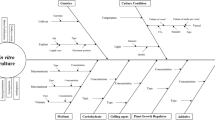
Abstract
Rauvolfia spp., also known as devil peppers, are a group of evergreen shrubs and trees. Among the ~ 76 various species, Rauvolfia serpentina is the most important one as it finds its use as an important medicinal plant. It is commonly known as the Indian snakeroot plant or Sarpagandha. The plant is rich in multiple secondary metabolites. Some of the well-known secondary metabolites are reserpine, ajmaline, ajmalicine, serpentine, yohimbine, etc. Alkaloids are also found in all parts of the plant but the richest sources are the roots. Since ancient times, roots (mainly due to reserpine) have been utilized in various Ayurvedic and Unani medicinal preparations for the treatment of diseases like hypertension, anxiety, insomnia and schizophrenia. Apart from this, there are many other pharmacological and ethnobotanical uses of this plant. There are a number of published reports regarding tissue culture techniques on Rauvolfia spp. The current review mainly illustrates and discusses the various in vitro biotechnological aspects such as direct regeneration, indirect regeneration via callus formation, somatic embryogenesis, synthetic seed production, hairy root culture, polyploidy induction and secondary metabolite estimation, which provides significant ideas regarding the ongoing research activities and future prospects related to the genetic improvement of this genus.



Similar content being viewed by others
References
Ahmad S, Amin MN, Azad MAK, Mosaddik MA (2002) Micropropagation and plant regeneration of Rauvolfia serpentina by tissue culture technique. Pak J Biol Sci 5:75–79
Ahmad N, Alatar AA, Faisal M, Khan MI, Fatima N, Anis M, Hagezy AK (2015) Effect of copper and zinc on the in vitro regeneration of Rauvolfia serpentina. Biol Plant 59:11–17
Akram M, Ilahi I (1986) Plantlet formation in root callus of Rauwolfia serpentina. Pak J Bot 18:15–19
Alatar AA (2015) Thidiazuron induced efficient in vitro multiplication and ex vitro conservation of Rauvolfia serpentina a potent antihypertensive drug producing plant. Biotechnol Biotechnol Equip 29:489–497
Alatar AA, Faisal M, Hegazy AK, Hend AA (2012) High frequency shoot regeneration and plant establishment of Rauvolfia serpentina: An endangered medicinal plant. J Med Plant Res 6:3324–3329
Ambasta SP, Ramchandran K, Kashyapa K, Chand R (1992) The useful plants of India. Council of Science and Industrial Research (CSIR), New Delhi
Aryal S, Joshi SD (2009) Callus induction and plant regeneration in Rauvolfia Serpentina (L.) Benth Ex. Kurz. J Nat Hist Mus 24:82–88
Bahuguna RN, Joshi R, Singh G, Shukla A, Gupta R, Bains G (2011) Micropropagation and total alkaloid extraction of Indian snake root (Rauwolfia serpentina). Indian J Agric Sci 81:1124–1129
Baksha R, Jahan MAA, Khatun R, Munshi JL (2007) In vitro rapid clonal propagation of Rauvolfia serpentina (Linn.) Benth. Bangladesh J Sci Ind Res 42:37–44
Benjamin BD, Roja G, Heble MR (1993) Agrobacterium rhizogenes mediated transformation of Rauvolfia serpentina: regeneration and alkaloid synthesis. Plant Cell Tissue Organ Cult 35:253–257
Bhadra SK, Bhowmik TK, Singh P (2008) In vitro micropropagation of Rauvolfia serpentina (L.) Benth through induction of direct and indirect organogenesis. Chittagong Univ J Biol Sci 3:1–9
Bhatt R, Arif M, Gaur AK, Rao PB (2008) Rauwolfia serpentina: protocol optimization for in vitro propagation. Afr J Biotechnol 7:4265–4268
Chitke SS, Dhakolkar P, Chikhale NJ, Burghate SK (2013) In vitro evaluation and comparison of differentiated roots for alkaloids of Rauwolfia serpentina. Scholarly J Agric Sci 3:573–575
Faisal M, Ahmed N, Anis M (2005) Shoot multiplication in Rauvolfia tetraphylla L. using thidiazuron. Plant Cell Tissue Organ Cult 80:187–190
Faisal M, Alatar AA, Ahmed N, Anis M, Hegazy AK (2012a) Assessment of genetic fidelity in Rauvolfia serpentina plantlets grown from synthetic (encapsulated) seeds following in vitro storage at 4 °C. Molecules 17:5050–5061
Faisal M, Alatar AA, Ahmed N, Anis M, Hegazy AK (2012b) An efficient and reproducible method for in vitro clonal multiplication of Rauvolfia tetraphylla L. and evaluation of genetic stability using DNA-based markers. Appl Biochem Biotechnol 168:1739–1752
Faisal M, Alatar AA, Hegazy AK (2013) Molecular and biochemical characterization in Rauvolfia tetraphylla plantlets grown from synthetic seeds following in vitro cold storage. Appl Biochem Biotechnol 169:408–417
Farooqi AA, Sreeramu BS (2001) Cultivation of medicinal and aromatic crops. Crop Science Reference. Universities Press, Banglore, India
Gajbhiye VR, Dhonukshe BL, Jambhale ND (2006) In vitro shoot regeneration from leaf derived callus of the Indian snakeroot (Rauwolfia serpentina (L.) Benth ex. Kurz). Indian J Genet Plant Breed 66:165–166
Gamborg OL, Miller RA, Ojima K (1968) Nutrient requirements of suspension cultures of soyabean root cells. Exp Cell Res 50:151–158
Gantait S, El-Dawayati MM, Panigrahi J, Labrooy C, Verma SK (2018) The retrospect and prospect of the applications of biotechnology in Phoenix dactylifera L.. Appl Microbiol Biotechnol 102:8229-8259
Gantait S, Kundu S (2017) Does synthetic seed storage at higher temperature reduce reserpine content of Rauvolfia serpentina (L.) Benth. Ex Kurz.? Rend Lincei Sci Fis Nat 28:679–686
Gantait S, Kundu S, Ali N, Sahu NC (2015) Synthetic seed production of medicinal plants: a review on influence of explants, encapsulation agent and matrix. Acta Physiol Plant 37:98
Gantait SS, Dutta K, Majumdar J (2017a) In vitro regeneration and conservation of endangered medicinal plant sarpagandha (Rauvolfia serpentina). J Hortic Sci 12:71–77
Gantait S, Kundu S, Yeasmin L, Ali MN (2017b) Impact of differential levels of sodium alginate, calcium chloride and basal media on germination frequency of genetically true artificial seeds of Rauvolfia serpentina (L.) Benth. Ex Kurz. J Appl Res Med Arom Plant 4:75–81
George S, Geetha SP, Balachandran I (2012) In vitro multiplication of Rauvolfia serpentina (L.) Benth. ex Kurz - An endangered medicinal plant. Med Plant 4:45–48
Goel MK, Mehrotra S, Kukreja AK, Shanker K, Khanuja SPS (2009) In vitro propagation of Rauwolfia serpentina using liquid medium, assessment of genetic fidelity of micropropagated plants, and simultaneous quantitation of reserpine, ajmaline, and ajmalicine. In: Jain SM, Saxena PK (eds) Protocols for in vitro cultures and secondary metabolite analysis of aromatic and medicinal plants. Methods in Molecular Biology (Methods and Protocols), vol 547. Humana Press, Totowa, pp 17–33
Goel MK, Goel S, Banerjee S, Shanker K, Kukreja AK (2010) Agrobacterium rhizogenes mediated transformed roots of Rauwolfia serpentina for reserpenr biosynthesis. Med Arom Plant Sci Biotechnol 4:8–14
Harisaranraj R, Suresh K, Babu SS (2009) Production of reserpine in somatic embryos of Rauwolfia serpentina cultured in bioreactors by the induction of elicitor (Methyl Jasmonate). Glob J Biotechnol Biochem 4:143–147
Hoagland DR, Arnon DI (1950) The water-culture method for growing plants without soil. Circ Calif Agric Exp Stn 347:1–32
Ilahi I, Rahim F, Jabeen M (2007) Enhanced clonal propagation and alkaloid biosynthesis in cultures of Rauwolfia. Pak J Plant Sci 13:45–56
Jain V, Singh D, Saraf S, Saraf S (2003) In-vitro micropropagation of Rauwolfia serpentina through multiple shoot generation. Anc Sci Life 23:44–49
Kataria V, Shekhawat NS (2005) Cloning of Rauvolfia serpentina – An Endangered Medicinal Plant. J Sustain For 20:53–65
Kaul KN (1956) Observations on the natural occurrence and cultural methods of Rauwolfia serpentina Benth. Indian J Pharm 18:127–131
Kaur S (2018) In vitro callus induction in inflorescence segments of medicinally important endangered plant Rauwolfia serpentina (L.) Benth. ex Kurz – a step towards ex situconservation. Ann Plant Sci 7:1988–1991
Khan S, Banu TA, Akhter S, Goswami B, Islam M, Hani U, Habib A (2018) In vitro regeneration protocol of Rauvolfia serpentina L. Bangladesh J Sci Ind Res 53:133–138
Kirillova NV, Smirnova MG, Komov VP (2001) Sequential isolation of superoxide dismutase and ajmaline from tissue culture of Rauwolfia serpentina Benth. Appl Biochem Microbiol 37:160–163
Kirtikar KR, Basu BD (1993) Indian Medicinal Plants, 2nd edn. Dehra Dun Publishers, Calcutta, p 289
Linsmaier EM, Skoog F (1965) Organic growth Factor Requirements of Tobacco Tissue Cultures. Physiol Plant 18:100–127
Lloyd G, McCown B (1981) Commercially feasible micropropagation of mountain laurel, Kalmia latifolia, by shoot tip culture. Int Plant Prop Soc Proc 30:421–427
Mallick SR, Jena RC, Samal KC (2012) Rapid in vitro multiplication of an endangered medicinal plant sarpgandha (Rauwolfia serpentina). Am J Plant Sci 3:437–442
Mallick SR, Samal P, Jena RC, Samal KC (2013) Alkaloid profiling of conventionally propagated and in vitro raised plants of Indian snake plant (Rauwolfia serpentina L.). Asian J Chem 25:6584–6586
Manandhar NP (2002) Plants and People of Nepal. Timber Press, Portland
Mathur A, Mathur AK, Kukreja AK, Ahuja PS, Tyagi BR (1987) Establishment and multiplication of colchi-tetraploids of Rauvolfia serpentina L. Benth. ex Kurz. through tissue culture. Plant Cell Tissue Organ Cult 10:129–134
Mishra Y, Usmani G, Mandal AK (2010) In vitro cloning of Rauvolfia serpentina (L.) Benth. var. CIM-Sheel and evaluation of its field performance. J Biol Res-Thessaloniki 13:85–92
Mitra GC (1976) Studies on the formation of viable and nonviable seeds in R. serpentine. Indian J Exp Biol 14:54–54
Mondal S, Texeirada da Silva JA, Ghosh PD (2011) In vitro flowering in Rauvolfia serpentina (L.) Benth. ex. Kurz. Int J Plant Dev Biol 5:75–77
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–495
Nandhini VS, Bai GV (2014) Secondary metabolites screening from in-vitro cultured Rauvolfia tetraphylla by HPTLC-MS: A special emphasises on their antimicrobial applications. Int J Adv Pharm Anal 4:81–87
Nurcahyani N, Solichatun, Anggarwulan E (2008) The reserpine production and callus growth of Indian snake root (Rauvolfia serpentina (L.) Benth. Ex Kurz) Culture by addition of Cu2+. Biodiversitas 9:177–179
Pan S, Neeraj A, Srivastava KS, Kishore P, Sarethy IP (2013) Effects of growth regulators on in vitro response and multiple shoot induction in some endangered medicinal plants. OA Biotechnol 2:3
Pandey VP, Kudakasseril J, Cherian E, Patani G (2007) Comparison of two methods for in vitro propagation of Rauwolfia serpentina from nodal explants. Indian Drugs 44:514–519
Pandey VP, Cherian E, Patani G (2010) Effect of growth regulators and culture conditions on direct root induction of Rauwolfia serpentina L. (Apocynaceae) Benth by leaf explants. Trop J Pharm Res 9:27–34
Pandey P, Kaur R, Singh S, Chattopadhyay SK, Srivastava SK, Banerjee S (2014) Long-term stability in biomass and production of terpene indole alkaloids by hairy root culture of Rauvolfia serpentina and cost approximation to endorse commercial realism. Biotechnol Lett 36:1523–1528
Pant KK, Joshi SD (2008) Rapid multiplication of Rauvolfia serpentina Benth. ex. Kurz through tissue culture. Sci World J 6:58–62
Pant KK, Joshi SD (2018) In-vitro somatic embryogenesis and organogenesis from the node induced calli of Rauvolfia serpentina Benth. ex Kurz. Nep J Agric Sci 17:167–173
Panwar GS, Guru SK (2015) Stimulation of reserpine production in the whole plant culture of Rauwolfia serpentina L. by elicitors and precursor feeding. J Plant Biochem Biotechnol 24:49–55
Panwar GS, Attitalla IH, Guru SK (2011) An efficient in vitro clonal propagation and estimation of reserpine content in different plant parts of Rauwolfia serpentina L. Am-Eur J Sci Res 6:217–222
Rajasekharan PE, Ambika SR, Ganeshan S (2007) In vitro regeneration and slow growth studies on Rauvolfia serpentina. IUP J Biotechnol 1:63–67
Rajbhandari KR (2001) Ethnobotany of Nepal. Ethnobotanical Society of Nepal (ESON), Kathmandu, p 189
Rama Subbu R, Sreekala AK, Kulloli SK (2008) Floral biology of Rauwolfia micrantha Hook. f.: a rare and endemic medicinal plant of Western Ghats, India. J Sci Trans Environ Technol 1:174–177
Rana SK, Sehrawat AR, Chowdhury VR (2015) Assessment of clonal fidelity in micropropagated plantlets of Rauwolfia serpentina Benth. Ex. Kurz. Med Plant 7:258–263
Rani A, Kumar M, Kumar S (2013) Rapid in vitro propagation of Serpegandha (Rauvolfia serpentina Linn.) Benth through young leaves. Adv Appl Res 5:131–134
Rani A, Kumar M, Kumar S (2014a) Effect of growth regulators on micropropagation of Rauvolfia serpentina (L.) Benth. J Appl Nat Sci 6:507–511
Rani A, Kumar M, Kumar S (2014b) Micropropagation of serpgandha (Rauvolfia serpentina L. Benth). An endangered medicinal plant. Appl Biol Res 16:114–118
Rashmi R, Trivedi MP (2016) Rapid in-vitro regeneration of an endangered medicinal plant sarpagandha (Rauvolfia serpentina L.). Eur J Pharm Med Res 3:276–284
Ray S, Majumdar A, Bandyopadhyay M, Jha S (2014a) Genetic transformation of sarpagandha (Rauvolfia serpentina) with Agrobacterium rhizogenes for identification of high alkaloid yielding lines. Acta Physiol Plant 36:1599–1605
Ray S, Samanta T, Majumdar A, Bandyopadhyay M, Jha S (2014b) Cytogenetic characterization of Agrobacterium rhizogenes transformed root lines of Rauvolfia serpentina. Nucleus 57:105–112
Reddy GS, Kumar DD, Srihari BR, Madhavi M (2006) Callus culture and synthetic seed production of Rauvolfia serpentina. Ind J Hort 62:102–103
Rohela GK, Bylla P, Kota S, Abbagani S, Chithakari R, Reuben TC (2013) In vitro plantlet regeneration from leaf and stem calluses of Rauwolfia tetraphylla (R. canescens) and confirmation of genetic fidelity of plantlets using the ISSR-PCR method. J Herb Spice Med Plant 19:66–75
Roja G, Heble MR (1996) Indole alkaloids in clonal propagules of Rauwolfia serpentina Benthe. ex Kurz. Plant Cell Tissue Organ Cult 44:111–115
Roja PC, Sipahimalani AT, Heble MR, Chadha MS (1987) Multiple shoot cultures of Rauvolfia serpentina: growth and alkaloid production. J Nat Prod 50:872–887
Salma U, Rahman MSM, Islam S, Haque N, Jubair TA, Haque AKMF, Mukti IJ (2008) The influence of different hormone concentration and combination on callus induction and regeneration of Rauwolfia serpentina L. Benth. Pak J Biol Sci 11:1638–1641
Santapau H (1956) Botanical aspect of Rauvolfia serpentina Benth. Indian J Pharm 18:117–126
Saravanan S, Sarvesan R, Vinod MS (2011) Identification of DNA elements involved in somaclonal variants of Rauvolfia serpentina (L.) arising from indirectorganogenesis as evaluated by ISSR analysis. Indian J Sci Technol 4:1241–1245
Sarker KP, Islam A, Islam R, Hoque A, Joarder OI (1996) In vitro propagation of Rauvolfia serpentina through tissue culture. Planta Med 62:358–359
Senapati SK, Lahere N, Tiwary BN (2014) Improved in vitro clonal propagation of Rauwolfia serpentina L. Benth – An endangered medicinal plant. Plant Biosyst 148:885–888
Sharma R (2004) Agro-Techniques of Medicinal Plants. Daya publishing house, Delhi, p 264
Shrestha UB, Shrestha S (2004) (2061 BS). Major Non-Timber Forest Products of Nepal. Bhundipuran Parkashan, Kathmandu, p 411
Siddiqui SS (1939) A note on the alkaloids Rauwolfia serpentina, Benth. J Indian Chem Soc 16:421–425
Singh P, Singh A, Shukla AK, Singh L, Pande V, Nailwal TK (2009) Somatic embryogenesis and in vitro regeneration of an endangered medicinal plant sarpgandha (Rauvolfia serpentina L.). Life Sci J 6:57–62
Sudha CG, Seeni S (2006) Spontaneous somatic embryogenesis on in vitro root segment cultures of Rauvolfia micrantha hook. f. – a rare medicinal plant. In Vitro Cell Dev Biol Plant 42:119–123
Sudha CG, Reddy BO, Ravishankar GA, Seeni S (2003) Production of ajmalicine and ajmaline in hairy root cultures of Rauvolfia micrantha Hook f., a rare and endemic medicinal plant. Biotechnol Lett 25:631–636
Sulochana CB (1959) Indian species of Rauvolfia. J Indian Bot Soc 38:575–594
Sumy O, Ved DK, Krishnan R (2000) Tropical medicinal plants - propagation methods. Foundation for Revitalization of Local Health Traditions, Banglore, India
Susila T, Reddy GS, Jyothsna D (2013) Standardization of protocol for in vitro propagation of an endangered medicinal plant Rauwolfia serpentina Benth. J Med Plant Res 7:2150–2153
Susila T, Reddy GS, Jyothsna D (2014) Standardization of protocol for in vitro propagation of Rauwolfia serpentina Benth. Progress Hortic 46:285–287
Uikey DS, Tripathi MK, Tiwari G, Patel RP, Ahuja A (2016) Embryogenic cell suspension culture induction and plantlet regeneration of Rauvolfia serpentina (L.) Benth.: influence of different plant growth regulator concentrations and combinations. Med Plant 8:153–162
White PR (1963) The cultivation of animal and plant cells, 2nd. edn. Ronald Press Co., New York, pp 228–229
Williams K, Kubelik AR, Rafalski JA, Tingey SV (1990) DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Res 18:1631–1635
Yahya AF, Hyun JO, Lee JH, Jung MS (2007) Effect of explants types, auxin concentration, and light condition on in vitro root production and alkaloid content of Rauvolfia serpentina (L.) Benth. ex. Kurz. J Korean For Soc 96:178–182
Yamamoto O, Yamada Y (1986) Production of Reserpine and its optimization in cultured Rauwolfia serpentina Benth. cells. Plant Cell Rep 5:50–53
Zafar N, Mujib A, Ali M, Tonk D, Gulzar B (2017) Aluminium chloride elicitation (amendment) improves callus biomass growth and reserpine yield in Rauvolfia serpentina leaf callus. Plant Cell Tissue Organ Cult 130:357–368
Zafar N, Mujib A, Ali M, Tonk D, Gulzar B, Malik M, Sayeed R, Mamgain J (2019) Genome size analysis of field grown and tissue culture regenerated Rauvolfia serpentina (L) by flow cytometry: Histology and scanning electron microscopic study for in vitro morphogenesis. Ind Crop Prod 128:545–555
Zietkiewics E, Rafalski A, Labuda D (1994) Genome fingerprinting by simple squence repeat (SSR) - anchored polymerase chain reaction amplification. Genomics 20:176–183
Acknowledgements
The authors acknowledge the e-library assistance from Bidhan Chandra Krishi Viswavidyalaya (BCKV), West Bengal, India and experimental assistance from Plant Tissue Culture laboratory at Regional Nuclear Agricultural Research Centre (RNARC), BCKV. We are further thankful to the anonymous reviewer(s) and the editor of this article for their critical comments and suggestions on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mukherjee, E., Gantait, S., Kundu, S. et al. Biotechnological interventions on the genus Rauvolfia: recent trends and imminent prospects. Appl Microbiol Biotechnol 103, 7325–7354 (2019). https://doi.org/10.1007/s00253-019-10035-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-019-10035-6