (Bugnicourt et al. 2014), but their inclusion also protects the cells from environmental stress conditions, such as high temperatures, freezing, osmotic shock, UV irradiation, and desiccation (Obruca et al. 2018). The wide-scale use of PHAs is currently prevented by their relatively high production costs and poor material processing properties (Surendran et al. 2020; Ingram and Winterburn 2021). The issue of cost can be helped by utilizing cheap carbon substrates, such as waste plant oils. Recently, oil extracted from SCGs has been demonstrated to be a suitable feedstock for the microbial production of PHAs by Cupriavidus necator (Cruz et al. 2014; Obruca et al. 2014; Bhatia et al. 2018; Ingram and Winterburn 2021, 2022). However, adding a plant oil to an aqueous growth medium forms a heterogeneous mixture, in which the low-density oil floats on the surface (Budde et al. 2011). This phenomenon creates a bottleneck in the process by limiting substrate availability and product recovery. Also, the quantification of the various metabolites must be performed offline, involving the use of hazardous organic solvents in multiple time-consuming stages. Furthermore, plant oil substrates often lead to varying lag times and difficulties in taking early representative samples (Budde et al. 2011; Cruz 2015).
Plant oils and animal fats predominantly consist of acylglycerides, which are esters of 1 to 3 fatty acids attached to a glycerol backbone. The acylglycerides can be saponified via reactions with sodium or potassium hydroxide to form fatty acid salts and glycerol. Unlike acylglycerides, the products of saponification are relatively water-soluble (Rustan and Drevon 2005; Ferre-Guell and Winterburn 2019), and can be rapidly absorbed to be metabolized by microorganisms, such as C. necator, without the need for enzymatic pretreatment (Obruca et al. 2014). In this context, saponification has often been used as a pretreatment method for fermentations involving Pseudomonads, as some species lack the lipases necessary for enzymatic hydrolysis (Tan et al. 1997; Allen et al. 2010; Mozejko and Ciesielski 2013; Bustamante et al. 2019; Boonyawanich et al. 2021). The content of free fatty acids (FFAs) in plant oil substrates has been demonstrated to strongly influence the production of P(3HB) by C. necator (Obruca et al. 2014). FFAs are natural surfactants that emulsify acylglycerides, which renders them more susceptible to the action of lipases through the increased contact area, solubility, and bioavailability (Budde et al. 2011; Lu et al. 2013; Ferre-Guell and Winterburn 2019). Hence, saponification could be used as a potential means to increase cell growth rates and PHA productivity of C. necator by increasing the FFA content of plant oil substrates.
High substrate concentrations can inhibit the cell growth rate due to the influence of ionic strength, osmotic pressure, or overloading of the membrane transport systems (Blanch and Clark 1997). In addition, surfactants can act as antimicrobial agents through lysis and solubilization of the cellular membrane (i.e., lipid bilayer) (Nazari et al. 2012; Ferre-Guell and Winterburn 2019). Therefore, this study assesses the effects of saponification of the crude SCG oil on the ability of C. necator DSM 545 to use the substrate for biomass and PHA production. An evaluation of the effect of the initial carbon concentration (C0) on the organism’s specific growth rate, μ, was also carried out in shake flasks to optimize the product yields. These results were then compared to several widely published kinetic growth models, extended or evolved from the Monod model. Further cultivations were performed, in which crude and saponified SCG oils were mixed in a range of different carbon ratios to evaluate if the saponified oil could act as a consumable surfactant. Finally, fermentations were carried out in 3-L bioreactors operated in batch mode, and the overall performances were assessed and compared with similar cultivations reported in the literature.
Materials and methods
Materials and microorganism
SCG oil, extracted with subcritical water, was purchased from Merck (product W530639, Darmstadt, Germany). All the other chemicals and reagents used in this study were of analytical grade and obtained from either Merck (Darmstadt, Germany) or Thermo Fisher Scientific (Loughborough, UK). C. necator DSM 545 (H1G+3) was obtained from the Leibniz Institute DSMZ (Braunschweig, Germany) in freeze-dried form and reactivated as previously described (Ingram and Winterburn 2021).
Saponification of SCG oil
Each gram of “crude” SCG oil was saponified with 10 mL of ethanolic KOH solution in 50 mL glass Duran™ bottles in a temperature-controlled incubator. A range of conditions was evaluated to optimize the process, including the concentration of KOH (0.50–1.00 mol/L), temperature (50–70 °C), and reaction time (30–90 min). Each sample in the optimization study used 2 g of SCG oil dissolved in 20 mL of ethanolic KOH solution. The resulting solutions contained a mixture of potassium salts of fatty acids, glycerol, and unsaponified substances, all dissolved in ethanol. The saponification value of each sample (SVsample) was directly measured and compared with that of the original oil sample (SVoil) to determine the extent of saponification, as per Eq. (1).
SVoil was previously determined to be 175.7 ± 2.3 mgKOH goil−1 (Ingram and Winterburn 2022). SVsample values were measured according to ISO 3657:2013, as previously described (ISO 2013; Ingram and Winterburn 2021).
Analysis of oil samples
The crude SCG oil was previously analyzed for its composition (%, w/w) of organic elements (C, H, N, S, and O) and fatty acids (Ingram and Winterburn 2022). The saponified oil samples were analyzed using the same methods as previously described (Ingram and Winterburn 2021). Briefly, a Flash 2000 Organic Elemental Analyser (Thermo Fisher Scientific, UK) was used to determine the composition of C, H, N, and S. The quantitative analysis of K in the samples was performed via inductively couple plasma-optical emission spectroscopy (ICP-AES) using an iCAP 6300 Duo ICP spectrometer (Thermo Fisher Scientific, UK). The remaining fraction of the organic elements was assumed to be oxygen. Fatty acids were quantified with gas chromatography equipped with a flame ionization detector (GC-FID) after their conversion to fatty acid methyl esters via methanolysis.
Fermentation media
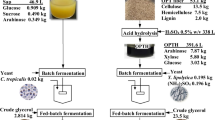
C. necator DSM 545 was cultivated in defined mineral media (modified DSMZ medium 81) at 30 °C and pH 6.8–7.0 for a duration of 24–72 h. Crude and saponified SCG oils were supplied as the sole carbon sources at concentrations later described. NH4Cl was added as the sole nitrogen source at an initial concentration of 2.17 g/L. Other media components were present in the following concentrations (g/L): KH2PO4 (2.32), Na2HPO4⋅2H2O (2.93), MgSO4⋅7H2O (0.51), CaCl2⋅2H2O (0.010), MnCl2⋅4H2O (0.005), ferric ammonium citrate (0.050), and 5 mL trace element solution. The composition of the trace element solution (g/L) was as follows: ZnSO4⋅7H2O (0.10), MnCl2⋅4H2O (0.03), H3BO3 (0.30), CoCl2⋅6H2O (0.20), CuCl2⋅2H2O (0.01), NiCl2⋅6H2O (0.02), Na2MoO4⋅2H2O (0.03). The crude and saponified oils, phosphate solutions, and trace element solutions were prepared and autoclaved separately (121 °C, 25 min) and then reconstituted aseptically at room temperature. A concentrated stock saponified oil solution was prepared using the previously determined conditions (0.50 molKOH/L, 50 °C, 90 min), subsequently dried to constant weight in a vacuum oven at 80 °C to remove the excess ethanol, and then dissolved in distilled water. 1 mol/L solutions of KOH and HCl were used to adjust the pH of the separate media solutions to 6.8–7.0 before autoclaving. Each fermentation was inoculated with 10% (v/v) of a preculture solution that was developed in shake flasks (30 °C and 200 rpm) containing rich media (DSMZ medium 1) over a 2-stage process, with each stage lasting 24 h (Ingram and Winterburn 2021) (Table 1).
Shake flask cultivations
Fermentations were initially carried out in 250-mL shake flasks containing 50 mL of DSMZ medium 81 to assess if saponification influences the ability of C. necator DSM 545 to consume the SCG oil in the first 24-h cultivation period. SCG oil was supplied as the sole carbon source at a range of initial concentrations of 3.72–30.00 g/L. For comparison, the conditions were repeated using saponified SCG oil (prepared with the optimized conditions), as the sole carbon source, at matching carbon concentrations of the crude SCG oil cultivations (Table 2). Hence, the initial C/N ratio (C/N0) ranged from 5.0 to 40.2 (g/g). Cultivation conditions were performed in duplicate. Triplicate samples were taken at the start (t = 0 h) and end (t = 24 h) of the cultivation period to determine the changes in the total biomass concentration, as measured by optical density at 600 nm, OD600.
Saponifying the SCG oil causes the potassium concentration in the aqueous media to increase to levels that potentially inhibit cell growth. Therefore, additional fermentations were carried out in shake flasks containing DSMZ medium 81, supplemented with 5 g/L of glucose and different concentrations of KCl ([K] = 0–20 g/L).
Estimation of kinetic growth model parameters
Nine Monod-based kinetic growth models (Table 1) were fitted to the shake flask cultivation data to evaluate the relationship between μ and C0. The parameters, function, and application of these models have been previously described in detail (Panikov 2010; Dutta et al. 2015; Muloiwa et al. 2020). Values of μ for the shake flask cultivations were calculated using Eq. (2).
The parameters of each model were estimated using non-linear regression analysis performed with a bespoke Python (ver. 3.8.0) program applying SciPy “curve_fit” (ver. 1.5.2), as seen at https://github.com/ristojm/Biofit. The coefficient of determination (R2) and the root mean squared error (RMSE) are both error functions used to determine the robustness of a model. R2 measures the degree of linear correlation between the observed and predicted values. In general, a value of R2 closer to 1 indicates a more valid model. Residuals are the difference between the observed and predicted values, and they describe the concentration of the data around the regression line. Furthermore, RMSE is the standard deviation of the residuals and serves to combine the scale of the errors in predictions into a single measure of predictive power. Values of RSME closer to 0 indicate a closer fit to the data. Values of R2 and RMSE for each model were determined using Eqs. (3) and (4), respectively.
where i and n are the index and upper limit of summation, respectively; and y, ŷ, and ȳ are observed, predicted, and mean of the observed values of µ, respectively.
Bioreactor cultivations
Bioreactor cultivations were performed as previously described (Ingram and Winterburn 2021) based on the optimal conditions surmised from the shake flask cultivation and accompanying analysis of the growth kinetics. Briefly, 3-L bioreactors (Applikon Biotechnology, UK) were operated in batch mode with a 2.2-L working volume (200 mL of the 2nd-stage preculture inoculated into 2 L of fresh mineral salt media). In the first batch experiment, crude SCG oil was initially supplied as the sole carbon source at 12.47 g/L (C0 = 9.57 g/L). Another batch cultivation was carried out in which a mixture of crude and saponified SCG oils was supplied at the same C0, but with a C-ratio between the crude and saponified oils of 75:25% (w/w). Air was supplied at 1.0 vvm, with dissolved oxygen (DO) maintained at 30% saturation level through variation of the rate of agitation (600–800 rpm). Process temperature was maintained at 30 ± 0.2 °C. pH was controlled 7.0 ± 0.1 via pulse additions of 3 mol/L solutions of HCl/NaOH. Propylene glycol, fed into the bioreactor via a pump connected to a level sensor, was used to suppress any foaming.
Analytical methods
Bioreactor samples taken for the determination of optical density at 600 nm (OD600), total biomass (g/L), and PHA (g/L) were prepared and analyzed as previously described (Ingram and Winterburn 2021). The cells were washed with n-hexane to remove any residual oils before analysis. Residual biomass was defined as the difference between the total biomass and PHA concentrations. For total nitrogen (TN), samples were initially prepared by taking 0.1 mL aliquots from the cell-free supernatants of the OD600 samples, diluted to a final volume of 20 ml with HPLC grade-water, and filtered through 0.45-μm pore nylon syringe filters into sample vials (Fisher Scientific, UK). Analysis was performed using a TOC-VCPH Total Organic Carbon analyzer, coupled with a TNM-1 TN analyzer unit (Shimadzu, UK), as previously described (Urbina et al. 2018; Wongsirichot 2020). Standards for the TN were made from NH4Cl.
Calculations
PHA accumulation, biomass, and PHA yield coefficients (with respect to initial oil, carbon and nitrogen concentrations) and volumetric productivities for each fermentation condition were all calculated as previously described (Ingram and Winterburn 2021).
Results
Saponification of SCG oil
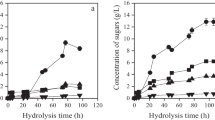
The saponification of SCG oil was carried out at different KOH concentrations (0.5–1.0 mol/L) and temperatures (50–70 °C) for varying lengths of time (30–90 min) to find the mildest conditions able to liberate the constituent fatty acids completely, as defined by the extent of saponification (%). The results of this process are summarized in Fig. 1. The mildest evaluated conditions (0.50 molKOH/L, 50 °C, 30 min) achieved the lowest extent of saponification, at 53.4 ± 2.6%. Increasing any of the three variables increased the overall conversion. At 90 min, conversions of ≥ 98% were achieved with all evaluated combinations of temperature and KOH concentration, except for the mildest condition (0.50 molKOH/L, 50 °C), which reached 81.7 ± 1.4%. Using 1.00 molKOH/L resulted in complete saponification, regardless of which temperature or time was used. Hence, the conditions selected to saponify the oil in subsequent fermentation experiments were 0.50 molKOH/L, 50 °C, and 90 min.
Analysis of crude and saponified SCG oil
The crude SGC oil was previously analyzed in terms of its elemental and fatty acid compositions and acid and saponification values (Ingram and Winterburn 2022). The same analyses were performed on the oil samples saponified using the previously established conditions (0.50 molKOH/L, 50 °C, 90 min). The results of these analyses are displayed in Table 2.
Effect of potassium on cell growth
Potassium-induced inhibition of C. necator DSM 545 was evaluated by cultivating the cells in shake flasks supplemented with different concentrations of KCl (0–38.1 g/L ([K] = 0–20 g/L)) and monitoring the change in OD600 after 24 h. All cultivations started with an initial OD600 value of 0.24. Cells grown in the absence of supplemented potassium reached an OD600 value of 2.1 ± 0.0. In comparison, OD600 increased to 2.3 ± 0.1 at [K] = 2.5, 5.0 or 10.0 g/L. However, cell growth was inhibited at [K] = 20.0 g/L, as OD600 reached only 0.9 ± 0.0.
Effect of SCG oil saponification on cell growth
C. necator DSM 545 was cultivated in shake flasks supplemented with either crude or saponified SCG oil as the sole carbon source (C0 = 2.9–23.0 g/L) to evaluate the effects of saponification on the organism’s ability to consume the substrate. All cultivations started with an initial OD600 value of 0.24. Figure 2 shows the values of OD600 obtained after 24 h. The effect of C0 on the OD600 values largely followed the same pattern for both substrates. For crude SCG oil, values of OD600 steadily rose with increasing C0, rising from 3.1 ± 0.2 (C0 = 2.9 g/L) to a peak of 7.4 ± 0.3 (C0 = 8.6 g/L). At the same concentrations, values of OD600 for cells grown on saponified SCG oil rose from 2.2 ± 0.2 to a higher peak of 7.9 ± 0.6. Further increases of C0 caused the resulting OD600 to decline for both substrates. Once the C0 reached 19.2 g/L, cell growth was entirely inhibited in the saponified SCG oil flasks, as no biomass could be detected. Similar results were reported by Bhatia et al. (2018) during their cultivations of C. necator Re2133 on SCG oil to synthesize poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) (P(3HB-co-3HHx)) copolymers. The authors observed biomass concentrations peaked at a C/N0 ratio of 20 g/g. In the present study, the peak OD600 values obtained at C0 = 8.6 g/L equated to an initial C/N0 ratio of 15.0 g/g.
Substrate inhibition growth kinetics
The relationship between μ and C0 was assessed by fitting the kinetic growth models (Table 1) to the results of the shake flask cultivations. The model parameters, estimated via non-linear regression, are shown in Table 3. All the models predicted the organism to have maximum specific growth rates, μmax, ranging between 0.140–0.145 and 0.147–0.152 when grown on the crude and saponified SCG oils, respectively. The Han-Levenspiel model had the closest fit to the experimental data for both substrates (Fig. 3), as indicated by the R2 and RMSE values. Additionally, the model correctly predicted that cell growth would be completely inhibited at C0 values higher than the critical substrate concentration (Cmax) of 19.1 g/L for the saponified oil. Hence, the Han-Levenspiel model is the most suitable to describe the specific growth rate of the organism under these fermentation conditions. According to the model, peak µ-values of 0.139 h−1 and 0.145 h−1 were reached with the crude and saponified SCG oil concentrations of 11.99 g/L (C0 = 9.20 g/L) and 17.40 g/L (C0 = 9.94 g/L), respectively. The mid-point of these two values (C0 = 9.57 g/L) was used in subsequent cultivations.
Comparison of µ (h.−1) values calculated experimentally (symbols) and from the Han-Levenspiel model (lines) for shake flask cultivations of C. necator DSM 545 grown on crude (black squares/line) and saponified (red circles/line) SCG oils for 24 h, at initial carbon concentrations, C0, of 2.9–23.0 g/L. Also shown are the corresponding initial carbon–nitrogen ratios, (C/N)0
Saponified SCG oil as a surfactant
A final set of shake flask cultivations was carried out in which crude and saponified SCG oils were mixed in incremental ratios to evaluate the resulting effects on cell growth; the results are shown in Table 4. By replacing 25% (w/w) of the initial carbon supplied from the crude SCG oil with the saponified substrate, the resulting OD600 value increased by 20.1%, from 7.8 ± 0.2 to 9.4 ± 0.1. Further substitution of the crude oil resulted in a slow decline of the OD600 values.
Bioreactor fermentations
C. necator DSM 545 was then cultivated in 3-L bioreactors (batch) for 72 h to evaluate if saponifying a portion (25% C, w/w) of the SCG oil helps to improve the overall fermentation process by emulsifying the remaining crude fraction to increase its bioavailability. Fermentations were also carried out with 100% crude SCG oil for comparison. Figure 4 shows the progression of substrate (N and DO) consumption and biomass (total and PHA) production.
Cells grown on only crude SCG oil experienced an initial lag phase (0–2 h) as they adapted to the new environmental conditions, synthesizing extracellular lipases to hydrolyze the acylglycerides present in the crude SCG oil (C0 = 9.57 g/L), forming free fatty acids and glycerol (Budde et al. 2011; Lu et al. 2013). The subsequent exponential growth phase (2–8 h) was characterized by a µ-value of 0.23 h−1. During the next unmeasured period (8–14 h), an essential nutrient for growth, such as P, K, or Mg, became depleted (neither N nor O were depleted as indicated by their measured values) as carbon remained in excess. In response, the cells adapted their metabolism to funnel the available carbon into P(3HB) production. Details of how C. necator shifts its metabolism from supporting cellular growth to PHA production has been described elsewhere in detail (Kessler and Witholt 2002; Ray and Kalia 2017; Ingram and Winterburn 2021). At this point, the total biomass concentration ([TB]) rapidly increased from 1.0 g/L (t = 8 h) to a peak value of 9.8 g/L at 28 h, plateauing thereafter. The corresponding concentration of P(3HB) at this peak was 7.0 g/L, equating to 71.4% (w/w) of the total biomass.
In the cultures employing the crude and saponified SCG oil mixture, the cells exhibited two distinct growth phases somewhat akin to diauxic growth, and a final PHA production period. Initially (0–8 h), cells consumed the saponified substrate ([C] = 2.39 g/L) for cellular growth without an observed initial lag phase, reaching a [TB] value of 1.2 g/L. The specific growth rate (µ) during this period was 0.35 h−1. This was followed by a lag phase (8–11 h, based on the O2 consumption profile) and a secondary growth phase (11–13 h) to reach a [TB] value of 2.4 g/L. After a second lag phase (13–22 h), the cells synthesized and accumulated 3HB monomers from the available carbon in the form of FFAs (22–72 h). The highest P(3HB) concentration of 6.8 g/L was reached at 52 h, corresponding to 84.2% (w/w) of the total biomass (8.0 g/L). Nitrogen was steadily consumed over the entire cellular growth phase, dropping from 0.59 to 0.13 g/L by the start of the PHA production phase and plateauing after that.
Polymer containing 3-hydroxyvalerate (3HV) was detected at the start in both cultivations and remained present throughout at concentrations in the range of 0.04–0.37 g/L. The precultures were developed from seed stocks stored in cryo-storage solutions containing 20% (v/v) glycerol which could be metabolized in such a way that led to the formation of 3HV-containing polymers (Ingram and Winterburn 2021, 2022). C. necator DSM 545 has been observed to produce 3HV distinctly after producing 3HB once the usable even-chained fatty acids in plant oils had been consumed (Ingram and Winterburn 2021). However, no such phase was observed in the present study (data not shown).
Table 5 compares the overall fermentation performances of both substrates with respect to the biomass (total and PHA) concentrations, yields, and volumetric productivities. The presented data is reflective of the time at which the fermentation productivity effectively stops. Cultures grown on the mixed substrates produced slightly more PHA (6.8 g/L) than those grown on just the SCG oil (6.6 g/L), but took over twice as long to do so; the volumetric productivities are reflective of that. The PHA yields are similar for both cultures.
Discussion
The relatively low cost and high abundance of waste plant oils make them attractive carbon substrates in PHA production. The immiscibility between plant oil substrates and aqueous fermentation media causes several issues, the chief among which is the induced limitation on substrate bioavailability. Surfactants can be used to form emulsified oil-in-water solutions to overcome this issue; this strategy has been successfully employed in highly reproducible bioreactor cultivations C. necator H16 to shorten the initial lag phase (Budde et al. 2011). However, surfactants are expensive and are thus unlikely to be used in the industrial production of PHAs where production costs remain high (Budde et al. 2011; Riedel et al. 2012; Surendran et al. 2020; Ingram and Winterburn 2021). The present study sought a similar solution, but via saponification of the oil instead. Optimizing the saponification reaction conditions (Fig. 1) is necessary to minimize the resources required in the additional processing step, which would otherwise add to the overall PHA production cost. Total saponification was achieved with relatively mild conditions (0.50 molKOH/L, 50 °C, 90 min), suggesting the saponification process could be further optimized. The saponification value of the SCG oil used in the current study was previously determined to be 175.7 ± 2.3 mgKOH goil−1 (Ingram and Winterburn 2022), meaning the lowest KOH concentration able to saponify the SCG oil under the proposed conditions entirely is 0.313 mol/L.
Using the selected saponification conditions, potassium comprised 12.92 ± 0.09% (w/w) of the saponified oil (Table 2). This figure is essential to consider when adding the substrate to the fermentation process, as cell growth is inhibited at [K] = 20.0 g/L. To introduce that much potassium would require the addition of 155 g/L of saponified SCG oil, which is substantially more than what was used in the present study (6.6–39.6 g/L). However, higher concentrations could easily be considered in the pursuit of greater PHA productivities, especially in fed-batch of continuous type fermentations. Sucrose, which has a C-fraction of 44.11% (w/w), has been previously used at a concentration of 900 g/L in a fed-batch fermentation of Alcaligenes latus DSM 1123 to produce the highest ever reported PHA volumetric productivity at 5.13 g/L h−1 (Wang and Lee 1997; Blunt et al. 2018). To supply the equivalent amount of carbon would require 683 g/L of saponified SCG oil, which would add 88.2 g/L of potassium. Thus, further improvements to the initial saponification step to lower the amount of potassium introduced would help to increase the maximum amount of substrate that could be supplied.
To improve the biopolymer yield and volumetric productivities from SCG oil, the present study experimentally evaluated the relationship between C0 and µ, and subsequently, nine kinetic growth models (Table 1) were curve-fitted to the results via non-linear regression analysis. Increasing the initial substrate concentration resulted in progressively higher cell densities for both crude and saponified SCG oils, but further increases began to inhibit cell growth (Fig. 2). Among the evaluated models, the Han-Levenspiel model resulted in the best curve fit to the experimental values (Fig. 3), with R2 and RMSE values of 0.885 and 0.003 for the crude SCG oil, and 0.947 and 0.004 for the saponified SCG oil, respectively (Table 3). This model extends the Monod model, accounting for inhibitory effects associated with cell, product, and substrate concentrations. The model also predicts cell growth will cease entirely at a critical inhibitory concentration of the substrate, Cmax, and describes the type of inhibition (competitive, non-competitive or uncompetitive) based on the values of the two constant parameters, n and m (Han and Levenspiel 1988; Dutta et al. 2015). The model predicted that Cmax would be 28.5 and 19.1 g/L (initial carbon concentration) for the crude and saponified oils, respectively. The latter was experimentally observed (Figs. 2 and 3). Based on the values of n and m (Table 3), the model suggests that the inhibition is uncompetitive (m > n > 0) for both substrates. Understanding the relationship between C0 and µ provides a rationale for designing suitable substrate feeding regimes. The subsequent shake flask cultivations—to determine the influence of mixing the crude and saponified SCG oils—were carried out at the optimal C0 value determined from the prior experimental modeling: C0 = 9.57 g/L.
Artificially increasing the FFA content of SCG oil—achieved by increasing the fraction of saponified oil in the mixtures—was confirmed in the present study to improve the bioavailability of the substrate (Table 4) up to a certain point, beyond which further substitutions of the crude SCG oil negatively influenced cell growth. The FFA content of plant oils has been shown to positively impact the growth and PHA production of C. necator as FFAs act to emulsify the remaining constituent triacylglycerides, making them more accessible to the action of lipases (Obruca et al. 2014). This influence would likely be more pronounced in other plant oils, which typically have much lower acid values than SCG oils (Al-Hamamre et al. 2012; Obruca et al. 2014; Ingram and Winterburn 2022). The option to effectively control the FFA content through altering the ratio of crude to saponified SCG oil is a powerful tool to allow the user to influence how the substrate ultimately behaves in the aqueous environment.
The findings of the shake flask experiments culminated in the final set of bioreactor cultivations. C. necator 545 was grown on a blend (75:25% C, w/w) of crude and saponified SCG oil (C0 = 9.57 g/L), benchmarked against 100% crude SCG oil at the same C0 (Fig. 4). The cells grew 1.52 times faster on the mixed substrates during the first exponential growth phase (µ = 0.35 h−1) than on just crude SCG oil (µ = 0.23 h−1). However, after 8 h, the saponified oil substrate was depleted, and the cells had to adapt to consuming the non-saponified lipids (triacylglycerides). This lag phase had the unfortunate effect of delaying the start of PHA production by approximately 12 h compared to the crude oil cultures. The ultimate effect of this is that cells grown on the mixed substrates took around twice as long (52 h) to produce a similar level of PHA (6.8 g/L) than those grown on just the crude SCG oil (6.6 g/L in 24 h). By comparison, these values remain significantly lower than authors who cultivated the organism’s wild-strain (C. necator H16) on SCG oil (13.1–49.4 g/L) (Cruz et al. 2014; Obruca et al. 2014).
Employing a feeding strategy whereby pulsed additions of saponified SCG oil are fed into the bioreactor at regular intervals could help to capitalize on the initial faster cell growth to improve the overall efficiency of PHA production from the mixed substrates. A fed-batch feeding strategy was previously demonstrated to greatly enhance P(3HB) production by C. necator H16 from 26.5 g/L (batch: 30 g/L SCG oil) to 49.4 g/L (fed-batch: 60 g/L SCG oil) in a shorter period (Obruca et al. 2014). As the organism begins to produce PHAs after an essential nutrient (e.g., N, P, K, O, and Mg) reaches a limiting concentration (Ray and Kalia 2017), the maximum PHA concentration is predetermined by the number of cells produced during the initial growth phase. So, the aim is to achieve a high-enough cell density in as little time as possible. The pulses should be frequent enough and of sufficient quantity to avoid total depletion of the saponified SCG oil, but also restricted as to prevent substrate-induced inhibition, as well as the potential foaming effects.
More so than other plant oils, SCG oil causes foaming when used as the sole carbon source for the biosynthesis of PHAs in a bioreactor (Obruca et al. 2014). This effect is greatly exasperated when the oil is entirely saponified, as observed in the present study when cultivations utilizing 100% saponified SCG oil was also attempted (data not shown). Foaming is a major issue because it can lead to a loss of cells, reduces the effective volume of the bioreactor, and increases the risk overflow and contamination (Li et al. 1995). Although foaming was suppressed in the present study through the addition of polypropylene glycol, the use of chemical antifoaming agents can interfere with the oil extraction process by artificially increasing the measured lipid concentration (Budde et al. 2011). While non-metabolisable antifoams, such as polyglycols and silicone oils, have little effect metabolism of the cells, they can cause a reduction in the oxygen transfer efficiency, as well as membrane fouling (Delvigne and Lecomte 2010). These issues could potentially be avoided through the use of other plant oils, such as rapeseed oil, as antifoaming agents (Obruca et al. 2014), or by mechanical means (e.g., foam breakers or foam centrifuges) (Budde et al. 2011; Riedel et al. 2012).
Overall, plant oil saponification presents an exciting opportunity for overcoming their immiscibility in aqueous cultures, which has long been a bottleneck in PHA biosynthesis. C. necator DSM 545 was successfully cultivated for the first time on saponified SCG oil, achieving a total biomass concentration of 8.0 g/L after 52 h, with a high PHA content of 84.2% (w/w). Further, the relationship between µ and C0 was accurately characterized by the Han-Levenspiel model, which provided insights into the optimal starting substrate concentration, as well as the potential inhibitory limits. Though the cells grew significantly (52%) faster on the mixed substrates during the first exponential growth phase (µ = 0.35 h−1) than on just crude SCG oil (µ = 0.23 h−1), additional work is required to capitalize on this for improved PHA volumetric productivity.
Data availability
Not applicable.
Code availability
The bespoke Python (ver. 3.8.0) program, which applied the SciPy “curve_fit” function (ver. 1.5.2) to estimate the parameters of the kinetic growth models, is available at https://github.com/ristojm/Biofit.
References
Aiba S, Shoda M, Nagatani M (1968) Kinetics of product inhibition in alcohol fermentation. Biotechnol Bioeng 10:845–864. https://doi.org/10.1002/bit.260100610
Al-Hamamre Z, Foerster S, Hartmann F, Kröger M, Kaltschmitt M (2012) Oil extracted from spent coffee grounds as a renewable source for fatty acid methyl ester manufacturing. Fuel 96:70–76. https://doi.org/10.1016/j.fuel.2012.01.023
Allen AD, Anderson WA, Ayorinde FO, Eribo BE (2010) Biosynthesis and characterization of copolymer poly(3HB-co-3HV) from saponified Jatropha curcas oil by Pseudomonas oleovorans. J Ind Microbiol Biotechnol 37:849–856. https://doi.org/10.1007/s10295-010-0732-7
Andrews JF (1968) A mathematical model for the continuous culture of microorganisms utilizing inhibitory substrates. Biotechnol Bioeng 10:707–723. https://doi.org/10.1002/bit.260100602
Aristizábal-Marulanda V, Chacón-Perez Y, Cardona Alzate CA (2017) The biorefinery concept for the industrial valorization of coffee processing by-products. In: Galanakis CM (ed) Handbook of Coffee Processing By-Products: Sustainable Applications. Academic Press, London, pp 63–92
Atabani AE, Al-Muhtaseb AH, Kumar G, Saratale GD, Aslam M, Khan HA, Said Z, Mahmoud E (2019) Valorization of spent coffee grounds into biofuels and value-added products: pathway towards integrated bio-refinery. Fuel 254:115640. https://doi.org/10.1016/j.fuel.2019.115640
Battista F, Zanzoni S, Strazzera G, Andreolli M, Bolzonella D (2020) The cascade biorefinery approach for the valorization of the spent coffee grounds. Renew Energy 157:1203–1211. https://doi.org/10.1016/j.renene.2020.05.113
Bhatia SK, Kim J-H, Kim M-S, Kim J, Hong JW, Hong YG, Kim H-J, Jeon J-M, Kim S-H, Ahn J, Lee H, Yang Y-H (2018) Production of (3-hydroxybutyrate-co-3-hydroxyhexanoate) copolymer from coffee waste oil using engineered Ralstonia eutropha. Bioprocess Biosyst Eng 41:229–235. https://doi.org/10.1007/s00449-017-1861-4
Blanch HW, Clark DS (1997) Microbial growth. Biochemical Engineering, 2nd edn. Marcel Dekker Inc., New York, pp 163–275
Blunt W, Levin DB, Cicek N (2018) Bioreactor operating strategies for improved Polyhydroxyalkanoate (PHA) Productivity. Polym 10:1197. https://doi.org/10.3390/polym10111197
Boonyawanich S, Tanikkul P, Thenchartanan P, Pisutpaisal N (2021) Productivity of Pseudomonas putida TISTR 1522 in polyhydroxyalkanoates (PHAs) production from saponified palm oil. Appl Biochem Biotechnol 193:1086–1098. https://doi.org/10.1007/S12010-020-03481-W/FIGURES/8
Budde CF, Riedel SL, Hübner F, Risch S, Popović MK, Rha C, Sinskey AJ (2011) Growth and polyhydroxybutyrate production by Ralstonia eutropha in emulsified plant oil medium. Appl Microbiol Biotechnol 89:1611–1619. https://doi.org/10.1007/s00253-011-3102-0
Bugnicourt E, Cinelli P, Lazzeri A, Alvarez V (2014) Polyhydroxyalkanoate (PHA): review of synthesis, characteristics, processing and potential applications in packaging. Express Polym Lett 8:791–808
Bustamante D, Tortajada M, Ramon D, Rojas A (2019) Camelina oil as a promising substrate for mcl-PHA production in Pseudomonas sp. cultures. Appl Food Biotechnol 6:61–70. https://doi.org/10.22037/afb.v6i1.21635
Caetano NS, Mata TM, Martins AA, Felgueiras MC (2017) New trends in energy production and utilization. Energy Procedia 107:7–14. https://doi.org/10.1016/j.egypro.2016.12.122
Cruz M v. (2015) Production of polyhydroxyalkanoates from oil-containing substrates. Doctoral dissertation, Universidade Nova de Lisboa, Portugal
Cruz MV, Paiva A, Lisboa P, Freitas F, Alves VD, Simões P, Barreiros S, Reis MAM (2014) Production of polyhydroxyalkanoates from spent coffee grounds oil obtained by supercritical fluid extraction technology. Bioresour Technol 157:360–363. https://doi.org/10.1016/j.biortech.2014.02.013
Delvigne F, Lecomte J (2010) Foam formation and control in bioreactors. In: Flickinger MC (ed) Encyclopedia of Industrial Biotechnology. Wiley Inc, Hoboken, NJ, USA, pp 1–13
Dutta K, Dasu VV, Mahanty B, Prabhu AA (2015) Substrate inhibition growth kinetics for cutinase producing pseudomonas cepacia using tomato-peel extracted cutin. Chem Biochem Eng Q 29:437–445. https://doi.org/10.15255/CABEQ.2014.2022
Ferre-Guell A, Winterburn J (2019) Increased production of polyhydroxyalkanoates with controllable composition and consistent material properties by fed-batch fermentation. Biochem Eng J 141:35–42. https://doi.org/10.1016/j.bej.2018.10.004
Girotto F, Pivato A, Cossu R, Nkeng GE, Lavagnolo MC (2018) The broad spectrum of possibilities for spent coffee grounds valorisation. J Mater Cycles Waste Manag 20:695–701. https://doi.org/10.1007/s10163-017-0621-5
Gómez-de la Cruz FJ, Cruz-Peragón F, Casanova-Peláez PJ, Palomar-Carnicero JM (2015) A vital stage in the large-scale production of biofuels from spent coffee grounds: the drying kinetics. Fuel Process Technol 130:188–196. https://doi.org/10.1016/j.fuproc.2014.10.012
Haldane JBS (1965) Enzymes. MIT Press, Cambridge, Massachusetts, USA
Han K, Levenspiel O (1988) Extended monod kinetics for substrate, product, and cell inhibition. Biotechnol Bioeng 32:430–447. https://doi.org/10.1002/bit.260320404
Ingram HR, Winterburn JB (2021) Anabolism of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) by Cupriavidus necator DSM 545 from spent coffee grounds oil. N Biotechnol 60:12–19. https://doi.org/10.1016/j.nbt.2020.08.001
Ingram HR, Winterburn JB (2022) Influence of emulsified plant oil composition on growth and biopolymer production of Cupriavidus necator DSM 545. Food Bioprod Process 132:23–34. https://doi.org/10.1016/j.fbp.2021.12.005
International Coffee Organisation (2020) Historical data on the global coffee trade: total production by all exporting countries (1990–2019). http://www.ico.org/historical/1990onwards/PDF/1a-total-production.pdf. Accessed 4 May 2020
ISO (2013) ISO 3657:2013 (E). Animal and vegetable fats and oils - determination of saponification value. Geneva, Switzerland
Karmee SK (2018) A spent coffee grounds based biorefinery for the production of biofuels, biopolymers, antioxidants and biocomposites. Waste Manag 72:240–254. https://doi.org/10.1016/j.wasman.2017.10.042
Kessler B, Witholt B (2002) Poly(3-Hydroxyalkanoates). In: Encyclopedia of Bioprocess Technology. Wiley Inc, Hoboken, NJ, USA
Kourmentza C, Economou ChN, Tsafrakidou P, Kornaros M (2018) Spent coffee grounds make much more than waste: exploring recent advances and future exploitation strategies for the valorization of an emerging food waste stream. J Clean Prod 172:980–992. https://doi.org/10.1016/j.jclepro.2017.10.088
Kovalcik A, Obruca S, Marova I (2018) Valorization of spent coffee grounds: a review. Food Bioprod Process 110:104–119. https://doi.org/10.1016/j.fbp.2018.05.002
Li GQ, Shin JH, Lee JM (1995) Mineral oil addition as a means of foam control for plant cell cultures in stirred tank fermenters. Biotechnol Tech 9:713–718. https://doi.org/10.1007/BF00159235
Lu J, Brigham CJ, Rha C, Sinskey AJ (2013) Characterization of an extracellular lipase and its chaperone from Ralstonia eutropha H16. Appl Microbiol Biotechnol 97:2443–2454. https://doi.org/10.1007/s00253-012-4115-z
Luong JHT (1987) Generalization of monod kinetics for analysis of growth data with substrate inhibition. Biotechnol Bioeng 29:242–248. https://doi.org/10.1002/bit.260290215
Machado EMS, Rodriguez-Jasso RM, Teixeira JA, Mussatto SI (2012) Growth of fungal strains on coffee industry residues with removal of polyphenolic compounds. Biochem Eng J 60:87–90. https://doi.org/10.1016/j.bej.2011.10.007
Massaya J, Prates Pereira A, Mills-Lamptey B, Benjamin J, Chuck CJ (2019) Conceptualization of a spent coffee grounds biorefinery: a review of existing valorisation approaches. Food Bioprod Process 118:149–166. https://doi.org/10.1016/j.fbp.2019.08.010
Mata TM, Martins AA, Caetano NS (2018) Bio-refinery approach for spent coffee grounds valorization. Bioresour Technol 247:1077–1084. https://doi.org/10.1016/j.biortech.2017.09.106
McNutt J, He QS (2019) Spent coffee grounds: a review on current utilization. J Ind Eng Chem 71:78–88. https://doi.org/10.1016/j.jiec.2018.11.054
Monod J (1949) The growth of bacterial cultures. Annu Rev Microbiol 3:371–394. https://doi.org/10.1146/annurev.mi.03.100149.002103
Moser H (1958) The dynamics of bacterial populations maintained in the chemostat. Carnegie Institution of Washington, Washington D.C., USA
Mozejko J, Ciesielski S (2013) Saponified waste palm oil as an attractive renewable resource for mcl-polyhydroxyalkanoate synthesis. J Biosci Bioeng 116:485–492. https://doi.org/10.1016/j.jbiosc.2013.04.014
Muloiwa M, Nyende-Byakika S, Dinka M (2020) Comparison of unstructured kinetic bacterial growth models. S Afr J Chem Eng 33:141–150. https://doi.org/10.1016/j.sajce.2020.07.006
Murthy PS, Naidu MM (2012) Sustainable management of coffee industry by-products and value addition—a review. Resour Conserv Recycl 66:45–58. https://doi.org/10.1016/j.resconrec.2012.06.005
Mussatto SI, Machado EMS, Martins S, Teixeira JA (2011) Production, composition, and application of coffee and its industrial residues. Food Bioproc Tech 4:661. https://doi.org/10.1007/s11947-011-0565-z
Nazari M, Kurdi M, Heerklotz H (2012) Classifying surfactants with respect to their effect on lipid membrane order. Biophys J 102:498–506. https://doi.org/10.1016/j.bpj.2011.12.029
Obruca S, Petrik S, Benesova P, Svoboda Z, Eremka L, Marova I (2014) Utilization of oil extracted from spent coffee grounds for sustainable production of polyhydroxyalkanoates. Appl Microbiol Biotechnol 98:5883–5890. https://doi.org/10.1007/s00253-014-5653-3
Obruca S, Benesova P, Kucera D, Petrik S, Marova I (2015) Biotechnological conversion of spent coffee grounds into polyhydroxyalkanoates and carotenoids. N Biotechnol 32:569–574. https://doi.org/10.1016/j.nbt.2015.02.008
Obruca S, Sedlacek P, Koller M, Kucera D, Pernicova I (2018) Involvement of polyhydroxyalkanoates in stress resistance of microbial cells: biotechnological consequences and applications. Biotechnol Adv 36:856–870
Panikov NS (2010) Kinetics, microbial growth. In: Flickinger MC (ed) Encyclopedia of Industrial Biotechnology: Bioprocess, Bioseparation, and Cell Technology. Wiley Inc, Hoboken, NJ, USA, pp 1513–1543
Rajesh Banu J, Kavitha S, Yukesh Kannah R, Dinesh Kumar M, Preethi AAE, Kumar G (2020) Biorefinery of spent coffee grounds waste: viable pathway towards circular bioeconomy. Bioresour Technol 302:122821. https://doi.org/10.1016/j.biortech.2020.122821
Ray S, Kalia VC (2017) Polyhydroxyalkanoate production and degradation patterns in Bacillus species. Indian J Microbiol 57:387–392. https://doi.org/10.1007/s12088-017-0676-y
Riedel SL, Bader J, Brigham CJ, Budde CF, Yusof ZAM, Rha C, Sinskey AJ (2012) Production of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) by Ralstonia eutropha in high cell density palm oil fermentations. Biotechnol Bioeng 109:74–83. https://doi.org/10.1002/bit.23283
Rustan AC, Drevon CA (2005) Fatty acids: structures and properties. Encyclopedia of Life Sciences. Wiley Ltd, Hoboken, NJ, pp 1–7
Salazar-López NJ, López-Rodríguez CV, Hernández-Montoya DA, Campos-Vega R (2020) Health benefits of spent coffee grounds. In: Campos-Vega R, Oomah BD, Vergara-Castaneda HA (eds) Food Wastes and By-products: Nutraceutical and Health Potential, 1st edn. Wiley Ltd, Chichester, UK, 327–371
Surendran A, Lakshmanan M, Chee JY, Sulaiman AM, van Thuoc D, Sudesh K (2020) Can polyhydroxyalkanoates be produced efficiently from waste plant and animal oils? Front Bioeng Biotechnol 8:169. https://doi.org/10.3389/fbioe.2020.00169
Tan IKP, Sudesh Kumar K, Theanmalar M, Gan SN, Gordon B (1997) Saponified palm kernel oil and its major free fatty acids as carbon substrates for the production of polyhydroxyalkanoates in Pseudomonas putida PGA1. Appl Microbiol Biotechnol 47:207–211. https://doi.org/10.1007/s002530050914
Urbina L, Wongsirichot P, Corcuera MÁ, Gabilondo N, Eceiza A, Winterburn J, Retegi A (2018) Application of cider by-products for medium chain length polyhydroxyalkanoate production by Pseudomonas putida KT2440. Eur Polym J 108:1–9. https://doi.org/10.1016/j.eurpolymj.2018.08.020
Wang F, Lee SY (1997) Poly(3-Hydroxybutyrate) Production with high productivity and high polymer content by a Fed-Batch Culture of Alcaligenes latus under Nitrogen Limitation. Appl Environ Microbiol 63:3703–3706. https://doi.org/10.1128/aem.63.9.3703-3706.1997
Webb J (1963) Enzyme and metabolic inhibitors. Academic Press, New York, USA
Wongsirichot P (2020) Rapeseed meal pretreatment for improved biopolymer production. Doctoral dissertation, The University of Manchester, UK
Yano T, Nakahara T, Kamiyama S, Yamada K (1966) Kinetic studies on microbial activities in concentrated solutions. Agric Biol Chem 30:42–48. https://doi.org/10.1080/00021369.1966.10858549
Zabaniotou A, Kamaterou P (2019) Food Waste valorization advocating circular bioeconomy - a critical review of potentialities and perspectives of Spent Coffee Grounds Biorefinery. J Clean Prod 211:1553–1566. https://doi.org/10.1016/j.jclepro.2018.11.230
Acknowledgements
The authors would like to kindly thank Martin Jennings and Carole Webb from the Department of Chemistry at the University of Manchester for their analytical help.
Funding
This work was funded by the Engineering and Physical Sciences Research Council (EPSRC) (EP/N509565/1).
Author information
Authors and Affiliations
Contributions
HRI conceived and designed the research. HRI developed the methodology and conducted the experiments. RJM developed the bespoke Python software program. HRI analyzed the data. HI wrote the manuscript. HRI developed the data visualizations. All authors read and approved the manuscript. Project funding and resources were acquired by JBW. Project administration was performed by HRI and JBW. Oversight and leadership were provided by JBW.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
Not applicable.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ingram, H.R., Martin, R.J. & Winterburn, J.B. Optimized cell growth and poly(3-hydroxybutyrate) synthesis from saponified spent coffee grounds oil. Appl Microbiol Biotechnol 106, 6033–6045 (2022). https://doi.org/10.1007/s00253-022-12093-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-022-12093-9